Abstract
Exopolysaccharides (EPSs) represent versatile biopolymers finding diverse applications in food, pharmaceuticals, and bioremediation industries. Extremophiles, thriving under extreme environmental conditions, have emerged as a promising source of novel EPSs with better stability and bioactivity. The present work reviews the complex influence of various abiotic factors and bioprocess parameters such as temperature, pH, carbon and nitrogen sources, C/N ratios, and oxygen transfer dynamics on the production of EPSs from extremophilic microorganisms. Results underline the important role of temperature for structural and functional properties of EPSs, from the synthesis of cryoprotective polymers in psychrophiles to the production of thermostable EPSs in thermophiles under cold stress. The pH has an extensive effect on enzymatic activities: optimal neutral to slightly acidic conditions exist for most strains. Carbon sources determine not only the yield of EPSs but also its structural features, while nitrogen sources and C/N ratios regulate the balance between biomass production and polymer biosynthesis. Besides that, oxygen transfer limitations—which may happen in particularly viscous or saline media—are overtaken by optimized bioreactor configuration and stirring strategies. These findings are highly relevant to the development of tailored cultivation conditions enabling the maximization of EPS yields and adaptation of its properties to comply with industrial requirements. This study provides a framework for enhancing EPS production by leveraging the adaptive traits of extremophiles. This approach supports the sustainable use of biopolymers, advances fermentation production processes, and helps uncover the underlying mechanisms involved.
1. Introduction
Polysaccharides are complex carbohydrates formed by the linkage of multiple monosaccharide units through glycosidic bonds, playing essential roles in structural support, energy storage, and cellular communication in living organisms. Based on the cellular location, polysaccharides can be categorized into cell wall polysaccharides, exopolysaccharides, and endopolysaccharides. In addition, they have been further classified into two categories based on the sugar composition: homopolysaccharides—excellent examples are starch and levan—and heteropolysaccharides, including xanthan and gellan, among others [1,2]. Exopolysaccharides (EPSs) are high-molecular-weight biopolymers synthesized by a variety of microorganisms: bacteria, fungi, and archaea. These biologically derived macromolecules have critical functions in microbial survival, biofilm formation, and stress response. EPSs have gained wide interest from the scientific community not only due to their natural origin but also due to the fact that they exhibit excellent properties such as nontoxicity, biodegradability, and versatility for many applications [3]. The structural diversity of EPSs, determined by their monosaccharide composition and branching patterns, gives them unique physicochemical properties. These properties include viscosity, emulsifying capabilities, and stability under extreme conditions. In fact, these properties make EPSs valuable in food, pharmaceutical, cosmetic, agricultural, and bioremediation applications. As such, despite great potential for industrial applications, the large-scale production of EPSs still represents a challenge due to the complex interplay of microbial physiology, substrate availability, and environmental factors impelling the biosynthesis processes. Therefore, addressing these challenges is essential to improve production processes and fully harness the commercial potential of these molecules.
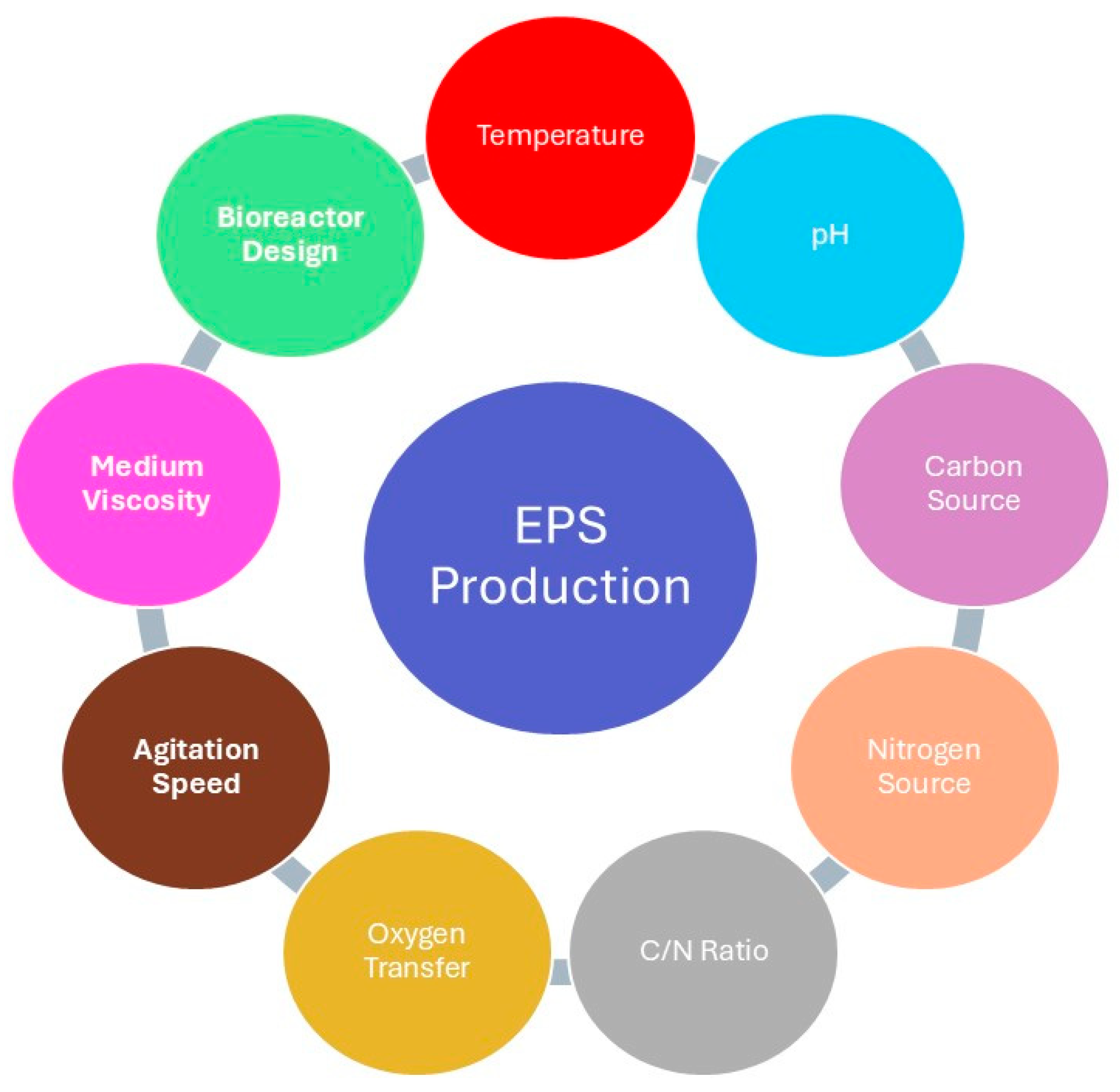
The synthesis of EPSs is highly influenced by both the microbial species and the environmental conditions under which they are cultivated. Surprisingly, optimum parameters for microbial growth may not be suitable conditions that will support the production of EPSs. Key factors that generally affect the yield, composition, and rheological properties of EPSs are sources of carbon and nitrogen, essential mineral salts, vitamins, temperature, pH, rates of agitation, and aeration (Figure 1). Apart from being available, the carbon source type, i.e., glucose, sucrose, and lactose, influences the rate of synthesis of the polymer and its structural attributes. On one hand, the nitrogen source such as ammonium sulfate or peptone, can provoke changes in metabolic pathways that will affect the overall yield and molecular weight of the produced EPS [4,5,6,7]. In the bioreactor systems, vessel design, the type of mixing device, and the strategy of aeration are critical parameters for the optimization of EPS production. For some, increased stirring rates enhance the dissolved oxygen availability, while, for others, oxygen limitation might be used to create stress responses, which turn on increased EPS synthesis. It further emphasizes that specific cultivation conditions have to be optimized with regard to the metabolic needs of the cultivated microorganism. The synthesis of EPS in some bacteria, such as Leuconostoc mesenteroides and Bacillus spp., is induced by specific substrates without requiring cellular uptake, thus showing a substrate-dependent biosynthetic pathway [8].
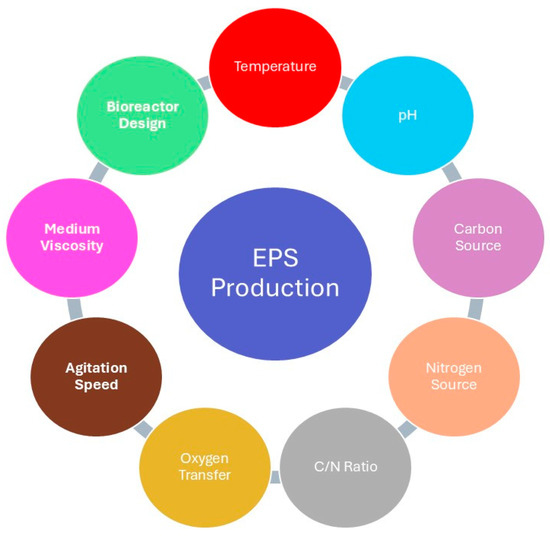
Figure 1.
Factors affecting EPS production.
