Influence of the Layout of Cells in a Traction Battery on the Evolution of a Fire in the Event of a Failure
Abstract
1. Introduction
2. Materials and Methods
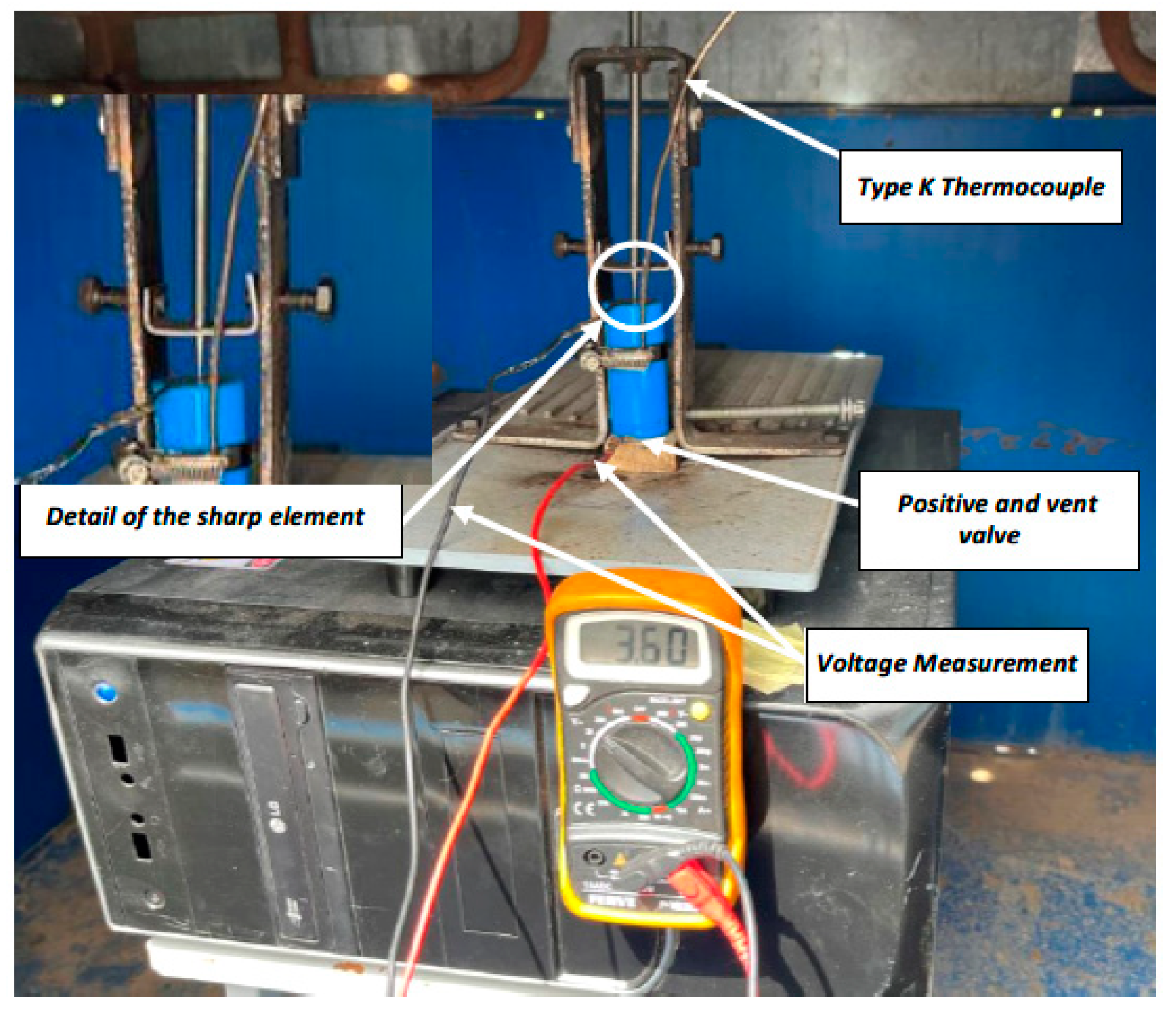
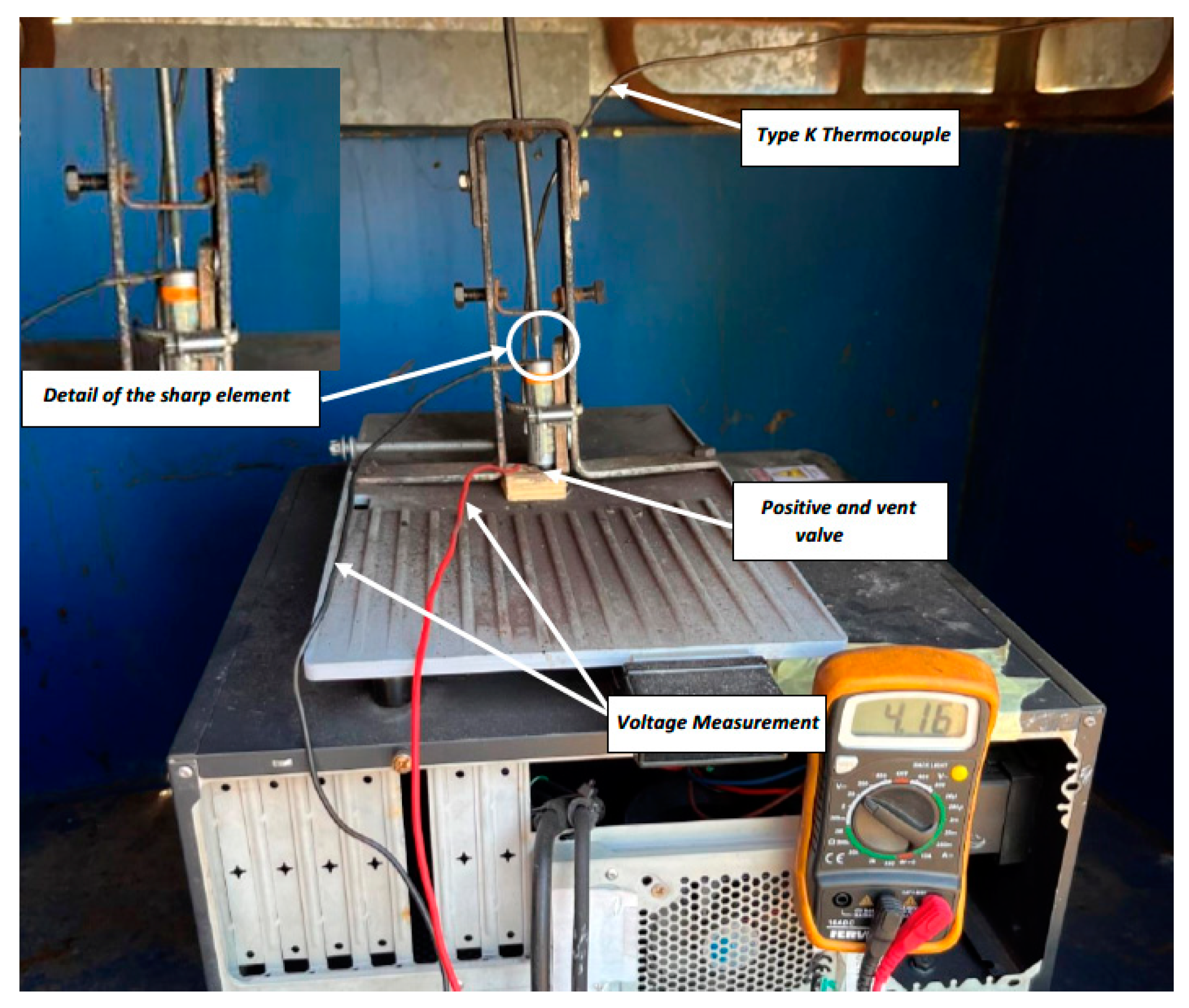
2.1. Mechanical Abuse Test
2.2. Electrical Abuse Test: Overcharge
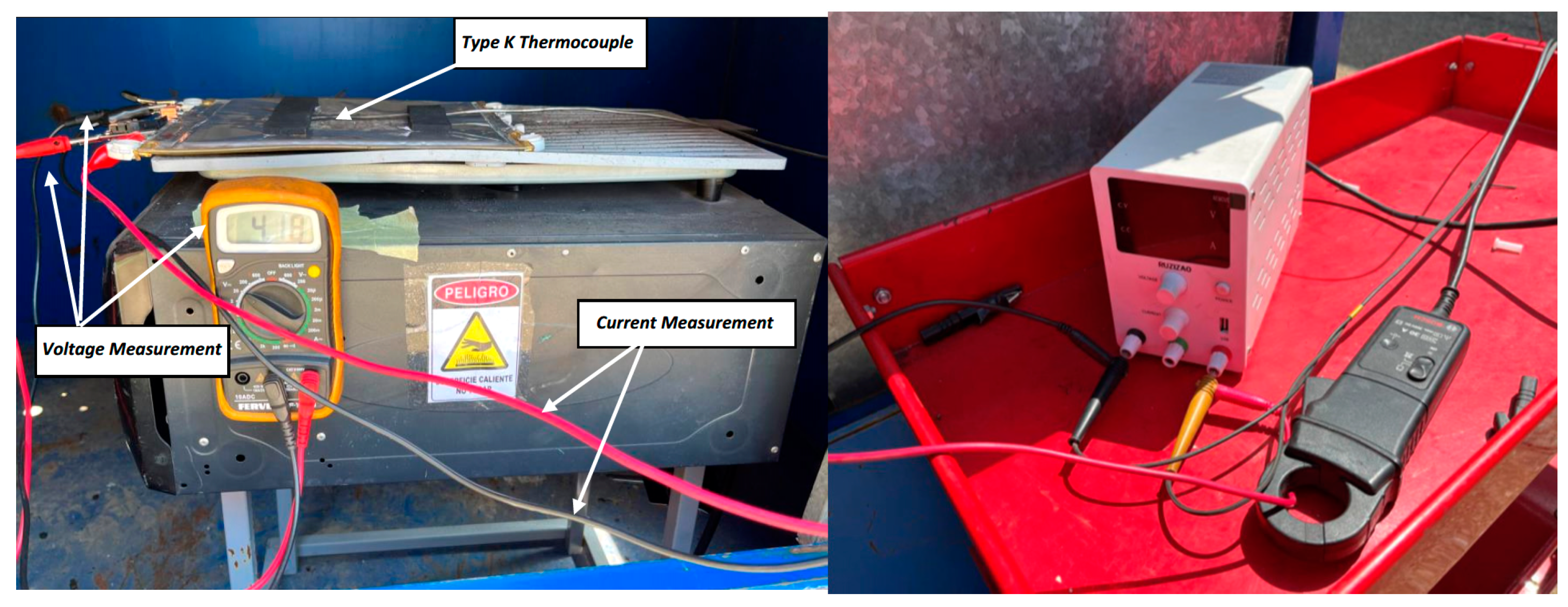
2.3. Thermal Abuse Test: Heating
3. Results
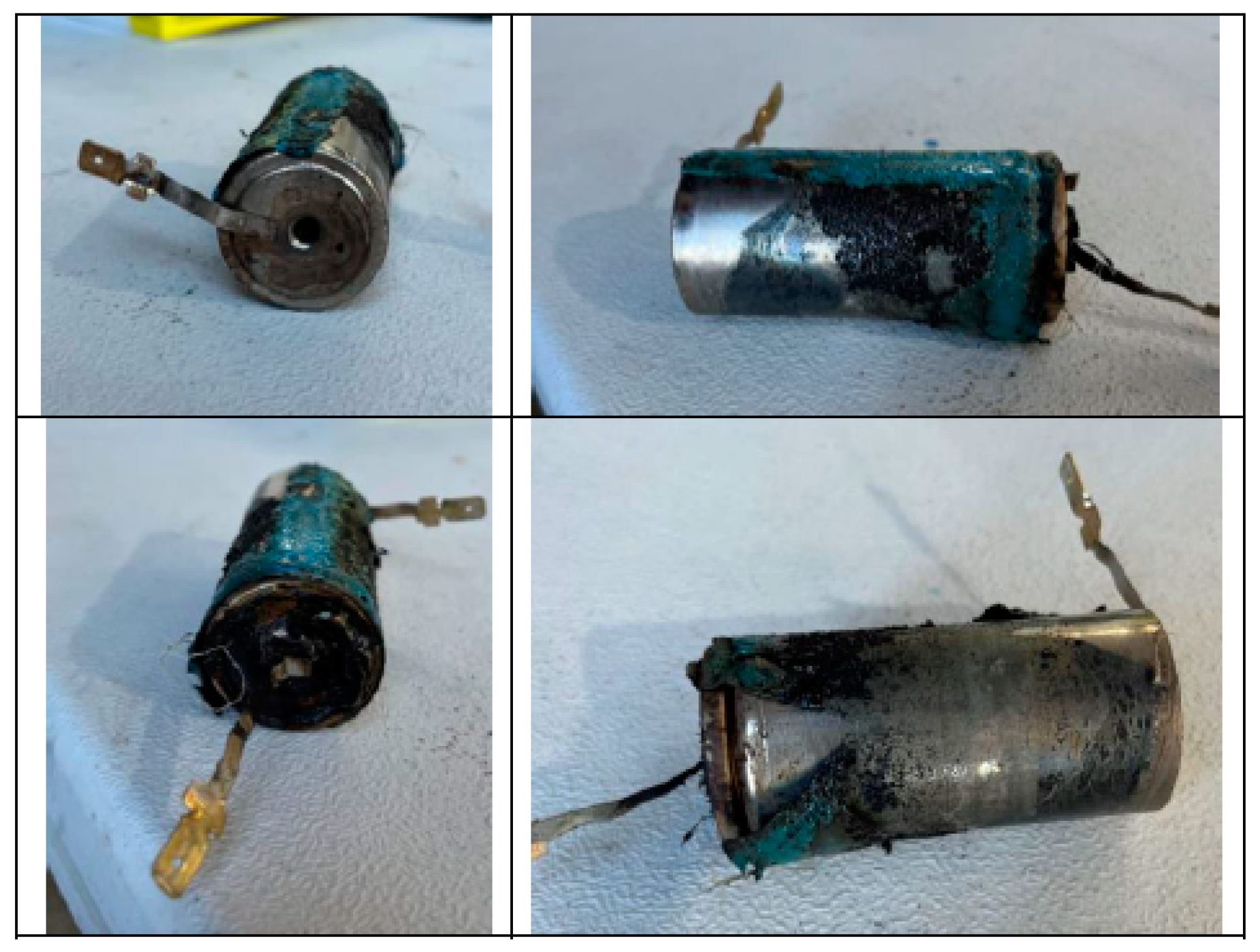
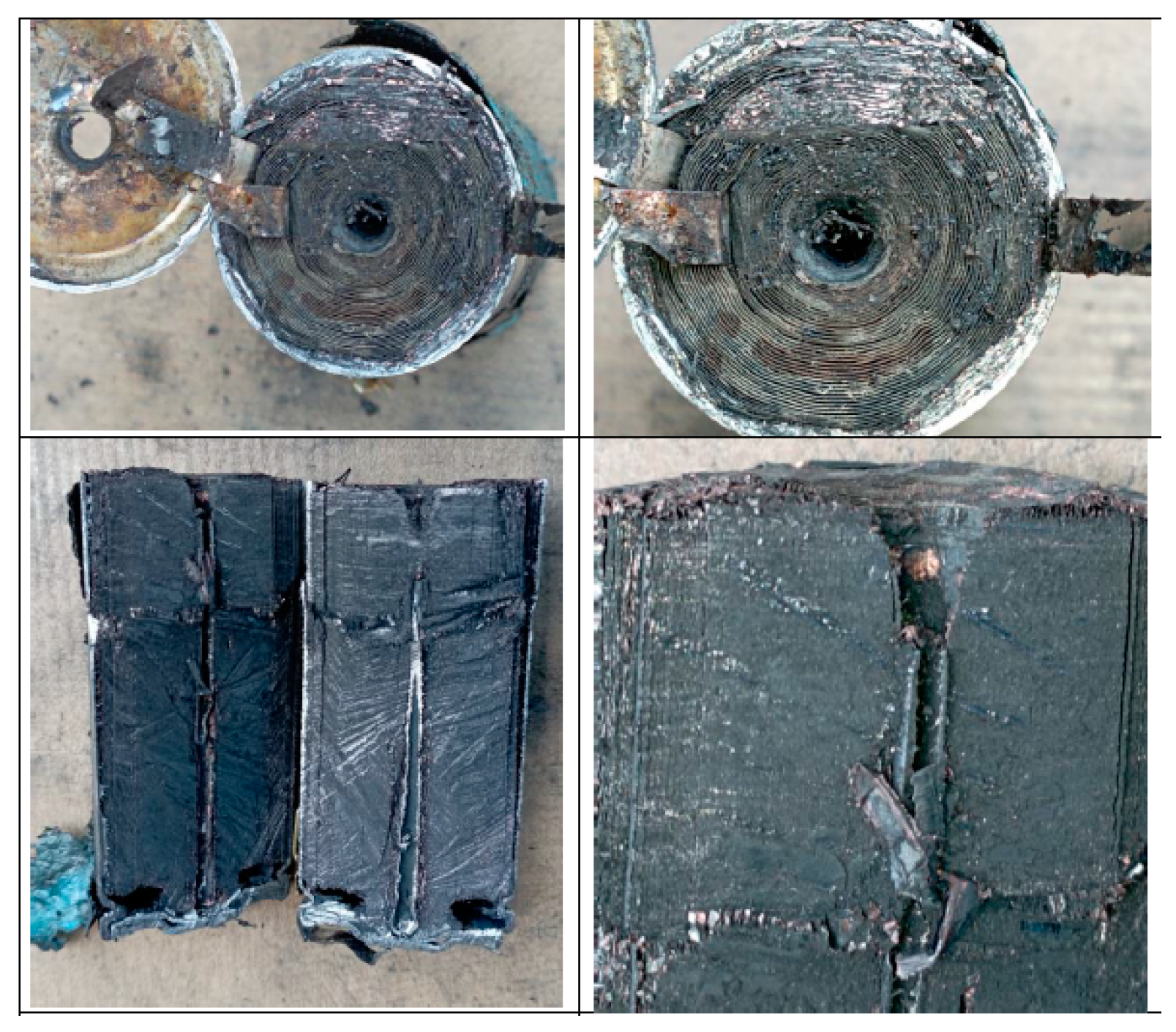
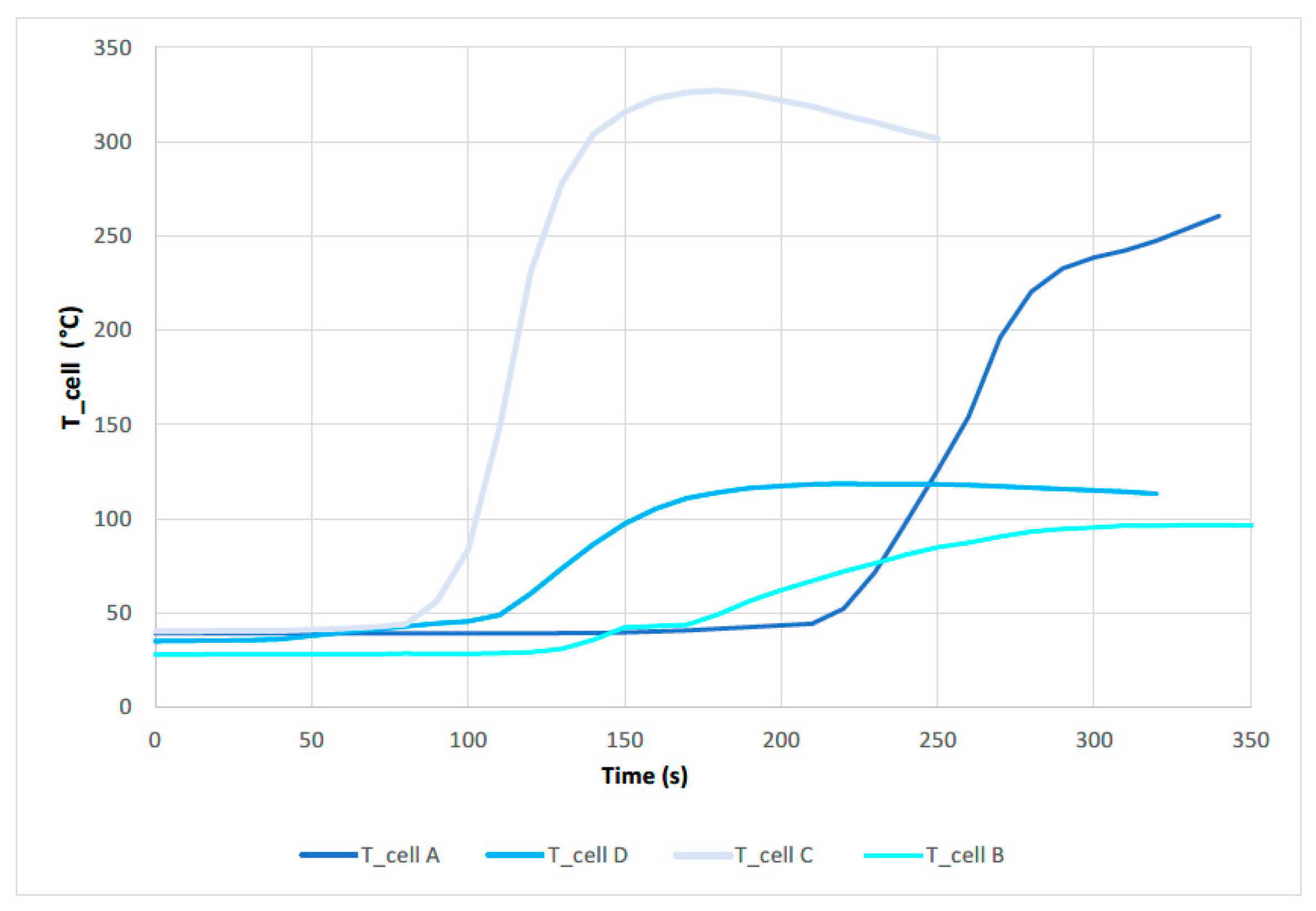
3.1. Mechanical Abuse Test Results
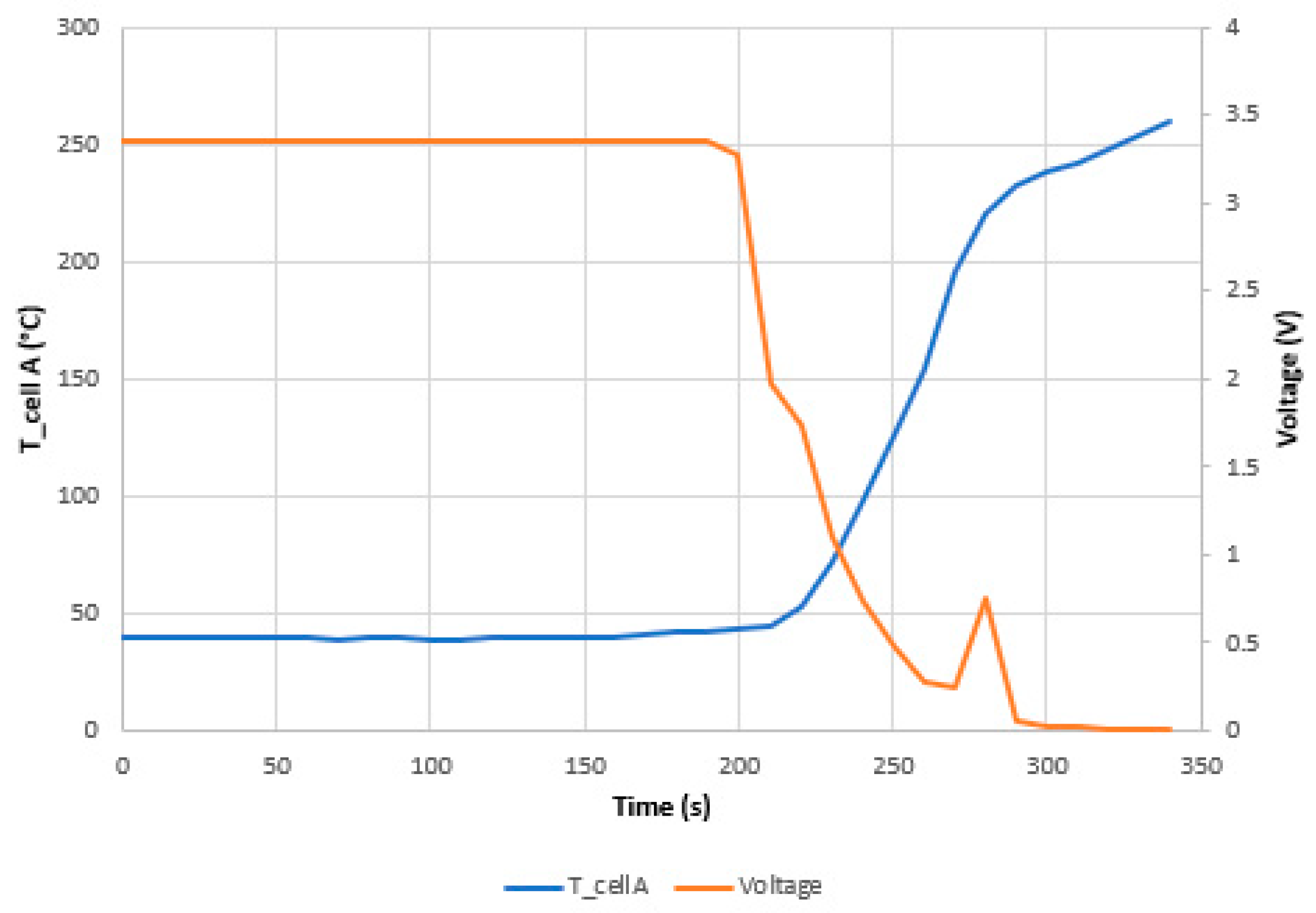
3.1.1. Test 1: Horizontal SoC 100% (Cell A)
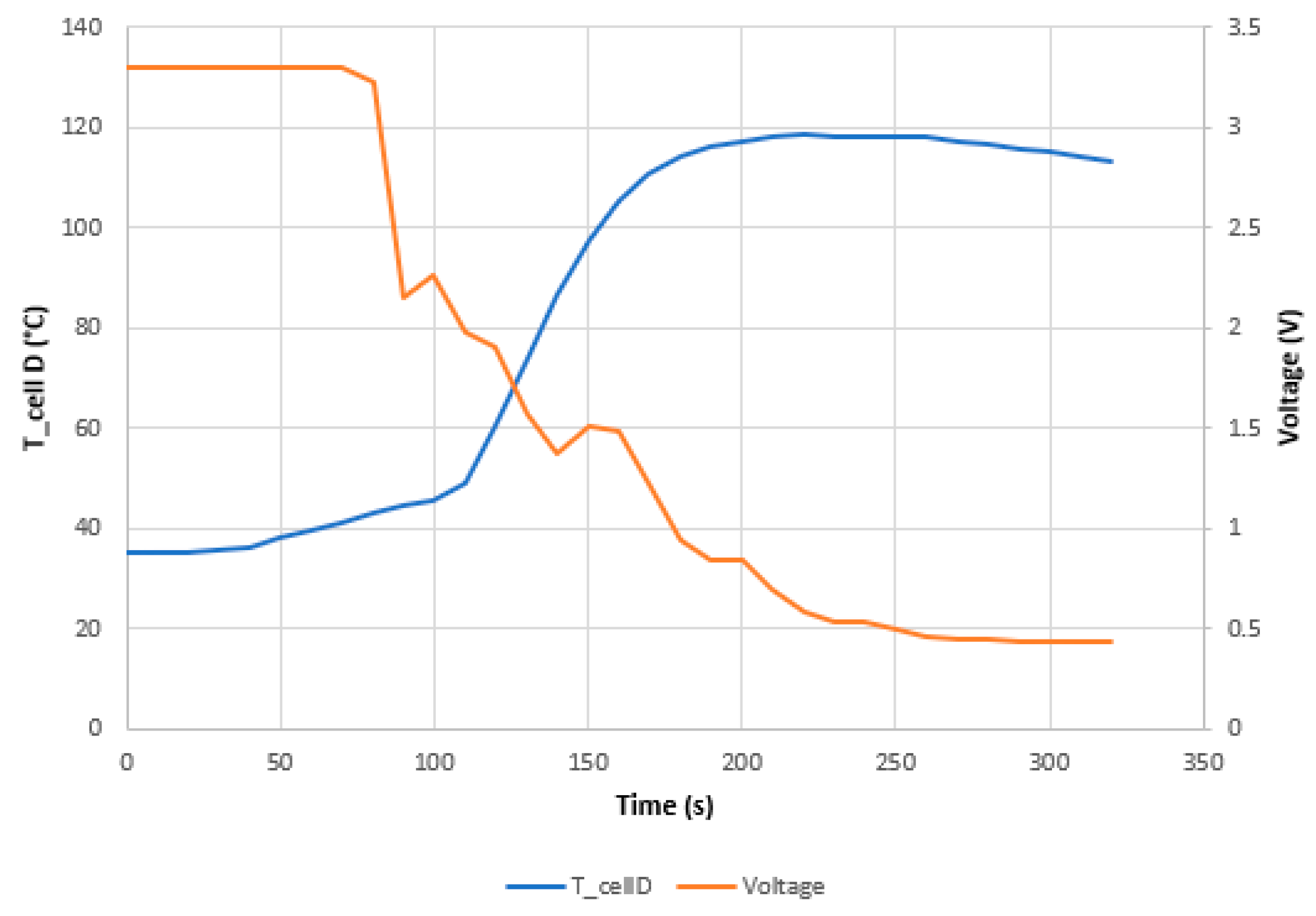
3.1.2. Test 2: Horizontal SoC 50% (Cell D)
