1. Introduction
Barium slag is a byproduct from the production of barium carbonate from barite, and its toxicity mainly originates from soluble barium ions, making it a hazardous waste [
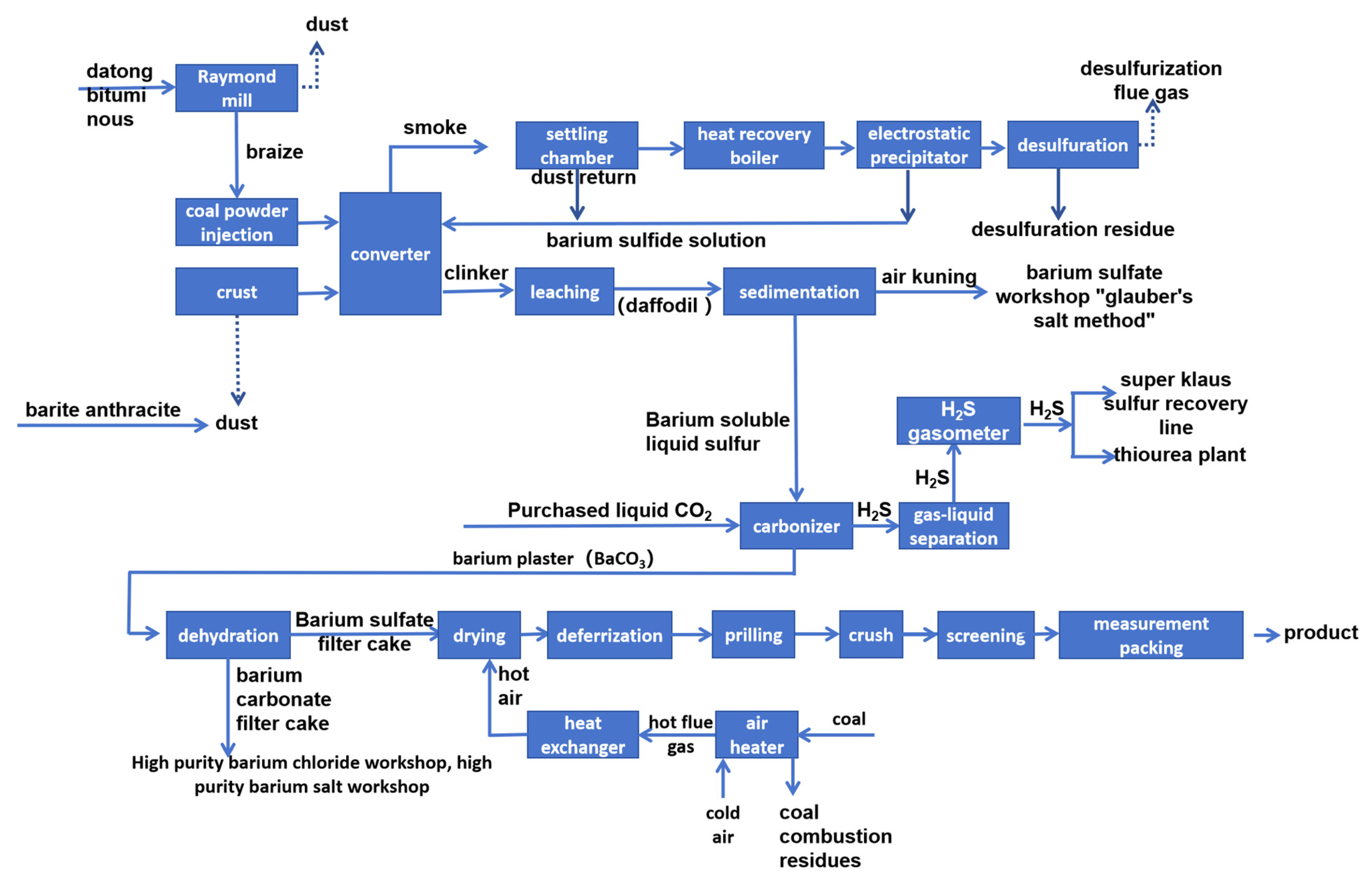
1]. The main production flow is shown in
Figure 1. In China’s “National Catalogue of Hazardous Wastes” (2021 Edition), barium slag is designated with the code HW47. The long-term storage of barium slag not only occupies a large amount of land resources, but also, the leaching of Ba
2+ ions in the barium slag commonly exceeds national standards by more than 10 times [
2]. Due to the erosion by rainwater, soluble barium salts such as barium sulfide can be leached from the stored barium slag, entering the soil and posing serious hazards to the environment and human health [
3,
4].