Extremophiles, microorganisms that thrive in extreme environments such as high temperatures, high salinity, low pH, or high radiation, represent a promising yet underexplored source of novel EPSs [9]. In general, the distinct structural features and functional robustness acquired through their evolution under extreme conditions make the extremophiles’ EPSs highly promising regarding enhanced stability and novel bioactivities [10]. Accordingly, thermophilic bacteria, which grow well at elevated temperatures, usually produce heat-resistant EPSs that could be of great importance in high-temperature-linked industrial applications. In the same manner, halophilic microorganisms originating from hypersaline environments are capable of synthesizing highly viscous and highly ionically strong EPSs, which could be of great value as thickening and stabilizing agents in the food and cosmetic industries. The synthesis of EPSs in extremophiles is not only influenced by standard cultivation parameters but also by special extremophilic features of the host organism [11]. Thus, for example, it has been observed that nutrient limitations, especially of nitrogen, phosphorus, or sulfur, increase EPS production in marine bacteria, showing a biosynthetic pathway induced by stress responses [12]. High salinity, osmotic pressure, desiccation, and temperature changes can also lead to increased EPS production, as these polymers often have protective functions against environmental stresses. The ability to produce EPS may be an important strategy toward survival in extremophiles, probably forming biofilms that could serve as physical barriers against adverse conditions and also participate in the maintenance of cell integrity. Furthermore, EPSs from extremophiles often contain characteristic chemical compositions, including less common sugars such as mannose, rhamnose, and fucose, and also non-carbohydrate substituents like acetyl, pyruvyl, and sulfate groups [13]. The unique functional properties, including increased thermal stability, the ability to form gels, and the ability to bind metal ions, further increase their biotechnological potential, aside from exhibiting several promising biomedical applications in drug delivery systems, wound healing, and as bioactive molecules with antiviral or antioxidant activities [14].
While the biotechnological potential of extremophiles’ EPSs is undoubted, some obstacles need to be resolved for large-scale production. The optimization of substrates, temperature, and pH variables of growth will be important for attaining the maximum recovery of EPSs. Similarly, the knowledge of regulatory mechanisms existing in biosynthesis of EPS in extremophiles would also help devise strategies of metabolic engineering for enhanced productivity. Advances in these omics techniques, like genomics, transcriptomics, and proteomics, are bound to increase the knowledge about genetic and enzymatic pathways of interest in the synthesis of EPS and, thus, will allow rational designs for microbial production systems.
EPSs represent a class of biopolymers with huge ecological and industrial relevance. The screening of extremophiles to produce novel EPSs has been highly promising so far as regards the discovery of new molecular structures featuring unique properties. Further studies on overcoming existing bottlenecks in their production and tapping the adaptive traits of extremophiles might open new horizons toward these sustainable, eco-friendly materials in most sectors.
To sum up, extremophilic microorganisms have been identified as valuable EPS producers due to their ability to survive in harsh environments. Various strains from different ecological niches have been studied for their EPS production under specific environmental conditions. Table 1 provides a summary of extremophilic microorganisms, their sources of isolation, testing conditions, EPS yields, and the effects of changing environmental factors on production.

Table 1.
Extremophilic microorganisms and their EPS production: sources, testing conditions, and influencing factors.
2. The Effect of Temperature on EPS Production in Extremophiles
Temperature is among the important environmental factors with a remarkable impact on extremophiles, which can produce significant changes in their pattern of growth, metabolic activities, as well as EPS yield [27]. Microbes are found to thrive under extreme conditions, and their EPS-synthesizing ability is always associated with their strategies to survive and adapt to an environment [28]. It is not just the amount but also the structural and functional characteristics of EPS that depend on the temperature at which it is synthesized [29]. While psychrophiles and psychrotolerant bacteria optimize EPS synthesis at low temperatures to cryoprotect themselves [30], thermophiles produce EPSs to withstand high temperatures and other associated stresses, such as pressure and salinity [31]. This section compiles information from a variety of studies in order to present an overview on the effect that temperature has on EPS production by extremophiles.
2.1. EPS Production at Low Temperatures
Low temperatures impose a variety of stresses on microbial life, including reduced enzymatic activity, changes in membrane fluidity, and the potential for ice formation. Consequently, many psychrophilic and psychrotolerant microorganisms produce EPS in response to low temperatures as a form of cryoprotection. These biopolymers perform very essential roles in the maintenance and preservation of cellular integrity under these harsh conditions imposed by the freeze–thaw cycles. They are also important in the stabilization of extracellular environments, which could be essential for the survival of cells. In addition, these biopolymers are capable of inhibiting the formation of ice through active modification of the microstructure of accumulated ice [32]. Besides these functions, EPSs play important roles in desalination processes occurring in icy habitats. This role enhances and improves further the survival conditions for organisms living in such extreme environments [33]. One of the EPSs from Leucosporidium antarcticum showed anti-freezing activity [34], indicating that these molecules play an important role in cryoprotection at very low temperatures. Additionally, there are two very vital adaptive mechanisms by which microorganisms survive under cold conditions. Two important adaptive mechanisms that enable microorganisms to survive at low temperatures are the overexpression of cold-shock proteins and the increased biosynthesis of extracellular polysaccharides [35,36]. These EPSs play a critical role not only in affording protection to microbial cells from the damaging effects of freezing, but they also play a significant role in ensuring that the cellular functions are maintained and retained effectively throughout the repeated freeze–thaw cycles that may be experienced. For instance, it has been reported in studies that the survival rates of the bacterial strain Escherichia coli K12 have been significantly enhanced when there is an increase in the concentration of EPSs from a low level of 1% to a much higher level of 5% (weight/volume) during the process of undergoing repeated freeze–thaw cycles [17]. This underlines the role of EPSs in increasing microbial resistance to temperature variation, by stabilization of their cellular environments and mitigation of injurious effects caused by the formation of ice.
2.1.1. Enhanced EPS Production Under Cold Stress
Psychrophilic bacteria produce more EPS at suboptimal growth temperatures. For example, Colwellia psychrerythraea 34H produced much more EPSs in the range of −8 °C and −14 °C, especially under stress conditions such as high salinity or pressure [15]. Casillo et al. [37] carried out a detailed study on how temperature variation affects EPS synthesis in the psychrophilic bacterium C. psychrerythraea 34H. Their comprehensive study showed that C. psychrerythraea 34H modulates the composition of EPS through unique and distinct biochemical pathways as a function of temperature variation. Such intricate modulation has significant implications in determining not only the structural components of fatty acids that make up the organism but also the quantity of biopolymers produced by the organism. Similarly, Pseudoalteromonas sp. CAM025 produced significantly higher amounts of EPS at lower temperatures, with a yield of approximately 97.2 mg/g (dry weight) of cell material at −2 °C and 99.9 mg/g (dry weight) at 10 °C, which is approximately 30-fold higher than the EPS yield at 20 °C (3.6 mg/g dry weight) [16]. Notably, the EPS produced at −2 °C and 10 °C exhibited a significantly higher uronic acid content, which may contribute to its polyanionic nature. This characteristic enhances its potential role in metal ion binding, particularly in environments like the Southern Ocean, where trace metal availability, such as iron, is a limiting factor for primary productivity. Additionally, the increased EPS production at suboptimal temperatures suggests a cryoprotective function, helping bacteria survive in the extreme salinity and freezing conditions of sea ice brine channels.
The effect of temperature on EPS production by cold-adapted Pseudomonas sp. BGI-2 was studied by another research group [17]. The maximum EPS production was at 15 °C, and higher temperatures (25–35 °C) stimulated growth but lowered EPS production, with no EPS produced at 35 °C and above. This suggests that the optimum temperature for growth is different from the optimum conditions required for EPS biosynthesis, characteristic of psychrotrophic and psychrophilic microorganisms. Both temperatures (4 °C and 15 °C) seem to elicit a stress response in Pseudomonas sp. BGI-2, resulting in higher productions of EPS. This may be concordant with the role of EPS as a cryoprotectant, enabling the bacterium to survive during freezing/thawing conditions in the glacial environment.
A recent study investigated the effect of temperature on EPS production by Tetragenococcus halophilus [18]. The low temperature (20 °C) remarkably increased the yield of EPS, while higher temperatures favored cell growth without increasing EPS accumulation, clearly indicating different optimal temperatures for growth and EPS synthesis. Low temperatures shifted the metabolic pathway to increase the key gene expression for EPS biosynthesis, as shown by transcriptomic analysis. This was associated with enhanced glycolysis and carbohydrate metabolism, which might increase nucleotide sugar production for EPS synthesis. Structural properties of EPS were altered at lower temperatures, which changed the molecular weight and monosaccharide composition, increased mannose, and decreased rhamnose glucuronic acid. The results indicated that temperature significantly influences the yield and characteristics of EPS. These findings suggest a temperature-shift strategy: cultivation at an initial high temperature increases biomass growth, and then, the shift to lower temperatures promotes EPS production. This increased the yield of EPS by 28% and hence gives insights into the optimization of the production of EPS in industries.
2.1.2. Structural and Functional Adaptations
Exopolysaccharides synthesized at a low temperature usually possess a number of structural features: higher molecular weight with resultant increased viscosity and higher enrichment in sugar monomers like glucose and mannose. With this concern, one may indicate a case of the microorganism Tetragenococcus halophilus. In culture at 20 °C, it showed big differences in the monosaccharide composition rate, especially with much higher mannose content, though giving a lower amount of rhamnose. In this respect, gene expression analyses performed within the framework of the study have revealed considerably increased expressions of genes directly related to glycolysis and carbohydrate metabolism under cold-stressed conditions. This factor is important to increase the amount of nucleotide sugar precursors required by the biosynthesis of EPS [34]. Similarly, the EPS of bacterium Pseudomonas sp. BGI-2 grown at 15 °C revealed very high cryoprotective properties [17]. Its production improved the survival of the bacteria during subsequent freeze–thaw cycles. These findings further establish a case for the dual role played by EPS in maintaining cellular stability apart from promoting survival in the bacterial cells undergoing freezing stress.
2.1.3. Industrial Implications of Cold-Derived EPSs
EPSs produced at low temperatures have some unique cryoprotective properties, with distinct structural features due to their low-temperature-producing conditions that significantly help them in gaining utility value in various biotechnological applications. High-viscosity EPSs produced by psychrophilic microorganisms, for instance, represent tremendous potential as far as cryopreservation is concerned. Such EPSs play an indispensable role of stabilizers in frozen systems and render biological tissues and cells long-term protection, thus safeguarding them against the negative effects imposed by both freezing and the succeeding thawing process [38].
These EPSs have this amazing characteristic of acting as stabilizers that greatly contribute to improving the texture and stability in a number of products, such as consumable products like ice cream and other frozen desserts [39]. In addition, inherent characteristics in these biopolymers make them suitable in various industrial applications. For these applications, the materials are supposed to maintain their functionality effectively below freezing temperatures and be used, for example, as lubricants and antifreeze solutions [40,41].
