The Use of Bioactive Edible Coatings Based on Pectin and Phenolic Acids for Enhancing Quality Attributes of Golden Delicious Apples During Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
Rheology of Coating Solutions
2.3. Film Preparation
2.3.1. Film Thickness
2.3.2. The Release Kinetic Measurements of Phenolic Acids
2.4. Coatings of Apples
3. Physicochemical Analyses
3.1. Weight Loss
3.2. Colour
3.3. Total Soluble Solids
3.4. Total Titratable Acidity
3.5. pH
3.6. Firmness
3.7. Respiration Rate
3.8. Sensory Analysis
3.9. Statistical Analysis
4. Results and Discussion
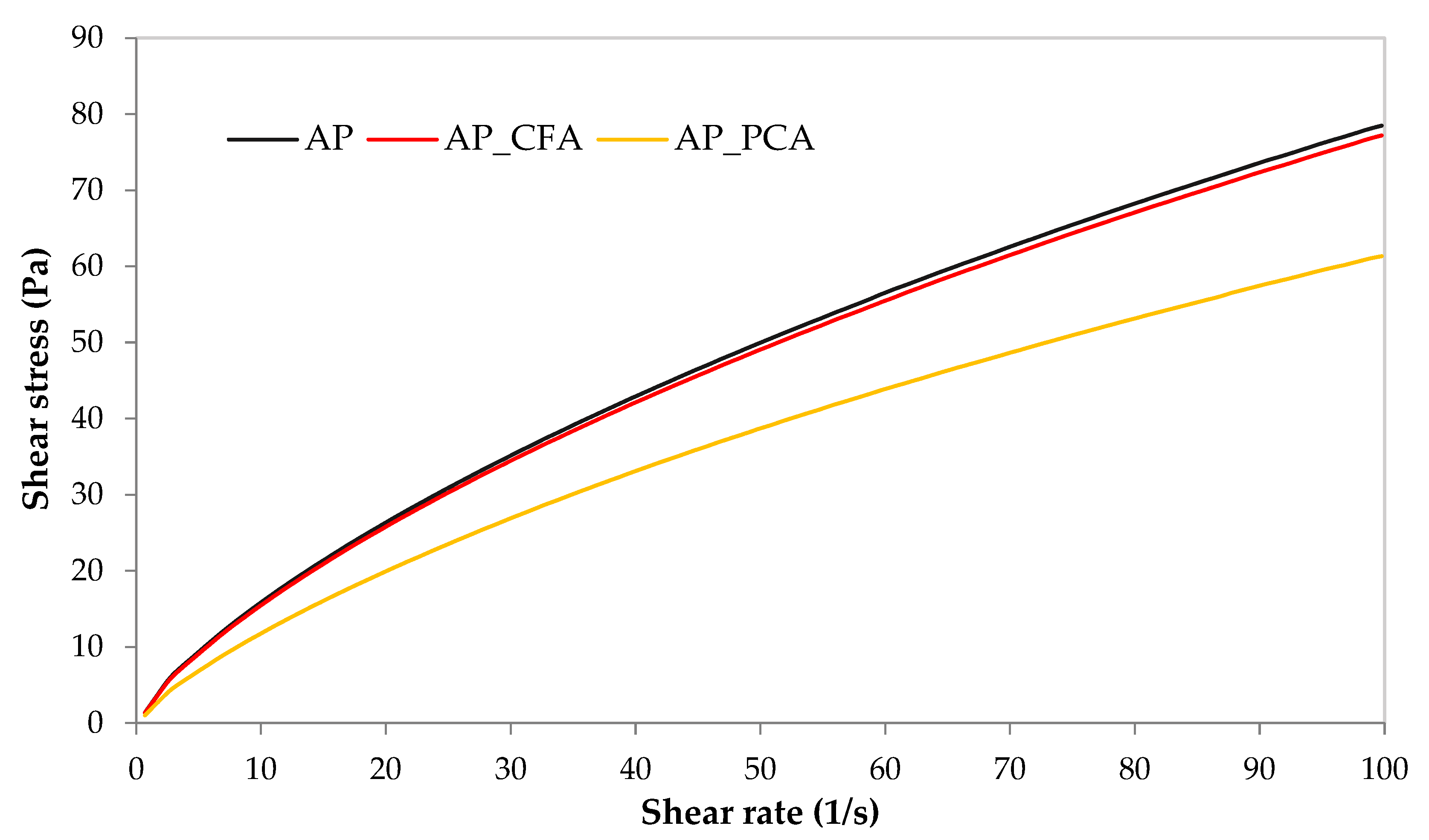
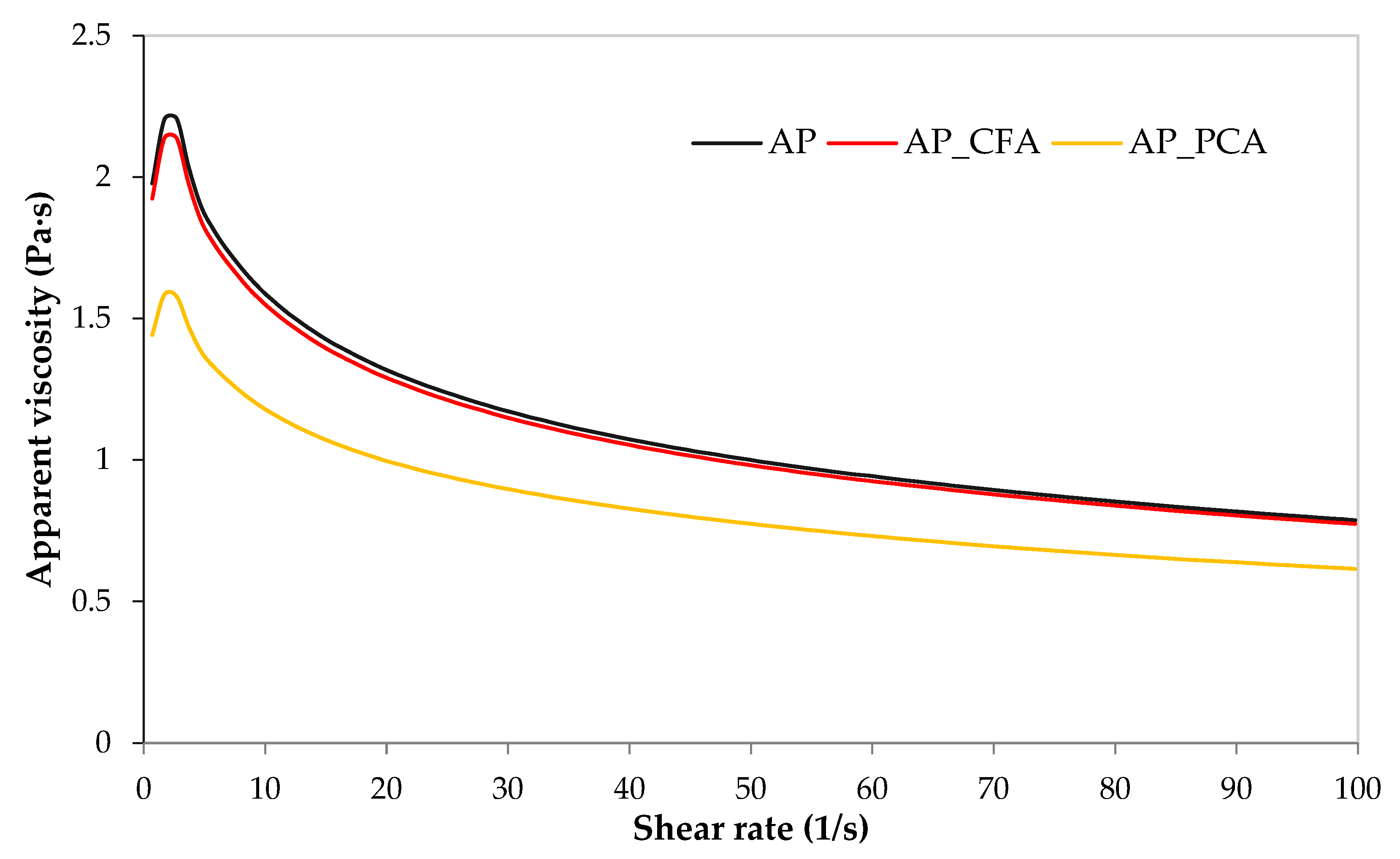
4.1. The Effect of Phenolic Acids on the Rheological Properties of Coating Solutions
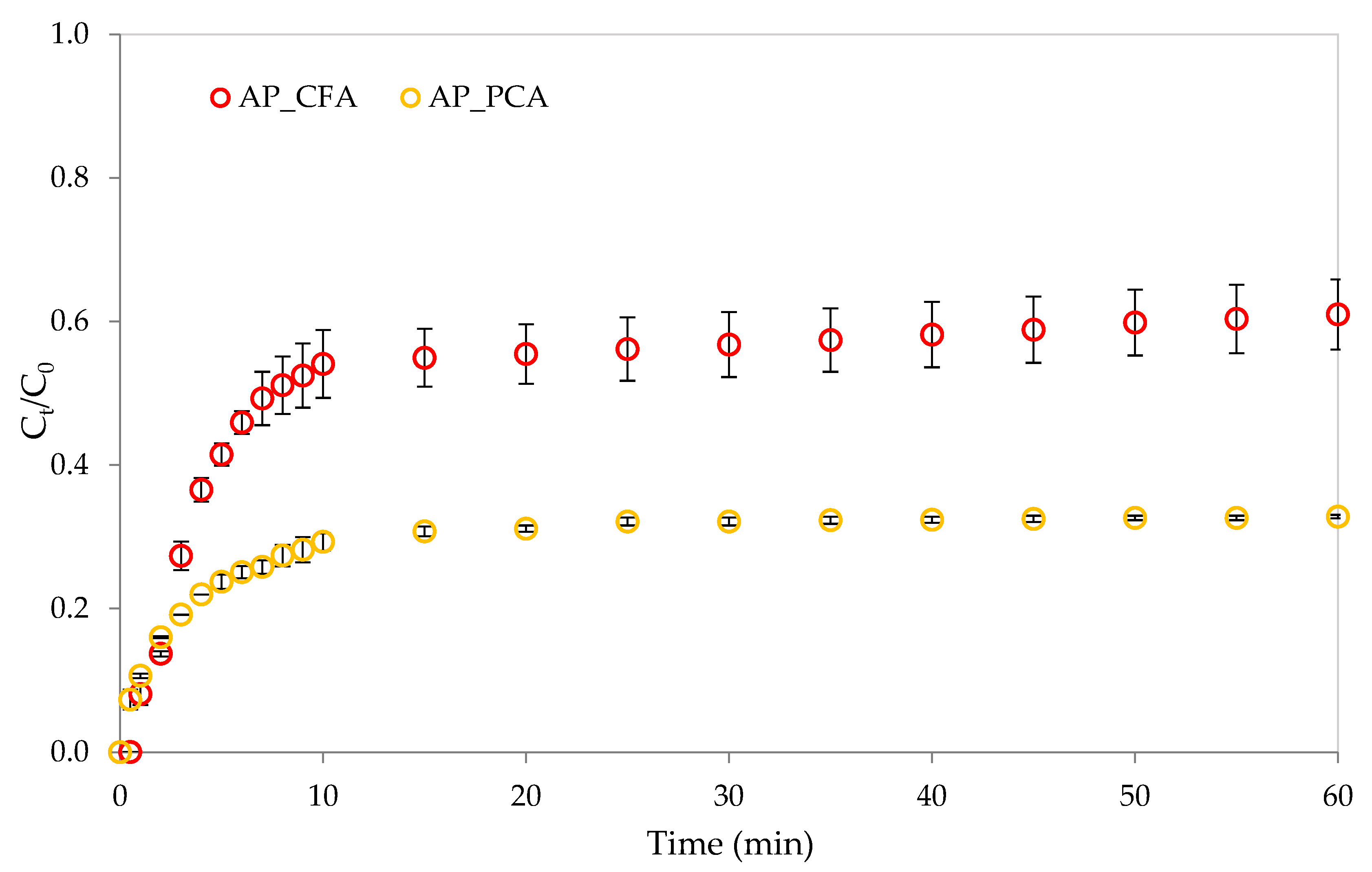
4.2. Release Kinetics of Caffeic and Protocatechuic Acids from Pectin Films
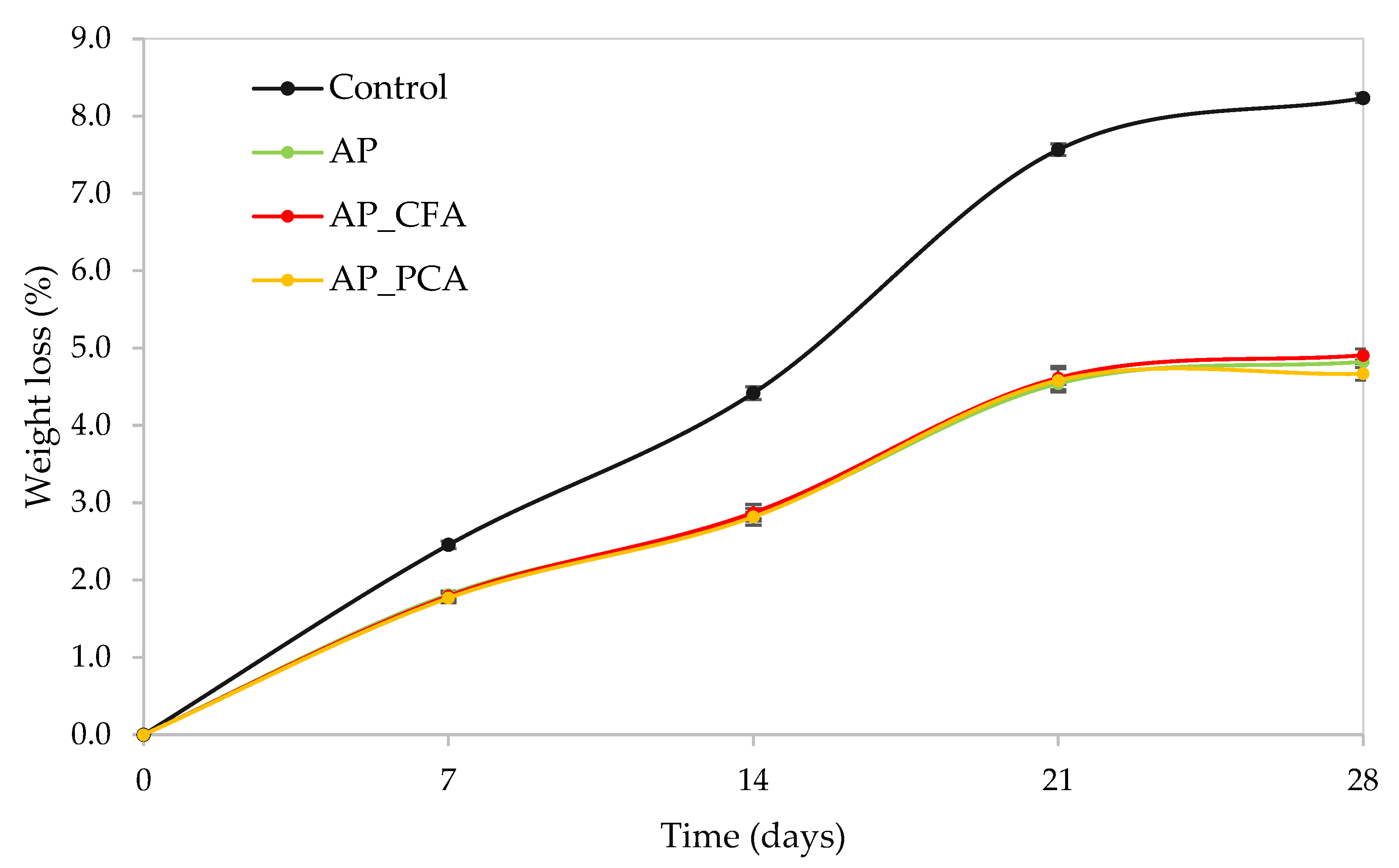
4.3. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Weight Loss of Apples During Storage
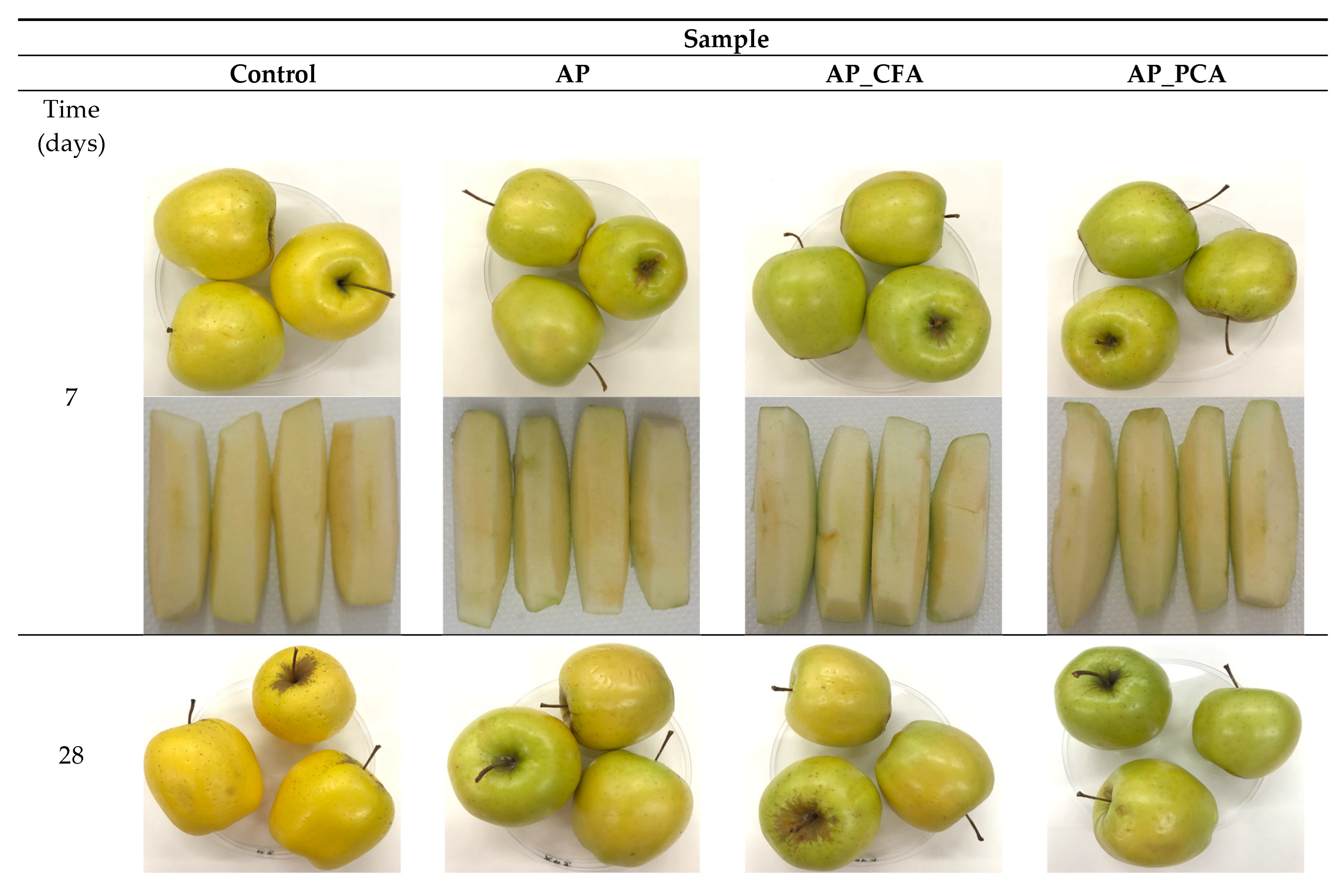
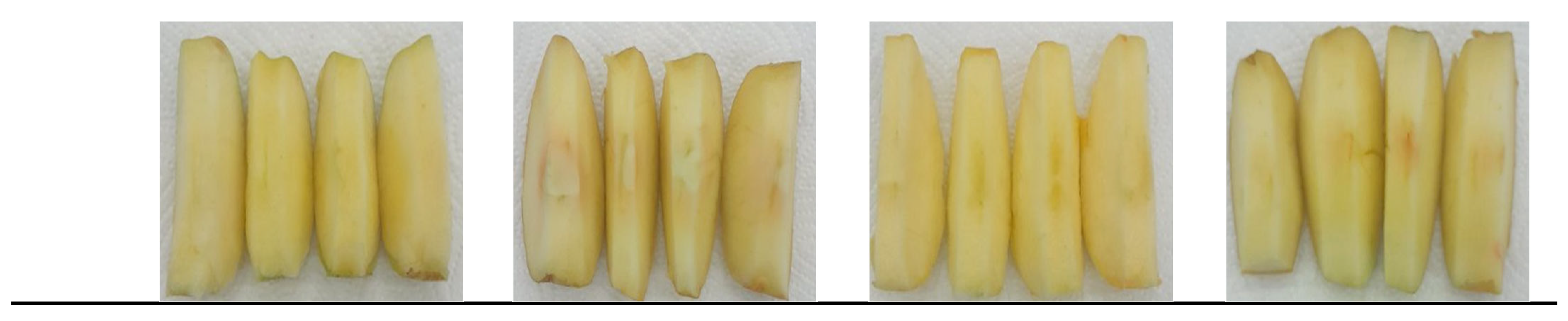
4.4. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Colour of Apples During Storage
4.5. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Total Soluble Solids of Apples During Storage
4.6. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Acidity of Apples During Storage
4.7. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the pH of Apples During Storage
4.8. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Firmness of Apples During Storage
4.9. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Respiration of Apples During Storage
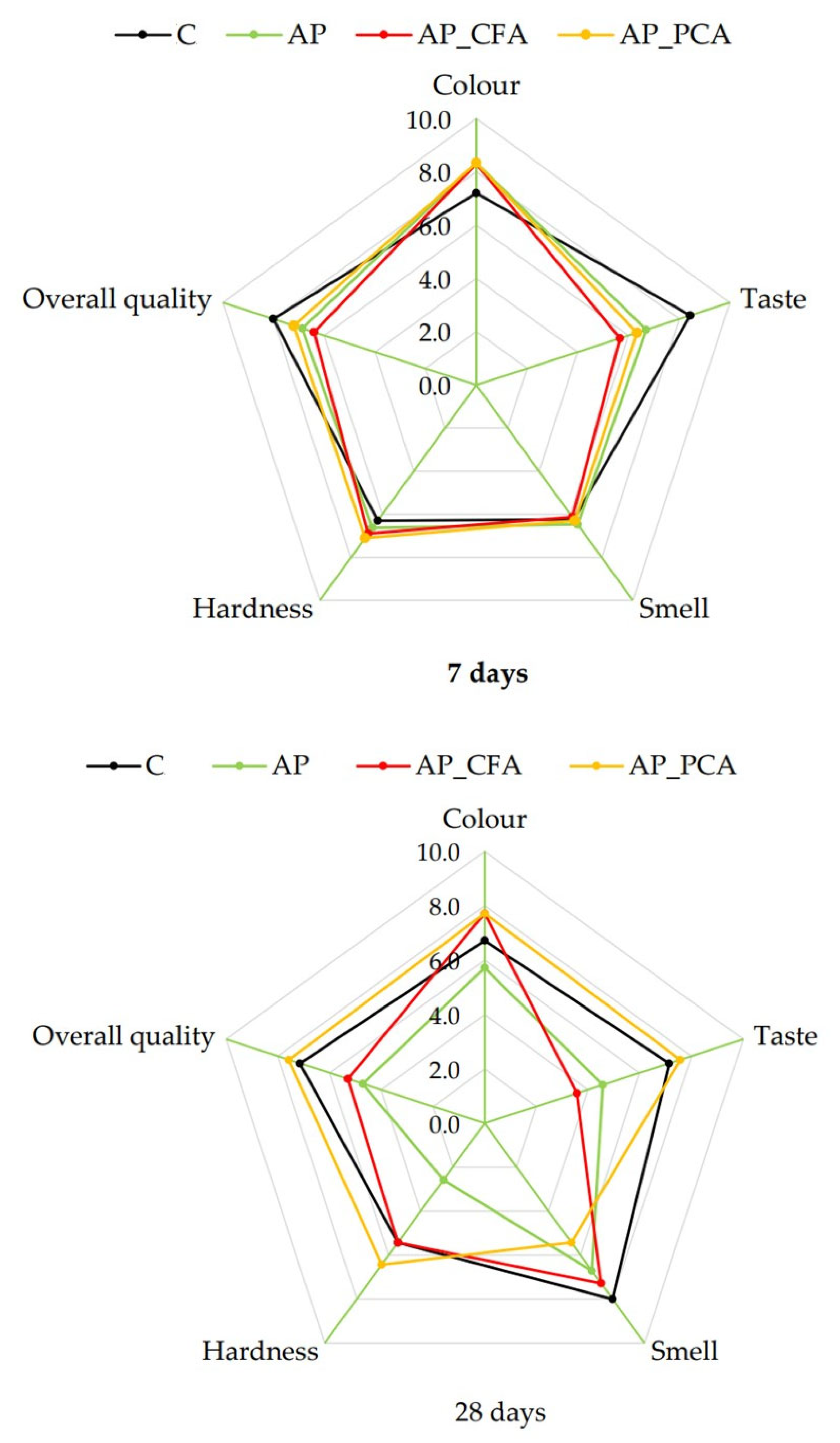
