Towards Sustainable Biopolymer Innovation: A Review of Opuntia ficus-indica Mucilage
Abstract
1. Introduction
1.1. Opuntia ficus-indica: Agronomic and Ethnobotanical Significance
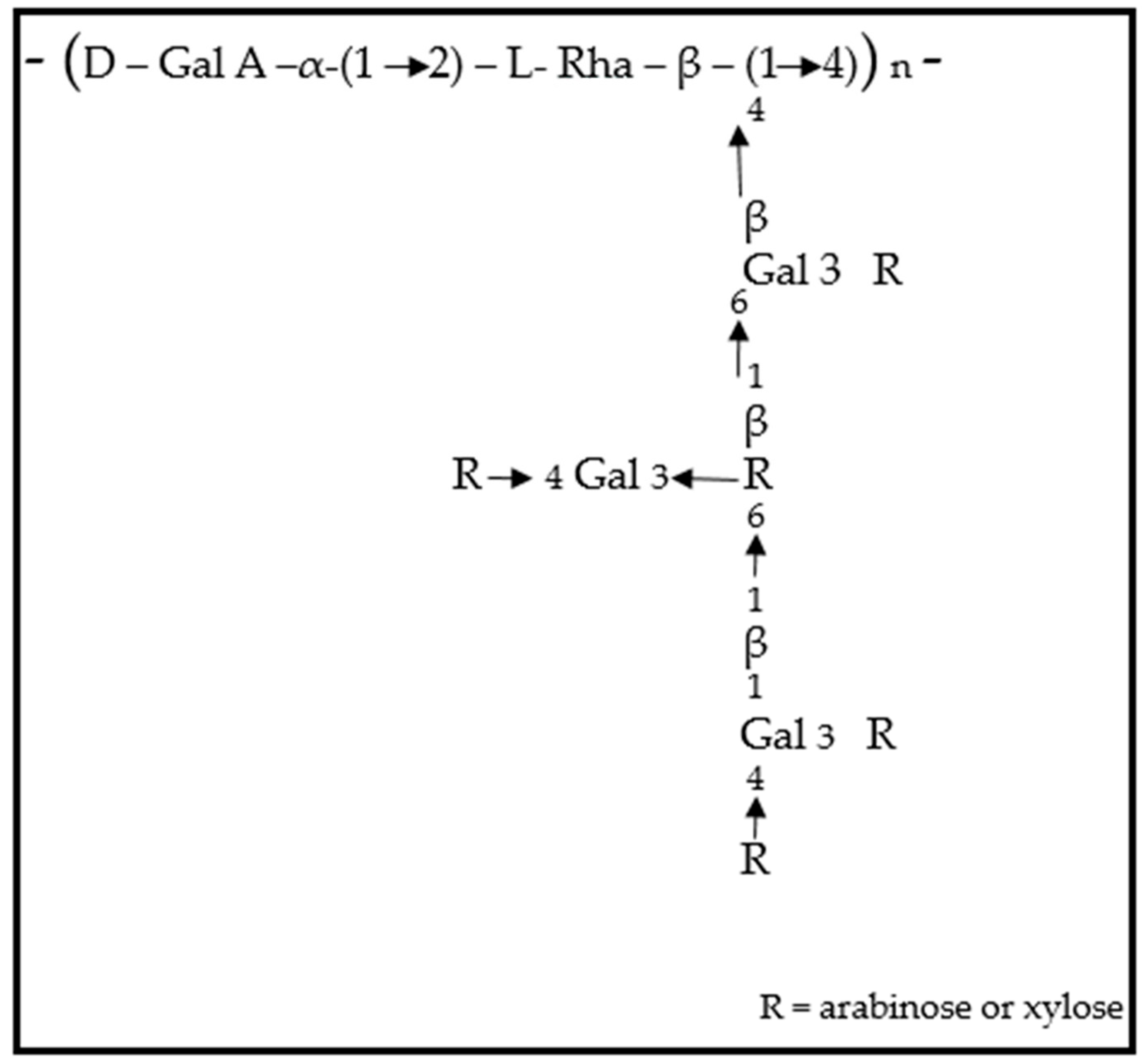
1.2. O. ficus-indica Mucilage: Composition and Functional Significance
1.3. Scope and Objectives of the Review
2. Materials and Methods
2.1. Data Sources
2.2. Search Terms
2.3. Inclusion and Exclusion Criteria
- Studies focusing on the chemical, functional, or application-related properties of mucilage derived specifically from OFI.
- Articles evaluating the use of OFI mucilage in food, pharmaceutical, biomedical, cosmetic, or environmental applications.
- Research papers published in peer-reviewed journals, as well as authoritative book chapters, patents, and technical reports.
- Studies written in English or with accessible English translations.
- Both experimental studies and review articles were included if they provided new insights or critical analysis relevant to the topic.
- Focused on other cactus species or plant gums not related to OFI.
- Did not clearly differentiate OFI mucilage from other components.
- They were duplicated, outdated, or lacked methodological clarity.
2.4. Data Extraction
- Publication details: Author(s), year, journal, country;
- Plant material: Source and part used (e.g., cladode medulla, peel, fruit);
- Extraction method: Technique, solvent, temperature, drying method;
- Characterization: Physicochemical properties (e.g., viscosity, thermal stability, functional groups);
- Application area: Food, pharmaceuticals, environmental, cosmetics, biomedicine;
- Key findings: Efficacy, performance, limitations, novelty.