Besides this, cold-derived EPSs hold immense promise in terms of ecological usage based on their cryoprotective nature alone: such substances have the ability to inhibit the formation of ice on a wide range of surfaces, which could be particularly useful in de-icing operations within the aviation and transport industries [42]. Moreover, in agricultural application, these biopolymers could be used in the desalination of frozen soils to obtain better quality of soil, increasing the likelihood of more improved plant growth during colder climates where, mostly, the conditions are not apt for such plant development.
The ability of psychrophilic microorganisms to synthesize such EPSs, maintaining functionality under such extreme conditions, really underscores their industrial and ecological relevance. This field of research and development has huge potential to develop new applications and improve processes in various other industries as well [9].
2.2. EPS Production at High Temperatures
Thermophilic microorganisms usually thrive at extremely high temperatures, generally above 45 °C, and are often combined with other stress factors such as high salinity, pressure, and heavy metal toxicity. Under such conditions, EPS production acts as a protective mechanism for the cells to resist environmental stresses and also stabilizes them in a way to make them more resistant.
2.2.1. Optimal Temperature for EPS Synthesis
For many thermophiles, the temperature that supports optimal growth also maximizes EPS production. For example, Rhodothermus marinus DSM 16675 grows best at about 65 °C, a condition required for good growth, effective substrate utilization (for example, glucose), and metabolic activity driving EPS synthesis [19]. This study showed that maximum EPS production occurred during the stationary phase, which is indicative of higher enzymatic activity and metabolic efficiency associated with high-temperature environments. These conditions not only optimize bacterial proliferation but also favor the biosynthesis and functional refinement of EPS, as attested to by the appearance of key structural features: O–H stretching and sulfate esters. The correlation of cell dry weight with EPS production underlines the pivotal role of temperature in supporting both bacterial growth and metabolite synthesis. These results, therefore, point out high temperatures as a very important factor in maximizing the efficiency and functionality of EPS production by R. marinus DSM 16675.
Similarly, Brevibacillus thermoruber exhibited maximum EPS production at 55 °C, with yields correlating strongly with biomass levels [20]. This study focuses on temperature influences on exopolysaccharide (EPS) production by B. thermoruber strain 423 during controlled fermentation. EPS production was found to be strictly temperature-dependent. The optimal temperature was observed at 55 °C for both maximum EPS yield and biomass production, indicating the strong interdependence of growth on EPS synthesis in this thermophilic bacterium. The results show that B. thermoruber appears to be growth-associated as its yield increases proportionally with biomass during exponential and stationary growth.
The effect of temperature on production and properties of EPS was studied in Geobacillus thermodenitrificans and related strains [21]. The optimal production of EPS occurred at 65 °C with maximum microbial biomass and yields of EPS at this temperature. This finding underscores the thermophilic nature of the bacteria, where elevated temperatures enhance metabolic activity and biosynthesis. On the other hand, it was observed that the production of EPS is significantly reduced when the temperature was either higher or lower than this optimum point. The observation of the study clearly shows that thermal precision plays a vital role in regulating the yield to its maximum potential.
Studies have shown that temperature also affects EPS production in other extremophiles. For instance, the influence of temperature was examined on exopolysaccharide (EPS) production during cobaltiferrous pyrite bioleaching by the BRGM-KCC microbial consortium [43]. The consortium consists of Leptospirillum ferriphilum BRGM1, Acidithiobacillus caldus BRGM3, and Sulfobacillus benefaciens BRGM2, as identified through 16S rRNA gene sequencing. These thermophilic and acidophilic microorganisms play crucial roles in bioleaching processes, contributing to metal solubilization and adaptation to harsh environmental conditions. EPS production, measured as free sugars, was rather insensitive to temperature changes in the first two reactors (R1 and R2), where sugar concentrations remained more or less stable within a range of 35 °C to 45 °C. In the last two reactors (R3 and R4), sugar concentrations dropped off significantly at higher temperatures, especially at 45 °C. This decrease in EPS concentration at high temperatures may be the outcome of a combination of factors, including reduced bacterial numbers, altered metabolic productivity, changes in bacterial community composition, precipitation with iron oxides, or consumption by heterotrophic bacteria. The last two would lead to an underestimation of actual EPS production. Overall, temperature had a small but noticeable effect on EPS production, more so in the later stages of the bioleaching process, thus further underlining the complex interactions between microbial activity and environmental conditions in continuous bioleaching systems [43].
2.2.2. Thermal Stability of EPSs
Among all those features, one of the truly defining and distinguishing ones that characterizes the EPSs produced by thermophilic organisms must have exceptional thermal stability. For instance, the EPSs synthesized by these specific strains, such as G. thermodenitrificans and some Bacillus sp. strains, have notably shown to remain intact at degradation temperatures even higher than an impressive threshold of 300 °C [44]. The reason for such a remarkable stability can thus be traced back to an incredibly special and unique composition, particularly with notably higher levels of certain essential sugars like mannose, galactose, and glucose. Through detailed analyses of the structure, quite a number of functional groups have been identified, including glycosidic linkages and sulfate esters. These specific groups contribute considerably to improving the resilience and increasing the durability of these kinds of biopolymers, as observed by another research group [23].
2.2.3. Metabolic Efficiency and Cost-Effectiveness
Thermophilic EPS production leverages the inherent metabolic efficiency of thermophiles, which are optimized to thrive and function at elevated temperatures. Compared to their mesophilic counterparts, these organisms do exhibit faster metabolic rates and, therefore, can make better use of substrates, synthesizing EPS in fermentation times much shorter than the latter [23]. This shortened production cycle not only increases productivity but also lowers operational expenses due to less energy and resource use per unit of product.
One more important benefit of thermophilic processes is their tolerance to contamination [45]. Most mesophiles are inhibited in the growth by high-temperature conditions, which therefore provide fewer requirements for sterilization and costly antimicrobial measures. This intrinsic robustness makes thermophilic EPS production very reliable and efficient under large-scale industrial operations.
Furthermore, thermophiles have enzymes and metabolic pathways stable under extreme temperatures; therefore, cells could exhibit the normal and required functionality when needed by processes with extended or long periods of production cycles [46]. All these make the thermophilic EPS process economically viable, which could meet the requirements of industries related to food, pharmaceutical, and biotechnological companies for high efficiency and productivity.
2.3. Comparative Insights and Ecological Relevance
The psychrophilic and thermophilic EPS production strategies underline the great ability of extremophiles to adapt to their particular environmental niches. In psychrophiles, exopolysaccharide (EPS) synthesis mainly constitutes a cryoprotective system. Such EPSs prevent the damaging actions of freezing temperatures by ice crystal formation inhibition, the preservation of cellular hydration, and the stabilization of enzymes and membranes [47]. The resulting polymers are usually characterized by specific structural features, such as hydrophilicity and specific arrangements of monosaccharides, which enhance their functional roles in icy environments. Such adaptations, in addition to sustaining single cells, can stabilize and protect entire microbial communities, permitting them to form biofilms and retain nutrients within subzero ecosystems.
This is specially marked in the case of thermophiles because the rapid biosynthesis of EPS—a heat-stable polymer—certainly attests to its adaptation in high-temperature environments. Higher temperatures favor the formation of more rigid molecular configurations. For instance, high-molecular-weight polysaccharides and special glycosidic linkages, like those described by Kambourova et al. [48], resist thermal depolymerization. Geothermal springs and industrial reactors are some other areas where the extra benefits of such EPSs can be conferred when the requirement for high heat resistance is necessary. Their thermostability is of interest in biotechnological applications where stable and functional biomaterials are felt to be needed.
In addition, the temperature-dependent structural and functional modulation of EPSs underlines their ecological and adaptive significance. In psychrophiles, EPSs are crucial to their life at freezing and desiccating conditions; hence, they ensure ecosystem stability in polar and glacial environments. In addition to providing protection against thermal stress to the cells, such polymers in thermophiles also contribute to biofilm formation, attachment to various surfaces, and the mediation of interactions between organisms in hot environments. That would be self-explanatory; this duality of functionality in the environmental resilience and structural versatility of EPSs defines their criticality for survival and ecological success at these extreme temperatures.
2.4. Industrial and Biotechnological Potential
The unique properties of temperature-adapted EPSs present diverse applications in biotechnology. Cold-derived EPSs, with their cryoprotective and stabilizing properties, are valuable in cryopreservation and ecological remediation. Conversely, thermophilic EPSs, with their thermal stability and rapid production, are ideal for industrial processes requiring heat-resistant polymers.
The ability to tailor cultivation conditions, for example, by applying temperature-shift strategies (first high temperature for growth and then low temperature for EPS production), provides further flexibility in the optimization of yield and functionality. This approach has improved the EPS yield up to 28% in the case of Tetragenococcus halophilus and thus holds potential for process improvement [18].
Moreover, the referenced study on T. halophilus highlighted the multifaceted role of temperature in modulating both the yield and structural properties of EPS. Clearly, EPS production was enhanced under low-temperature stress (20 °C), likely due to a metabolic shift that increased the expression of key genes involved in EPS biosynthesis. Transcriptional analysis showed that the upregulation in glycolysis and carbohydrate metabolism pathways made more nucleotide sugars available—the required precursors for EPS synthesis. Furthermore, this study showed that temperature affects the structural characteristics of EPS. Lower temperatures increased the molecular weight and altered the monosaccharide composition, with higher levels of mannose and reduced levels of rhamnose and glucuronic acid. Such compositional change would further expand the potential application of EPS in industries requiring specific structural properties for food stabilization, bioremediation, and biopolymer development. Based on these findings, integrating temperature-shift cultivation strategies will enable industries to fine-tune their processes, enhancing both the yield and the structural and functional properties of EPS to meet the specific demands of various biotechnological and industrial applications.