It is estimated that China emits about 1 million tons of Ba annually, and this amount is still accumulating [
5]. Barium metal is primarily used in industries such as electronics and electrical engineering, oil and gas, glass and ceramics, metallurgy, chemical engineering, and new energy. For example, barium slag can be used in the production of ceramic capacitors, piezoelectric materials, ceramic glazes, and television screens. Currently, the accumulated stock of barium slag in our country has exceeded tens of millions of tons [
2]. The industrial technologies for the disposal of barium slag mainly include comprehensive utilization, resource recovery, and safe landfilling, etc. The harmless treatment of barium slag is a prerequisite for its emission, storage, and comprehensive utilization. The main resourceful methods for barium slag include the preparation of building materials [
6,
7,
8], and the extraction of high-purity barium salts in the chemical industry [
9,
10,
11], etc. The main harmless disposal methods include reducing toxicity through incineration, barium ion extraction, and stabilization of barium ions [
12,
13,
14,
15,
16], etc. Industrially, sodium sulfate and ferrous sulfate are mainly used for the harmless treatment of barium slag. The treated harmless barium slag can be used for the production of building materials [
17,
18,
19], cement additives [
2], road base, and building material bricks [
8,
20,
21], etc. Additionally, hydrometallurgy represents a technology that transfers metal ions into the liquid phase, followed by their recovery in elemental form or as other compounds. Wu et al. [
22] used hydrometallurgical methods to solve the scientific problems of flocculation and precipitation of soluble barium in the leach solution of acid treatment of barium slag. The experimental results showed that more than 98.5% of the barium-containing compounds were successfully converted into BaCO
3 and BaSO
4, achieving high purities of 98.7% and 79.4%, respectively. Furthermore, the use of flocculants significantly improved the flocculation efficiency and rate of the BaCO
3 turbid fluid.
Currently, the main application of barium slag has enabled these research efforts to achieve value-added utilization of barium. However, the introduction of these technologies also brings certain challenges. These factors include the gradual increase in barium slag stockpiles, the growing complexity of its composition, the low recovery rate of valuable components, high energy consumption, and non-compliance with sustainable development standards, particularly regarding the use of organic reagents [
23]. Therefore, there is an urgent need to develop and implement innovative technologies for the effective removal of Ba to mitigate the resulting environmental pollution.
Studies have indicated that mining methods can be employed to comprehensively recover barium resources from barium slag, addressing the environmental pollution caused by barium slag and simultaneously recovering the barium contained within. Research into the properties of barium slag allows for the investigation of barium slag shaking table gravity concentration tests, barium slag flotation tests, barium slag acid leaching—preparation of barium sulfate from leachate tests, barium slag acid leaching residue flotation tests, and the study of flotation mechanisms of barium slag, among others. However, research on barium slag shaking table gravity concentration tests has shown that barium content in finer particle sizes of barium slag is higher than in coarser particle sizes. Through shaking table separation, it is difficult to effectively recover barium from barium slag, and multiple rounds of concentration cannot significantly improve the grade of the concentrate. Studies on the screening of barium slag flotation tailings have indicated that flotation has only a slight enriching effect on fine particle barium-containing minerals [
24]. Flotation reagents and inevitable ions have a significant impact on the flotation of barite and quartz, with high concentrations of barium ions consuming a large amount of oleate, inhibiting barite [
25]. Calcium and magnesium ions have a strong inhibitory effect on barite but can effectively activate quartz, making the floatability of the two very close, which makes flotation separation difficult. There is now an urgent need for more detailed mineral processing work to enhance the utilization of barium in barium slag.
In this study, a method was proposed to first reduce the state of barium slag and then separate and recover barium salts (barium sulfate, barium oxide) from barium slag through shaking table gravity concentration. The study primarily focused on the barium compounds in barium slag, with an emphasis on how to revert the agglomerated barium slag raw material back to its original state and separate the barium compounds, among other related issues.
2. Materials and Methods
2.1. Raw Material
The raw material used in this study is barium slag produced from the production of barium carbonate from barite, sourced from a company in Guizhou that manufactures barium carbonate. The raw material was dried and prepared for analysis.