3. Results and Discussion
3.1. Extraction of Mucilage from O. ficus-indica
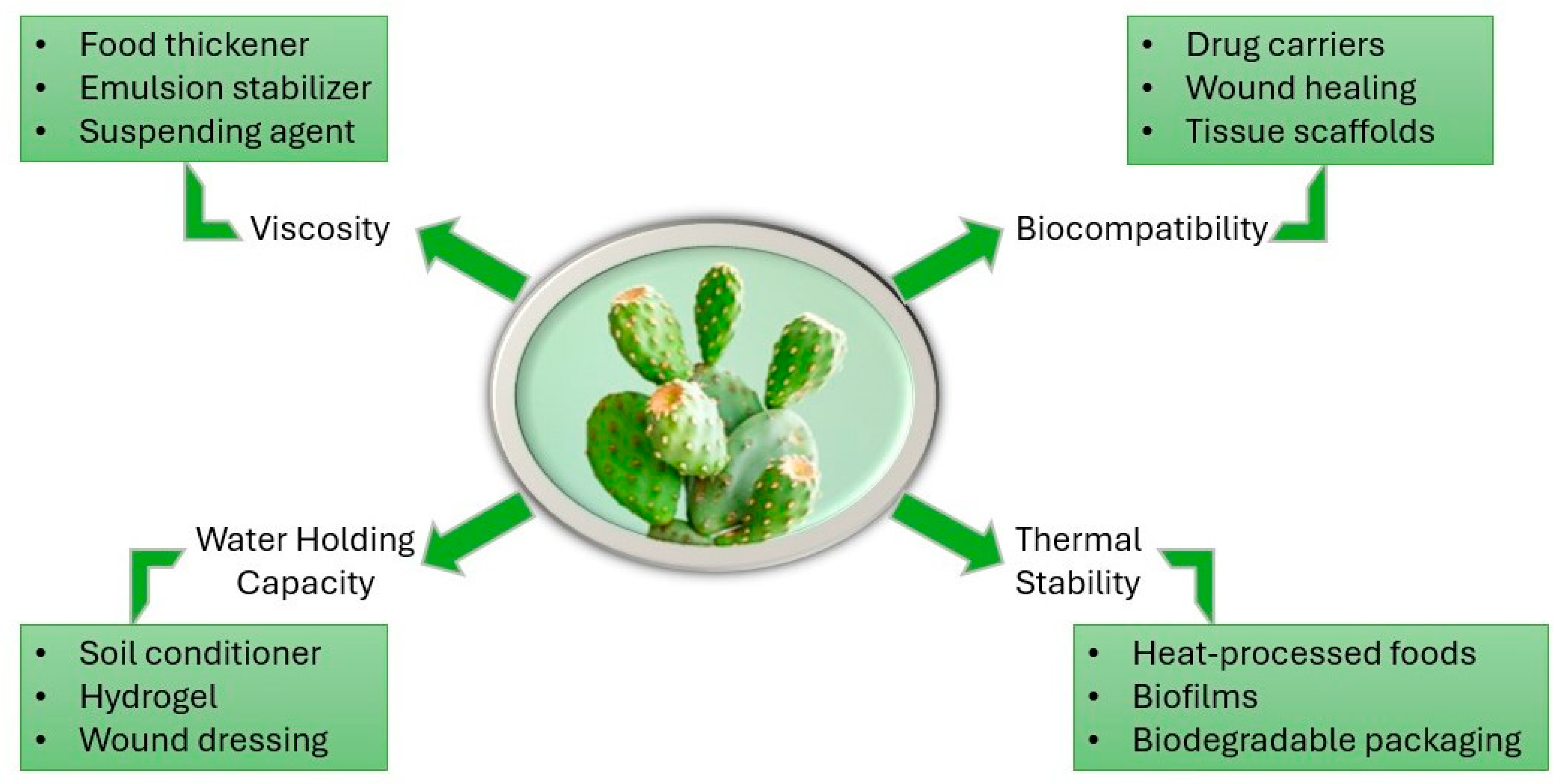
3.2. Key Physicochemical Properties of O. ficus-indica Mucilage
3.3. Comparative Advantages of Mucilage from OFI over Other Common Plant Mucilages
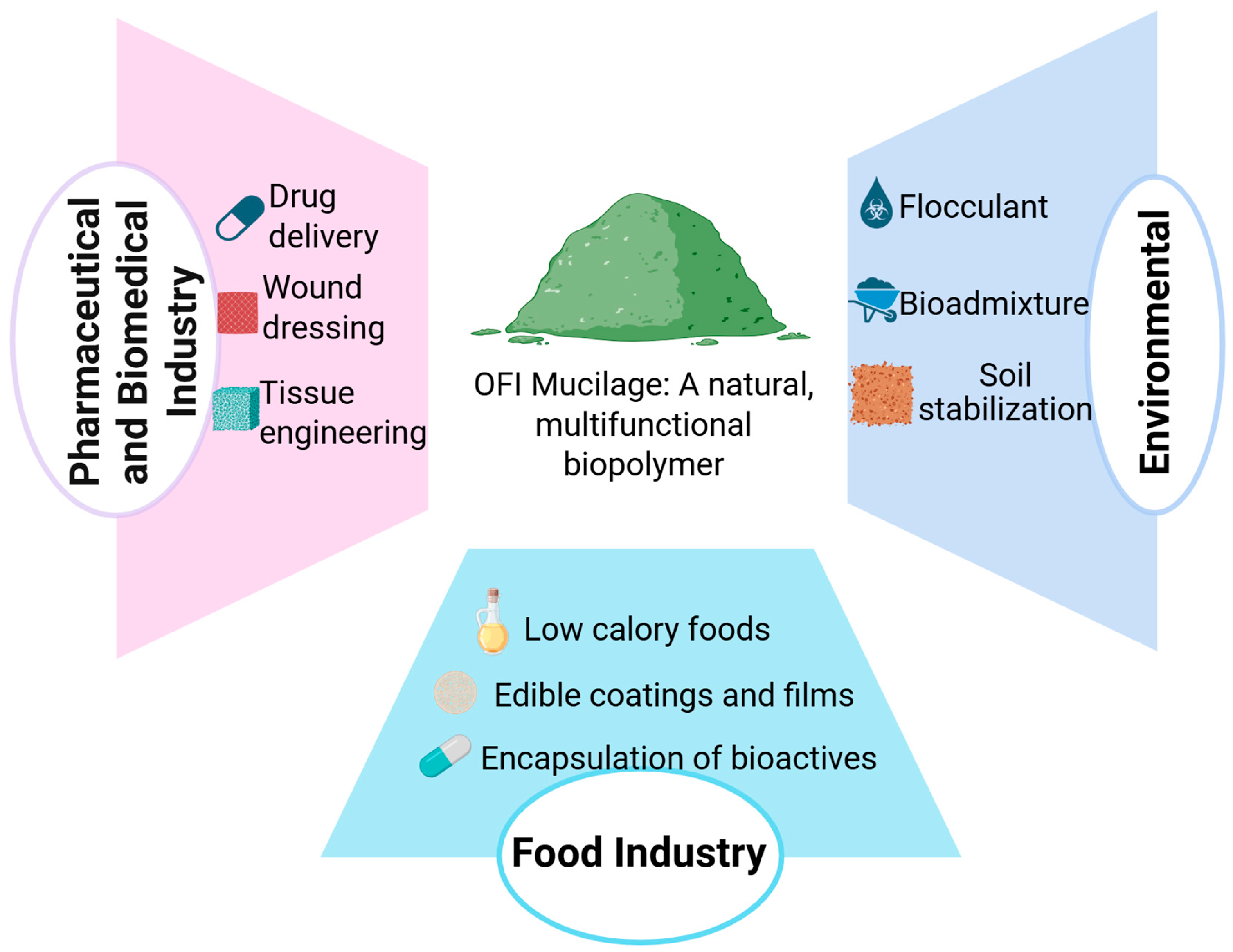
3.4. Current Applications of O. ficus-indica Mucilage
3.4.1. Food Industry
3.4.2. Pharmaceuticals and Biomedicine
3.4.3. Environmental Applications
3.5. Future Prospects and Research Gaps
3.6. Integration with Circular Economy
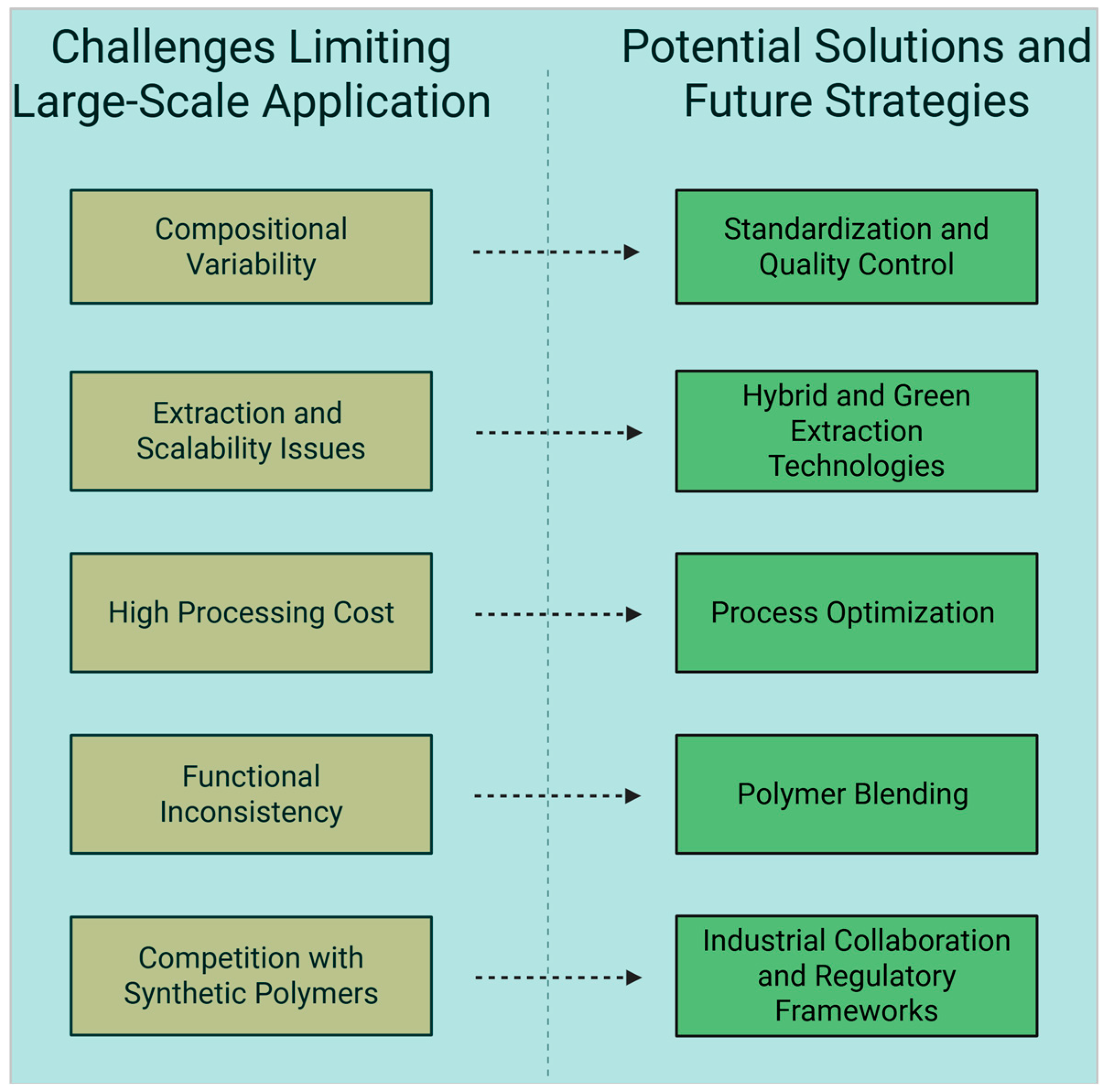
3.7. Challenges and Potential Solutions for the Application of O. ficus-indica Mucilage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFI | Opuntia ficus-indica |
References
- Bansal, K.K.; Rosenholm, J.M. Synthetic polymers from renewable feedstocks: An alternative to fossil-based materials in biomedical applications. Ther. Deliv. 2020, 11, 297–300. [Google Scholar] [CrossRef]
- UNEP. UNEP Annual Report 2024. Available online: https://www.unep.org/annualreport/ (accessed on 18 June 2025).
- Khanra, A.; Vasistha, S.; Rai, M.P.; Cheah, W.Y.; Khoo, K.S.; Chew, K.W. Green bioprocessing and applications of microalgae-derived biopolymers as a renewable feedstock: Circular bioeconomy approach. Environ. Technol. Innov. 2022, 1, 102872. [Google Scholar] [CrossRef]
- Asimakopoulou, E.; Giotis, C.; Andreadis, I.I.; Fatouros, D.G.; Ritzoulis, C. Stability and rheology of plant-derived hydrocolloid–mucin mixtures. J. Texture Stud. 2022, 53, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Biopolymer Packaging Market Size, Share and Industry Report 2032. Available online: https://www.fortunebusinessinsights.com/biopolymer-packaging-market-109414 (accessed on 18 June 2025).
- Naorem, A.; Patel, A.; Hassan, S.; Louhaichi, M.; Jayaraman, S. Global research landscape of cactus pear (Opuntia ficus-indica) in agricultural science. Front. Sustain. Food Syst. 2024, 22, 1354395. [Google Scholar] [CrossRef]
- Feugang, J.M. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci. 2006, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
- Al-Alam, J.; Harb, M.; Hage, T.G.; Wazne, M. Assessment of Opuntia ficus-indica (L.) Mill. extracts for the removal of lead from soil: The role of CAM plant harvest phase and soil properties. Environ. Sci. Pollut. Res. 2023, 30, 798–810. [Google Scholar] [CrossRef]
- Barba, F.J.; Cyrielle, G.; Amandine, F.; Paulo, E.S.M.; Jose, M.L.; Aouatif, A. Opuntia ficus-indica edible parts: A food and nutritional security perspective. Food Rev. Int. 2020, 38, 930–952. [Google Scholar] [CrossRef]
- Gebremariam, T.; Melaku, S.; Yami, A. Effect of different levels of cactus (Opuntia ficus-indica) inclusion on feed intake, digestibility and body weight gain in tef (Eragrostis tef) straw-based feeding of sheep. Anim. Feed Sci. Technol. 2006, 131, 43–52. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Akkol, E.K.; Ilhan, M.; Karpuz, B.; Genç, Y.; Sobarzo-Sánchez, E. Sedative and anxiolytic activities of Opuntia ficus-indica (L.) Mill.: An experimental assessment in mice. Molecules 2020, 25, 1844. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica fruit: A systematic review of its phytochemicals and pharmacological activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J. Cactus pear mucilage (Opuntia spp.) as a novel functional biopolymer: Mucilage extraction, rheology and biofilm development. Polymers 2024, 16, 1993. [Google Scholar] [CrossRef]
- Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Muller, G.; Grisel, M. Prickly pear nopals pectin from Opuntia ficus-indica physico-chemical study in dilute and semi-dilute solutions. Carbohydr. Polym. 2001, 46, 69–79. [Google Scholar] [CrossRef]
- Elshewy, A.; Blando, F.; Bahlol, H.; El-Desouky, A.; De Bellis, P.; Khalifa, I. Egyptian Opuntia ficus-indica (OFI) residues: Recovery and characterization of fresh mucilage from cladodes. Horticulturae 2023, 9, 736. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp mucilage’s: A functional component with industrial perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; Valderrama-Bravo, M.d.C.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and rheological characterization of Opuntia ficus mucilage at three different maturity stages of cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Quintero-García, M.; Gutiérrez-Cortez, E.; Bah, M.; Rojas-Molina, A.; Cornejo-Villegas, M.d.l.A.; Del Real, A. Comparative analysis of the chemical composition and physicochemical properties of the mucilage extracted from fresh and dehydrated Opuntia ficus indica cladodes. Foods 2021, 10, 2137. [Google Scholar] [CrossRef]
- Scalisi, A.; Morandi, B.; Inglese, P.; Lo Bianco, R. Cladode growth dynamics in Opuntia ficus-indica under drought. Environ. Exp. Bot. 2016, 122, 158–167. [Google Scholar] [CrossRef]
- Puligundla, P.; Lim, S. A review of extraction techniques and food applications of flaxseed mucilage. Foods 2022, 11, 1677. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.C.; Jiménez-Martínez, C.; Jaime-Fonseca, M.R.; Alamilla-Beltrán, L. Extraction of purple prickly pear (Opuntia ficus-indica) mucilage by microfiltration, composition, and physicochemical characteristics. Polymers 2024, 16, 3383. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carranza, P.; Rivadeneyra-Mata, M.; Ramos-Cassellis, M.E.; Aparicio-Fernández, X.; Navarro-Cruz, A.R.; Ávila-Sosa, R. Characterization of red prickly pear peel (Opuntia ficus-indica L.) and its mucilage obtained by traditional and novel methodologies. J. Food Meas. Charact. 2019, 13, 1111–1119. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Cifuentes, G.R.; Gómez Castaño, J.A. Use of Opuntia ficus-indica fruit peel as a novel source of mucilage with coagulant physicochemical/molecular characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Felkai-Haddache, L.; Dahmoune, F.; Remini, H.; Lefsih, K.; Mouni, L.; Madani, K. Microwave optimization of mucilage extraction from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 84, 24–30. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M. Patent PA153178P A Process for Extracting Mucilage from Opuntia ficus-indica, Aloe barbadensis and Agave americana. Ph.D. Thesis, University of Free State, Bloemfontein, South Africa, 2021. [Google Scholar]
- Reyes-Ocampo, I.; Córdova-Aguilar, M.S.; Guzmán, G.; Blancas-Cabrera, A.; Ascanio, G. Solvent-free mechanical extraction of Opuntia ficus-indica mucilage. J. Food Process Eng. 2019, 42, e12954. [Google Scholar] [CrossRef]