In conclusion, temperature is one of the pivotal factors responsible for EPS production in extremophiles. Psychrophiles and thermophiles reveal different strategies related to EPS synthesis, obviously reflecting their adaptation to stresses in the environment. From these insights, industries and researchers could devise optimal cultivation processes to achieve the maximum yield of EPS with the desired properties of biopolymers for a wide range of applications, starting from cryoprotection to high-temperature industrial processes.
3. The Effect of pH on EPS Production in Extremophiles
pH is one of the most important parameters that generally influences microbial growth and metabolism. The synthesis and properties of exopolysaccharides are affected by pH. The optimization of pH supports enzymatic activity and metabolic pathways involved in the production of EPSs, besides maintaining the structural and functional integrity of produced biopolymers [49]. This section discusses effects of pH on the synthesis of EPS in various microorganisms and highlights optimal conditions with challenges posed by extreme pH values.
3.1. pH Sensitivity and Optimal Ranges for EPS Production
pH has a direct influence on microbial growth and metabolic pathways contributing to EPS production [50]. Most studies have defined an optimal pH range for maximum EPS production.
Alkalibacillus sp. w3: This alkaliphilic strain showed maximum growth and EPS yield at pH 9–11, while growth and EPS synthesis were completely inhibited below pH 8. The result underlines the strong pH dependence of polymer synthesis in alkaliphilic microbes [51].
Pseudomonas sp. BGI-2, a psychrotrophic bacterium isolated from the Batura Glacier, Pakistan, showed maximum EPS production at pH 6 with a considerable yield of 273 mg/L. Further, the results revealed that production significantly reduced upon the exposure of bacterium under extreme pH values at 4 and 11, respectively. The finding clearly explains that both acidic as well as highly alkaline conditions can inactivate enzymatic activities as well as disrupt cell membrane integrity [17].
Tetragenococcus halophilus: The results of this particular study have clearly shown that the highest yield of EPS is obtained by maintaining the pH at 6. This would indicate that mild acidification could increase the enzyme activities and the metabolic ones. It was further noted that EPS synthesis was grossly reduced when the pH deviated from the range, especially below pH 4 and above pH 7. Such results emphasize the importance of precise pH control in fermentation processes [18].
Brevibacillus thermoruber 423: In this thermophilic strain, detailed studies have indicated that the pH value for optimal exopolysaccharide production was 6.5, with a yield of 650 mg/L. Of interest, whereas the biomass levels had relatively stable measurements between pH 6.0 and 7.0, the EPS yields were very sensitive to changes in pH, and a sharp decrease was observed when the pH dropped out of the mentioned range. Such a finding strongly suggests that the metabolic pathways involved in polysaccharide biosynthesis are more sensitive to pH conditions than those pathways related to cellular growth [20].
Rhodothermus marinus DSM 16675, a notable bacterial strain, showed the best cultivation results when cultivated at a neutral pH value of 7.2, which effectively supported not only robust bacterial growth but also the regular and reliable production of EPS. However, any deviation from this carefully maintained pH level resulted in various challenges, including issues like nutrient precipitation and a striking reduction in enzymatic efficiency, further underlining the critical importance of keeping stable pH conditions to achieve optimal yields in bacterial cultivation [19].
3.2. Mechanisms Influencing EPS Production Under Different pH Conditions
The relationship between pH and EPS production is mediated by several factors that influence microbial physiology and enzyme activity:
Enzymatic Activity: Within optimum pH, key enzymes responsible for the metabolism of carbohydrates and the biosynthesis of polysaccharides are optimally active. Under this condition, enhanced enzymatic activity strongly favors the catalysis of conversion of the substrates to EPSs. For instance, during the pH range between 6 and 7, the enzymes contained in Pseudomonas sp. BGI-2 and in T. halophilus are most efficiently active, thereby facilitating higher yields of target products [17,18].
Cell Membrane Permeability: The more extreme values of pH actually cause considerable damage to cellular membranes and, as such, greatly reduce the permeability of cells to capture the required nutrients, thereby becoming metabolically inefficient. Particularly for Pseudomonas sp. BGI-2, when the culture medium had highly acidic conditions at pH 4 or highly alkaline conditions at pH 11, conditions completely inhibited EPS production [17].
Metabolic Pathways: Change in pH can also inactivate biosynthetic pathway regulation. For instance, the optimal synthesis of EPS by B. thermoruber 423 was at pH 6.5, whereby the metabolic pathways for the production of polysaccharide are at their most optimal state [20].
Environmental Stability: In the case of acidophiles and alkaliphiles, EPS performs protective functions in the form of resistance to stress caused by low or high pH. These biopolymers contribute to maintaining cellular stability and reducing environmental damage; however, the extreme conditions still limit their production efficiency [11].
Considering all this information, pH plays a fundamental role in EPS production, influencing microbial growth, metabolic activity, and the structural properties of the biopolymers. While optimal pH ranges vary among microorganisms, near-neutral to slightly acidic conditions are often favored for maximum yield and quality. Understanding the interplay between pH and microbial physiology is crucial for optimizing industrial fermentation processes, ensuring consistent and high-quality EPS production tailored to specific applications.
4. The Effect of Carbon Sources on EPS Production in Extremophiles
The carbon source is important because it will be directly impacted on the final yield and structural features of the synthesized EPSs from the extremophiles. Originally, extremophiles are organisms adapted to live under very harsh environmental conditions; thus, they possess tremendous metabolic diversity. This enormous adaptability allows them to use numerous types of substrates for their metabolic activity. It is very important to point out, however, that not only the type of carbon source but also its concentration used in the fermentation will strongly affect the efficiency of production of EPS and the features of produced polymers.
4.1. Effect on EPS Yield
Carbon source selection is one of the most influential parameters affecting EPS yield in extremophilic microorganisms. For instance, Pseudomonas sp. BGI-2, isolated from a glacier environment, produced the highest yield of 2.01 g/L EPS when cultivated on glucose under optimized conditions [17]. T. halophilus showed maximum EPS concentration at 100 g/L of glucose; however, higher concentrations resulted in inhibition due to the accumulation of lactic acid, hence the importance of balancing substrate concentration to avoid metabolic stress [18].
For thermophilic strains, such as G. thermodenitrificans and Geobacillus toebii, fructose was the most effective carbon source, producing 76 mg/L and 80 mg/L of EPS, respectively [21]. This shows that substrate-specific pathways may play important roles in the maximization of yield. In addition, B. thermoruber 423 preferred maltose and produced EPS at a high yield of 202 mg/L under optimized conditions [20]. In continuous cultures of hyperthermophilic strains such as Thermococcus litoralis and Thermotoga maritima, maltose also supported improved EPS production at higher dilution rates, with more synthesis as excess carbon was redirected into polymer biosynthesis rather than biomass growth [52].
4.2. Influence on Structural Properties
The carbon source not only affects EPS yield but also has a critical role in defining the structural features of the polymer. For instance, the type of carbon source significantly impacts the composition of EPS produced by B. thermoruber 423. Maltose promotes the production of glucose-rich heteropolymers with minor contributions from galactose and mannose, yielding stable and predictable polymers ideal for industrial use.
In contrast, other carbon sources like xylose and fructose only alter the monosaccharide profile by a shift in metabolic flux. These substrate-dependent changes enable the tailoring of EPS properties for particular applications, thus highlighting the importance of optimization of the carbon source for the production of EPS [53]. Conversely, sucrose is known to support the levan-type polysaccharide produced by Pseudomonas extremaustralis, mainly consisting of fructose residues, while the functional properties of this polymer, including viscosity and emulsification, depend precisely on such residues [54]. Similar studies in R. marinus DSM 16675 have indicated that the substrate control of glucose concentration mainly affected the structural features related to glycosidic linkages and sulfate esters in EPS, thus hinting at most precise substrate control [19]. In Geobacillus sp. strain WSUCF1, carbon sources have been found to have a major impact on both the production and structural characteristics of EPS. While glucose emerged as optimal substrate due to the presence of both PTS and permease transport systems, simpler pathways, like fructose-based NDP-sugar biosynthesis, exhibited lower EPS yields likely due to reduced transporter activity. This highlights the critical role of substrate-specific transport mechanisms and metabolic pathways in optimizing EPS production and tailoring polymer characteristics [55].
4.3. Economic and Industrial Relevance
The choice of carbon sources is also closely related to the economic feasibility of EPS production. Representing industrial by-products, molasses has so far been one of the possible substitutes at a lower cost than pure sugars. In this respect, for instance, Pseudomonas sp. BGI-2 grown on molasses produced 675 mg/L, a very good option for the lower cost of production, since the yields remain competitive [17]. Similarly, fructose and maltose proved to be other substrates that were very amenable to thermophilic varieties of microorganisms and hence could fit in perfectly with broader industrial purposes toward scalability and efficiency in production.
In addition, customized exopolysaccharide production will allow the design of biopolymers with specific properties for particular industrial and biotechnological applications. For instance, the capability to produce EPSs with pre-determined structural and functional properties could be made possible by using chemically modified carbon feedstocks and/or biosynthetic pathways. Such customized modes of production have great potential to enhance product quality for various industrial applications.
EPSs produced by extremophiles, due to the stability of their structure and functional diversity, could have proper applications, not only as an antioxidant but also in the emulsification and flocculation properties of foods, in waste-water treatments, and in pharmaceuticals. The optimization of carbon sources in view of specific industry needs will ensure the quality and widen the range of applications for such biopolymers.
5. The Effect of Nitrogen Sources on EPS Production in Extremophiles
The type of nitrogen source significantly affects EPS synthesis. For example, Rhizobium meliloti demonstrated the highest EPS production with sodium glutamate, particularly at intermediate concentrations (0.1–0.2%). Excess nitrogen levels favored biomass growth at the expense of EPS synthesis, while nitrogen limitation redirected metabolic flux toward polysaccharide production. This trend underscores the importance of maintaining an optimal nitrogen balance to enhance EPS yields without compromising polymer quality [56].