2.2. Methods
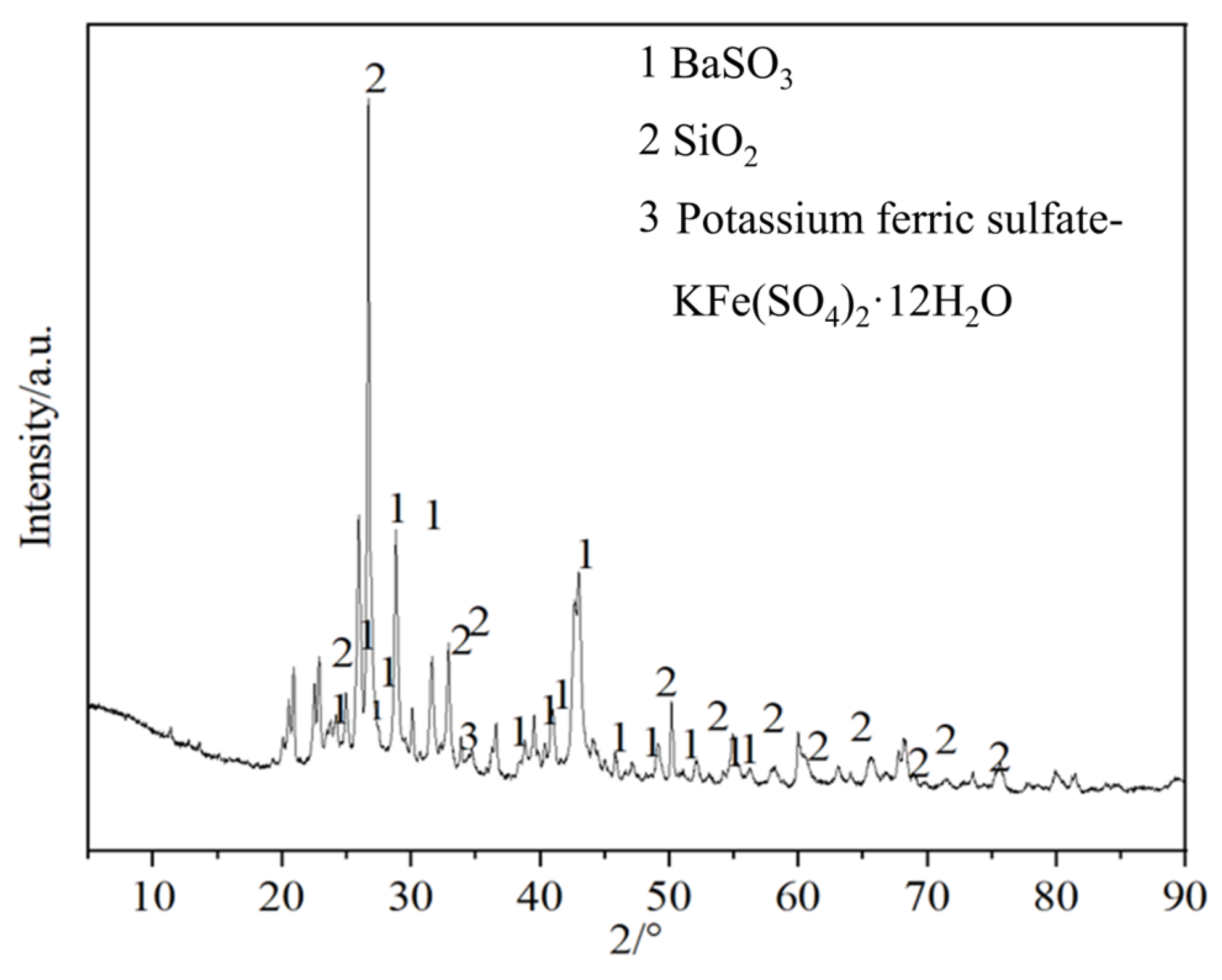
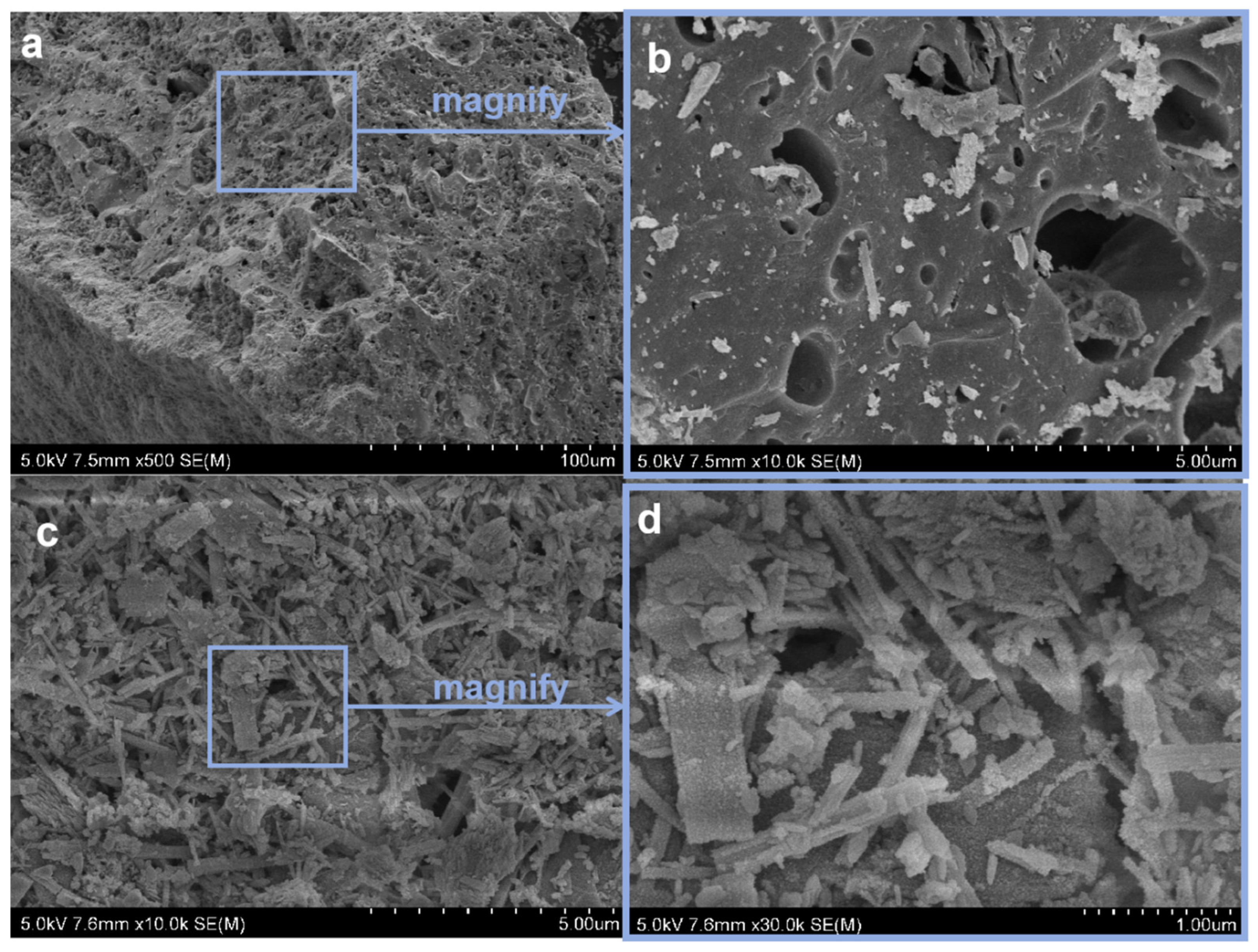
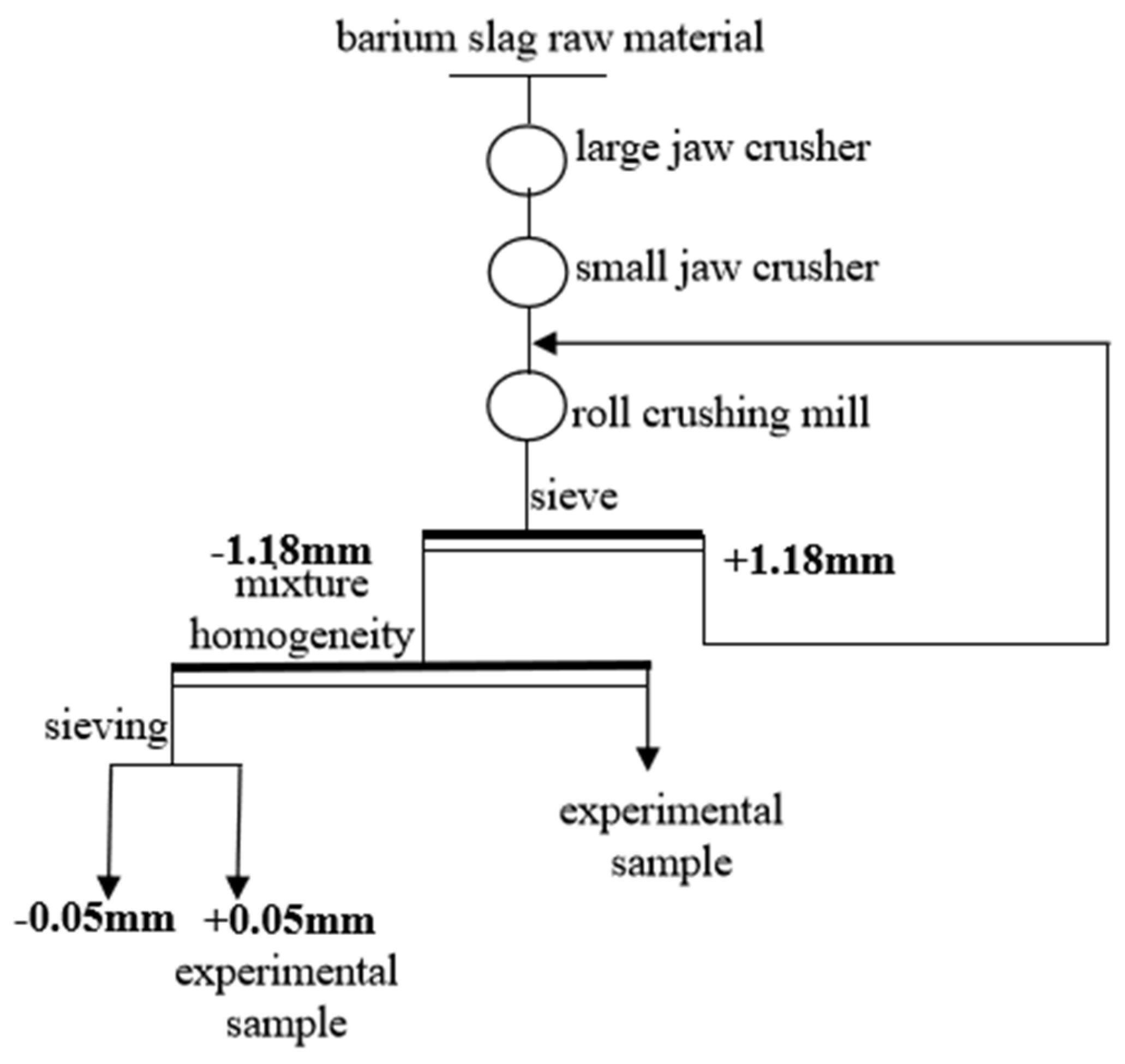
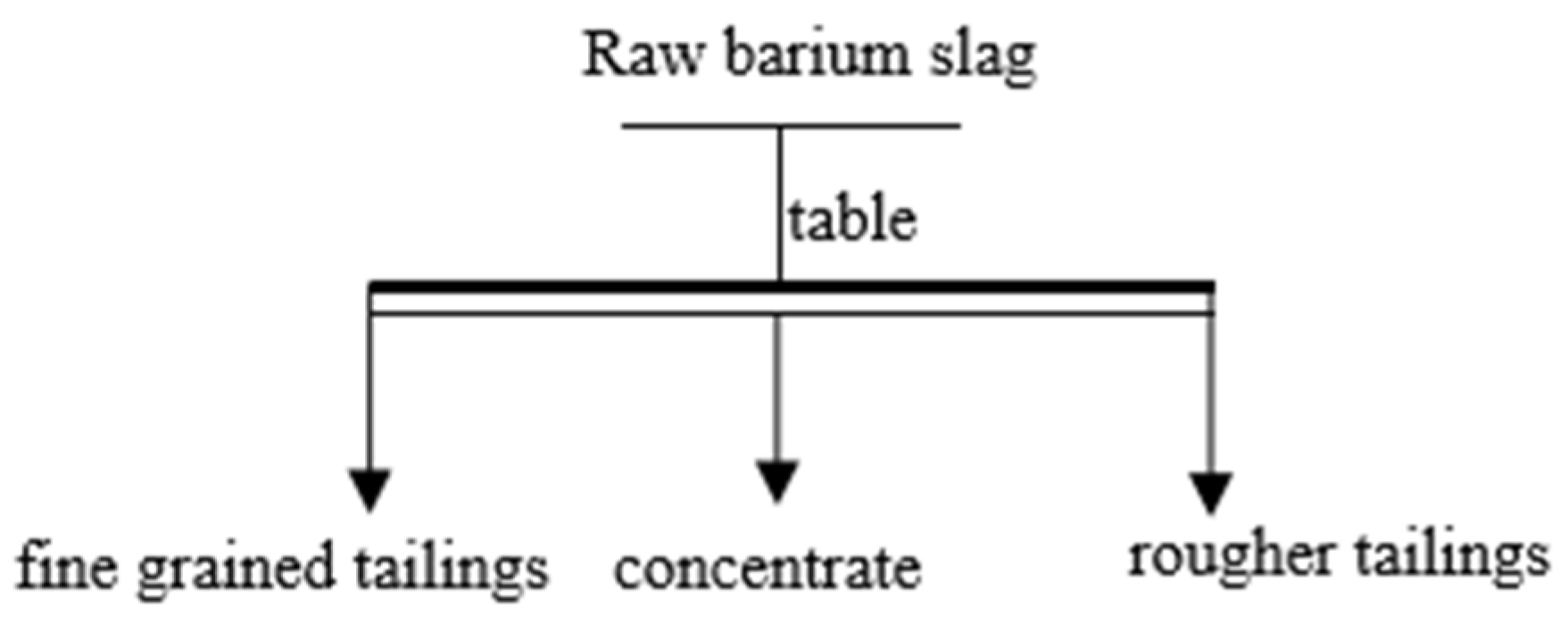
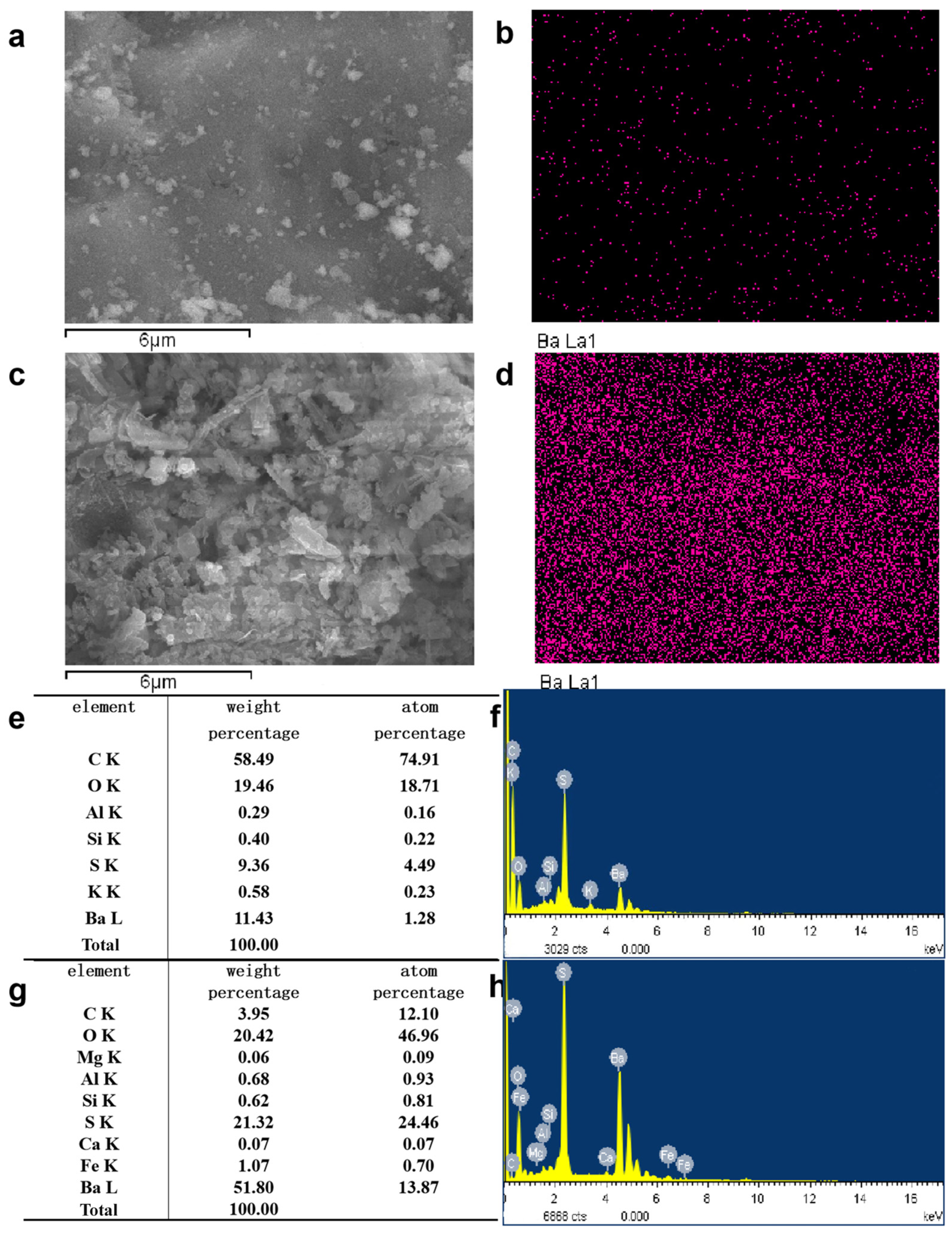
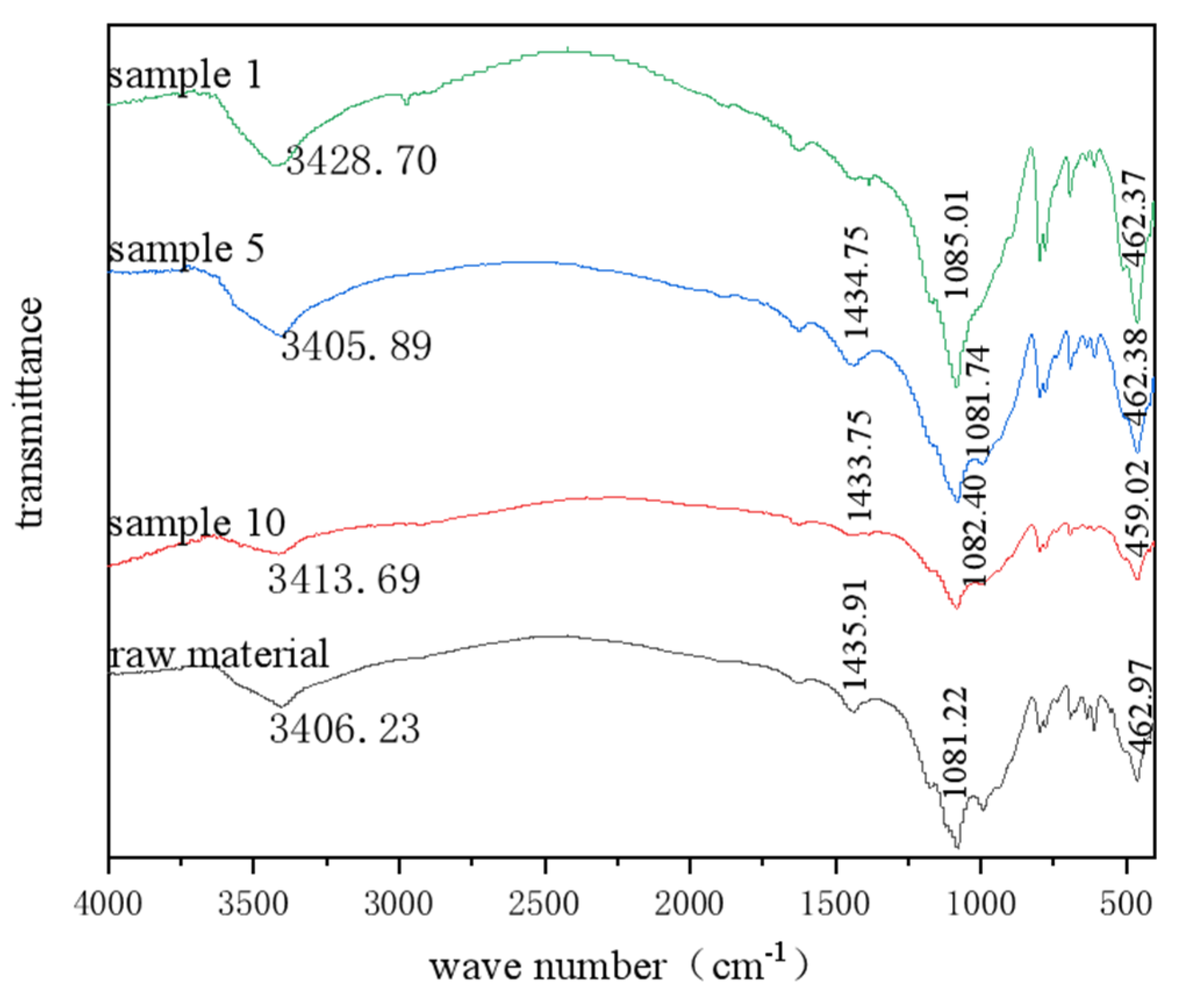
In order to analyze the composition of the samples after the heavy media separation of barium slag, this study first pretreats the barium slag to a prereduced state and then uses the density difference to separate it into samples 1–16 using a shaking table, separating and selecting the compounds with high barium content. The model of the shaker table was LY-1100 × 500 (Shaker Manufacturing Co., Shaoxing, China). Macroscopic and microscopic analytical methods such as XRD (X-ray