4.10. The Effect of Bioactive Coatings Based on Apple Pectin and Phenolic Acids on the Sensory Attributes of Apples During Storage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarawneh, H.; Obeidat, H.; Shawaqfeh, S.; Al-massad, M.; Alrabadi, N.; Hiary, M.; Talhouni, M.; Alrosan, M. Effectiveness of edible coating in extending shelf life and enhancing quality properties of golden delicious apple. Discov. Food 2025, 5, 137. [Google Scholar] [CrossRef]
- Martínez, A.; Hernández, A.; Moraga, C.; Tejero, P.; Córdoba, M.d.G.; Martín, A. Detection of volatile organic compounds associated with mechanical damage in apple cv. ‘Golden Delicious’ by headspace solid-phase microextraction (HS-SPME) and GC-MS analysis. LWT—Food Sci. Technol. 2022, 172, 114213. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Extending the Shelf Life of Apples After Harvest Using Edible Coatings as Active Packaging—A Review. Appl. Sci. 2025, 15, 767. [Google Scholar] [CrossRef]
- Waghmode, B.; Masoodi, L.; Kushwaha, K.; Mir, J.I.; Sircar, D. Volatile components are non-invasive biomarkers to track shelf-life and nutritional changes in apple cv. ‘Golden Delicious’ during low-temperature postharvest storage. J. Food Compos. Anal. 2021, 102, 104075. [Google Scholar] [CrossRef]
- Pillai, A.R.S.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide-Based Edible Biopolymer-Based Coatings for Fruit Preservation: A Review. Foods 2024, 13, 1529. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent advances in polysaccharide-based edible coatings for preservation of fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 3823–3838. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Rezek Jambrak, A.; Sharma, S.; Roy, S.; Sinhmar, A.; Thory, R.; Pal Singh, G.; Aayush, K.; et al. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Omid Jeivan, A.; Galus, S. Edible Pouch Packaging for Food Applications—A Review. Processes 2025, 13, 2910. [Google Scholar] [CrossRef]
