1. Introduction
Distillation remains one of the most widely applied separation processes in the chemical and process industries, accounting for a significant proportion of the total energy consumed in chemical manufacturing [
1]. Its prevalence is due to its versatility and ability to separate components based on volatility differences, enabling the production of fuels, solvents, and high-purity intermediates that are essential for industrial and consumer products [
2]. However, the thermodynamic constraints imposed by azeotropes often limit the effectiveness of conventional distillation, necessitating the development of alternative techniques to address these challenges [
3,
4]. The need for more efficient separation technologies has grown particularly urgent in the context of rising energy costs, increasing environmental concerns, and the global drive toward sustainable industrial practices [
5].
The separation of highly non-ideal azeotropic mixtures has long been a challenge for chemical engineers, continually stimulating research into new approaches [
6]. Efforts in this area typically focus on the development of novel solvents [
7], advanced optimization strategies, and innovative process design solutions [
8,
9]. However, the introduction of entirely new unit operation concepts is comparatively rare. A novel and notable development is Extractive Heterogeneous Azeotropic Distillation (EHAD), first proposed by Szanyi [
10], which represents a significant innovation in separation technology. EHAD combines the advantages of extractive distillation and heterogeneous azeotropic distillation, offering a promising new pathway for overcoming the limitations of conventional methods. The principle of EHAD relies on the ability of the autoentrainer to induce phase heterogeneity [
11], creating an additional degree of freedom that allows the separation of components that would otherwise be locked in azeotropic equilibrium.
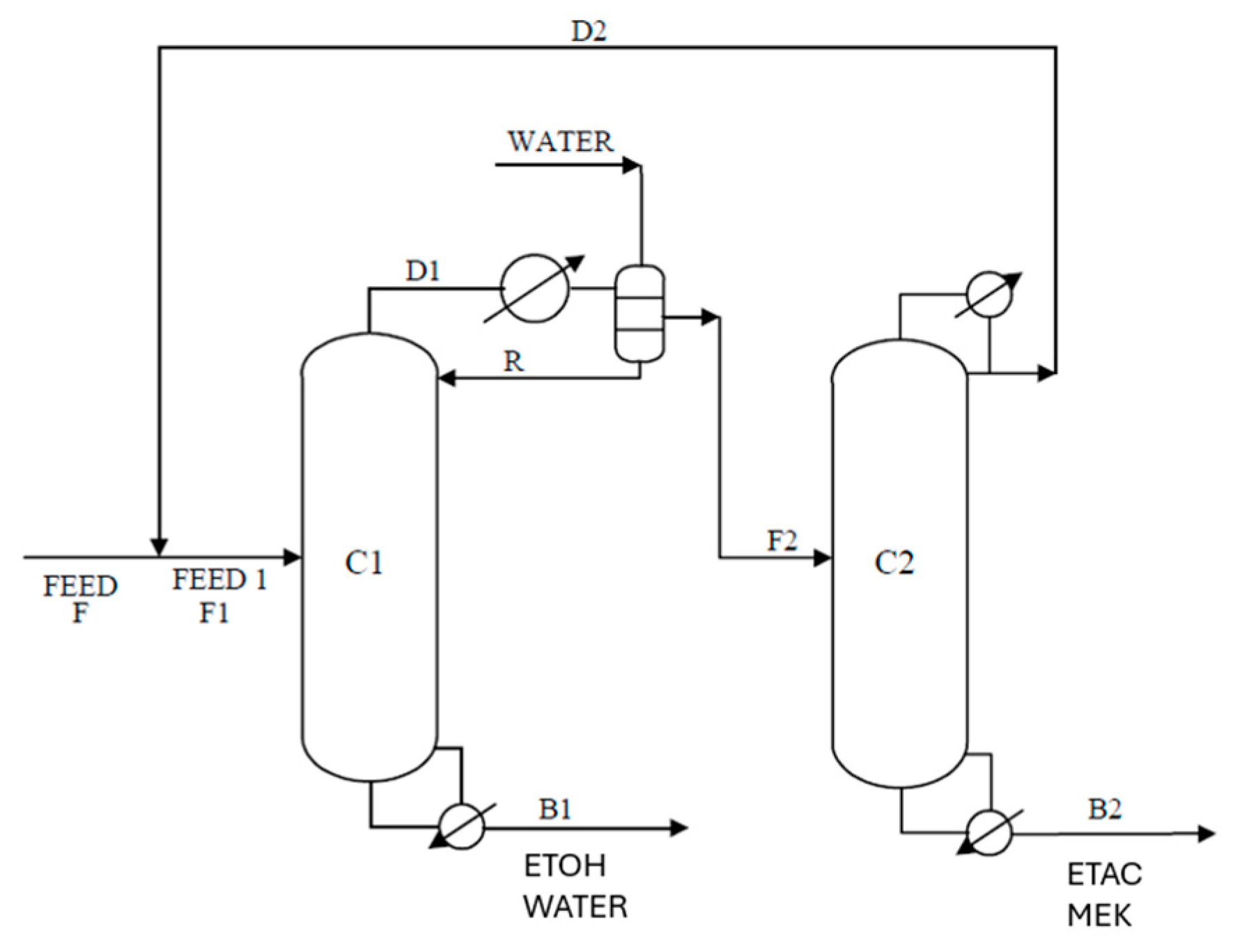
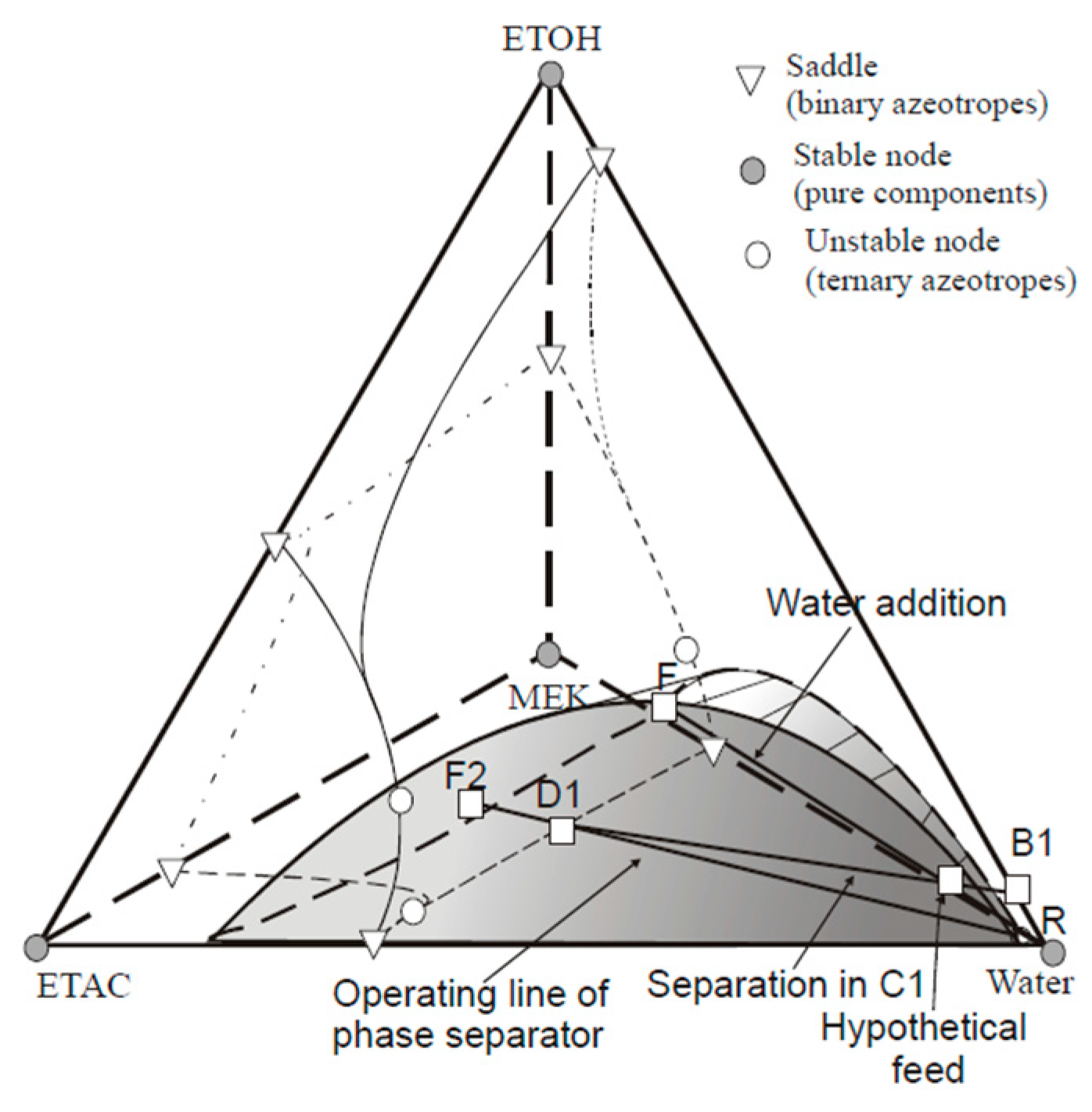
Several studies have reported that the idea of EHAD, as a hybrid separation technology, can achieve effective separation without the addition of an external entrainer when the original feed itself is capable of forming a heterogeneous azeotrope. Zhao et al. [