- Mannai, F.; Elhleli, H.; Yılmaz, M.; Khiari, R.; Belgacem, M.N.; Moussaoui, Y. Precipitation solvents effect on the extraction of mucilaginous polysaccharides from Opuntia ficus-indica (Cactaceae): Structural, functional and rheological properties. Ind. Crops Prod. 2023, 202, 117072. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Castaño, J.A.G. Extraction and physicochemical characterization of dried powder mucilage from Opuntia ficus-indica cladodes and Aloe vera leaves: A comparative study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef]
- Adjeroud, N.; Elabbas, S.; Merzouk, B.; Hammoui, Y.; Felkai-Haddache, L.; Remini, H. Effect of Opuntia ficus indica mucilage on copper removal from water by electrocoagulation-electroflotation technique. J. Electroanal. Chem. 2018, 811, 26–36. [Google Scholar] [CrossRef]
- Mannai, F.; Elhleli, H.; Mosbah, M.B.; Khiari, R.; Nacer, S.N.; Belgacem, M.N. Comparative study of conventional and combined ultrasound-assisted methods on the quality of mucilage extracted from Opuntia ficus-indica cladodes. Ind. Crops Prod. 2024, 214, 118566. [Google Scholar] [CrossRef]
- García-Barradas, O.; Esteban-Cortina, A.; Mendoza-Lopez, M.R.; Ortiz-Basurto, R.I.; Díaz-Ramos, D.I.; Jiménez-Fernández, M. Chemical modification of Opuntia ficus-indica mucilage: Characterization, physicochemical, and functional properties. Polym. Bull. 2023, 80, 8783–8798. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Morghese, M.; Santulli, A.; Liguori, G.; Inglese, P. Seasonal characterization of nutritional and antioxidant properties of Opuntia ficus-indica [(L.) Mill.] mucilage. Food Hydrocoll. 2021, 111, 106398. [Google Scholar] [CrossRef]
- Shinga, M.H.; Fawole, O.A. Opuntia ficus indica mucilage coatings regulate cell wall softening enzymes and delay the ripening of banana fruit stored at retail conditions. Int. J. Biol. Macromol. 2023, 245, 125550. [Google Scholar] [CrossRef] [PubMed]
- Mannai, F.; Mechi, L.; Alimi, F.; Alsukaibi, A.K.D.; Belgacem, M.N.; Moussaoui, Y. Biodegradable composite films based on mucilage from Opuntia ficus-indica (Cactaceae): Microstructural, functional and thermal properties. Int. J. Biol. Macromol. 2023, 252, 126456. [Google Scholar] [CrossRef] [PubMed]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and physicochemical characterization of mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal degradation of bioactive compounds during drying process of horticultural and agronomic products: A comprehensive overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Du Toit, A. Selection, Extraction, Characterization and Application of Mucilage from Cactus Pear (Opuntia ficus-indica and Opuntia robusta) Cladodes. Ph.D. Thesis, University of Free State, Bloemfontein, South Africa, 2021. Available online: http://hdl.handle.net/11660/6514 (accessed on 13 April 2025).
- Shinga, M.H.; Fawole, O.A.; Pareek, S. Opuntia ficus-indica mucilage coating prolongs the shelf life of bananas (Musa spp.) by enhancing antioxidant activity and modulating antioxidant enzyme systems. Food Biosci. 2025, 65, 106039. [Google Scholar] [CrossRef]
- Martins, M.; Ribeiro, M.H.; Almeida, C.M.M. Physicochemical, nutritional, and medicinal properties of Opuntia ficus-indica (L.) Mill. and its main agro-industrial use: A review. Plants 2023, 12, 1512. [Google Scholar] [CrossRef]
- Ullah, A.; Ahmed, S. Green Biopolymers for Packaging Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; Available online: https://www.taylorfrancis.com/books/9781003455356 (accessed on 14 April 2025).