- Kozakiewicz, G.; Małajowicz, J.; Szulc, K.; Karwacka, M.; Ciurzyńska, A.; Żelazko, A.; Janowicz, M.; Galus, S. The Effect of a Pectin Coating with Gamma-Decalactone on Selected Quality Attributes of Strawberries During Refrigerated Storage. Coatings 2025, 15, 903. [Google Scholar] [CrossRef]
- Bashir, O.; Amin, T.; Hussain, S.Z.; Naik, H.R.; Goksen, G.; Wani, A.W.; Manzoor, S.; Malik, A.R.; Wani, F.J.; Proestos, C. Development, characterization and use of rosemary essential oil loaded water-chestnut starch based nanoemulsion coatings for enhancing post-harvest quality of apples var. Golden delicious. Curr. Res. Food Sci. 2023, 7, 100570. [Google Scholar] [CrossRef]
- Galus, S.; Kowalska, H.; Ignaczak, A.; Kowalska, J.; Karwacka, M.; Ciurzyńska, A.; Janowicz, M. Effects of Polysaccharide-Based Edible Coatings on the Quality of Fresh-Cut Beetroot (Beta vulgaris L.) During Cold Storage. Coatings 2025, 15, 583. [Google Scholar] [CrossRef]
- Soppelsa, S.; Van Hemelrijck, W.; Bylemans, D.; Andreotti, C. Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life. Agronomy 2023, 13, 822. [Google Scholar] [CrossRef]
- Gal, T.E.; Alexa, E.C.; Șumălan, R.M.; Dascălu, I.; Iordănescu, O.A. Factors Affecting Patulin Production by Penicillium expansum in Apples. Foods 2025, 14, 2310. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Y.; Jiang, Y.; Zhang, Z. Advances in control technologies and mechanisms to treat peel browning in postharvest fruit. Sci. Hortic. 2023, 311, 111798. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Mi, H.; Pristijono, P.; Ge, Y.; Lv, J.; Li, Y.; Liu, B. Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy 2022, 12, 2794. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M.; Synowiec, A.; Przybył, J.L.; Bączek, K.; Węglarz, Z. The use of pullulan coating enriched with plant extracts from Satureja hortensis L. to maintain pepper and apple quality and safety. Postharvest Biol. Technol. 2014, 90, 63–72. [Google Scholar] [CrossRef]