Similarly, studies on Aeribacillus pallidus 418 revealed that inorganic nitrogen sources, such as ammonium chloride (NH4Cl), were more effective for EPS production compared to organic sources like peptone or yeast extract. Ammonium salts supported the synthesis of heteropolysaccharides with high mannose content, making them suitable for applications requiring structural stability and thermal resistance. In contrast, organic nitrogen sources promoted better biomass growth but yielded lower EPS [25].
Moreover, Geobacillus tepidamans V264, isolated from the Mizinka hot spring in Bulgaria, exhibited optimal EPS production when ammonium phosphate ((NH4)2HPO4) was used as the nitrogen source at a concentration of 3 g/L, highlighting the effectiveness of inorganic nitrogen compounds in supporting biosynthetic pathways [23]. In another study, ammonium chloride has been identified as a superior nitrogen source for certain microorganisms. For instance, Zunongwangia profunda SM-A87 demonstrated significant EPS production (6.47 g/L) in the presence of lactose as the carbon source and NH4Cl as the nitrogen source [24]. These findings emphasize the dual role of nitrogen sources in supporting cell growth and enhancing EPS biosynthesis while also highlighting that excess nitrogen can sometimes redirect metabolic resources toward biomass accumulation at the expense of EPS production [57,58]. Therefore, optimizing nitrogen availability and balancing it with carbon supply are essential for achieving high yields and desired polymer properties in extremophilic microorganisms.
5.1. Nitrogen Availability and Metabolic Flux
Nitrogen presence and its availability are two very critical factors that have an effect on the metabolic pathway producing EPS. In particular, for Pseudomonas alcaligenes Med1, an EPS yield of up to 6.8 g/L was achieved under optimized conditions with a nitrogen concentration of 30 g/L at neutral pH. The addition of a 30 g/L nitrogen source likely enhanced EPS production by increasing microbial biomass and activating key metabolic pathways involved in polysaccharide biosynthesis. High nitrogen availability may have promoted precursor molecule synthesis, ultimately leading to an increase in EPS yield. This proves the crucial role that nitrogen can play in regulating biosynthetic processes in EPS production. On the other hand, when nitrogen concentration goes to the higher side, there is a diversion of metabolic priorities with the biomass growth increasing rather than that of EPS, thereby decreasing the yield of the polymer produced. Thus, an optimum amount of nitrogen availability must be maintained to avoid metabolic inefficiency, which is crucial to gain maximum output from the polymer production process [59].
In the case of hyperthermophiles, which are T. litoralis and T. maritima, there are organism-specific effects of nitrogen sources: whereas T. litoralis utilized amino acids more readily and even showed an inhibitory response towards ammonium chloride, its counterpart T. maritima depends upon NH4Cl for its N-source, reflecting a balanced growth and EPS production in its moderate concentration. This reflects the metabolic adaptiveness of the extremophiles with particular N-sources [52].
5.2. Structural and Functional Implications of Nitrogen Sources
Nitrogen sources not only influence EPS yield but also determine its structural properties, such as monosaccharide composition and functional groups. In Chroococcidiopsis spp., nitrate-supported EPS was more effective in biofilm formation and showed higher resistance to desiccation and hence could realize better performance in extremely adverse environmental conditions. Urea and ammonium, though less effective, also contributed to variations in the composition of monosaccharides in EPS, testifying to the adaptability of these cyanobacteria to nitrogen supply [26].
Nitrogen type affects not only quantity but also functional properties of the synthesized biopolymer. Sodium glutamate in R. meliloti favored a glucose-rich polymer, while other nitrogen sources changed the ratios of constituent monosaccharides. This flexibility allows for tailoring EPS characteristics for specific industrial applications such as emulsification, viscosity modulation, and environmental remediation [56].
Nitrogen source selection and optimization are central points of interest when the aim is to maximize EPS production or alter its structural properties for specific industrial applications. Knowledge on how nitrogen availability, metabolic pathways, and polymer synthesis interact will lead researchers to improve yields further and tailor the characteristics of EPS for use in biomedicine, food technology, and environmental biotechnology.
6. The Effect of C/N Ratio on EPS Production in Extremophiles
The carbon-to-nitrogen (C/N) ratio is among the most basic and critical parameters in the highly controlled processes of EPS production by extremophilic microorganisms. This highly affects the different metabolic pathways of such microorganisms because of the division of resource allocation between biomass production, which is very important for the growth of the organisms, and polymer synthesis necessary for exopolysaccharide production. Such a division depends on the nutrient availability present in their surrounding medium. Various vast studies on a wide range of extremophiles mentioned that this particular C/N ratio has to be optimized as highly important for the purpose of improving the yield of EPS and engineering its properties for a wide range of applications.
6.1. Influence of C/N Ratio on EPS Yield
High C/N ratios favor EPS production by re-routing excess carbon toward polysaccharide synthesis under nitrogen-limiting conditions. For example, Haloferax mucosum had an optimal C/N ratio of 9 and reached an EPS yield of 7.15 g/L. In the case of a higher ratio, excess carbon gets channeled towards biomass rather than EPS, which restricts polymer output [60]. Similarly, under nitrogen-limited conditions, a better Ypx means a better use of the carbon source by Halomonas smyrnensis AAD6T under such conditions of stress was observed [22].
On the other hand, low C/N ratios favor high biomass growth at the expense of EPS synthesis. For instance, the thermophilic A. pallidus showed optimum growth and EPS production at a C/N ratio of 4.5:1; a lower C/N ratio prioritized cell proliferation over polymer generation [25]. These observations underscore the necessity of carefully optimizing the C/N ratio for obtaining the best yields.
6.2. Impact on EPS Composition and Functional Properties
The C/N ratio highly influences the structural property of EPS. Generally, nitrogen-limited conditions relate to the production of high-molecular-weight polymers with improved functional properties. For instance, Halomonas sp. S19 produced under optimized C/N conditions synthesized EPS constituted by 65% carbohydrates, 4.07% proteins, 8.08% uronic acids, and 6.39% sulfur. Such components make it possess properties such as emulsification and stability, among others, which are suitable for industrial application. As opposed to the equal C/N values, an imbalanced C/N ratio impairs the quality of overall polymers internally and, importantly, modifies the structural features so that they cannot be used or are less usable in various industrial purposes [61].
6.3. Practical Applications and Industrial Relevance
The C/N ratio’s influence extends to practical applications, where adjusting this parameter can enhance cost-effectiveness and scalability. In this regard, low-priced nutrient sources, including industrial wastewater, when combined with a highly manipulated C/N ratio, lowered their costs and further produced high-quality EPSs, as demonstrated in studies with Rhizobium leguminosarum [62]. Furthermore, in the context of the C/N ratio, the form of nitrogen source used impacts the properties of the produced EPS, in terms of molecular weight—the organic nitrogen source is known to result in high-molecular-weight EPSs, while the inorganic source produces simpler kinds of polymers. Hence, the process lends itself to manipulation for generating these biopolymers in relation to particular industrial applications [63].
The possibility of effectively manipulating the C/N ratio indeed is a very strong and effective methodology of optimization of the production process of EPSs. It enhances both yield and polymer functionality to meet the demands of diverse biotechnological applications [64].
7. Oxygen Transfer and Hydrodynamics in EPS Production by Extremophiles
Exopolysaccharide (EPS) production in laboratory, semi-industrial, and industrial settings is profoundly influenced by various factors, including the geometry of bioreactors, dissipation devices, aeration systems, operational parameters, and the physicochemical properties of the cultivation medium. The presence of oxygen-consuming cells further complicates these dynamics. These factors collectively impact the hydrodynamic environment in fermenters, significantly influencing polymer synthesis. However, oxygen transfer limitations often emerge as a bottleneck in aerobic bioprocesses due to the low solubility of oxygen in viscous liquid media, especially when EPS increases the medium’s viscosity. This effect is particularly critical for extremophilic microorganisms, such as thermophiles and halophiles, where oxygen solubility and diffusion rates are naturally limited due to extreme environmental conditions [65].