12] investigated the separation of ethanol, toluene, and water by heterogeneous azeotropic distillation, using toluene, which was already present in the mixture as the entrainer. Similarly, Wu et al. [
13] designed a comparable configuration for the separation of a hexane/methanol mixture. Luyben [
14] demonstrated this principle using a simple two-column distillation system to separate an n-butanol/water mixture, and found that the extractive distillation process was more economical than pressure-swing distillation (PSD). Cui et al. [
15] and Tsai et al. [
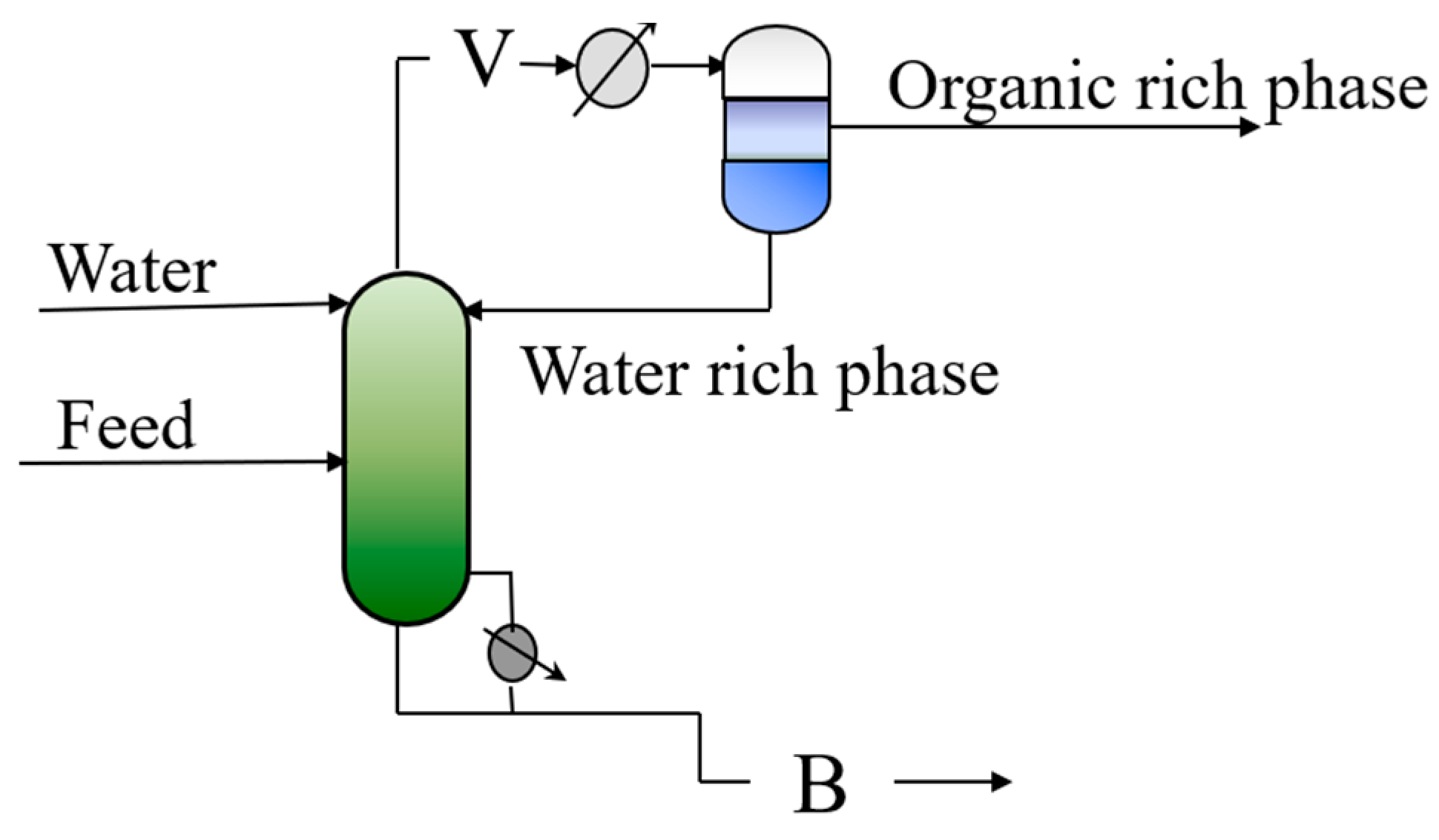
16] also studied the energy integration possibilities in the case of a hybrid separation system, containing multiple distillation columns. The separation of a benzene/isopropanol/water system with varying feed compositions in a hybrid separation system confirmed the feasibility and efficiency of the energy integration alternatives. However, they did not describe the conditions of the alternatives, and their system contained multiple distillation columns. These studies collectively demonstrate the potential of utilizing system-inherent components as autoentrainers. EHAD is illustrated in
Figure 1.