- Kassebi, S.; Farkas, C.; Székely, L.; Géczy, A.; Korzenszky, P. Late Shelf Life Saturation of Golden Delicious Apple Parameters: TSS, Weight, and Colorimetry. Appl. Sci. 2023, 13, 159. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Ribeiro, I.S.; Maciel, G.M.; Bortolini, D.G.; Fernandes, I.d.A.A.; Maroldi, W.V.; Pedro, A.C.; Rubio, F.T.V.; Haminiuk, C.W.I. Sustainable innovations in edible films and coatings: An overview. Trends Food Sci. Technol. 2024, 143, 104272. [Google Scholar] [CrossRef]
- Felicia, W.X.L.; Rovina, K.; Mamat, H.; Aziz, A.H.A.; Lim, L.S.; Jaziri, A.A.; Nurdiani, R. Advancements in fruit preservation technologies: Harnessing chitosan, aloe vera gel, and plant-based essential oils for coating applications. Appl. Food Res. 2024, 4, 100439. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Singh, R.; Selvaraj, M.; Malik, T.; Al Tawaha, A.R.M. Natural biopolymers in edible coatings: Applications in food preservation. Food Chem. X 2025, 25, 102171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, H.; Hu, L. Recent Advances of Proteins, Polysaccharides and Lipids-Based Edible Films/Coatings for Food Packaging Applications: A Review. Food Biophys. 2023, 19, 29–45. [Google Scholar] [CrossRef]
- Bajaj, K.; Adhikary, T.; Gill, P.P.S.; Kumar, A. Edible coatings enriched with plant-based extracts preserve postharvest quality of fruits: A review. Prog. Org. Coat. 2023, 182, 107669. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.; Gonzalez-Martinez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Raghav, P.; Agarwal, N.; Saini, M. Herbal Edible Coatings of Fruits & Vegetables: A Newer Concept. Int. J. Adv. Res. 2016, 4, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-based edible films and coatings: From extraction to application on food packaging towards circular economy—A review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Food coating—Materials, methods and application in the food industry. Food Sci. Technol. Qual. 2020, 125, 5–24. [Google Scholar] [CrossRef]