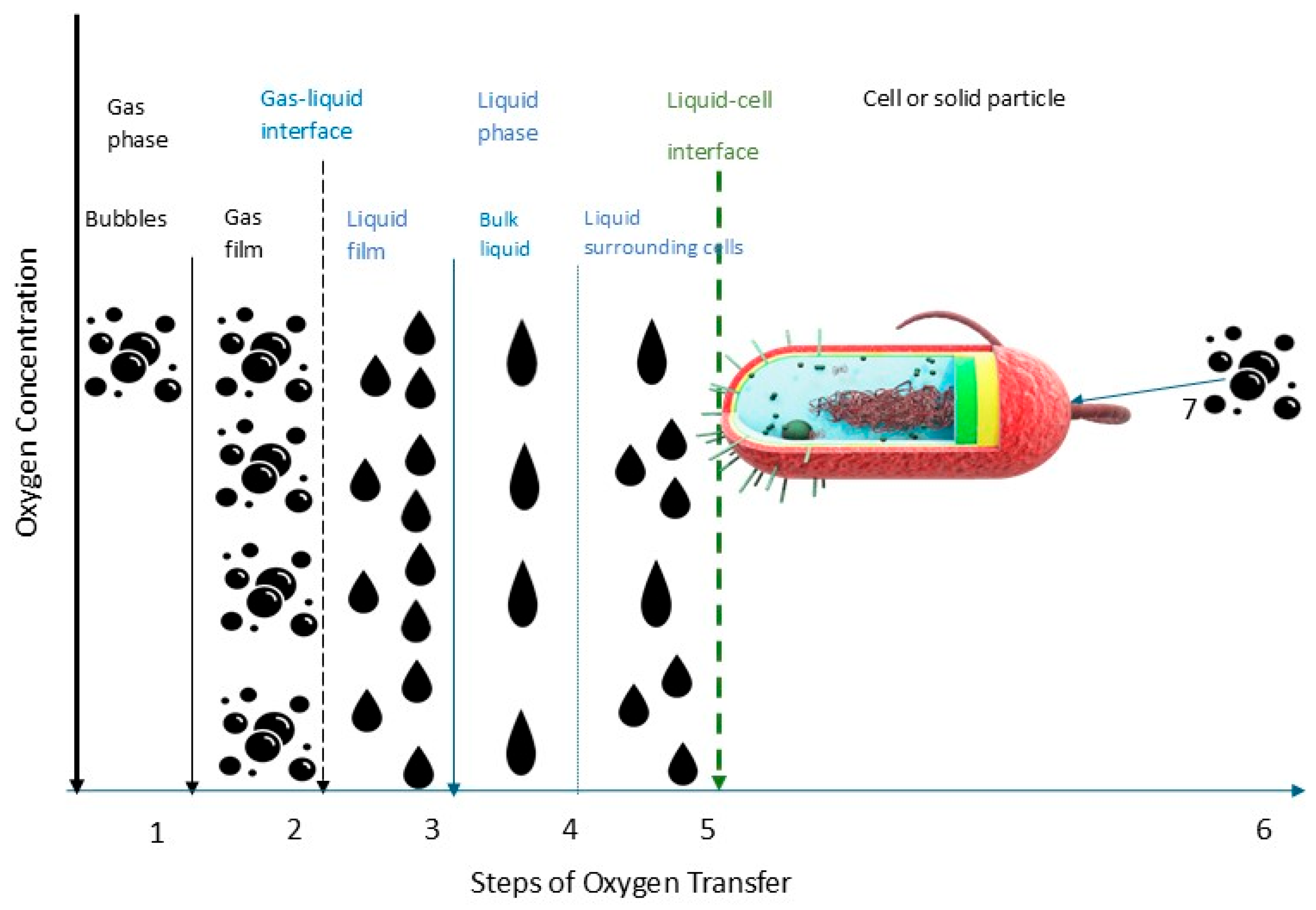
Accurately predicting and optimizing the oxygen transfer rate (OTR) is essential to ensure adequate oxygen supply for microbial growth and polymer synthesis. For extremophiles, the oxygen demand can vary significantly depending on their metabolic adaptations. Thermophilic EPS producers, such as R. marinus, require optimized aeration strategies to balance oxygen availability and shear stress, while halophilic bacteria, such as H. smyrnensis, must cope with reduced oxygen solubility in hypersaline environments. Insufficient oxygen can negatively impact or, in some cases, have no significant effect on polymer synthesis, depending on the microorganism’s metabolic requirements [65]. In parallel with this, maximum synthesis under oxygen-limiting conditions within 2 h at a stirring rate of 900 rpm was observed for the thermophilic bacterium A. pallidus 418 [66]. A similar effect was observed also in the strain of Sphingomonas elodea ATCC 3146, where it was found that maximum gelan synthesis occurs when oxygen restriction precedes the onset of gelan formation [67]. This duality highlights the need for precise control over oxygen delivery, often requiring an enhanced power supply to overcome mass transfer limitations. The balance between oxygen transport and the metabolic reaction rate is critical; when transport becomes slower than biochemical reaction rates, it governs the overall process dynamics. In aerobic bioprocesses, oxygen is transferred from gas bubbles into the liquid phase and subsequently delivered to the cell’s interior through oxidative phosphorylation. This oxygen transport process can be conceptualized as a sequential series of steps, each contributing to the efficient delivery of oxygen to support cellular metabolism (Figure 2).
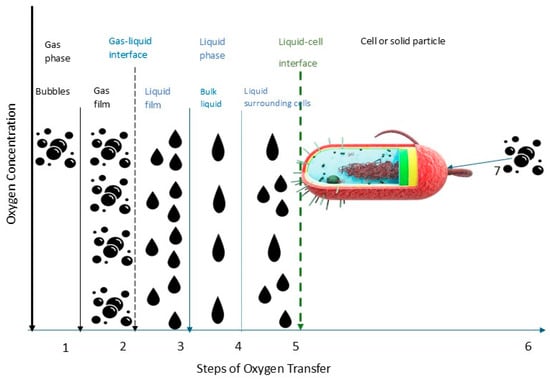
Figure 2.
Oxygen transfer from the gas bubbles to the cell–steps: (1) transfer from the interior of the bubble and gas film; (2) movement across the gas–liquid interface; (3) diffusion through the relatively stagnant liquid film surrounding the bubble; (4) transport through the bulk liquid; (5) diffusion through the relatively stagnant liquid film surrounding the cells; (6) movement across the liquid–cell interface; and (7) transport through the cytoplasm to the site of the biochemical reaction.
7.1. Factors Influencing Oxygen Transfer in Extremophiles
The OTR is affected by cultivation conditions, bioreactor geometry, the oxygen demand of cells, physical properties of the gases and liquids, and biomass concentration. The volumetric mass transfer coefficient (kLa) is a critical parameter for quantifying the OTR and predicting metabolic pathways. For extremophilic bacteria, optimizing the kLa is crucial due to their adaptation to unique environments. In thermophiles, excessive aeration can lead to oxidative stress and polymer degradation, whereas halophiles benefit from controlled oxygenation to maintain biofilm integrity. Equation (1) serves as a fundamental model for calculating oxygen transfer dynamics in bioreactors.
Therein, kL is the mass-transfer coefficient (cm/h), a denotes the interfacial area available for mass transfer (cm−1), and (C∗ − C) represents the driving force (mmol/L). Hence, the OTR is expressed as mmol/(L/h).
In most bioprocessing applications, it is difficult to estimate the interfacial area, a. For example, when air or oxygen is sparged in a bioreactor, a depends on the size and number of bubbles present—and those depend on many other factors, such as medium density and viscosity, power input (stirrer speed), and gas flow rate. For EPS-producing extremophiles, high-viscosity media further complicates this process, necessitating specialized bioreactor configurations to optimize mass transfer efficiency. Because such parameters also influence kL, the terms a and kL usually are combined, yielding the kLa. Several algorithms and methods have been proposed to determine the kLa under various operational scenarios. Studies on bubble column reactors and air-lift reactors (ALRs) have shown their effectiveness in cultivating shear-sensitive and filamentous cells, such as certain halophiles and thermophiles, due to low shear environments and efficient heat transfer. In contrast, stirred tank bioreactors (STRs) provide high mass transfer rates and excellent mixing but require the careful control of agitation to minimize shear stress, which can degrade product quality [66,67,68,69].
Stirring intensity is a fundamental parameter in microbial bioprocesses, as it directly influences the hydrodynamic regime—laminar, transient, or turbulent. This intensity is determined by the liquid medium’s properties (e.g., viscosity and density), operating conditions (e.g., rotational speed), and the agitator’s geometry (e.g., diameter and type). Initially, Van’t Riet [70] suggested that the geometry and number of agitators did not significantly affect kLa values in non-viscous systems. However, subsequent studies, such as those conducted by Puthli et al. [71], demonstrated that the type and configuration of agitators could significantly influence kLa values. For example, replacing conventional Rushton turbines with hydro-streamlined Narcissus impellers improved oxygen transfer efficiency and reduced shear stress, particularly in non-Newtonian fluids like EPS-rich media [72]. In such media, excessive shear stress can degrade product quality by causing mechanical damage to cells and breaking polymer chains. Additionally, modifications to stirring speed have been shown to affect biomass distribution and oxygen transfer. For instance, speeds above 400 rpm have been found optimal for achieving uniform homogenization in psychrophilic Sporobolomyces salmonicolor AL1 cultures, while lower speeds result in uneven flow fields [73].
7.2. Oxygen Transfer in Saline and High-Viscosity Media
For halophilic organisms, oxygen transfer is particularly challenging due to the reduced solubility of oxygen in saline media. Studies indicate that low stirring speeds in saline environments can achieve a kLa value up to 12% higher than in non-saline media, likely due to reduced bubble coalescence in the presence of salts [74]. The highest EPS synthesis for C. canadensis was observed at low kLa values (0.00398 s−1) in a 2 L STR bioreactor under saline conditions, emphasizing the importance of tailored oxygen transfer strategies for specific microbial and environmental conditions [75].
High-viscosity environments further complicate oxygen transfer by forming a diffusion barrier around cells, converting dissolved oxygen into a limiting nutrient. This effect is particularly evident in processes with high oxygen demand, such as xanthan gum production. Effective agitation and aeration strategies are critical for overcoming these barriers while minimizing shear-induced cell damage. For instance, modifying impeller types, such as replacing conventional Rushton turbines with hydro-streamlined Narcissus impellers, has been shown to improve oxygen transfer efficiency and reduce shear stress in EPS-rich non-Newtonian fluids [76].
7.3. Measurement and Optimization of kLa
Dynamic methods, such as those developed by Taguchi and Humphrey, are widely used to measure the kLa by monitoring oxygen uptake rates (OURs) during microbial respiration [77]. These methods calculate the kLa based on the decline and recovery rates of dissolved oxygen when aeration is stopped and resumed. Such measurements are essential for assessing aeration efficiency and adjusting bioreactor parameters to optimize EPS production. Real-time kLa monitoring in extremophilic cultures can provide insights into oxygen transfer limitations and enable better control strategies for optimizing polymer yields.
7.4. Industrial Implications
In industrial bioprocesses, the optimization of oxygen transfer through careful control of the kLa and hydrodynamic conditions is essential for scaling up EPS production. Bioreactor configurations, agitator designs, and aeration rates must be tailored to the specific requirements of the microorganism and medium composition.
The interactive influence of engineering parameters such as bioreactor configurations, agitator designs, blade spacing, and aeration rates must be tailored to the specific requirements of the microorganism and medium composition.
The insights gained from studies on halophilic and thermophilic strains provide valuable frameworks for improving oxygen transfer efficiency and ensuring consistent polymer yields across different production scales. For example, in the large-scale production of thermophilic EPSs, gradual aeration adjustments have been implemented to maintain metabolic stability, preventing oxidative stress and unwanted polymer degradation.
8. Conclusions
This study provides an overall analysis of the factors determining EPS production in extremophiles, underlining their great adaptability to adverse environments and their potential in industry. The results indicate that temperature, pH, carbon and nitrogen sources, and C/N ratios are important factors in optimizing EPS yield and functionality. Tailored temperature strategies, for instance, a shift from optimal growth to stress conditions, enhance polymer synthesis, while precise pH regulation supports enzymatic efficiency and structural integrity.