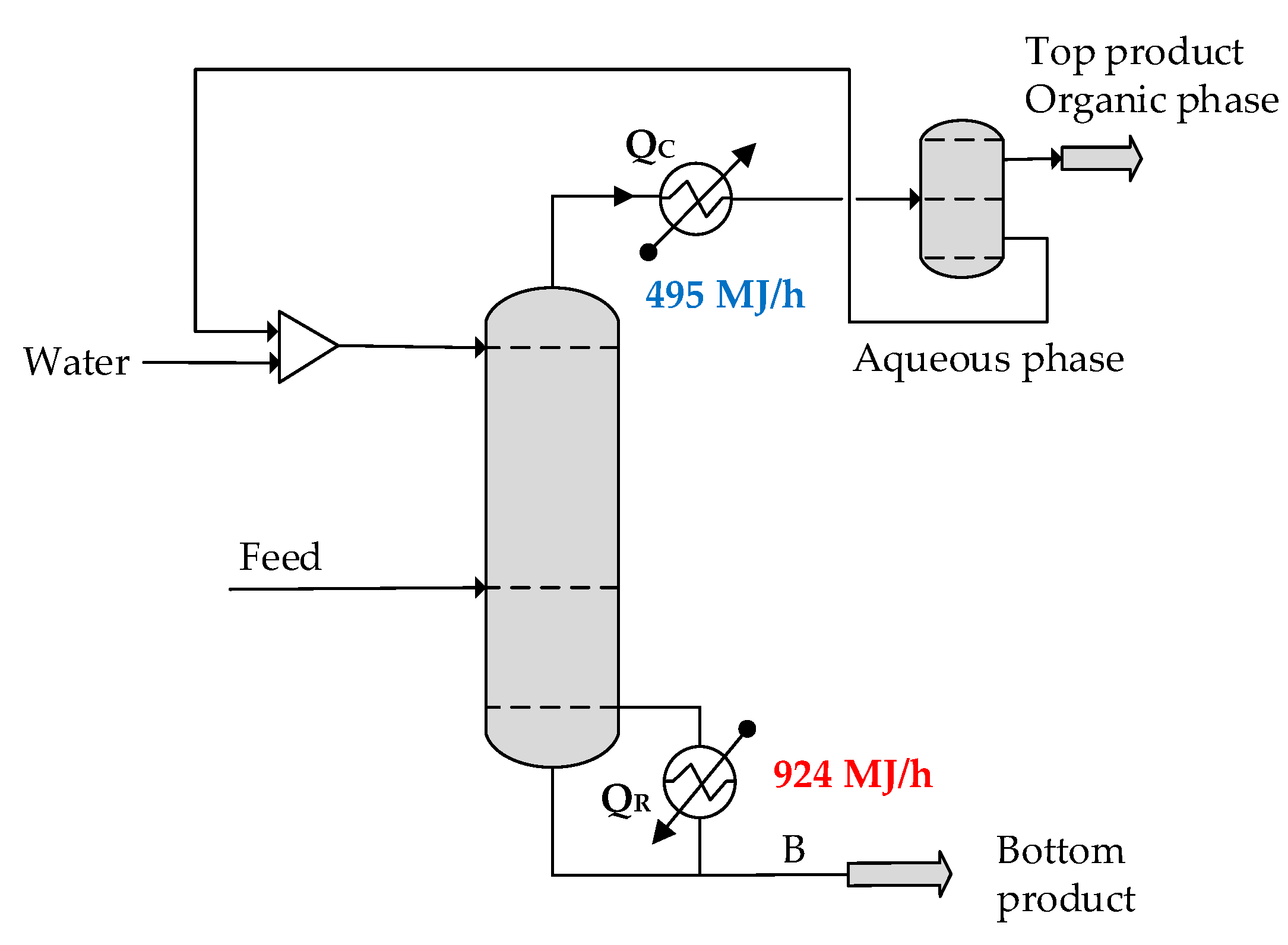
In EHAD, a component is introduced at the top of the column that simultaneously acts as both entrainer and extractive agent. A distinctive feature of this method is that no external compound is required, in line with the principles of green chemistry. Another advantage of EHAD is that no new azeotrope is formed, allowing the process to maintain thermodynamic simplicity. The entrainer, water, is intentionally added at the top of the column so that it exerts its extractive effect along the entire length of the column. This configuration eliminates the need for a separate enrichment section. Introducing water at the top stage, in addition to its presence in the main feed, optimizes the entrainer concentration profile along the entire length of the column and enhances both separation efficiency and energy performance of the EHAD process. After condensation of the overhead vapor, the liquid undergoes phase separation in a condenser-decanter system: the organic-rich phase is withdrawn as distillate, while the water-rich phase is recycled back into the column as reflux. Thus, the water entrainer serves a dual role, acting not only as the extractive agent but also as the reflux stream, thereby simplifying the process and enhancing its efficiency. In contrast, the bottom product is a single homogeneous phase.