Diffraction), XRF (X-ray Fluorescence), FTIR (Fourier Transform Infrared Spectroscopy), SEM (Scanning Electron Microscopy), and EDS (Energy-dispersive X-ray Spectroscopy) are employed to analyze the barium compounds separated from the barium slag. The XRD analysis was conducted using a Japanese Rigaku SmartLab SE X-ray diffractometer (An Electronic Technology Co., Shenzhen, China), employing copper potassium radiation, with a voltage of 40 kV and a current of 40 mA. The scanning range was from 5° to 90°, at a scanning speed of 5° per minute, with a step size of 0.02, to perform X-ray dual-component (XRD) measurements on the powder samples and to observe and analyze the phases present in the samples. The XRF spectrometer used was the Japanese Rigaku ZSX Primus III+ (Rigaku Ltd., Tokyo, Japan), which uses high-energy X-rays or gamma rays to bombard materials, exciting secondary X-rays that are used to determine the types and amounts of elements in the materials. FTIR analysis was performed using a Thermo Scientific Nicolet iS20 Fourier Transform Infrared Spectrometer (Thermo Fisher Scientific, Shanghai, China), which is a U.S. NICOLET 470 model. The analysis was conducted with a resolution of 4 cm−1, 32 scans, and within the wavenumber range of 400 to 4000 cm−1. The FTIR spectra of the samples were obtained and recorded, and the characteristic peaks of the hydrated products were determined by analyzing chemical bonds and functional groups. SEM-EDS was employed using