3.1.3. Test 2: Vertical SoC 100% (Cell C)
3.1.4. Test 4: Vertical SoC 50% (Cell B)
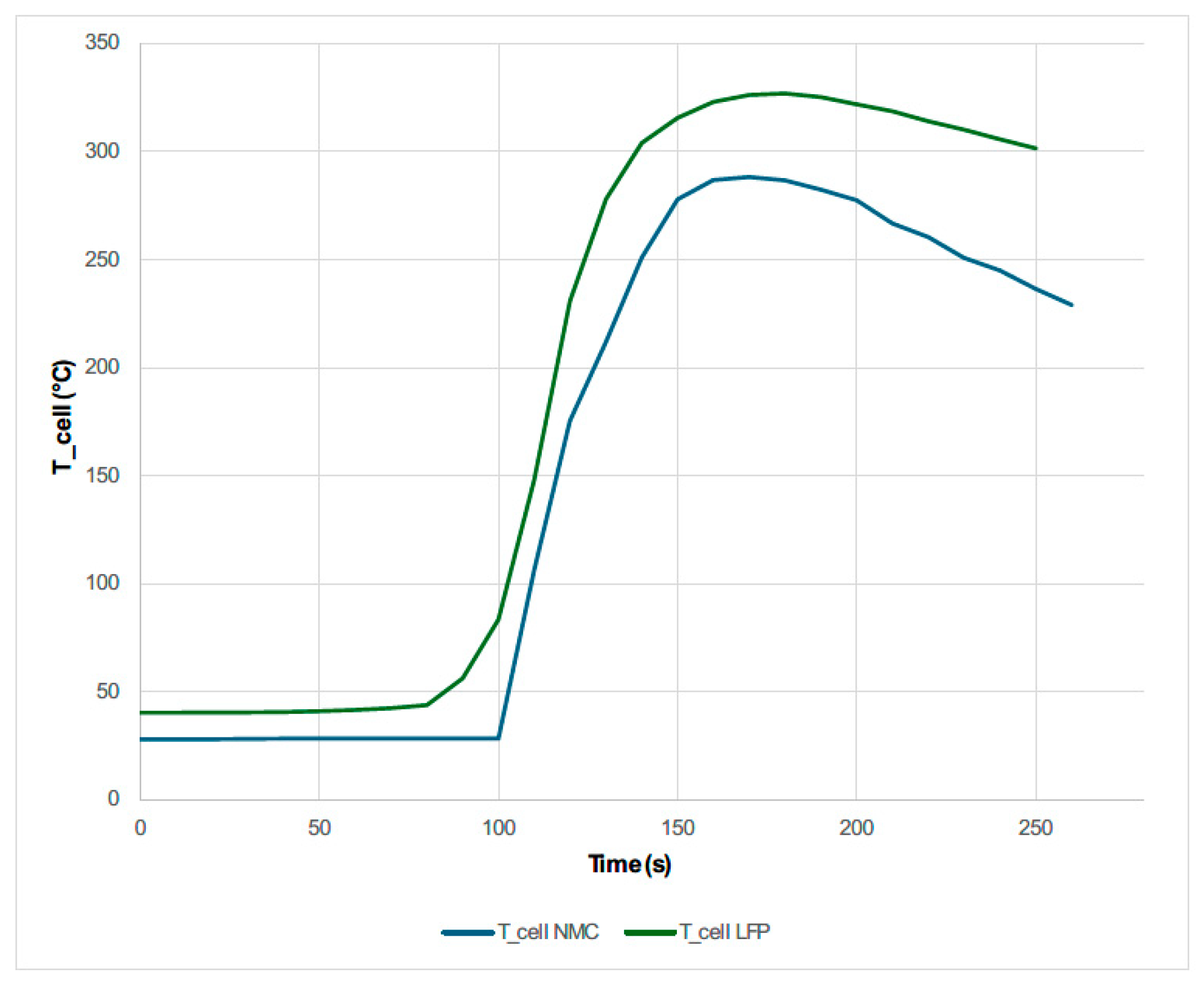
3.1.5. Test 5: Vertical SoC 50% (Lithium-Ion Cell NMC)
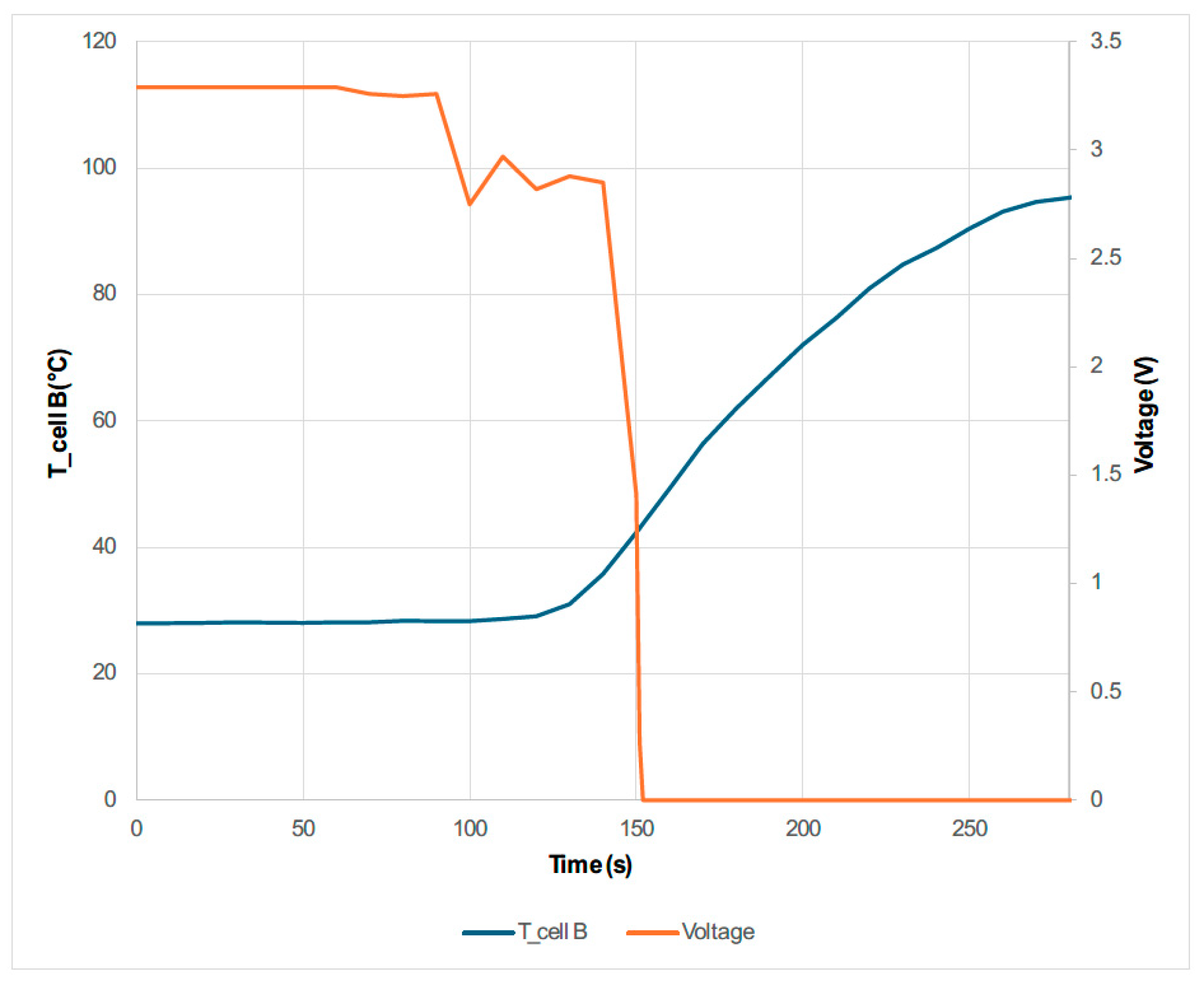
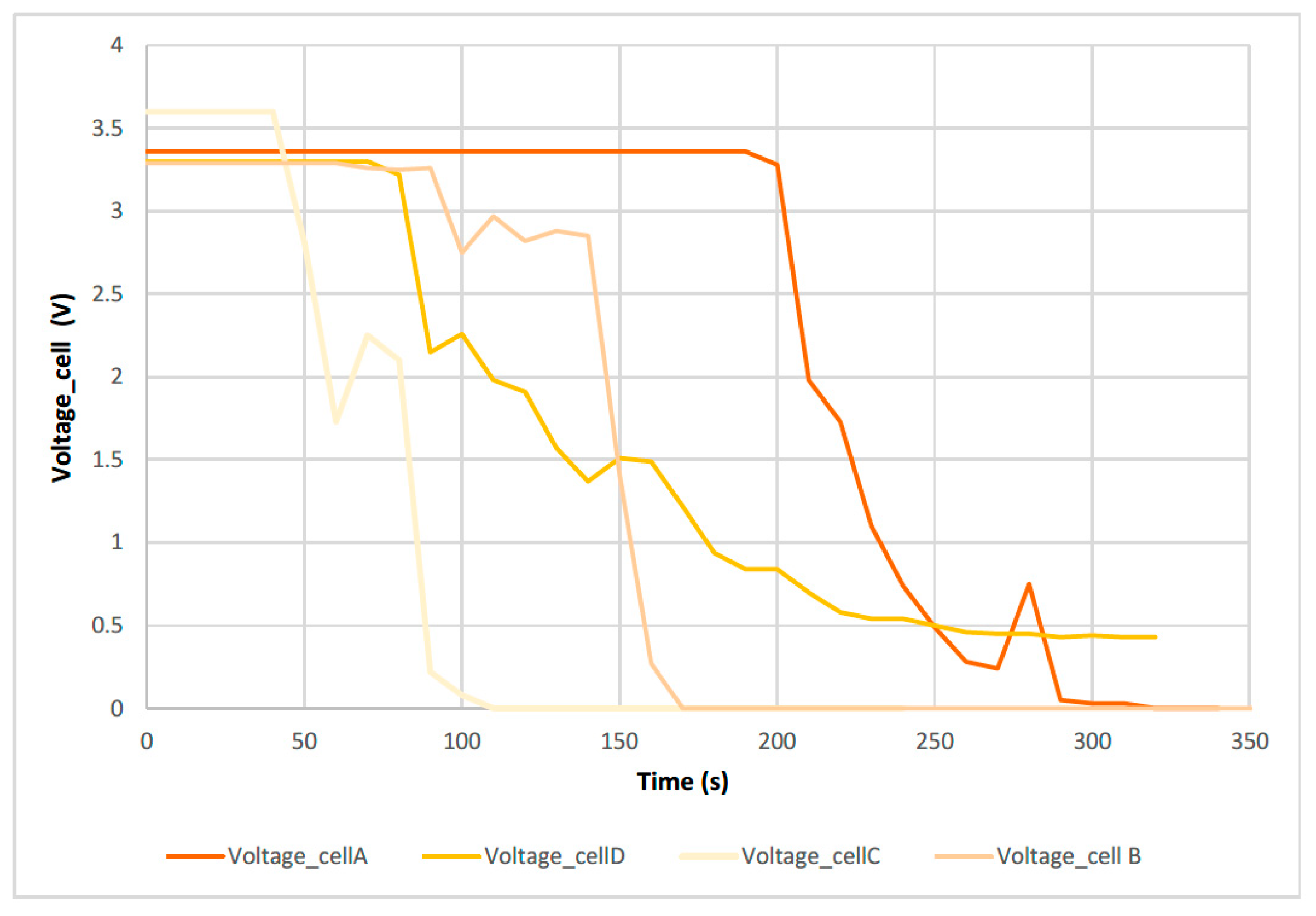
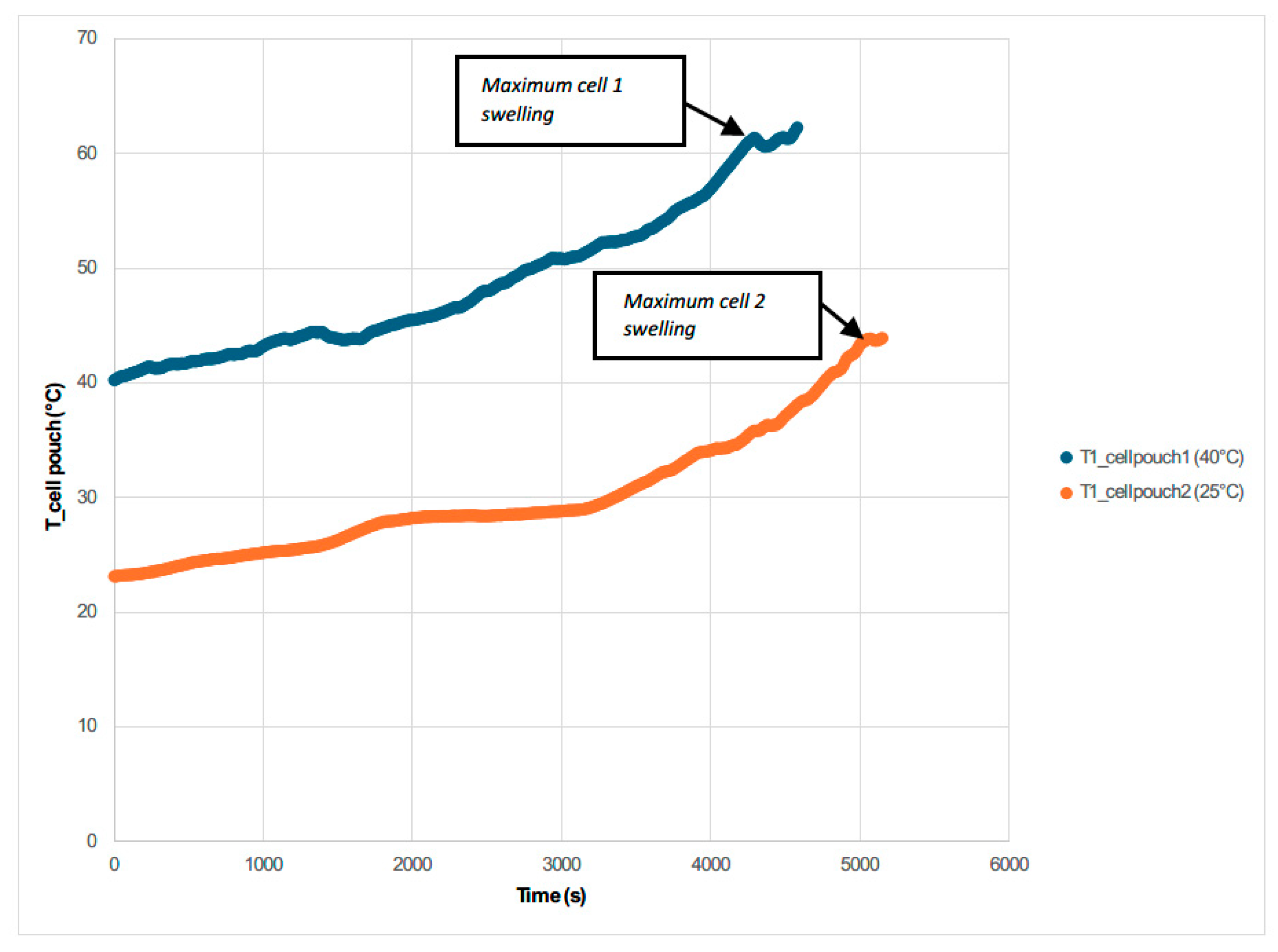
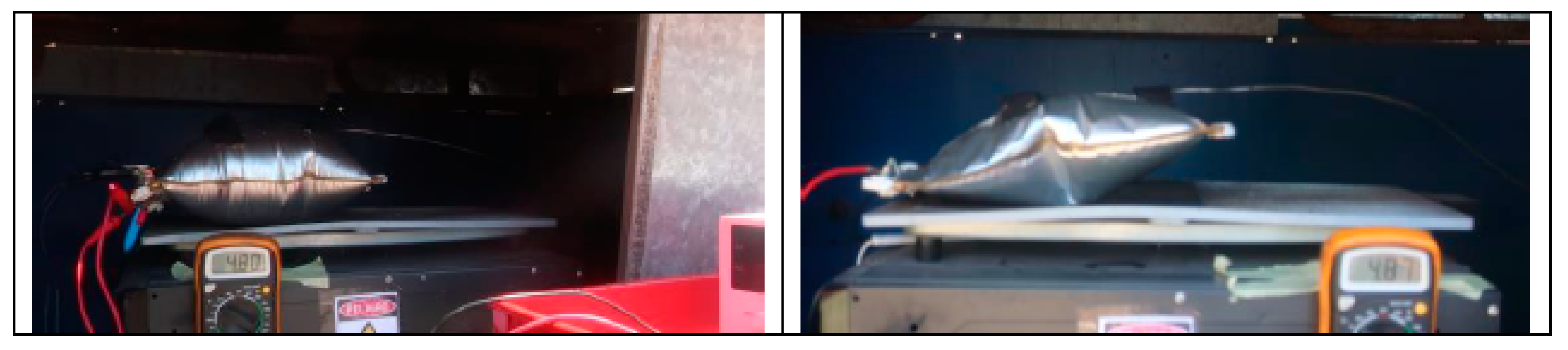
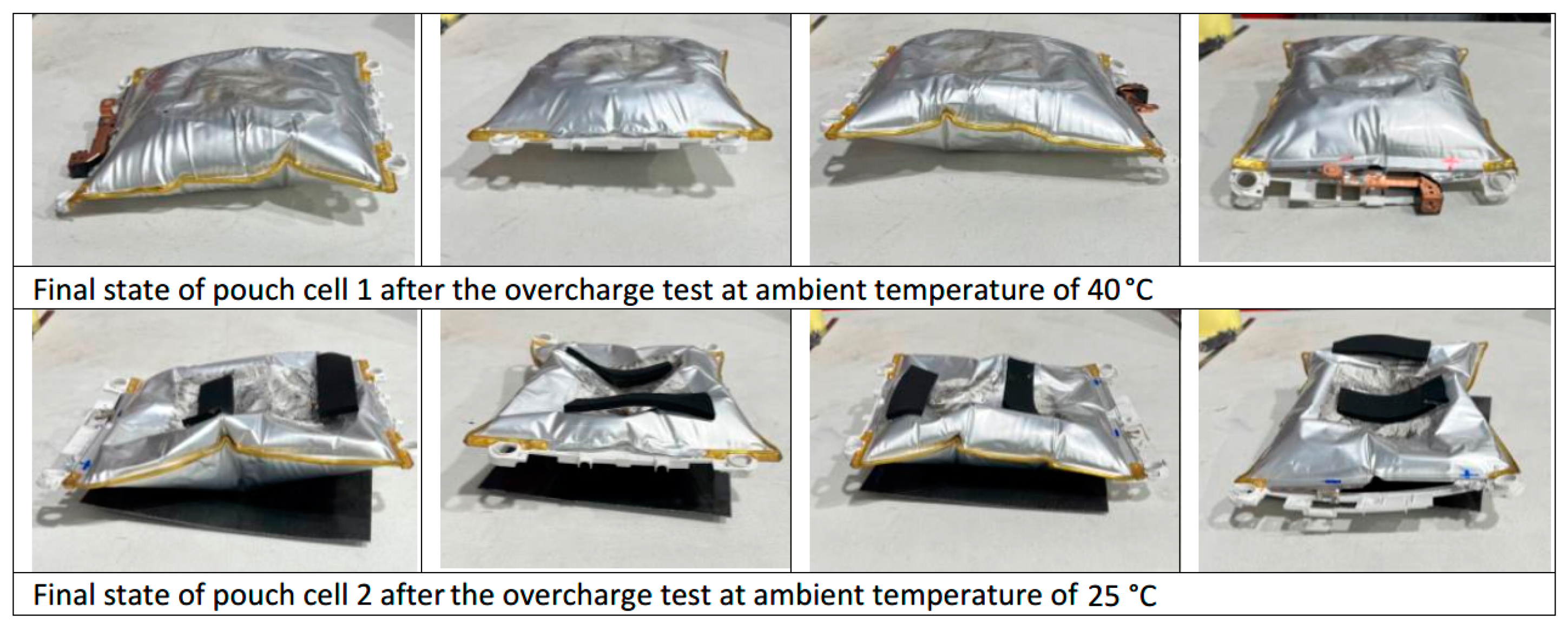
3.2. Electrical Abuse Test Results: Overcharge
3.3. Thermal Abuse Test Result: Heating
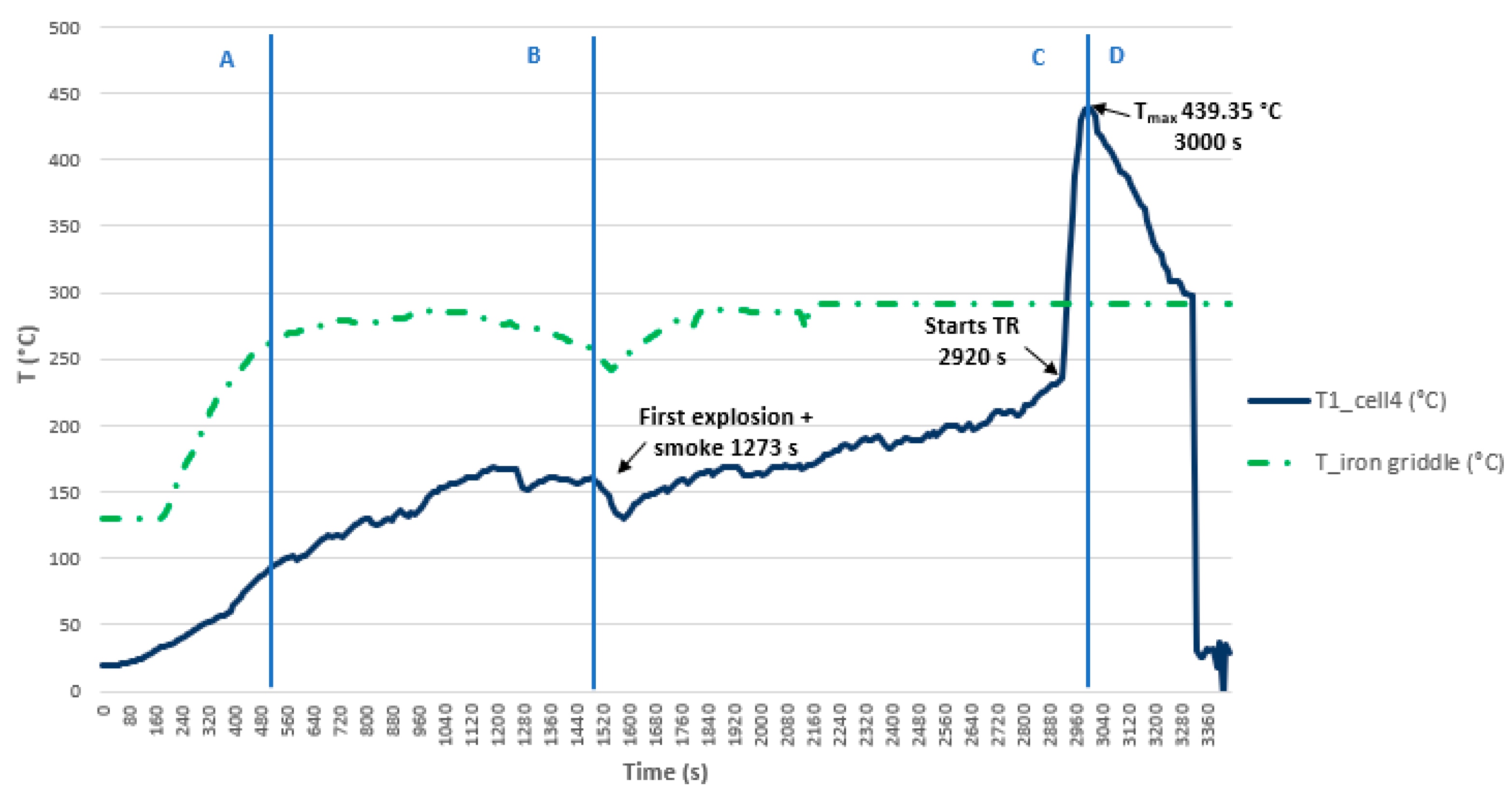
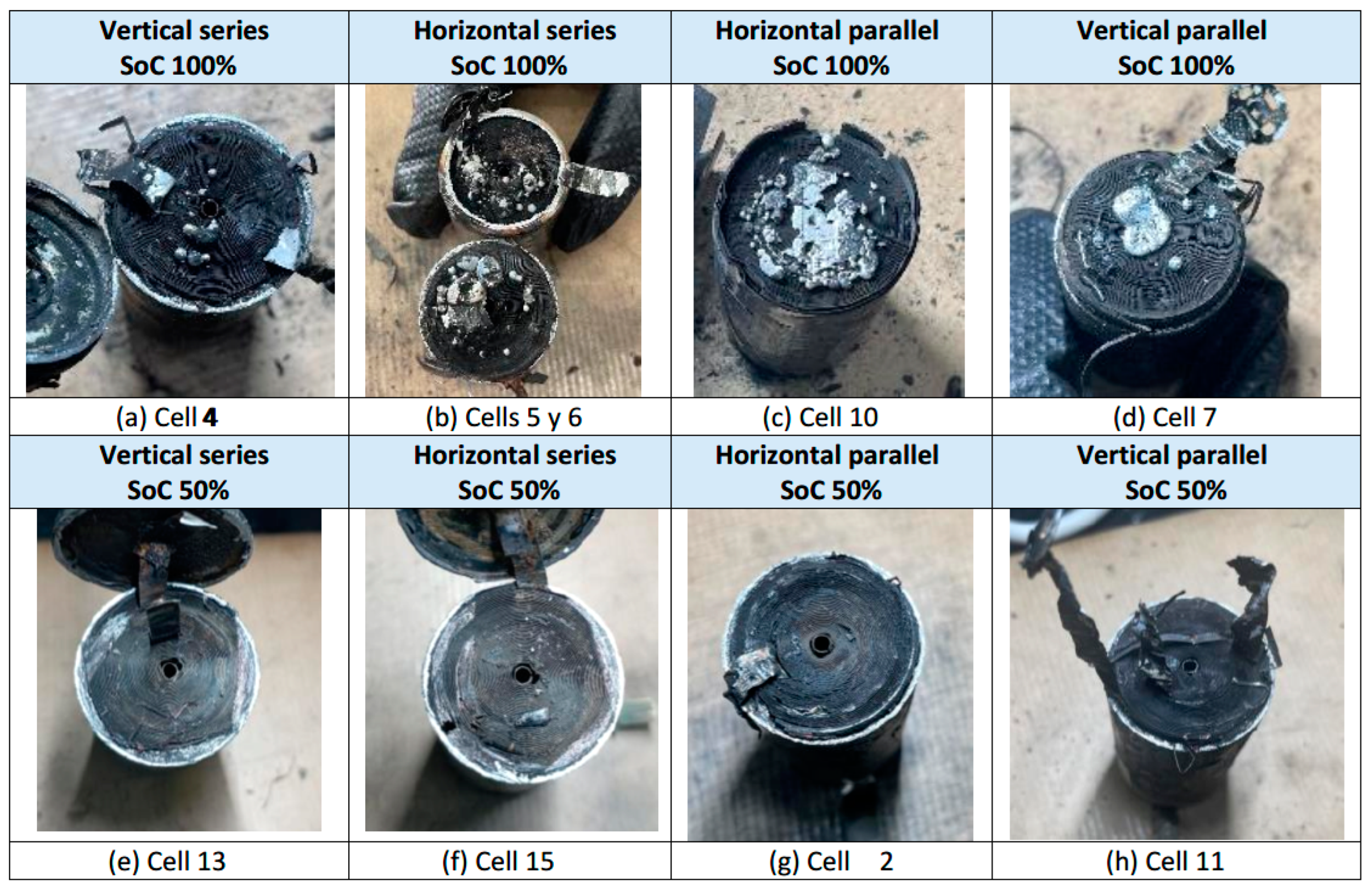
3.3.1. Test 1: Vertical, Series Connection and SoC 100% (Cell 4 Below and Cell 3 Above)
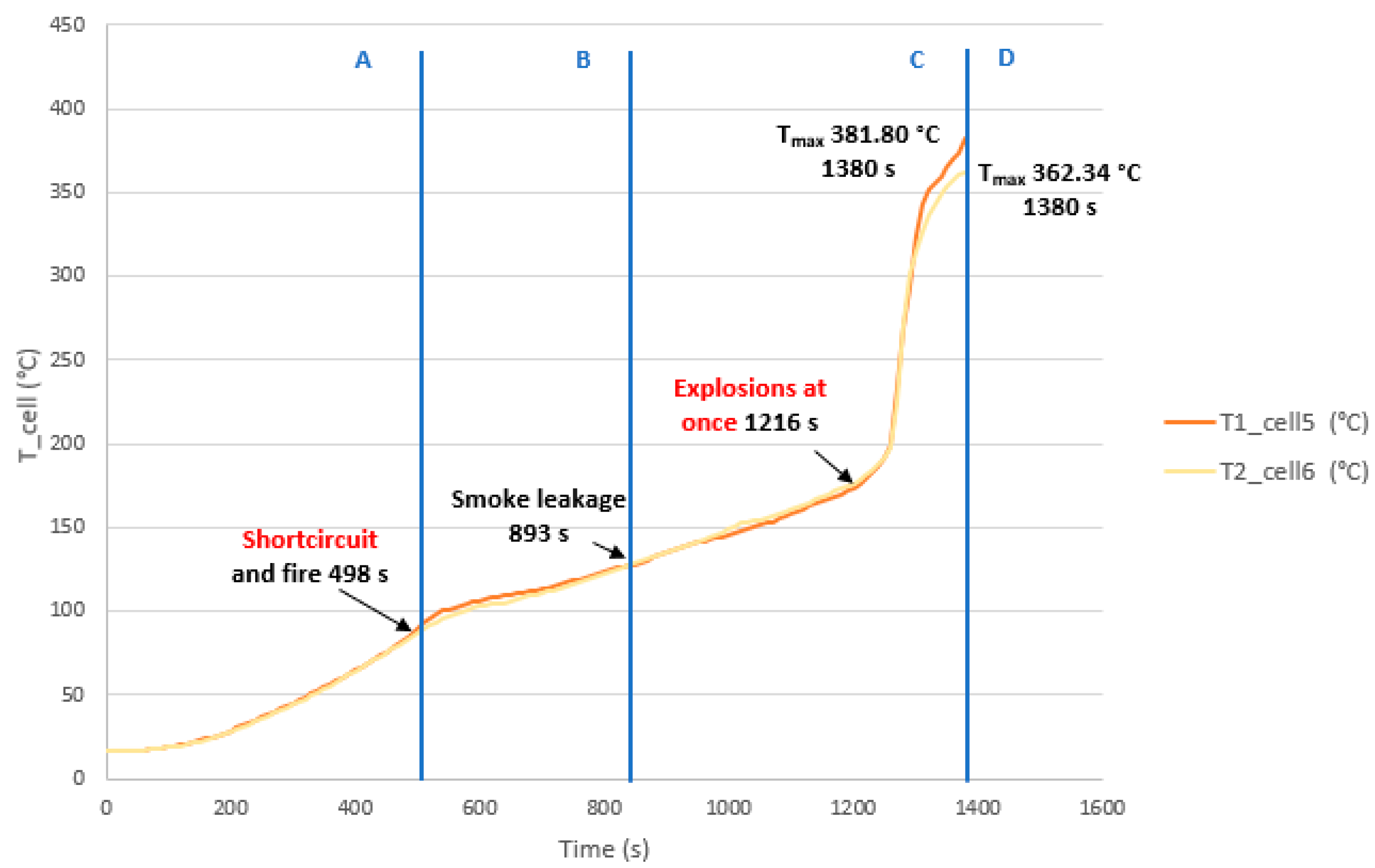