EHAD has proved its capability in separating both ternary and quaternary mixtures in which water is one of the components, thereby making water the natural choice as the entrainer. Extensive studies have confirmed the efficiency and reliability of EHAD in handling complex separations. In addition, recent vapor-liquid–liquid equilibrium analyses have provided new insights into the classification of ternary azeotropes when a fourth component is introduced.
Szanyi et al. [
17] proposed a systematic strategy for separating mixtures of varying complexity, categorized according to their azeotropic behavior. This strategy, which is illustrated in
Figure 2, emphasizes the optimal positioning of EHAD within separation schemes to maximize its applicability and efficiency.
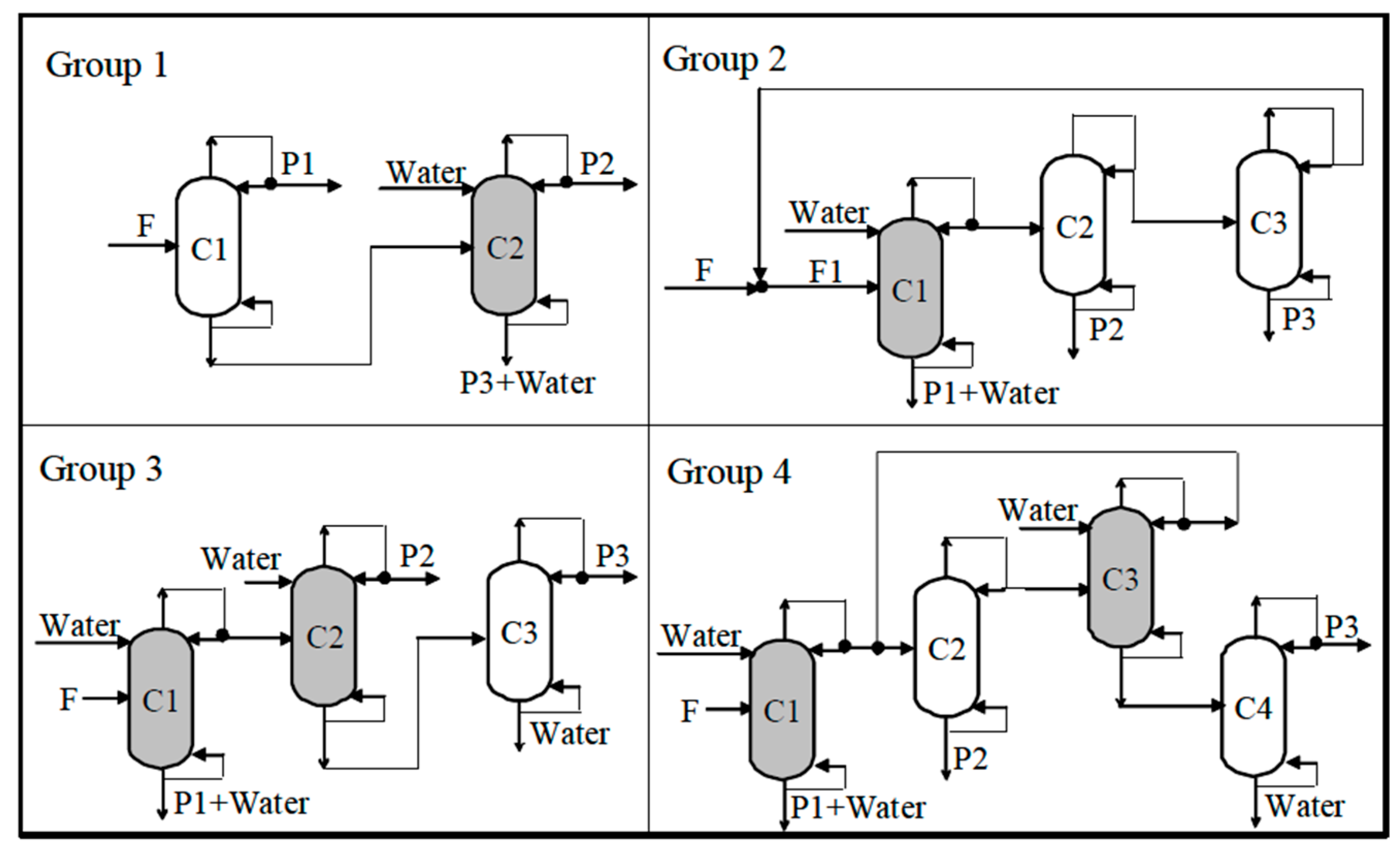
The complexity of the EHAD-based separation structure strongly depends on the thermodynamic complexity of the quaternary mixtures [
18]. As the number of binary or ternary azeotropes increases, the separation becomes increasingly difficult but feasible. The different liquid mixtures are classified into Groups depending on their azeotropic behavior.
EHAD has demonstrated the capability to split quaternary mixtures into their binary components and to separate ternary mixtures when azeotropy exists among their constituents. For mixtures classified as Group 3 or Group 4, successful separation requires the application of two EHAD columns strategically positioned within the process flowsheet.