- Medina-Torres, L. Rheological properties of the mucilage gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G. Gelling potential of native cactus pear mucilage. In Acta Horticulturae, Proceedings of the X International Congress on Cactus Pear and Cochineal, João Pessoa, Brazil, 26–29 September 2022; ISHS Series; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2022; pp. 489–496. [Google Scholar]
- Elhleli, H.; Mannai, F.; Khiari, R.; Moussaoui, Y. The use of mucilage extracted from Opuntia ficus indica as a microencapsulating shell. J. Serb. Chem. Soc. 2021, 86, 25–38. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; Alancay, M.; de Mishima, B.L.; Iturriaga, L. Physicochemical and rheological properties of mucilage extracted from Opuntia ficus indica (L. Miller). Comparative study with guar gum and xanthan gum. J. Food Meas. Charact. 2018, 12, 459–470. [Google Scholar] [CrossRef]
- Luna-Zapién, E.A.; Zegbe, J.A.; Meza-Velázquez, J.A.; Contreras-Esquivel, J.C.; Morales-Martínez, T.K. Mucilage yield, composition, and physicochemical properties of cultivated cactus pear varieties as influenced by irrigation. Agronomy 2023, 13, 419. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Majdoub, H.; Picton, L.; Le Cerf, D.; Roudesli, S. Water retention capacity of polysaccharides from prickly pear nopals of Opuntia ficus indica and Opuntia litoralis: Physical–chemical approach. J. Polym. Environ. 2010, 18, 451–458. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist wound healing with commonly available dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.K.; Tareq, A.Z.; Kamal, I.M. Optimization of swelling, drug loading and release from natural polymer hydrogels. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012017. [Google Scholar] [CrossRef]
- Pierdomenico, M.; Giardullo, P.; Bruno, G.; Bacchetta, L.; Maccioni, O.; Demurtas, O.C. The mucilage from the Opuntia ficus-indica (L.) mill. cladodes plays an anti-inflammatory role in the lps-stimulated hepg2 cells: A combined in vitro and in silico approach. Mol. Nutr. Food Res. 2025, 69, e202400479. [Google Scholar] [CrossRef]
- Trombetta, D.; Puglia, C.; Perri, D.; Licata, A.; Pergolizzi, S.; Lauriano, E.R. Effect of polysaccharides from Opuntia ficus-indica (L.) cladodes on the healing of dermal wounds in the rat. Phytomedicine 2006, 13, 352–358. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P. A comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef]
- Haseeb, M.T.; Muhammad, G.; Hussain, M.A.; Bukhari, S.N.A.; Sheikh, F.A. Flaxseed (Linum usitatissimum) mucilage: A versatile stimuli–responsive functional biomaterial for pharmaceuticals and healthcare. Int. J. Biol. Macromol. 2024, 278, 134817. [Google Scholar] [CrossRef]
- Dantas, T.L.; Alonso Buriti, F.C.; Florentino, E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants 2021, 10, 1683. [Google Scholar] [CrossRef]
- Öncü Glaue, Ş.; Akcan, T.; Tavman, Ş. Thermal properties of ultrasound-extracted okra mucilage. Appl. Sci. 2023, 13, 6762. [Google Scholar] [CrossRef]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide-based films of cactus mucilage and agar with antioxidant properties for active food packaging. Polym. Bull. 2022, 79, 11369–11388. [Google Scholar] [CrossRef]
- Agrawal, R. Psyllium: A source of dietary fiber. In Dietrary Fibres; IntechOpen: London, UK, 2022. [Google Scholar]
- Geremew Kassa, M.; Alemu Teferi, D.; Asemu, A.M.; Belachew, M.T.; Satheesh, N.; Abera, B.D. Review on psyllium husk: Nutritional, functional, health benefits, food industry applications, waste treatment, and potential negative effects. CyTA-J. Food 2024, 22, 2409174. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; Ćirić, A.; Silva, S.P.; Coelho, E. Compositional features and bioactive properties of Aloe vera leaf (fillet, mucilage, and rind) and flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Cobbinah-Sam, E.; Ekaette, I.; Yu, Z.; Onyeaka, H. Plant seed hydrocolloids: Extraction methods and techno-functional properties—A review. Food Bioprocess. Technol. 2025, 18, 6010–6034. [Google Scholar] [CrossRef]
- Du Toit, L. Gelling Properties of Cactus Pear Mucilage-Hydrocolloid Combinations in a Sugar-Based Confectionery. Ph.D. Thesis, University of Free State, Bloemfontein, South Africa, 2018. [Google Scholar]
- Du Toit, L.; Bothma, C.; De Wilt, M.; Hugo, A. Replacement of gelatin with liquid Opuntia ficus-indica mucilage in marshmallows. Part 1: Physical parameters. J. Prof. Assoc. Cactus Dev. 2020, 18, 25–39. [Google Scholar] [CrossRef]
- Rodrigues, P.D.; Fernandes, I.A.A.; de Marins, A.R.; Feihrmann, A.C.; Gomes, R.G. Use of mucilage from Opuntia ficus-indica in the manufacture of probiotic cream cheese. Processes 2024, 12, 2289. [Google Scholar] [CrossRef]
- Shiam, M.A.H.; Islam, M.S.; Ahmad, I.; Haque, S.S. A review of plant-derived gums and mucilages: Structural chemistry, film forming properties and application. J. Plast. Film Sheet. 2025, 41, 195–237. [Google Scholar] [CrossRef]
- López-Díaz, A.S.; Méndez-Lagunas, L.L. Mucilage-based films for food applications. Food Rev. Int. 2022, 39, 6677–6706. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A review on the encapsulation of bioactive components using spray-drying and freeze-drying techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Utrilla-Coello, R.G.; Soto-Castro, D. Effect of Opuntia ficus-indica mucilage in the ecological extraction, drying, and storage of eggplant anthocyanins. J. Food Process. Preserv. 2018, 42, e13439. [Google Scholar] [CrossRef]
- Amiri, M.S.; Mohammadzadeh, V.; Yazdi, M.E.T.; Barani, M.; Rahdar, A.; Kyzas, G.Z. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, J.M.; Singh, R.K.; Kupski, L.; Fernandes, S.S.; Otero, D.M. Techno-functional properties of Cactaceae polysaccharides for food packaging application: A review. Int. J. Biol. Macromol. 2025, 312, 144099. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Silipo, A.; Molinaro, A.; Parrilli, M.; Schiraldi, C.; D’Agostino, A. The polysaccharide and low molecular weight components of Opuntia ficus indica cladodes: Structure and skin repairing properties. Carbohydr. Polym. 2017, 157, 128–136. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H. Antioxidant, antibacterial and in vivo dermal wound healing effects of Opuntia flower extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S. Flavonoids as potential wound-healing molecules: Emphasis on pathways perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Gaviria-Bedoya, A.; Rubio-Clemente, A. Coagulant and flocculant effect of Opuntia ficus-indica in water treatment. J. Chem. Eng. Theor. Appl. Chem. 2024, 81, 112–122. [Google Scholar] [CrossRef]
- Javed, S.; Zulfiqar, Z.; Fatima, Z.; Muhammad, G.; Hussain, M.A.; Mushtaq, M. A comprehensive review of plant-based mucilages as promising candidates for water remediation. J. Environ. Chem. Eng. 2024, 12, 114035. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; de Lima Stebbins, D.M.; Moldes, A.B.; Cruz, J.M.; Alcantar, N.A. Evaluation of a cactus mucilage biocomposite to remove total arsenic from water. Environ. Technol. Innov. 2016, 6, 69–79. [Google Scholar] [CrossRef]
- Vargas-Solano, S.V.; Rodríguez-González, F.; Martínez-Velarde, R.; Morales-García, S.S.; Jonathan, M.P. Removal of heavy metals present in water from the Yautepec River Morelos México, using Opuntia ficus-indica mucilage. Environ. Adv. 2022, 7, 100160. [Google Scholar] [CrossRef]
- Matos, T.; Martins, M.S.; Henriques, R.; Goncalves, L.M. A review of methods and instruments to monitor turbidity and suspended sediment concentration. J. Water Process Eng. 2024, 64, 105624. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Figueroa Márquez, I.F.; Leigue Fernández, M.A.; Angulo Reyes, M.R. Assessment of the efficiency as coagulant-flocculant of mucilage obtained from prickly pear (Opuntia ficus-indica). In Proceedings of the International Congress on Project Management and Engineering, Donostia-San Sebastian, Spain, 10–13 July 2023; pp. 1306–1317. [Google Scholar]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Gómez Castaño, J.A.; Cifuentes, G.R. Evaluation of turbidity and color removal in water treatment: A comparative study between Opuntia ficus-indica fruit peel mucilage and FeCl3. Polymers 2023, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.C.; Lim, J.W.; Ho, Y.C. Garden cress mucilage as a potential emerging biopolymer for improving turbidity removal in water treatment. Process. Saf. Environ. Prot. 2018, 119, 233–241. [Google Scholar] [CrossRef]
- Fox, D.I.; Pichler, T.; Yeh, D.H.; Alcantar, N.A. Removing Heavy Metals in Water: The Interaction of Cactus Mucilage and Arsenate (As (V)). Environ. Sci. Technol. 2012, 46, 4553–4559. [Google Scholar] [CrossRef]
- Wan, J.; Chakraborty, T.; Xu, C.; Ray, M.B. Treatment train for tailings pond water using Opuntia ficus-indica as coagulant. Sep. Purif. Technol. 2019, 211, 448–455. [Google Scholar] [CrossRef]
- Tesfay, H. Investigation of Removal Efficiency of Blended Cactus Mucilage and Alum Coagulants in Textile Wastewater Treatment. 2019. Available online: https://etd.aau.edu.et/items/9dd2f648-4f75-4e5c-b265-f885ab5d663a (accessed on 14 April 2025).