3.3.2. Test 2: Horizontal, Series Connection and SoC 100% (Cell 5 and Cell 6)
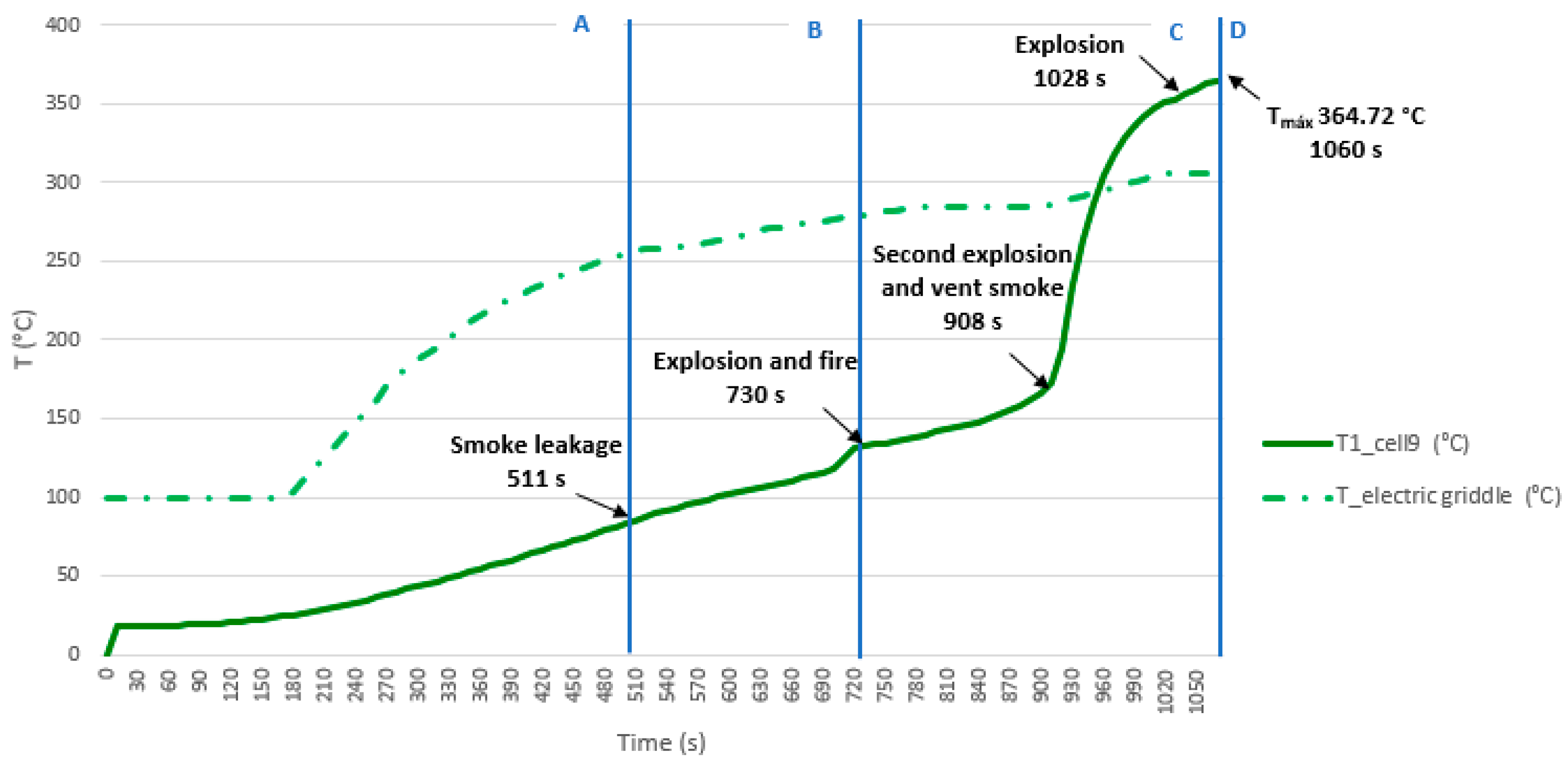
3.3.3. Test 3: Horizontal, Parallel Connection and SoC 100% (Cell 9 and Cell 10)
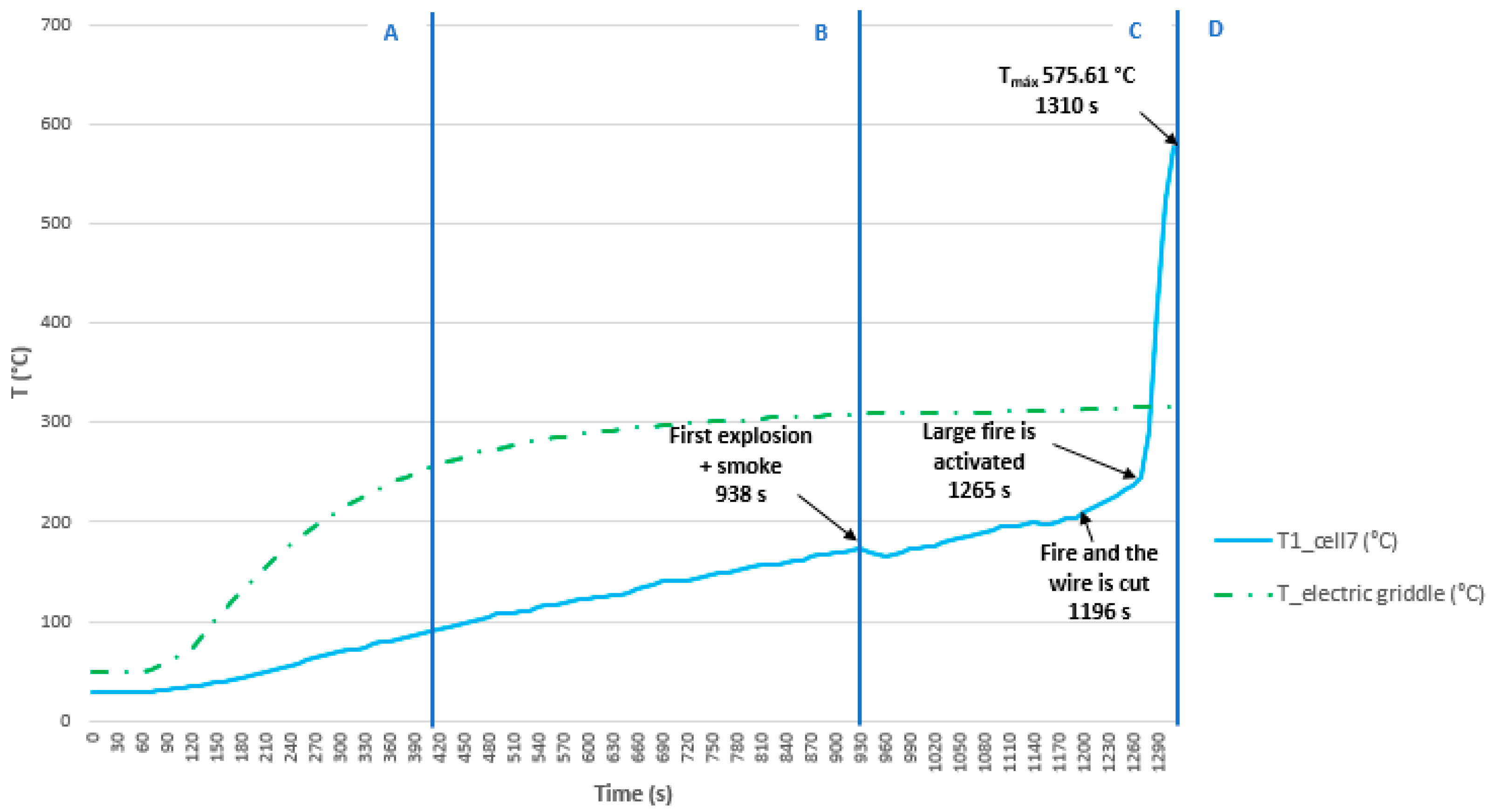
3.3.4. Test 4: Vertical, Parallel Connection and SoC 100% (Cell 7 (Below) and Cell 8)
3.3.5. Test 5: Vertical, Parallel Connection and SoC 50% (Cell 11 (Below) and Cell 12)
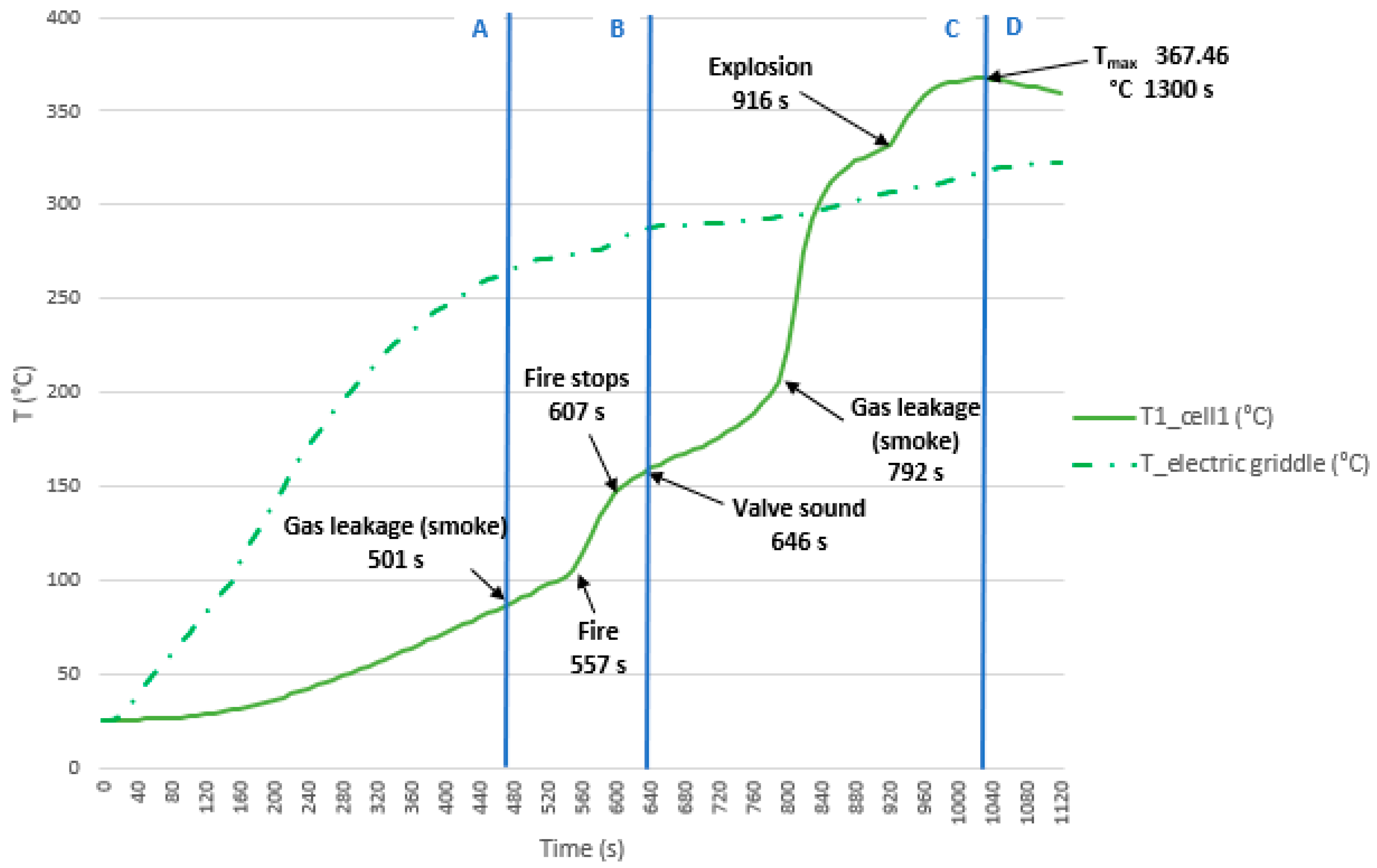
3.3.6. Test 6: Horizontal, Parallel Connection and SoC 50% (Cell 1 and Cell 2)
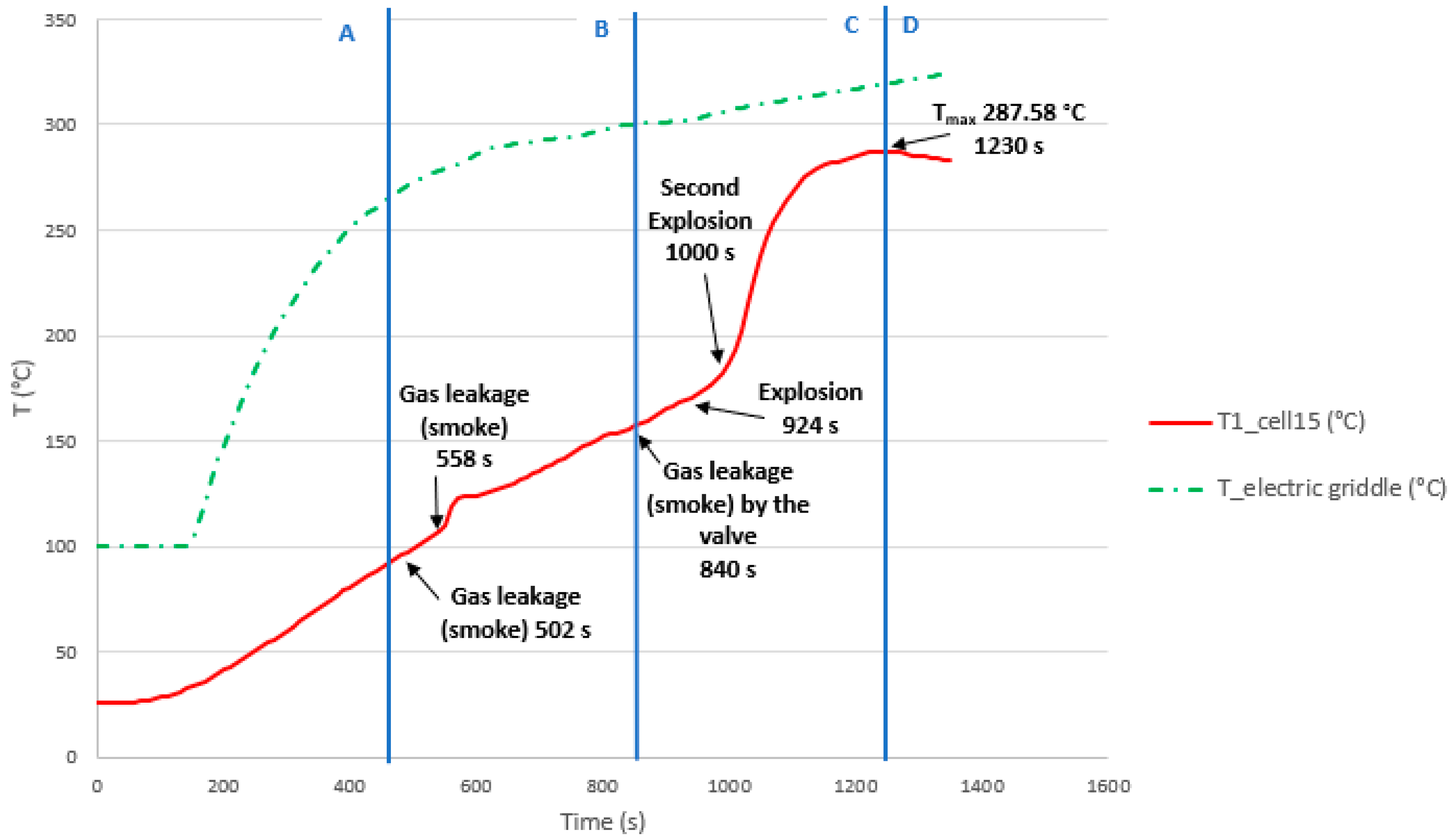
3.3.7. Test 7: Horizontal, Series Connection and SoC 50% (Cell 15 and Cell 14)
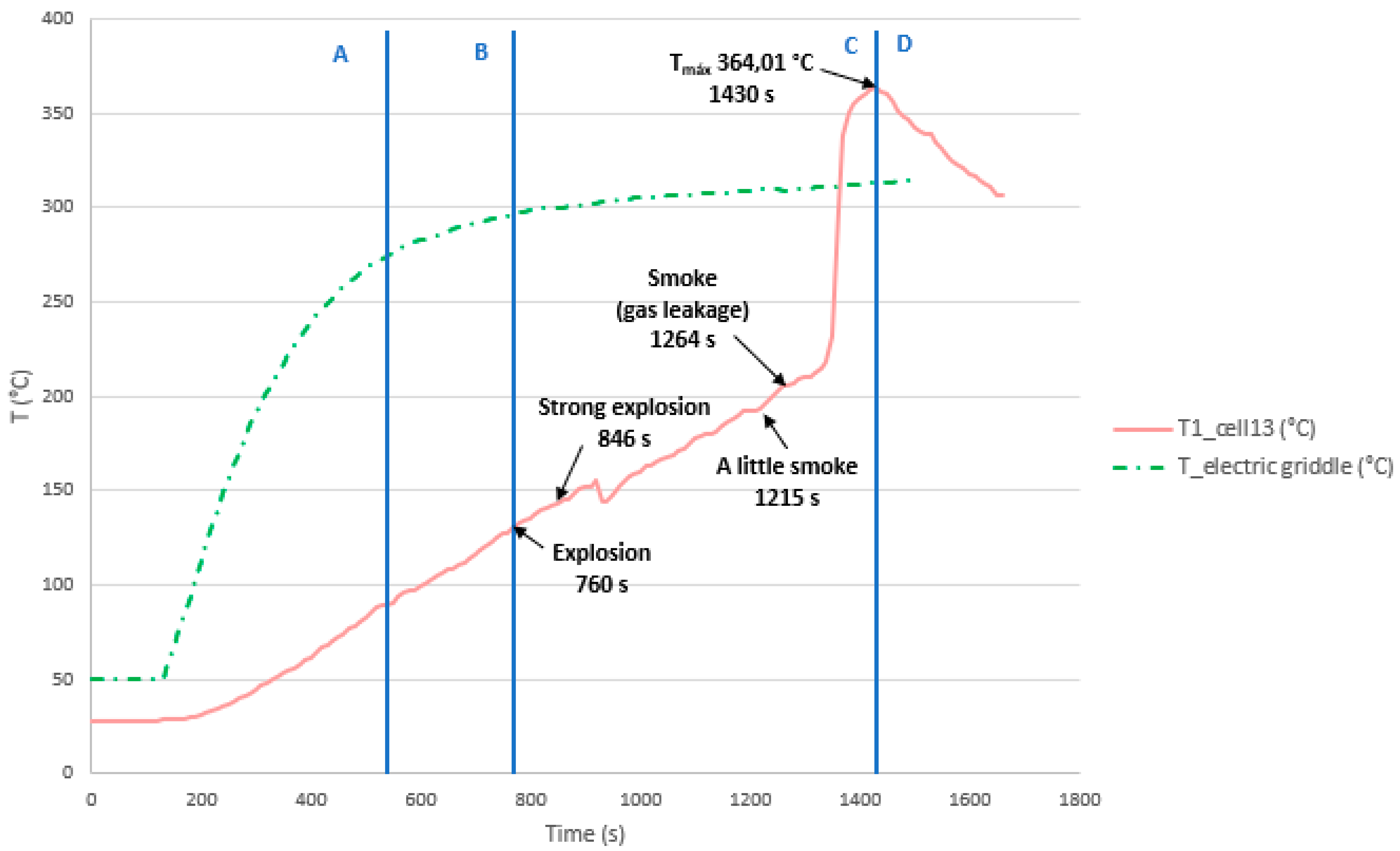
3.3.8. Test 8: Vertical, Series Connection and SoC 50% (Cell 13 (Below) and Cell 17)