a JSM-6701F field emission scanning electron microscope (JEOL Nippon Electronics Co., Akishima, Japan) to observe the microstructure of the samples. The operating voltage was set at 10 kV, with a beam (spot size 6) and an aperture of 3. The scanning electron microscope was used to observe the microstructure of the hydrated products in the samples.
4. Conclusions
The content of barite in barium slag is mostly around 20%, especially for the partially unreacted and larger particles of barite, as well as the partially sintered symbiotic bodies, where the content can reach over 30%. By employing a jigging process to reuse the incompletely reacted barite, the recovery rate of the barite component, barium sulfate, in sample 1 can reach up to 82.46% or higher.
The process pioneers the delamination of sintered ore and symbiotic bodies from waste slag generated in the production of barium sulfate. It utilizes the differences in densities of various components to sort the waste slag and employs a jigging process, a physical method, to recover the incompletely reacted barite for reuse. The recovered barite component, barium sulfate, can have a content of over 50%. The residual slag undergoes harmless treatment, and high-purity barium sulfate is chemically recovered. The method for processing the unburnt large particles or incompletely reacted sintered symbiotic bodies in the remaining waste slag may depend on specific experimental conditions and sample properties. This approach not only enhances the utilization rate of resources but also contributes to environmental protection by reducing the environmental impact of waste slag.
Therefore, the pretreatment of barium slag by shaking table beneficiation experiments can separate and sort out barium compounds, obtaining a barite concentrate with a grade of 82.46%, which is a significant improvement in the index. This concentrate can be used locally for the production of barium carbonate, allowing for the recycling and reuse of resources. At the same time, the soluble barium and other toxic substances in the tailings after beneficiation are essentially removed, making the tailings more suitable for use as building materials and road construction materials.