- González-Avilez, E.; Rodríguez-González, F.; Vargas-Solano, S.V.; Osorio-Ruiz, A.; Jonathan, M.P.; Campos-Villegas, L.E. Effect of the concentration of uronic acids in Opuntia mucilage on the removal of heavy metals and water quality of the Yautepec River, Mexico. Arab. J. Chem. 2024, 17, 105636. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Cano-Barrita, P.F.D.J. Cactus mucilage: A review of its rheological and physicochemical properties and use as bio-admixture in building materials. Int. J. Biol. Macromol. 2024, 279, 135111. [Google Scholar] [CrossRef]
- Alisi, C.; Bacchetta, L.; Bojorquez, E.; Falconieri, M.; Gagliardi, S.; Insaurralde, M. Sustainable additives from Opuntia mucilage in restoration mortars. Acta Hortic. 2022, 1343, 435–442. [Google Scholar] [CrossRef]
- Ventolà, L.; Vendrell, M.; Giraldez, P.; Merino, L. Traditional organic additives improve lime mortars: New old materials for restoration and building natural stone fabrics. Constr. Build. Mater. 2011, 25, 3313–3318. [Google Scholar] [CrossRef]
- Alisi, C.; Bacchetta, L.; Bojorquez, E.; Falconieri, M.; Gagliardi, S.; Insaurralde, M. Mucilages from different plant species affect the characteristics of bio-mortars for restoration. Coatings 2021, 11, 75. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Cano-Barrita, P.F.d.J.; Castellanos, F.; Luna-Vicente, K.B.; Ramírez-Arellanes, S.; Gómez-Yáñez, C. Carbonation of high-calcium lime mortars containing cactus mucilage as additive: A spectroscopic approach. J. Mater. Sci. 2021, 56, 3778–3789. [Google Scholar] [CrossRef]
- Ravi, R.; Selvaraj, T.; Sekar, S.K. Characterization of hydraulic lime mortar containing Opuntia ficus-indica as a bio-admixture for restoration applications. Int. J. Archit. Herit. 2016, 10, 714–725. [Google Scholar] [CrossRef]
- Fernandez, F.; Forte, A.; Badalamenti, S.; Lombardo, A. Use of prickly pear (nopal) mucilage in construction applications: Research and results. Life Saf. Secur. 2024, 12, 1. [Google Scholar]
- de Souza, G.F.A.; Pereira, M.M.L.; Palma e Silva, A.A.; Capuzzo, V.M.S.; Machado, F. Opuntia ficus-indica mucilage: A sustainable bio-additive for cementitious materials. Constr. Build. Mater. 2024, 456, 139254. [Google Scholar] [CrossRef]
- Lorika, D.A.; Hamad, B.; Yehya, A.; Salam, D.A. Evaluating the use of mucilage from Opuntia ficus-indica as a bio-additive in production of sustainable concrete. Constr. Build. Mater. 2023, 396, 132132. [Google Scholar] [CrossRef]
- Durán-Herrera, A.; De-León, R.; Juárez, C.A.; Valdez-Tamez, P. Opuntia ficus indica mucilage (ofim) as internal curing enhancer in self consolidating concrete. Rev. Rom. Mater. 2017, 47, 532. [Google Scholar]
- Akinwumi, I.I.; Ukegbu, I. Soil modification by addition of cactus mucilage. Geomech. Eng. 2015, 8, 649–661. [Google Scholar] [CrossRef]
- Borah, D.; Mishra, V.; Debnath, R.; Ghosh, K.; Gogoi, D.; Rout, J. Facile green synthesis of highly stable, water dispersible carbohydrate conjugated Ag, Au and Ag-Au biocompatible nanoparticles: Catalytic and antimicrobial activity. Mater. Today Commun. 2023, 37, 107096. [Google Scholar] [CrossRef]
- Li, R.; He, M.; Cui, Y.; Ji, X.; Zhang, L.; Lan, X. Silver-palladium bimetallic nanoparticles stabilized by elm pod polysaccharide with peroxidase-like properties for glutathione detection and photothermal anti-tumor ability. Int. J. Biol. Macromol. 2024, 264, 130673. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Tagad, C. Biosynthesis of polysaccharides-stabilized metal nanoparticles for chemical and biosensing applications. In Polysaccharide Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2022; pp. 553–583. [Google Scholar] [CrossRef]
- Lee, H.B.; Son, S.U.; Lee, J.E.; Lee, S.H.; Kang, C.H.; Kim, Y.S. Characterization, prebiotic and immune-enhancing activities of rhamnogalacturonan-I-rich polysaccharide fraction from molokhia leaves. Int. J. Biol. Macromol. 2021, 175, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Akl, E.M.; Mohamed, R.S.; Abdelgayed, S.S.; Fouda, K.; Abdel-Wahhab, M.A. Characterization and antioxidant activity of flaxseed mucilage and evaluation of its dietary supplementation in improving calcium absorption in vivo. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100444. [Google Scholar] [CrossRef]
- Guevara-Arauza, J.C.; De Jesús Ornelas-Paz, J.; Pimentel-González, D.J.; Rosales Mendoza, S.; Soria Guerra, R.E.; Paz Maldonado, L.M.T. Prebiotic effect of mucilage and pectic-derived oligosaccharides from nopal (Opuntia ficus-indica). Food Sci. Biotechnol. 2012, 21, 997–1003. [Google Scholar] [CrossRef]