4. Conclusions
- -
- The mechanical test of a cell does not faithfully reproduce the conditions of failure but shows the behavior of the cell under abusive conditions. It should be noted that cells are manufactured to be resistant or safe to some mechanical abuse, but it should be noted that a battery installed in a vehicle can suffer significant mechanical deformation during a traffic accident.
- -
- Mechanical abuse techniques remain a widely accepted method for safety and evaluation of the response of lithium-ion cells to abuse. However, it should be noted that changes in test conditions and even changes in cell construction can result in significantly different responses. Future lines of research should analyze the interdependence between test conditions and cell construction. Controlled and programmed mechanical abuse tests will be proposed to control certain test parameters.
- -
- The aim is to analyze how the cell structure influences the mechanical test and how the loading state of the cell influences the mechanical test.
- -
- In the case of mechanical abuse by perforation tests, it is concluded that a certain level of damage to the internal components of the cell (electrodes) is necessary for the short-circuit to occur.
- -
- It can be stated that, under the same mechanical abuse by perforation in a direction perpendicular to the longitudinal axis of the cell, the behavior of cells with higher SoC is more dangerous.
- -
- Both from the final state of the cell and from the analysis of the ΔDCIR (mΩ), it can be stated that the cell that suffers greater damage and is more degraded and deteriorated is the cell with an SoC of 100% subjected to a perforation test in the direction of the longitudinal axis of the cell, in the negative pole. The cell that suffers less damage and is less degraded is the cell subjected to a perforation test in the direction perpendicular to the longitudinal axis of the cell, with an SoC of 50%.
- -
- It is concluded that the loading condition is one of the most influential factors in the occurrence of Thermal Runaway after mechanical abuse by perforation.