- Magri, A.; Rega, P.; Capriolo, G.; Petriccione, M. Impact of Novel Active Layer-by-Layer Edible Coating on the Qualitative and Biochemical Traits of Minimally Processed ‘Annurca Rossa del Sud’ Apple Fruit. Int. J. Mol. Sci. 2023, 24, 8315. [Google Scholar] [CrossRef]
- Cazón, P.; Mateus, A.R.; Silva, A.S. Advances in active packaging using natural biopolymers and fruit by-products for enhanced food preservation. Food Res. Int. 2025, 213, 116439. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, R.; Vaidya, D.; Sharma, N.; Thakur, D.; Suhag, R. Effect of Slice Thickness and Pretreatments on the Quality of Dried Apple Slices (Golden Delicious). J. Food Biochem. 2024, 2024, 1711150. [Google Scholar] [CrossRef]
- Starzec, A.; Raj, D.; Fecka, I. Recent advances on health properties of orchard apple fruits (Malus × domestica Borkh.). Pharmacognosy 2020, 76, 137–148. [Google Scholar] [CrossRef]
- Yi, M.; Kong, J.; Yu, Z. Effect of heat treatment on the quality and energy metabolism in “Golden Delicious” apple fruit. J. Food Biochem. 2021, 45, e13759. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Aleem, M.; Sultan, M.; Sajjad, U.; Ibrahim, S.M.; Shamshiri, R.R.; Farooq, M.; Usman Khan, M.; Bilal, M. Evaluating Evaporative Cooling Assisted Solid Desiccant Dehumidification System for Agricultural Storage Application. Sustainability 2022, 14, 1479. [Google Scholar] [CrossRef]
- Farkas, A.; Ficzek, G.; Veres, A. The Examination of Apple Shelf Life from Consumer Storage Perspective. Analecta Tech. Szeged. 2024, 18, 1–7. [Google Scholar] [CrossRef]