The separation structure must not only be technically feasible but also economically and environmentally competitive. Reducing the environmental footprint requires lowering energy consumption, which is directly linked to emissions. Although membrane-based techniques offer alternatives, distillation remains the most practical and reliable option for separating complex multicomponent mixtures.
From an energy perspective, distillation can be more competitive if supported by energy integration strategies that typically reduce energy consumption. Such energy integration strategies include:
feed preheating with internal streams,
heat integration,
the use of heat pump,
thermally coupled columns, and
the application of side heaters or side coolers.
Energy integration also demonstrates clear benefits in hybrid separation systems [
19]. Mtogo et al. [
20] investigated several alternatives for integrating heat into distillation processes designed for azeotropic mixtures. They demonstrated that the thermodynamic efficiency of distillation can be nearly doubled through proper selection of the energy integration alternative. More recently, Pimentel et al. [
21] conducted a detailed energetic optimization of EHAD, completed with P-graph theory, and concluded that EHAD represents the most effective structure for the separation of quaternary mixtures, which underlines its efficiency.
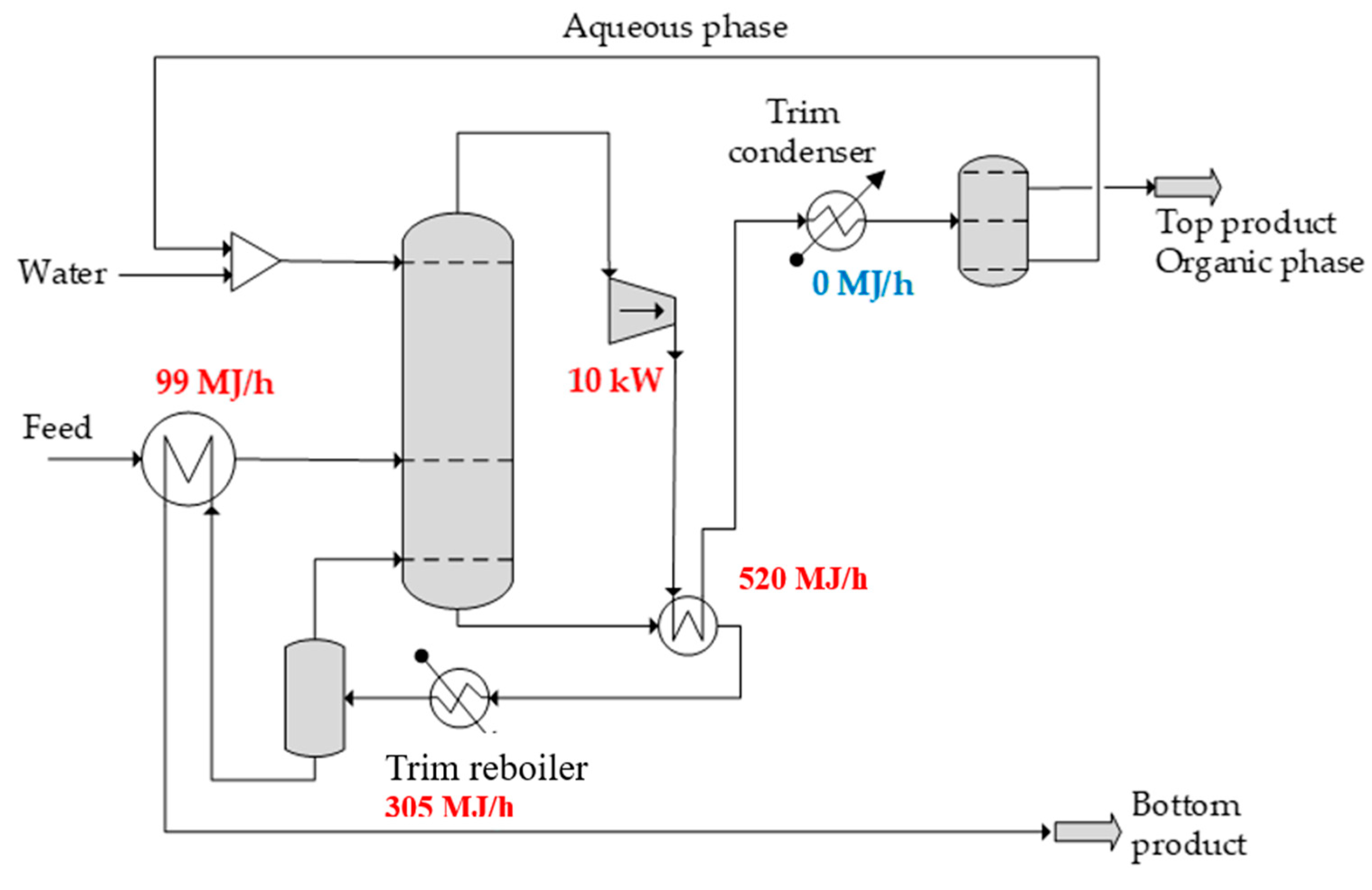
The vapor recompression heat pump technique represents a promising energy-saving strategy in so called stand-alone distillation systems. It compresses the overhead vapor of the stand-alone distillation column to increase its temperature making it capable of being used as a heating medium to supply heat to the reboiler. Besides saving heating energy, cooling energy can also be saved because the overhead vapor condenses, effectively reusing energy within the system [
22,
23,
24,
25]. With the growing emphasis on “green electricity,” this approach has gained significant attention as a promising pathway toward fully electrified and sustainable distillation processes [
26,
27,
28].
Therefore, the evaluation of energy integration further increases the attractiveness of EHAD and establishes its practical applicability as an industrial solution. Simulation studies based on reliable thermodynamic and process-design models provide quantitative estimates of energy savings and separation performance. However, experimental validation must also be part of industrial design to verify the most attractive alternative obtained through modeling. By coupling theoretical predictions with empirical data, researchers can determine the robustness of EHAD and its readiness for large-scale implementation.
The attractiveness of EHAD is demonstrated through a real industrial separation problem that can be solved in different ways. The solutions are experimentally verified. The industrial attractiveness of EHAD can be further improved if energy integration alternatives are also applied. Since EHAD is a stand-alone system in this case, the feed preheating and heat pumping can be considered.