- -
- It is therefore concluded that the worst case scenario for a failure due to mechanical abuse by perforation is that the cell/battery is 100% charged and the perforation occurs in the direction of the longitudinal axis.
- -
- After comparing the NMC cell and the LFP cell subjected to the same mechanical abuse by perforation, it is concluded that the NMC cell catches fire while the LFP cell does not; however, the maximum temperature reached by the LFP cell is higher than that reached by the NMC cell. This may have occurred because the tests were carried out at room temperature, and on the day the LFP cell was tested, the ambient temperature was higher than when the NMC cell was tested. On the other hand, the fact that the maximum temperature reached is lower in the case of the LFP cell than in the case of the NMC cell could be since the cells tested by mechanical abuse by perforation have different diameters, and there are studies that indicate that the size of the cells influences their behavior in the event of a thermal release. Therefore, a comparison should be made between NMC and LFP cells of the same diameter, subjected to the same perforation mechanical abuse test to analyze the temperature profile reached.
- -
- Overcharging an electric vehicle with a pouch cell battery pack (tested cells) at high temperatures, for example on a summer day at midday, is critical and dangerous. The same overcharge tests should be repeated at different ambient temperatures and with different cell types.
- -
- The maximum voltage is reached due to the overcharge is when the maximum temperature is reached and when the maximum swelling of the two cells is reached.
- -
- The results of the analysis of the overcharge tests with pouch cells give very important information from a safety point of view and can be useful to generate safe charging procedures for electric vehicle batteries to prevent possible dangerous misuse conditions.
- -
- The loss of internal material of the cells due to thermal tests by heating is variable according to different experimental conditions. It is observed that more internal material is melted in the case of cells with 100% SoC than in the case of cells with 50% SoC. In the case of 100% SoC, material ejection occurs with complete detachment of solid internal material, whereas in the case of 50% SoC, the amount of molten aluminum is very small.
- -
- The most dangerous situation for TR propagation due to thermal failure by heating is the vertical arrangement with the cells connected in parallel and with a 100% SoC. The safest situation is the horizontal arrangement with the cells connected in series and with an SoC of 50%.
- -
- The tests carried out allow conclusions about the maximum temperature reached during the TR, but not the time required to reach it, as they are conducted at ambient temperature. It is concluded that the ambient temperature influences TR propagation due to thermal failure by heating.
- -
- LFP cells react more slowly and at significantly higher temperatures to thermal abuse than other chemistries, making this cell chemistry considerably safer.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| TR | Thermal Runaway |
| SoC | State of Charge |
| BMS | Battery Management System |
| SEI | Solid Electrolyte Interface |
| EC | Cyclic Ethylene Carbonate |
| PC | Propylene Carbonate |
| BC | Butene Carbonate |
| DMC | Chain Dimethyl Carbonate |
| DEC | Diethyl Carbonate |
| EMC | Methyl Ethyl Carbonate |
| PP | Polypropylene |
| PE | Polyethylene |
| BTMS | Battery Thermal Management System |
| PCM | Phase Change Material |
| NiB | Sodium-ion battery |
| LFP | Lithium-ion phosphate battery |
| LFL | Lower Flammability Limit |
| LPB | Lithium-ion Prismatic Batteries |
| DC | Direct Current |
| CV | Constant Voltage |
| PID | Proportional Integral Derivative Control |
| DCIR | Direct Current Internal Resistance |
References
- Musk, E. Model S Fire; Tesla Blog: Austin, TX, USA, 2013; Available online: https://www.businessinsider.com/elon-musk-blog-post-on-tesla-fires-2013-11 (accessed on 30 January 2025).
- Voelcker, J.; Yanks, C. Plug-In Hybrid Test Fleet Off Roads, Will Replace Batteries, Green Car Reports. 2012. Available online: http://www.greencarreports.com/news/1079368_chrysler-yanks-plug-inhybrid-test-fleet-off-roads-will-replace-batteries (accessed on 30 January 2025).
- Lowy, J. Overcharging Batteries Eyed in Boeing 787 Mishaps; Phys.org: Douglas, GA, USA, 2013. [Google Scholar]
- He, D.; Wang, J.; Peng, Y.; Li, B.; Feng, C.; Shen, L.; Ma, S. Research advances on thermal runaway mechanism of lithium-ion batteries and safety improvement. Sustain. Mater. Technol. 2024, 410, e01017. [Google Scholar] [CrossRef]
- Richard, M.N.; Dahn, J.R. Accelerating Rate Calorimetry Study on the Thermal Stability of Lithium Intercalated Graphite in Electrolyte. I. Experimental. J. Electrochem. Soc. 1999, 146, 2068. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Larcher, D.; Dahn, J.R. Comparison of the Reactivity of Various Carbon Electrode Materials with Electrolyte at Elevated Temperature. J. Electrochem. Soc. 2019, 146, 3596–3602. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal stability of LiPF6/EC+DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta 2005, 437, 12–16. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2013, 4, 3633–3642. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes—Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Qin, P.; Jia, Z.; Wu, J.; Jin, K.; Duan, Q.; Jiang, L.; Sun, J.; Ding, J.; Shi, C.; Wang, Q. The thermal runaway analysis on LiFePO4 electrical energy storage packs with different venting areas and void volumes. Appl. Energy 2022, 313, 118767. [Google Scholar] [CrossRef]
- Wong, A.C.-Y.; Lam, F. Study of selected thermal characteristics of polypropylene/polyethylene binary blends using DSC and TGA. Polym. Test. 2002, 21, 691–696. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, L.; Feng, X.; He, X. Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries. J. Power Sources 2018, 378, 527–536. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Park, S.H.; Woo, S.I. Additional doping of phosphorus and/or sulfur into nitrogen-doped carbon for efficient oxygen reduction reaction in acidic media. Phys. Chem. Chem. Phys. 2013, 15, 1802–1805. [Google Scholar] [CrossRef]
- Rui, X.; Feng, X.; Wang, H.; Yang, H.; Zhang, Y.; Wan, M.; Wei, Y.; Ouyang, M. Synergistic effect of insulation and liquid cooling on mitigating the thermal runaway propagation in lithium-ion battery module. Appl. Therm. Eng. 2021, 199, 117521. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xiaoming, X.; Sun, X.; Li, R.; Zhang, Y.; Jiaqi, F. Numerical study on the inhibition control of Lithium-ion battery Thermal runaway. ACS Omega 2020, 5, 18254–18261. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge. J. Power Sources 2003, 119–121, 330–337. [Google Scholar] [CrossRef]
- Yang, H.; Bang, H.; Amine, K.; Prakash, J. Investigations of the Exothermic Reactions of Natural Graphite Anode for Li-Ion Batteries during Thermal Runaway. J. Electrochem. Soc. 2004, 152, A73. [Google Scholar] [CrossRef]
- Yang, H.; Zhuang, G.V.; Ross, P.N. Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Tangqin, W.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Botte, G.G.; White, R.E.; Zhang, Z.J. Thermal stability of LiPF6-EC:EMC electrolyte for lithium ion batteries. J. Power Sources 2001, 97–98, 570–575. [Google Scholar] [CrossRef]
- Abd-El-Latif, A.A.; Sichler, P.; Kasper, M.; Waldmann, T.; Mehrens, M.W. Insights into thermal runaway of Li–ion cells by accelerating rate calorimetry coupled with external sensors and online gas analysis. Batter. Supercaps 2021, 4, 1135–1144. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.; Jinmei, X.; Ning, F.; Wang, D. Investigation on gas generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Wendel, C.H. Design and Analysis of Reversible Solid Oxide Cell Systems for Electrical Energy Storage. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2015. [Google Scholar]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A Review of Lithium Ion Battery Failure Mechanisms and Fire Prevention Strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, M.; Li, Y.; Li, C.; Zhang, Y.; Chen, S.; Shen, H.; Qian, F.; Feng, X.; et al. Experimental study on Thermal runaway behavior of Lithium-ion battery and analysis of combustible limit of gas production. Batteries 2022, 8, 250. [Google Scholar] [CrossRef]
- Hewson, J.C.; Domino, S.P. Thermal Runaway of Lithium-Ion Batteries and Hazards of Abnormal Thermal Environments. In Proceedings of the 9th U.S. National Combustion Meeting, Cincinnati, OH, USA, 17–20 May 2015. [Google Scholar]
- Wang, Q.; Jiang, L.; Yu, Y.; Sun, J. Progress of enhancing the safety of lithium ion battery from the electrolyte aspect. Nano Energy 2019, 55, 93–114. [Google Scholar] [CrossRef]
- Kriston, A.; Kersys, A.; Antonelli, A.; Ripplinger, S.; Holmstrom, S.; Trischler, S.; Döring, H.; Pfrang, A. Initiation of thermal runaway in lithium-ion cells by inductive heating, 2020. J. Power Sources 2020, 454, 227914. [Google Scholar] [CrossRef]
- Chancelier, L.; Diallo, A.; Santini, C.; Marlair, G.; Gutel, T.; Mailley, S.; Len, C. Thermal Stability and Fire Safety of Ionic Liquid-Based Electrolytes for Lithium-Ion Batteries. ECS Meeting Abstracts 2014, MA2014-01, 41. [Google Scholar] [CrossRef]
- Mallick, S.; Gayen, D. Thermal behaviour and thermal runaway propagation in lithium-ion battery systems—A critical review. J. Energy Storage 2023, 62, 106894. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Feng, X.; Wang, L.; Ouyang, M.; Zhang, Q. Thermal stability of ionic liquids for lithium-ion batteries: A review. Renew. Sustain. Energy Rev. 2024, 207, 114949. [Google Scholar] [CrossRef]
- Dahn, J.R.; Ehrlich, G.M. Linden’s Handbook of Batteries; Reddy, T.B., Ed.; McGraw Hill: New York, NY, USA, 2011; pp. 26.1–26.79. [Google Scholar]
- Chai, Z.; Liu, Z.; Xue, Q.; Xiao, Y.; Tan, P.; Qiu, M.; Li, J. Efficient coupled mechanical-electrical-thermal modeling and safety assessment of lithium-ion battery under mechanical abuse. J. Energy Storage 2025, 114, 115917. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Gao, P.; Jiang, J.; Huang, A. Novel composite electrolyte additive for enhancing the thermal and cycling stability of SiO/C anode Li-ion battery. Process Saf. Environ. Prot. 2024, 189, 756–767. [Google Scholar] [CrossRef]
- Wang, H.; Lara-Curzio, E.; Rule, E.T.; Winchester, C.S. Mechanical abuse simulation and thermal runaway risks of large format Li-ion batteries. J. Power Sources 2017, 342, 913–920. [Google Scholar] [CrossRef]
- Jhu, C.-Y.; Wang, Y.-W.; Wen, C.-Y.; Chiang, C.-C.; Shu, C.-M. Self-reactive rating of thermal runaway hazards on 18650 lithium-ion batteries. J. Therm. Anal. Calorim. 2011, 106, 159–163. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, F.; Gao, Z.; Liu, M.; Wang, J.; Kou, Z.; Lin, Y.; Li, Y.; Gao, L.; Chen, Y.; et al. Review of mechanical abuse related thermal runaway models of lithium-ion batteries at different scales. J. Energy Storage 2023, 64, 107145. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kirston, A.; Omar, N.; Van den Bossche, P.; Boon-Brett, L. A review of international abuse testing standards and regulations for lithium ion batteries in electric and hybrid electric vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Z.; Mei, M.; Qiu, H.; Wang, Y. Numerical simulation study on two-phase flow of thermal runaway evolution and jet fire of 18650 lithium-ion battery under thermal abuse. Case Stud. Therm. Eng. 2024, 53, 103726. [Google Scholar] [CrossRef]
- Xia, Q.; Ren, Y.; Wang, Z.; Yang, D.; Yan, P.; Wu, Z.; Sun, B.; Feng, Q.; Qian, C. Safety risk assessment method for thermal abuse of lithium-ion battery pack based on Multiphysics simulation and improved bisection method. Energy 2023, 264, 126228. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Steele, L.A.M.; Spangler, S.W. Failure propagation in multi-cell lithium-ion batteries. J. Power Sources 2015, 283, 517–523. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, N.; Jiang, F. Study of the effect on spacing on thermal runaway propagation for lithium-ion batteries. J. Therm. Anal. Calorim. 2020, 140, 2849–2863. [Google Scholar] [CrossRef]
- Huang, P.; Wang, Q.; Li, K.; Ping, P.; Sun, J. The combustion behavior of large scale lithium titanate battery. Sci. Rep. 2015, 5, 7788. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, S.; Li, K.; Liu, C.; Cheng, X.; Zhang, H. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter. Power Sources 2015, 273, 216–222. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, D.; Chen, X.; Zhang, W.; Liu, J.; Yuen, R.; Wang, J. Investigation on the thermal hazards of 18650 lithium ion batteries by fire calorimeter. J. Therm. Anal. Calorim. 2015, 122, 755–763. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Roth, E.P.; Doughty, D.H. Thermal abuse performance of high-power 18650 Li-ion cells. J. Power Sources 2004, 128, 308–318. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal behavior of lithiated graphite with electrolyte in lithium-ion batteries. J. Electrochem. Soc. 2006, 153, A329–A333. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Zhang, S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard. Mater. 2020, 381, 11. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal Runaway Caused Fire and Explosion of Lithium Ion Battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Feng, Z.; Li, Y.; Ding, Y. Thermal management performances of PCM/water cooling-plate using for lithium-ion battery module based on non-uniform internal heat source. Appl. Therm. Eng. 2017, 126, 17–27. [Google Scholar] [CrossRef]
- Garg, M.; Tanim, T.R.; Rahn, C.D.; Bryngelsson, H.; Legnedahl, N. Elevated temperature for life extension of lithium ion power cells. Energy 2018, 159, 716–723. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. Mechanism of gases generation during lithium-ion batteries cycling. J. Electrochem. Soc. 2019, 166, A897–A908. [Google Scholar] [CrossRef]
- Wang, G.; Kong, D.; Ping, P.; He, X.; Lu, H.; Zhao, H.; Hong, W. Modeling venting behaviour of lithium-ion batteries during thermal runaway propagation by coupling cfd and thermal resistance network. Appl. Energy 2023, 334, 120660. [Google Scholar] [CrossRef]
- Fang, J.; Cai, J.; He, X. Experimental study on the vertical thermal runaway propagation in cylindrical Lithium-ion batteries: Effects of spacing and state of charge. Appl. Therm. Eng. 2021, 197, 11739. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Ouyang, M.; Wang, F.; He, X.; Lu, L.; Peng, H. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module. J. Power Source 2015, 275, 261–273. [Google Scholar] [CrossRef]
- Feng, X.; Lu, L.; Ouyang, M.; Li, J.; He, X. A 3D thermal runaway propagation model for a large format lithium ion battery module. Energy 2016, 115, 194–208. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental analysis of thermal runaway and propagation in lithium-ion battery modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Gao, S.; Feng, X.; Lu, L.; Kamyab, N.; Du, J.; Comsn, P.; White, R.E.; Ouyang, M. An experimental and analytical study of thermal runaway propagation in a large format lithium ion battery module with NMC pouch-cells in parallel. Int. J. Heat Mass Transf. 2019, 135, 93–103. [Google Scholar] [CrossRef]
- Gharehghani, A.; Rabiei, M.; Mehranfar, S.; Saeedipour, S.; Mahmoudzadeh-Andwari, A.; García, A.; Reche, C.M. Progress in battery thermal management systems technologies for electric vehicles. Renew. Sustain. Energy Rev. 2024, 202, 114654. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Yang, B.; Cai, S. A review of battery thermal management systems using liquid cooling and PCM. J. Energy Storage 2024, 76, 109836. [Google Scholar] [CrossRef]
- Qi, C.; Wang, H.; Li, M.; Li, C.; Li, Y.; Shi, C.; Wei, N.; Wang, Y.; Zhang, H. Research on the Thermal Runaway Behavior and Flammability Limits of Sodium-Ion and Lithium-Ion Batteries. Batteries 2025, 11, 24. [Google Scholar] [CrossRef]
- Yu, D.; Ren, D.; Dai, K.; Zhang, H.; Zhang, J.; Yang, B.; Ma, S.; Wang, X.; You, Z. Failure mechanism and predictive model of lithium-ion batteries under extremely high transient impact. J. Energy Storage 2021, 43, 103191. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J. Evaluation of mechanical abuse techniques in lithium ion batteries. J. Power Sources 2014, 247, 189–196. [Google Scholar] [CrossRef]
- Available online: https://www.agu.ch/1.0/crashtest-datenbank/ (accessed on 15 April 2024).