- Janowicz, M.; Galus, S.; Ciurzyńska, A.; Nowacka, M. The Potential of Edible Films, Sheets, and Coatings Based on Fruits and Vegetables in the Context of Sustainable Food Packaging Development. Polymers 2023, 15, 4231. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packag. Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Kausar, T.; Nadeem, M.; Ainee, A.; Mehmood, T. Optimization of fenugreek and flax polysaccharides-based edible coating formulation to preserve the quality and storability of apple after harvesting. J. Food Process. Preserv. 2020, 44, e14812. [Google Scholar] [CrossRef]
- Nicolau-Lapeña, I.; Aguiló-Aguayo, I.; Kramer, B.; Abadias, M.; Viñas, I.; Muranyi, P. Combination of ferulic acid with Aloe vera gel or alginate coatings for shelf-life prolongation of fresh-cut apples. Food Packag. Shelf Life 2021, 27, 100620. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants: A comprehensive review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef]
- Sun, W.; Hu, Y.; Pandi, A.; Yi, G.; Tan, Z. Caffeic acid-integrated biopolymer systems: Advancing sustainable active packaging for food preservation. Food Chem. X 2025, 29, 102763. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meng, C.-g.; Wang, X.-c.; Chen, Y.; Kan, J.; Jin, C.-h. Effect of Protocatechuic Acid-Grafted-Chitosan Coating on the Postharvest Quality of Pleurotus eryngii. J. Agric. Food Chem. 2016, 64, 7225–7233. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; An, F.; He, H.; Geng, F.; Song, H.; Huang, Q. Structural and rheological characterization of pectin from passion fruit (Passiflora edulis f. flavicarpa) peel extracted by high-speed shearing. Food Hydrocoll. 2021, 114, 106555. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. The Effect of Phenolic Acids on the Sorption and Wetting Properties of Apple Pectin-Based Packaging Films. Molecules 2025, 30, 1960. [Google Scholar] [CrossRef]