- Patel, A.R.; Nicholson, R.A.; Marangoni, A.G. Applications of fat mimetics for the replacement of saturated and hydrogenated fat in food products. Curr. Opin. Food Sci. 2020, 33, 61–68. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.; Fouché, H.J.; Taljaard, M.; Venter, S.L.; Hugo, A. Mucilage powder from cactus pears as functional ingredient: Influence of cultivar and harvest month on the physicochemical and technological properties. J. Food Sci. Technol. 2019, 56, 2404–2416. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An overview and update on the chemical composition and potential health benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Soltani, M.; Bordes, C.; Ariba, D.; Majdoub, M.; Majdoub, H.; Chevalier, Y. Emulsifying properties of biopolymer extracts from Opuntia ficus indica cladodes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133005. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Rashad, M.; Pari, L.; Outzourhit, A.; Fernando, A.L. Mucilage extraction from Opuntia spp for production of biofilms. In Proceedings of the 27th European Biomass Conference and Exhibition, Lisbon, Portugal, 27–30 May 2019. [Google Scholar]
- Colín-Chávez, C.; Soto-Valdez, H.; Turrado-Saucedo, J.; Rodríguez-Félix, A.; Peralta, E.; Saucedo-Corona, A.R. Papermaking as potential use of fibers from Mexican Opuntia ficus-indica waste. Biotecnia 2021, 23, 141–150. [Google Scholar] [CrossRef]
- Marin-Bustamante, M.Q.; Chanona-Pérez, J.J.; Güemes-Vera, N.; Cásarez-Santiago, R.; PereaFlores, M.J.; Arzate-Vázquez, I. Production and characterization of cellulose nanoparticles from nopal waste by means of high impact milling. Procedia Eng. 2017, 200, 428–433. [Google Scholar] [CrossRef]
- Luna-Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez-Fuentes, H.; Pastrana, L.M.; Azevedo, A.G.; González-Sandoval, D.C. Extraction and characterization of mucilage from Opuntia ficus-indica cultivated on hydroponic system. Not. Bot. Horti Agrobot. 2022, 50, 12460. [Google Scholar] [CrossRef]
- Loretta, B.; Oliviero, M.; Vittorio, M.; Bojórquez-Quintal, E.; Franca, P.; Silvia, P. Quality by design approach to optimize cladodes soluble fiber processing extraction in Opuntia ficus indica (L.) Miller. J. Food Sci. Technol. 2019, 56, 3627–3634. [Google Scholar] [CrossRef]
- Nkoi, V.; De Wit, M.; Van Biljon, A.; Van Niekerk, J.A. Comparison and integration of cactus mucilage protein and soy protein in functional food systems. Acta Hortic. 2022, 1343, 425–434. [Google Scholar] [CrossRef]
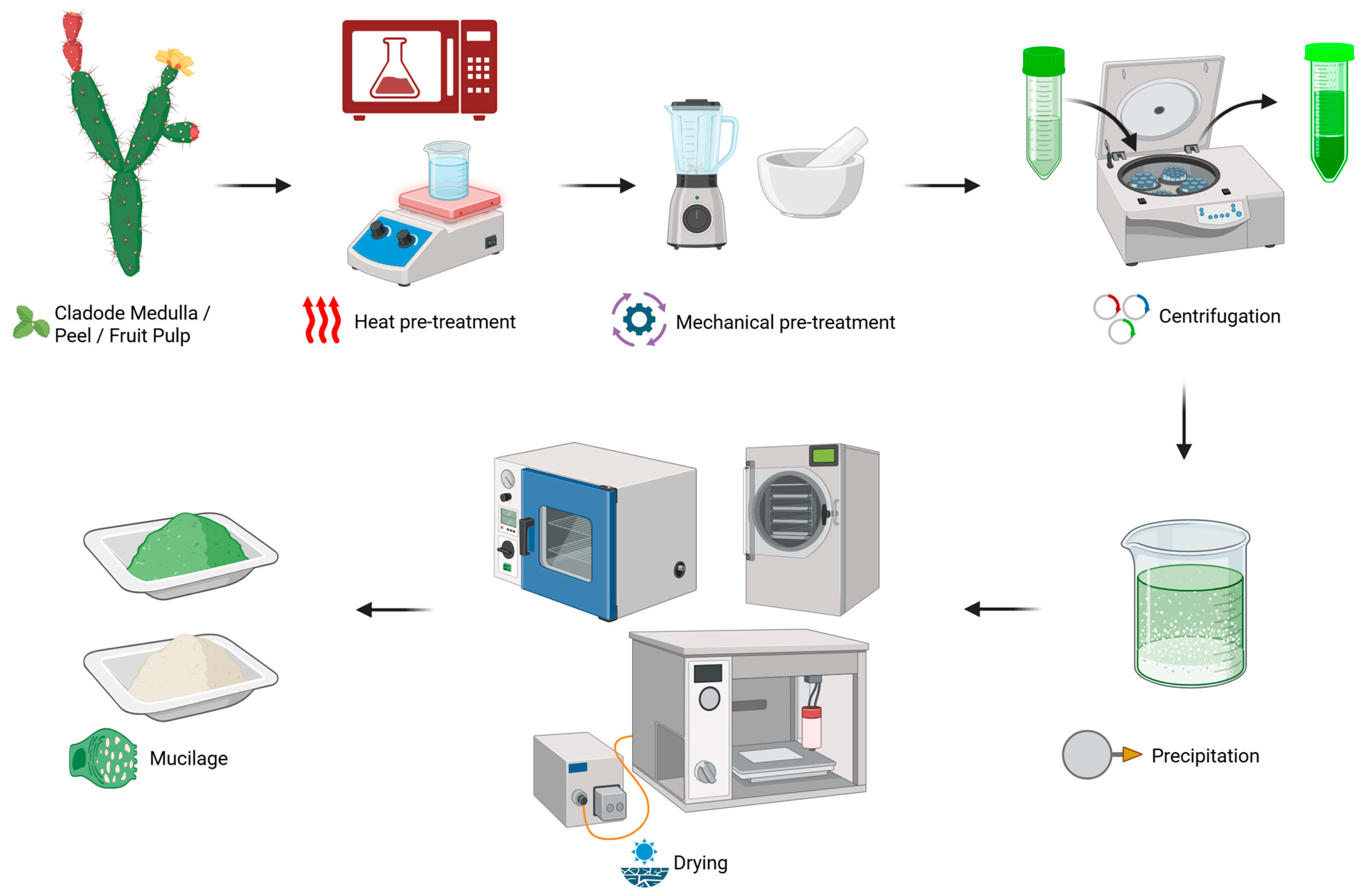
| Plant Part | Thermal Pre-Treatment | Mechanical Disintegration | Centrifugation | Precipitation | Drying | Yield | References |
|---|---|---|---|---|---|---|---|
| Cladode | None | Pressing | 10,000 rpm for 10 min | Ethanol (1:3) | Oven-dried (105 °C for 24 h) | NA | [26] |
| Cladode | None | Pressing | No | Ethanol (2:3) | Oven-dried (50 °C for 24 h) | 14% | [19] |
| Cladode | None | Blending | 7000 rpm for 1 h | None | Freeze-dried (NA) | NA | [15] |
| Cladode | Microwave | Milling | 4000 rpm for 15 min | Ethanol (1:3) | Freeze-dried (−55 °C for 12 h) | Up to 25.6% | [27] |
| Cladode | Microwave | Blending | Yes (NA) | None | Freeze-dried (NA) | Up to 20.9% | [28] |
| Cladode | None | Blending | No | None | Spray-dried (inlet temp. of 135 °C) | 1.17% | [29] |
| Cladode | None | Milling | Yes (NA) | Ethanol (1:2) | Oven-dried (35 °C for 40 min) | 15.69% | [20] |
| Cladode | Hot water | None | Yes (4000 rpm for 20 min) | Ethanol or isopropanol (2:3) | Oven-dried (40 °C for 24 h) | Isopropanol (23.2%) Ethanol (19%) | [30] |
| Fruit | Hot water | None | Yes (2500 rpm for 15 min) | Ethanol (1:3) | Oven-dried (30 °C for 48 h) | 9.92% | [23] |
| Cladode | None | Pressing | Yes (10,000 rpm for 30 min) | Ethanol (1:3) | Oven-dried (105 °C for 24 h) | NA | [31] |
| Fruit peel | None | Pressing | No | Ethanol (1:3) | Oven-dried (50 °C for 3 h) | NA | [25] |
| Fruit peel | Hot water | Ultrasound sonication | No | NA | NA | Up to 41.7% Up to 33.6% | [24] |
| Cladodes | Microwave | Blending | No | Ethanol (1:3) | Oven-dried (NA) | 18.8% | [32] |
| Cladodes | Hot water | Ultrasound sonication | Yes (4000 rpm for 20 min) | NA | Oven-dried (45 °C for 24 h) | 19 to 22.8% | [33] |
| Cladodes | None | Crushing | Yes (4500 rpm for 30 min) | Ethanol (1:3) | Freeze-dried (NA) | NA | [34] |
| Cladodes | Microwave | Blending | Yes (8117 rpm for 15 min) | None | NA | Up to 26% | [35] |
| Cladodes | Microwave | None | Yes (10,100 rpm for 15 min) | None | Freeze-dried (72 h) | NA | [36] |
| Mucilage Source | Key Properties | Limitations | Advantages of OFI Mucilage | References |
|---|---|---|---|---|
| Flaxseed (Linum usitatissimum) | Good water-binding; high viscosity | Lower uronic acid content; less WHC | Higher WHC due to uronic acids and branched heteropolysaccharides | [22,49,56] |
| Okra (Abelmoschus esculentus) | Emulsifying and thickening | Low thermal stability (Tg = 50 °C, Mp = 166 °C) | Maintains viscosity and structural integrity near 200 °C | [19,57,58] |