Furthermore, the type and balance of sources of carbon and nitrogen are very important for guiding metabolic pathways toward polysaccharide production to tailor EPS properties for the desired applications. The oxygen transfer impact of bioprocessing can often be alleviated with advanced bioreactor designs and optimized agitation strategies for saline media and those with high viscosity.
Compared to eubacterial EPS producers, extremophiles exhibit distinct advantages that enhance their biotechnological potential: (i) Higher stability under extreme conditions, including high temperature, salinity, and pressure, which ensure better performance in industrial and environmental applications. (ii) Unique biochemical compositions, such as highly sulfated EPS, uronic acid-enriched structures, and rare sugar components, which enhance their applicability in biomedicine, food technology, and bioremediation. (iii) Intrinsic resilience, reducing the need for genetic modifications or extensive bioprocess adjustments to maintain yield and quality. (iv) Superior functionality in specialized applications, such as biofilm formation for metal sequestration, oil spill recovery, and extreme environmental bioremediation.
Given these advantages, extremophilic EPS-producing microorganisms represent a valuable resource for the development of novel biomaterials with high industrial relevance. Future research should focus on enhancing bioprocess optimization strategies, scaling up production, and exploring novel extremophilic strains to further expand the potential applications of these biopolymers.
Author Contributions
All authors contributed equally to all stages of this review. The conceptualization, literature review, analysis, writing, and revision of the manuscript were carried out collaboratively. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no competing interests.
References
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides producing Bacteria: A review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Siddharth, T.; Sridhar, P.; Vinila, V.; Tyagi, R. Environmental applications of microbial extracellular polymeric substance (EPS): A review. J. Environ. Manag. 2021, 287, 112307. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, S.H.; Lee, J.H.; Lee, H.K. Effect of aeration rates on production of extracellular polysaccharide, EPS-R, by marine bacterium Hahella chejuensis. Biotechnol. Bioprocess Eng. 2001, 6, 359–362. [Google Scholar] [CrossRef]
- Moscovici, M.; Ionescu, C.; Oniscu, C.; Fotea, O.; Protopopescu, P.; Hanganu, L.D. Improved exopolysaccharide production in fed-batch fermentation of Aureobasidium pullulans, with increased impeller speed. Biotechnol. Lett. 1996, 18, 787–790. [Google Scholar] [CrossRef]
- Rau, U.; Gura, E.; Olszewski, E.; Wagner, F. Enhanced glucan formation of filamentous fungi by effective mixing, oxygen limitation and fed-batch processing. J. Ind. Microbiol. 1992, 9, 19–25. [Google Scholar] [CrossRef]
- Yang, F.-C.; Liau, C.-B. The influence of environmental conditions on polysaccharide formation by Ganoderma lucidum in submerged cultures. Process Biochem. 1998, 33, 547–553. [Google Scholar] [CrossRef]
- Sutherland, I. Microbial Exopolysaccharide Synthesis; ACS Publications: Washington, DC, USA, 1977. [Google Scholar]
- Mouro, C.; Gomes, A.P.; Gouveia, I.C. Microbial exopolysaccharides: Structure, diversity, applications, and future frontiers in sustainable functional materials. Polysaccharides 2024, 5, 241–287. [Google Scholar] [CrossRef]
- Kiran, N.S.; Yashaswini, C.; Singh, S.; Prajapati, B.G. Revisiting microbial exopolysaccharides: A biocompatible and sustainable polymeric material for multifaceted biomedical applications. 3 Biotech 2024, 14, 95. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I. A review of extracellular polysaccharides from extreme niches: An emerging natural source for the biotechnology. From the adverse to diverse! Int. J. Biol. Macromol. 2021, 177, 559–577. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-T.; Pham, T.-T.; Nguyen, P.-T.; Le-Buanec, H.; Rabetafika, H.N.; Razafindralambo, H.L. Advances in Microbial Exopolysaccharides: Present and Future Applications. Biomolecules 2024, 14, 1162. [Google Scholar] [CrossRef]
- Marx, J.G.; Carpenter, S.D.; Deming, J.W. Production of cryoprotectant extracellular polysaccharide substances (EPS) by the marine psychrophilic bacterium Colwellia psychrerythraea strain 34H under extreme conditions. Can. J. Microbiol. 2009, 55, 63–72. [Google Scholar] [CrossRef]
- Nichols, C.M.; Bowman, J.P.; Guezennec, J. Effects of incubation temperature on growth and production of exopolysaccharides by an Antarctic sea ice bacterium grown in batch culture. Appl. Environ. Microbiol. 2005, 71, 3519–3523. [Google Scholar] [CrossRef] [PubMed]
- Ali, P.; Shah, A.A.; Hasan, F.; Hertkorn, N.; Gonsior, M.; Sajjad, W.; Chen, F. A glacier bacterium produces high yield of cryoprotective exopolysaccharide. Front. Microbiol. 2020, 10, 3096. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, M.; Wang, Z.; Jiao, X.; Wu, C. Temperature stress improved exopolysaccharide yield from Tetragenococcus halophilus: Structural differences and underlying mechanisms revealed by transcriptomic analysis. Bioresour. Technol. 2023, 390, 129863. [Google Scholar] [CrossRef]
- Mukti, I.J.; Sardari, R.R.; Kristjansdottir, T.; Hreggvidsson, G.O.; Karlsson, E.N. Medium development and production of carotenoids and exopolysaccharides by the extremophile Rhodothermus marinus DSM16675 in glucose-based defined media. Microb. Cell Factories 2022, 21, 220. [Google Scholar] [CrossRef]
- Yasar Yildiz, S.; Anzelmo, G.; Ozer, T.; Radchenkova, N.; Genç, S.; Di Donato, P.; Nicolaus, B.; Toksoy Oner, E.; Kambourova, M. Brevibacillus themoruber: A promising microbial cell factory for exopolysaccharide production. J. Appl. Microbiol. 2014, 116, 314–324. [Google Scholar] [CrossRef]
- Panosyan, H.; Di Donato, P.; Poli, A.; Nicolaus, B. Production and characterization of exopolysaccharides by Geobacillus thermodenitrificans ArzA-6 and Geobacillus toebii ArzA-8 strains isolated from an Armenian geothermal spring. Extremophiles 2018, 22, 725–737. [Google Scholar] [CrossRef]
- Tohme, S.; Hacıosmanoğlu, G.G.; Eroğlu, M.S.; Kasavi, C.; Genç, S.; Can, Z.S.; Oner, E.T. Halomonas smyrnensis as a cell factory for co-production of PHB and levan. Int. J. Biol. Macromol. 2018, 118, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kambourova, M.; Mandeva, R.; Dimova, D.; Poli, A.; Nicolaus, B.; Tommonaro, G. Production and characterization of a microbial glucan, synthesized by Geobacillus tepidamans V264 isolated from Bulgarian hot spring. Carbohydr. Polym. 2009, 77, 338–343. [Google Scholar] [CrossRef]
- Liu, S.-B.; Qiao, L.-P.; He, H.-L.; Zhang, Q.; Chen, X.-L.; Zhou, W.-Z.; Zhou, B.-C.; Zhang, Y.-Z. Optimization of fermentation conditions and rheological properties of exopolysaccharide produced by deep-sea bacterium Zunongwangia profunda SM-A87. PLoS ONE 2011, 6, e26825. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and properties of two novel exopolysaccharides synthesized by a thermophilic bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef]
- Baldanta, S.; Arnal, R.; Blanco-Rivero, A.; Guevara, G.; Navarro Llorens, J.M. First characterization of cultivable extremophile Chroococcidiopsis isolates from a solar panel. Front. Microbiol. 2023, 14, 982422. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Dey, P. Bacterial exopolysaccharides as emerging bioactive macromolecules: From fundamentals to applications. Res. Microbiol. 2023, 174, 104024. [Google Scholar] [CrossRef]
- Dhaulaniya, A.S.; Balan, B.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Cold survival strategies for bacteria, recent advancement and potential industrial applications. Arch. Microbiol. 2019, 201, 1–16. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular polymeric substances (EPS) as microalgal bioproducts: A review of factors affecting EPS synthesis and application in flocculation processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Purwar, S.; Srivastava, S. Adaptations of Psychrophilic Microorganism to Low-Temperature Environments. Appl. Microbiol. Theory Technol. 2024, 5, 168–188. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Zammuto, V.; Gugliandolo, C.; Madeleine-Perdrillat, C.; Spanò, A.; Magazù, S. Thermal restraint of a bacterial exopolysaccharide of shallow vent origin. Int. J. Biol. Macromol. 2018, 114, 649–655. [Google Scholar] [CrossRef]
- Krembs, C.; Eicken, H.; Junge, K.; Deming, J. High concentrations of exopolymeric substances in Arctic winter sea ice: Implications for the polar ocean carbon cycle and cryoprotection of diatoms. Deep. Sea Res. Part I 2002, 49, 2163–2181. [Google Scholar] [CrossRef]
- Krembs, C.; Eicken, H.; Deming, J.W. Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. Proc. Natl. Acad. Sci. USA 2011, 108, 3653–3658. [Google Scholar] [CrossRef]
- Tsuji, M.; Fujiu, S.; Xiao, N.; Hanada, Y.; Kudoh, S.; Kondo, H.; Tsuda, S.; Hoshino, T. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctica. FEMS Microbiol. Lett. 2013, 346, 121–130. [Google Scholar] [CrossRef]
- Phadtare, S. Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 2004, 6, 125–136. [Google Scholar] [CrossRef]