- Benbettaieb, N.; Cox, R.; Gilbert, M.; Debeaufort, F. How the temperature and salt content of food simulant affect the release of tyrosol or phenolic acids from bioactive films? Future Foods 2021, 4, 100040. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975. [Google Scholar]
- Szulc, K.; Galus, S. Structural and Rheological Characterization of Vegetable Crispbread Enriched with Legume Purée. Molecules 2024, 29, 1880. [Google Scholar] [CrossRef]
- Janowicz, M.; Kadzińska, J.; Ciurzyńska, A.; Szulc, K.; Galus, S.; Karwacka, M.; Nowacka, M. The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée. Appl. Sci. 2023, 13, 6959. [Google Scholar] [CrossRef]
- Shuai, X.; Chen, J.; Liu, Q.; Dong, H.; Dai, T.; Li, Z.; Liu, C.; Wang, R. The Effects of Pectin Structure on Emulsifying, Rheological, and In Vitro Digestion Properties of Emulsion. Foods 2022, 11, 3444. [Google Scholar] [CrossRef]
- Jiao, X.; Yang, B.; Li, F.; Zhang, L.; Wei, Y.; Zhao, J.; Li, Q. Effect of the degree of methyl esterification on the physicochemical properties and hypoglycaemic potential of pectin. Food Chem. 2025, 484, 144348. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Muniglia, L.; Humeau, C.; Jasniewski, J. Physicochemical characterization of pectin grafted with exogenous phenols. Food Hydrocoll. 2016, 60, 486–493. [Google Scholar] [CrossRef]
- Karaki, N.; Aljawish, A.; Muniglia, L.; Bouguet-Bonnet, S.; Leclerc, S.; Paris, C.; Jasniewski, J.; Humeau-Virot, C. Functionalization of pectin with laccase-mediated oxidation products of ferulic acid. Enzym. Microb. Technol. 2017, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochem. 2013, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable active packaging: Components, preparation, and applications in the preservation of postharvest perishable fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come into Contact with Food (Text with EEA Relevance); European Commission: Brussels, Belgium, 2009.