| Chia Seed (Salvia hispanica) | Excellent gelation; emulsion stabilization | Less flexible films, poor transparency | Forms flexible, and transparent films with higher elongation at break | [38,59,60] |
| Psyllium (Plantago ovata) | High fiber, gelling agent | Allergenic reactions due to protein contaminants | Hypoallergenic, non-toxic, highly biocompatible | [44,61,62] |
| Aloe vera | Hydrating, bioactive-rich mucilage | High cultivation inputs (controlled growth and water requirements) | Thrives in arid zones; sustainable, high-yield; rapid cladode regrowth | [6,21,63] |
| Process Type | Efficiency | Mechanism | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Turbidity removal | 80–95% | Polymer bridging, charge neutralization | Renewable, biodegradable | Efficiency varies with ionic strength | [83,84,85] |
| Heavy metal removal | 60–100% (Cu2+ > Fe2+ > Cr6+) | Chelation via −COOH and −OH groups | High selectivity | Sensitive to pH, competitive ions | [32,81,87] |
| Soil stabilization | Increased unconfined compressive strength, reduced permeability | Mineral-polysaccharide film formation | Improved durability | Requires drying uniformity | [101] |
| Lime mortar admixture | 60–70% strength increase | Pore refinement, carbonation control | Eco-friendly binder | Scalability and consistency | [92,93,95] |
| Application Area | Scientific Basis | Research Gaps | Potential Impact |
|---|---|---|---|
| Metal nanoparticle synthesis | Reducing and capping properties via hydroxyl/carboxyl groups | Optimization of synthesis conditions; nanoparticle characterization | Eco-friendly nanomaterials |
| Prebiotic functional ingredient | Non-digestible polysaccharides support gut microbiota; good encapsulation of probiotics | In vivo studies on fermentation, microbiome modulation | Gut health, functional foods |
| Fat replacer in foods | High viscosity, gel-forming, and creamy texture | Sensory evaluation; compatibility with food matrices | Low-calorie food formulations |
| Drug delivery systems | High encapsulation efficiency, mucoadhesion, and biodegradability | Pharmacokinetics; release modeling; biocompatibility assays | Targeted, sustained drug delivery |
| Tissue engineering scaffolds | Biocompatible, porous, moisture-retaining, supports cell growth | In vivo studies; mechanical property optimization | Regenerative medicine |
| Antimicrobial/antioxidant carriers | Natural antimicrobial and antioxidant activity; film-forming capability | Controlled release profiling; synergy with antibiotics | Topical agents, active dressings |
| Cosmetics and personal care | Emulsifying, moisturizing, antimicrobial, antioxidant properties | Long-term skin compatibility studies; stability testing | Natural ingredient in personal care |
| Challenges | Description | Implication | References |
|---|---|---|---|
| Variability in Composition | Affected by cultivar, environment, and season | Limits standardization and reproducibility of product functionality | [65,115] |
| Extraction Challenges | Influenced by extraction method, water ratio, temperature, drying technique | Affects yield, purity, and scalability | [33,116] |
| Environmental Impact of Extraction | Use of solvents in traditional methods | Necessitates greener extraction approaches | [23,71] |
| Functional Inconsistencies | Differences in viscosity, emulsification, protein content | Reduces performance predictability in food and cosmetic formulations | [43,117] |
| Cost and Scalability | High production cost, need for specialized equipment | Limits industrial application; requires optimization for economic viability | [23,116] |
| Competition with Synthetic Polymers | Synthetic polymers offer more consistency and engineered properties | Challenges adoption unless mucilage is functionally enhanced | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukaila, Y.O.; Adeyemi, J.O.; Fawole, O.A. Towards Sustainable Biopolymer Innovation: A Review of Opuntia ficus-indica Mucilage. Processes 2025, 13, 3837. https://doi.org/10.3390/pr13123837
Mukaila YO, Adeyemi JO, Fawole OA. Towards Sustainable Biopolymer Innovation: A Review of Opuntia ficus-indica Mucilage. Processes. 2025; 13(12):3837. https://doi.org/10.3390/pr13123837
Chicago/Turabian StyleMukaila, Yusuf O., Jerry O. Adeyemi, and Olaniyi A. Fawole. 2025. "Towards Sustainable Biopolymer Innovation: A Review of Opuntia ficus-indica Mucilage" Processes 13, no. 12: 3837. https://doi.org/10.3390/pr13123837
APA StyleMukaila, Y. O., Adeyemi, J. O., & Fawole, O. A. (2025). Towards Sustainable Biopolymer Innovation: A Review of Opuntia ficus-indica Mucilage. Processes, 13(12), 3837. https://doi.org/10.3390/pr13123837