3. Results and Discussion
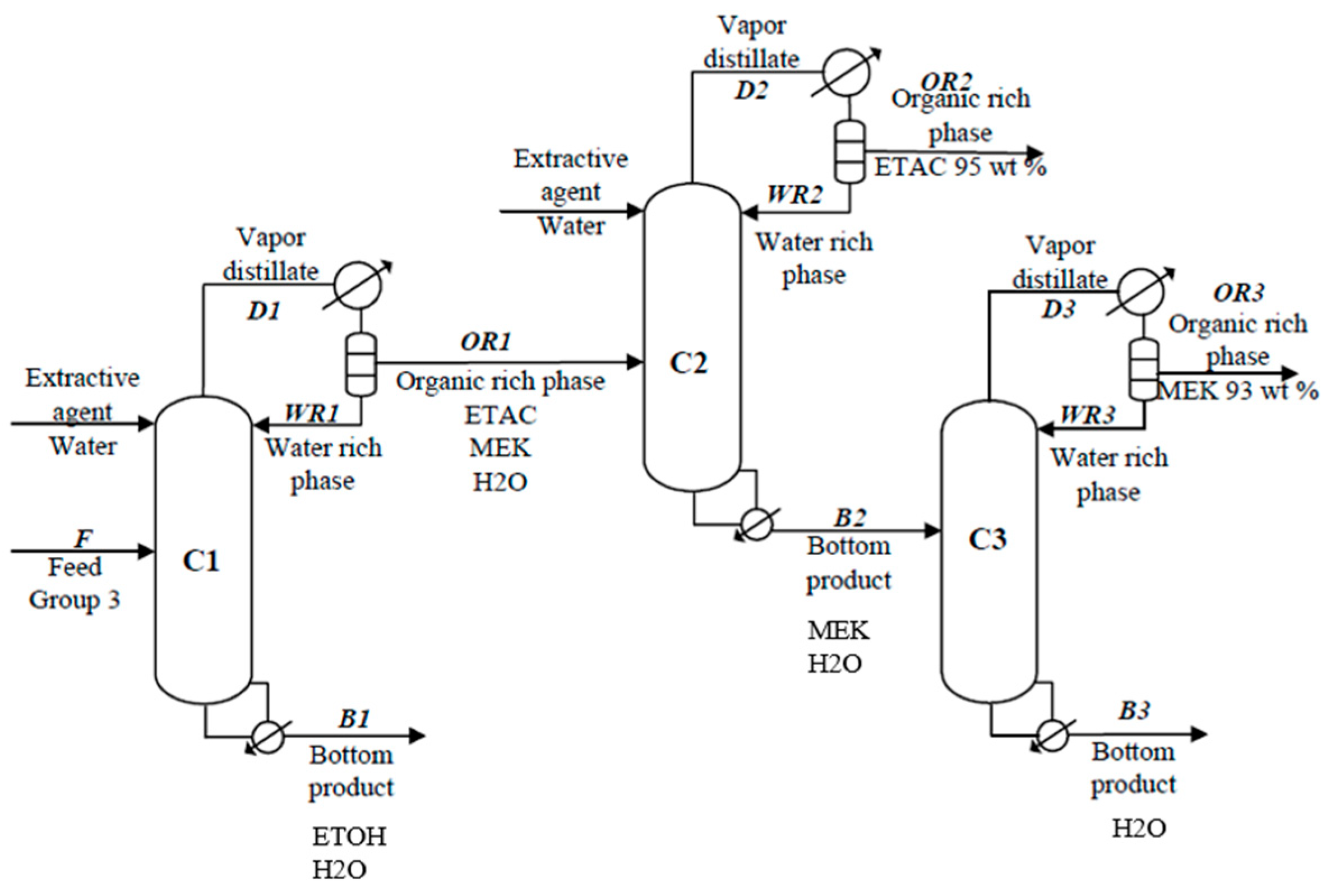
The separation of non-ideal azeotropic mixtures usually requires high energy, and the solution is also a challenging engineering task. A novel innovation, Extractive Heterogeneous Azeotropic Distillation (EHAD), is a suitable process for the efficient separation of ternary and quaternary azeotropic mixtures containing water.
The solution to an industrial separation problem, that is, the separation of a quaternary mixture of a printing company, demonstrates the effectiveness of EHAD. If the previously designed two-column configuration is applied to this separation problem, the quaternary mixture of a printing company can be successfully split into two binary subsystems. However, EHAD offers clear advantages over the two-column system since it accomplishes the same separation in a single column, thereby simplifying the process structure. Moreover, comparative energy analysis reveals that EHAD requires significantly less energy than the two-column system, with reductions ranging from 20% to 40% depending on the mixture.
The energy-saving potential of EHAD can be further enhanced through energy integration alternatives. Two alternatives are investigated: (i) feed preheating with the bottom product and (ii) heat pump application. The first option can save about 10% of the total energy, while the heat pump enables a further 54% reduction in input energy and eliminates all cooling requirements.
The findings can be summarized as flows:
the novel separation process, the EHAD is capable of separating highly nonideal quaternary azeotropic mixtures containing also water in an efficient way,
EHAD accomplishes the separation in one column instead of two or more,
EHAD requires less energy than the previously designed two-column system,
experimental data verify the accuracy of the modeling results,
the separation of non-ideal azeotropic mixtures has the typical feature that the top and bottom temperatures are close to each other, making them attractive for the use of heat pump
EHAD is significantly more attractive when combined with a heat pump.
These findings highlight the strong potential for EHAD in sustainable and energy-efficient industrial separations.