- Available online: https://euroncap.newsmarket.com/images-and-videos/all/nio-el6---euro-ncap-2024-results---5-stars/s/71368fe6-5ad6-49d2-bc72-ca95a589c4c9 (accessed on 15 April 2024).
- Wang, L.; Li, J.; Chen, J.; Duan, X.; Li, B.; Li, J. Revealing the internal short circuit mechanisms in lithium-ion batteries upon dynamic loading based on Multiphysics simulation. Appl. Energy 2023, 351, 121790. [Google Scholar] [CrossRef]
- Xi, S.; Zhao, Q.; Chang, L.; Huang, X.; Cai, Z. The dynamic failure mechanism of a lithium-ion battery at different impact velocity. Eng. Fail. Anal. 2020, 116, 104747. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Wang, Q. Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter. J. Energy Storage 2020, 28, 101232. [Google Scholar] [CrossRef]
- Williams, F.W.; Back, G.G. Lithium Battery Fire Tests and Mitigation; Naval Research Laboratory: Washington, DC, USA, 2014. [Google Scholar]
- Ouyang, D.; Chen, M.; Wang, J. Fire behaviours study on 18650 batteries pack using cone-calorimeter. J. Therm. Anal. Calorim. 2018, 136, 2281–2294. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Zhu, X.; Wang, H.; Huang, L.; Wang, Y.; Xu, S. Overchargeto-thermal-runaway behavior and safety assessment of commercial lithium-ion cells with different cathode materials: A comparison study. J. Energy Chem. 2021, 55, 484–498. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J.; Gao, T.; Mao, N. Heat generation and thermal runaway mechanisms induced by overcharging of aged lithium-ion battery. Appl. Therm. Eng. 2022, 212, 118565. [Google Scholar] [CrossRef]
- Ye, J.; Chen, H.; Wang, Q.; Huang, P.; Sun, J.; Lo, S. Thermal behavior and failure mechanism of lithium ion cells during overcharge under adiabatic conditions. Appl. Energy 2016, 182, 464–474. [Google Scholar] [CrossRef]
- Lalinde, I.; Berrueta, A.; Valera, J.J.; Arza, J.; Sanchis, P.; Ursúa, A. Perspective Chapter: Thermal Runaway in Lithium-Ion Batteries. In Lithium-Ion Batteries—Recent Advanced and Emerging Topics; Lamblin, D.G., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Kemeny, M.; Ondrejka, P.; Mikolasek, M. Comprehensive Degradation Analysis of NCA Li-Ion Batteries via Methods of Electrochemical Characterisation for Various Stress-Inducing Scenarios. Batteries 2023, 9, 33. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Theng, J.-H.; Chen, C.-C.; Kao, C.-S. Comparative study on thermal runaway of commercial 14500, 18650 and 26650 LiFePO4 batteries used in electric vehicles. J. Energy Storage 2020, 31, 101580. [Google Scholar] [CrossRef]
- Roth, E.P. Abuse response of 18650 Li-ion cells with different cathodes using EC:EMC/LiPF6 and EC:PC:DMC/LiPF6 Electrolytes. ECS Trans. 2008, 11, 19–41. [Google Scholar] [CrossRef]
- Lei, B.; Zhao, W.; Ziebert, C.; Uhlmann, N.; Rohde, M.; Seifert, H.J. Experimental analysis of thermal runaway in 18650 cylindrical Li-ion cells using an accelerating rate calorimeter. Batteries 2017, 3, 14. [Google Scholar] [CrossRef]
- Bugryniec, P.J.; Davidson, J.N.; Cumming, D.J.; Brown, S.F. Pursuing safer batteries: Thermal abuse of LiFePO4 cells. J. Power Sources 2019, 414, 557–568. [Google Scholar] [CrossRef]
- Lebkowski, A. Temperature, Overcharge and Short-Circuit Studies of Batteries Used in Electric Vehicles; Department of Ship Automation, Gdynia Maritime University: Gdynia, Poland, 2017. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, 1611–1617. [Google Scholar] [CrossRef]
- Feng, X.; Weng, C.; Ouyang, M.; Sun, J. Online internal short circuit detection for a large format lithium ion battery. Appl. Energy 2016, 161, 168–180. [Google Scholar] [CrossRef]
- Nedjalkov, A.; Meyer, J.; Kohring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic gas emissions from damaged lithium ion batteries, analysis and safety enhancement solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Sources 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Leising, R.A.; Palazzo, M.J.; Takeuchi, E.S.; Takeuchi, K.J. Abuse testing of lithium-ion batteries: Characterization of the overcharge reaction of LiCoO2/graphite cells. J. Electrochem. Soc. 2001, 148, A838–A844. [Google Scholar] [CrossRef]
- Fernandes, Y.; Bry, A.; De Persis, S. Identification and quantification of gases emitted during abuse tests by overcharge of a commercial Li-ion battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Yuan, Q.F.; Zhao, F.; Wang, W.; Zhao, Y.; Linag, Z.; Yan, D. Overcharge failure investigation of lithium-ion batteries. Electrochim. Acta 2015, 178, 682–688. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Endler, E.; Li, R.; Liu, L.; Li, Y.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Mele, M.L.; Bracciale, M.P.; Ubaldi, S.; Santarelli, M.L.; Mazzaro, M.; Di Bari, C.; Russo, P. Thermal Abuse Tests on 18650 Li-Ion Cells Using a Cone Calorimeter and Cell Residues Analysis. Energies 2022, 15, 2628. [Google Scholar] [CrossRef]
- Mikolajczak, C.; Kahn, M.; White, K.; Long, R.T. Lithium-Ion Batteries Hazard and Use Assessment. Exponent Failure Analysis Associates, Inc.: Menlo Park, CA, USA. Available online: https://content.nfpa.org/-/media/Project/Storefront/Catalog/Files/Research/Research-Foundation/Reports/Hazardous-materials/RFLithiumIonBatteriesPhaseIII.pdf?rev=a511861eecaa43b88a9cbc80d8395387 (accessed on 17 March 2024).
- Ohneseit, S.; Finster, P.; Floras, C.; Lubenau, N.; Uhlmann, N.; Seifert, H.J.; Ziebert, C. Thermal and Mechanical Safety Assessment of Type 21700 Lithium-Ion Batteries with NMC, NCA and LFP Cathodes–Investigation of Cell Abuse by Means of Accelerating Rate Calorimetry (ARC). Batteries 2023, 9, 237. [Google Scholar] [CrossRef]
- Brand, M.; Gläser, S.; Geder, J.; Menacher, S.; Obpacher, S.; Jossen, A.; Quinger, D. Electrical safety of commercial Li-ion cells based on NMC and NCA technology compared to LFP technology. World Electr. Veh. J. 2013, 6, 572–580. [Google Scholar] [CrossRef]
































| Cell Type | Parameters | Image |
|---|---|---|
| 32700 Cylindrical cell | 6.0 Ah, LiFePO4 3.2 V 19.2 Wh 32700 Format Manufacturer: HAIDI |  |
| Pouch AESC Nissan Leaf (2018) Battery structure: 24 modules 8 cells for each module | 56.3 Ah, NMC 523 3.65 V 205.49 Wh Manufacturer: Envision AESC |  |
| Cell Tested | Location of Perforation | State of Charge, SoC (%) |
|---|---|---|
| 32700 Cylindrical | Lateral zone | 100 |
| 32700 Cylindrical | Lateral zone | 50 |
| 32700 Cylindrical | Upper zone | 100 |
| 32700 Cylindrical | Upper zone | 50 |
| Cell Tested | Crate | SoC (%) | Tamb (°C) |
|---|---|---|---|
| Pouch cell | 1/5.6 C | 100 | Aprox 40 °C |
| Pouch cell | 1/5.6 C | 100 | Aprox 25 °C |
| Cell Tested | Layout | Type of Connection | State of Charge (SoC %) |
|---|---|---|---|
| 32700 Cylindrical | Horizontal | 2 connected in parallel | 100% |
| 32700 Cylindrical | Horizontal | 2 connected in parallel | 50% |
| 32700 Cylindrical | Horizontal | 2 connected in series | 100% |
| 32700 Cylindrical | Horizontal | 2 connected in series | 50% |
| 32700 Cylindrical | Vertical | 2 connected in parallel | 100% |
| 32700 Cylindrical | Vertical | 2 connected in parallel | 50% |
| 32700 Cylindrical | Vertical | 2 connected in series | 100% |
| 32700 Cylindrical | Vertical | 2 connected in series | 50% |
| Cell Tested | Place Where the Perforation Takes Place | State of Charge (SoC %) | Time in Which the TR Takes Place | Temperature at Which the Test Is Carried Out (Tamb) |
|---|---|---|---|---|
| 32700 Cylindrical Cell A | Side area | 100 | 210 s | 39 °C |
| 32700 Cylindrical Cell A | Side area | 50 | 80 s | 45 °C |
| 32700 Cylindrical Cell C | Upper area | 100 | 75 s | 44 °C |
| 32700 Cylindrical Cell B | Upper area | 50 | 140 s | 28 °C |
| Test | Start of the Test (mΩ) | End of the Test (mΩ) | ∆DCIR (mΩ) |
|---|---|---|---|
| Test 1_cell A Horizontal SoC 100% | 7.199 | 1145 | 1137.80 |
| Test 2_cell D Horizontal SoC 50% | 7.280 | 953.3 | 946.02 |
| Test 3_cell C Vertical SoC 100% | 6.986 | 2320 | 2313.014 |
| Test 4_cell B Vertical SoC 50% | 7.500 | 1505 | 1497.500 |
| Type of Cell | Parameters | Image |
|---|---|---|
| Cylindrical 18650 BT-4000 | 4.0 Ah, NMC 4.2 V 16.8 Wh 18650 format Manufacturer: DigiVolt |  |
| Cell Tested | Crate | State of Charge (SoC %) | Tamb | Maximum Cell Temperature Tmax |
|---|---|---|---|---|
| Pouch Cell | 1/5.6 C | 100 | 40 °C | 62.30 °C |
| Pouch Cell | 1/5.6 C | 100 | 25 °C | 43.92 °C |
| Test | Start of the Test (mΩ) | End of the Test (mΩ) | ∆DCIR (mΩ) |
|---|---|---|---|
| Test 1_pouch cell 1 Tamb = 40 °C | 1.61 | 191.4 | 189.79 |
| Test 2_pouch cell 2 Tamb = 25 °C | 1.52 | 50.62 | 49.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olona, A.; Castejón, L. Influence of the Layout of Cells in a Traction Battery on the Evolution of a Fire in the Event of a Failure. Processes 2025, 13, 889. https://doi.org/10.3390/pr13030889
Olona A, Castejón L. Influence of the Layout of Cells in a Traction Battery on the Evolution of a Fire in the Event of a Failure. Processes. 2025; 13(3):889. https://doi.org/10.3390/pr13030889
Chicago/Turabian StyleOlona, Ana, and Luis Castejón. 2025. "Influence of the Layout of Cells in a Traction Battery on the Evolution of a Fire in the Event of a Failure" Processes 13, no. 3: 889. https://doi.org/10.3390/pr13030889
APA StyleOlona, A., & Castejón, L. (2025). Influence of the Layout of Cells in a Traction Battery on the Evolution of a Fire in the Event of a Failure. Processes, 13(3), 889. https://doi.org/10.3390/pr13030889