- Donot, F.; Fontana, A.; Baccou, J.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Casillo, A.; D’Angelo, C.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. Membrane and extracellular matrix glycopolymers of Colwellia psychrerythraea 34H: Structural changes at different growth temperatures. Front. Microbiol. 2022, 13, 820714. [Google Scholar] [CrossRef] [PubMed]
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034. [Google Scholar] [CrossRef]
- Arserim Ucar, D.K.; Konuk Takma, D.; Korel, F. Exopolysaccharides in food processing industrials. In Microbial Exopolysaccharides as Novel and Significant Biomaterial; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 201–234. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, D.; Cong, B.; Liu, S.; Zhang, P. Structure characterization and cryoprotective activity of an exopolysaccharide from Pseudoalteromonas sp. LP6-12-2. Food Biosci. 2024, 57, 103557. [Google Scholar] [CrossRef]
- de Lemos, E.A.; da Silva, M.B.F.; Coelho, F.S.; Jurelevicius, D.; Seldin, L. The role and potential biotechnological applications of biosurfactants and bioemulsifiers produced by psychrophilic/psychrotolerant bacteria. Polar Biol. 2023, 46, 397–407. [Google Scholar] [CrossRef]
- Fraser, A.; Wongpan, P.; Langhorne, P.; Klekociuk, A.; Kusahara, K.; Lannuzel, D.; Massom, R.; Meiners, K.; Swadling, K.; Atwater, D. Antarctic landfast sea ice: A review of its physics, biogeochemistry and ecology. Rev. Geophys. 2023, 61, e2022RG000770. [Google Scholar] [CrossRef]
- Michel, C.; Bény, C.; Delorme, F.; Poirier, L.; Spolaore, P.; Morin, D.; d’Hugues, P. New protocol for the rapid quantification of exopolysaccharides in continuous culture systems of acidophilic bioleaching bacteria. Appl. Microbiol. Biotechnol. 2009, 82, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Yaşar Yildiz, S. Exploring the hot springs of golan: A source of thermophilic bacteria and enzymes with industrial promise. Curr. Microbiol. 2024, 81, 101. [Google Scholar] [CrossRef]
- Arbab, S.; Ullah, H.; Khan, M.I.; Khattak, M.N.; Zhang, J.; Li, K.; Hassan, I.U. Diversity and distribution of thermophilic microorganisms and their applications in biotechnology. J. Basic Microbiol. 2022, 62, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Krembs, C.; Deming, J.W. The role of exopolymers in microbial adaptation to sea ice In Psychrophiles: From Biodiversity to Biotechnology; Margesin, R., Schinner, F., Marx, J.C., Gerday, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 247–264. [Google Scholar] [CrossRef]
- Kambourova, M.; Radchenkova, N.; Tomova, I.; Bojadjieva, I. Thermophiles as a promising source of exopolysaccharides with interesting properties. In Biotechnology of Extremophiles: Advances and Challenges; Rampelotto, P., Ed.; Springer: Cham, Switzerland, 2016; pp. 117–139. [Google Scholar] [CrossRef]
- Wu, J.; Ding, Z.-Y.; Zhang, K.-C. Improvement of exopolysaccharide production by macro-fungus Auricularia auricula in submerged culture. Enzym. Microb. Technol. 2006, 39, 743–749. [Google Scholar] [CrossRef]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Arayes, M.A.; Mabrouk, M.E.; Sabry, S.A.; Abdella, B. Exopolysaccharide production from Alkalibacillus sp. w3: Statistical optimization and biological activity. Biologia 2023, 78, 229–240. [Google Scholar] [CrossRef]
- Rinker, K.D.; Kelly, R.M. Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyperthermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol. Bioeng. 2000, 69, 537–547. [Google Scholar] [CrossRef]
- Yaşar Yildiz, S.; Nikerel, E.; Toksoy Öner, E. Genome-scale metabolic model of a microbial cell factory (Brevibacillus thermoruber 423) with multi-industry potentials for exopolysaccharide production. OMICS J. Integr. Biol. 2019, 23, 237–246. [Google Scholar] [CrossRef]
- Finore, I.; Vigneron, A.; Vincent, W.F.; Leone, L.; Di Donato, P.; Schiano Moriello, A.; Nicolaus, B.; Poli, A. Novel psychrophiles and exopolymers from permafrost thaw lake sediments. Microorganisms 2020, 8, 1282. [Google Scholar] [CrossRef]
- Wang, J.; Goh, K.M.; Salem, D.R.; Sani, R.K. Genome analysis of a thermophilic exopolysaccharide-producing bacterium-Geobacillus sp. WSUCF1. Sci. Rep. 2019, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Dudman, W. Immune diffusion analysis of the extracellular soluble antigens of two strains of Rhizobium meliloti. J. Bacteriol. 1964, 88, 782–794. [Google Scholar] [CrossRef]
- Torres, C.A.; Marques, R.; Ferreira, A.R.; Antunes, S.; Grandfils, C.; Freitas, F.; Reis, M.A. Impact of glycerol and nitrogen concentration on Enterobacter A47 growth and exopolysaccharide production. Int. J. Biol. Macromol. 2014, 71, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sá-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Cabrera-Barjas, G.; Singh, R.N.; Fabi, J.P.; Breig, S.J.M.; Tapia, J.; Sani, R.K.; Banerjee, A. Unveiling a novel exopolysaccharide produced by Pseudomonas alcaligenes Med1 isolated from a Chilean hot spring as biotechnological additive. Sci. Rep. 2024, 14, 25058. [Google Scholar] [CrossRef]
- López-Ortega, M.A.; Escalante-Avilés, M.; Rodríguez-Hernández, A.I.; del Rocio López-Cuellar, M.; Aguirre-Loredo, R.Y.; Martínez-Juárez, V.M.; Pérez-Guevara, F.; Hernández-Valdepeña, M.Á.; Chavarría-Hernández, N. Co-production of polyhydroxyalkanoates (PHAs) and exopolysaccharides (EPSs) by halophilic archaeon Haloferax mucosum. New J. Chem. 2024, 48, 20188–20200. [Google Scholar] [CrossRef]
- Karuppiah, P.; Venkatasamy, V.; Viswaprakash, N.; Ramasamy, T. A statistical approach on optimization of exopolymeric substance production by Halomonas sp. S19 and its emulsification activity. Bioresour. Bioprocess 2015, 2, 48. [Google Scholar] [CrossRef]
- Sellami, M.; Oszako, T.; Miled, N.; Ben Rebah, F. Industrial wastewater as raw material for exopolysaccharide production by Rhizobium leguminosarum. Braz. J. Microbiol. 2015, 46, 407–413. [Google Scholar] [CrossRef]
- Soto, L.R.; Thabet, H.; Maghembe, R.; Gameiro, D.; Van-Thuoc, D.; Dishisha, T.; Hatti-Kaul, R. Metabolic potential of the moderate halophile Yangia sp. ND199 for co-production of polyhydroxyalkanoates and exopolysaccharides. Microbiol. Open 2021, 10, e1160. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; de Andrade Alves, A.; da Costa e Silva, P.E.; de Barros, M.P.S.; Houllou, L.M. Polyhydroxyalkanoates and exopolysaccharides: An alternative for valuation of the co-production of microbial biopolymers. Biotechnol. Prog. 2024, 40, e3412. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Wu, J.-Y.; Ho, K.-P. Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem. Eng. J. 2006, 27, 331–335. [Google Scholar] [CrossRef]
- Garcia-Calvo, E. Fluid dynamics of airlift reactors: Two-phase friction factors. AIChE J. Am. Inst. Chem. Eng. 1992, 38, 1662–1666. [Google Scholar] [CrossRef]
- Kawase, Y.; Halard, B.; Moo-Young, M. Liquid-phase mass transfer coefficients in bioreactors. Biotechnol. Bioeng. 1992, 39, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ochoa, F.; Gomez, E. Theoretical prediction of gas–liquid mass transfer coefficient, specific area and hold-up in sparged stirred tanks. Chem. Eng. Sci. 2004, 59, 2489–2501. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Prediction of gas-liquid mass transfer coefficient in sparged stirred tank bioreactors. Biotechnol. Bioeng. 2005, 92, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Van’t Riet, K. Review of measuring methods and results in nonviscous gas-liquid mass transfer in stirred vessels. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 357–364. [Google Scholar] [CrossRef]
- Puthli, M.S.; Rathod, V.K.; Pandit, A.B. Gas–liquid mass transfer studies with triple impeller system on a laboratory scale bioreactor. Biochem. Eng. J. 2005, 23, 25–30. [Google Scholar] [CrossRef]
- Vlaev, S.D.; Martinov, M. Shape effects on impeller power characteristic for mixing and gassing power law fluids. In Institution of Chemical Engineers Symposium Series; Hemsphere Publishing Corporation: London, UK, 1999; Volume 146, pp. 253–262. [Google Scholar]
- Vlaev, S.; Rusinova-Videva, S.; Pavlova, K.; Kuncheva, M.; Panchev, I.; Dobreva, S. Submerged culture process for biomass and exopolysaccharide production by Antarctic yeast: Some engineering considerations. Appl. Microbiol. Biotechnol. 2013, 97, 5303–5313. [Google Scholar] [CrossRef]
- Mahler, N.; Tschirren, S.; Pflügl, S.; Herwig, C. Optimized bioreactor setup for scale-up studies of extreme halophilic cultures. Biochem. Eng. J. 2018, 130, 39–46. [Google Scholar] [CrossRef]
- Radchenkova, N.; Boyadzhieva, I.; Hasköylü, M.E.; Atanasova, N.; Yıldız, S.Y.; Kuncheva, M.J.; Panchev, I.; Kisov, H.; Vassilev, S.; Oner, E.T. High bioreactor production and emulsifying activity of an unusual exopolymer by Chromohalobacter canadensis 28. Eng. Life Sci. 2020, 20, 357–367. [Google Scholar] [CrossRef]
- Bandaiphet, C.; Prasertsan, P. Effect of aeration and agitation rates and scale-up on oxygen transfer coefficient, kLa in exopolysaccharide production from Enterobacter cloacae WD7. Carbohydr. Polym. 2006, 66, 216–228. [Google Scholar] [CrossRef]
- Taguchi, H. Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. J. Ferment. Technol. 1966, 44, 881–889. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).