- Peerzada Gh, J.; Sinclair, B.J.; Perinbarajan, G.K.; Dutta, R.; Shekhawat, R.; Saikia, N.; Chidambaram, R.; Mossa, A.-T. An overview on smart and active edible coatings: Safety and regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Du, H.; Sun, X.; Chong, X.; Yang, M.; Zhu, Z.; Wen, Y. A review on smart active packaging systems for food preservation: Applications and future trends. Trends Food Sci. Technol. 2023, 141, 104200. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Mehany, T.; Shyu, D.J.H.; Assadpour, E.; Malekjani, N.; Castro-Muñoz, R.; Jafari, S.M. Release of Encapsulated Bioactive Compounds from Active Packaging/Coating Materials and Its Modeling: A Systematic Review. Colloids Interfaces 2023, 7, 25. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Active Packaging with Controlled Release: Principles, Progress, and Prospects. ACS Food Sci. Technol. 2022, 2, 1166–1183. [Google Scholar] [CrossRef]
- Lavoine, N.; Guillard, V.; Desloges, I.; Gontard, N.; Bras, J. Active bio-based food-packaging: Diffusion and release of active substances through and from cellulose nanofiber coating toward food-packaging design. Carbohydr. Polym. 2016, 149, 40–50. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Packag. Shelf Life 2022, 33, 100894. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Li, H.; Wang, Y. Nanocomplexes film composed of gallic acid loaded ovalbumin/chitosan nanoparticles and pectin with excellent antibacterial activity: Preparation, characterization and application in coating preservation of salmon fillets. Int. J. Biol. Macromol. 2024, 259, 128934. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A. Water Loss: A Postharvest Quality Marker in Apple Storage. Food Bioprocess Technol. 2024, 17, 2155–2180. [Google Scholar] [CrossRef]
- Ali, M.; Ali, A.; Ali, S.; Chen, H.; Wu, W.; Liu, R.; Chen, H.; Ahmed, Z.F.R.; Gao, H. Global insights and advances in edible coatings or films toward quality maintenance and reduced postharvest losses of fruit and vegetables: An updated review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70103. [Google Scholar] [CrossRef]
- Jurić, M.; Maslov Bandić, L.; Carullo, D.; Jurić, S. Technological advancements in edible coatings: Emerging trends and applications in sustainable food preservation. Food Biosci. 2024, 58, 103835. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, J.; Chen, L.; Ban, Z.; Zheng, Y.; Jiang, Y.; Jiang, Y.; Li, X. Effects of Fruit Storage Temperature and Time on Cloud Stability of Not from Concentrated Apple Juice. Foods 2022, 11, 2568. [Google Scholar] [CrossRef]
- Trebar, M.; Žalik, A.; Vidrih, R. Assessment of ‘Golden Delicious’ Apples Using an Electronic Nose and Machine Learning to Determine Ripening Stages. Foods 2024, 13, 2530. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Kassebi, S.; Korzenszky, P. Examination of weight loss and colour changes in Golden Delicious apples under light and dark storage conditions. Prog. Agric. Eng. Sci. 2024, 20, 199–215. [Google Scholar] [CrossRef]
- Schifferstein, H.N.J. Changes in appearance during the spoilage process of fruits and vegetables: Implications for consumer use and disposal. Clean. Responsible Consum. 2024, 12, 100184. [Google Scholar] [CrossRef]
- Sahraei Khosh Gardesh, A.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT—Food Sci. Technol. 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Sapper, M.; Palou, L.; Pérez-Gago, M.B.; Chiralt, A. Antifungal Starch–Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings 2019, 9, 333. [Google Scholar] [CrossRef]
- Jahanshahi, B.; Jafari, A.; Vazifeshenas, M.R.; Gholamnejad, J. A novel edible coating for apple fruits. J. Hortic. Postharvest Res. 2018, 1, 63–72. [Google Scholar] [CrossRef]
- Kraciński, P.; Stolarczyk, P.; Czerwińska, W.; Nosecka, B. Fruit Consumption Habits and Apple Preferences of University Students in Poland. Foods 2025, 14, 2073. [Google Scholar] [CrossRef]
- Harker, F.; Gunson, F.A.; Jaeger, S. The case for fruit quality: An interpretive review of consumer attitudes, and preferences for apples. Postharvest Biol. Technol. 2003, 28, 333–347. [Google Scholar] [CrossRef]
- Fernández-Cancelo, P.; Iglesias-Sanchez, A.; Torres-Montilla, S.; Ribas-Agustí, A.; Teixidó, N.; Rodriguez-Concepcion, M.; Giné-Bordonaba, J. Environmentally driven transcriptomic and metabolic changes leading to color differences in “Golden Reinders” apples. Front. Plant Sci. 2022, 13, 913433. [Google Scholar] [CrossRef]
- Gorfer, L.M.; Vestrucci, L.; Grigoletto, V.; Lazazzara, V.; Zanella, A.; Robatscher, P.; Scampicchio, M.; Oberhuber, M. Chlorophyll breakdown during fruit ripening: Qualitative analysis of phyllobilins in the peel of apples (Malus domestica Borkh.) cv. ‘Gala’ during different shelf life stages. Food Res. Int. 2022, 162, 112061. [Google Scholar] [CrossRef]
- Anjani, A.M.; Setiawan, C.K.; Utama, N.A. Effect of CaCl2 and Alginate-Essential Oil Edible Coating in Maintaining Quality and Antioxidant Content in Rose Apple cv. Dalhari. IOP Conf. Ser. Earth Environ. Sci. 2021, 752, 012004. [Google Scholar] [CrossRef]
- Ali, N.; Dina, E.; Tas, A.A. Bioactive Edible Coatings for Fresh-Cut Apples: A Study on Chitosan-Based Coatings Infused with Essential Oils. Foods 2025, 14, 2362. [Google Scholar] [CrossRef]
- Shu, C.; Liu, B.; Zhao, H.; Cui, K.; Jiang, W. Effect of Near-Freezing Temperature Storage on the Quality and Organic Acid Metabolism of Apple Fruit. Agriculture 2024, 14, 1057. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S.; Arshad, M. Postharvest quality assessment of apple during storage at ambient temperature. Heliyon 2021, 7, e07714. [Google Scholar] [CrossRef] [PubMed]
- Rashid, F.; Ahmed, Z.; Ferheen, I.; Mehmood, T.; Liaqat, S.; Ghoneim, M.M.; Rahman, A. Effect of fenugreek and flaxseed polysaccharide-based edible coatings on the quality attributes and shelf life of apple fruit during storage. Food Sci. Nutr. 2024, 12, 2093–2103. [Google Scholar] [CrossRef]
- Ghidelli, C.; Perez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2018, 58, 662–679. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef]
- Han, Y.; Su, Z.; Du, J. Effects of apple storage period on the organic acids and volatiles in apple wine. LWT—Food Sci. Technol. 2023, 173, 114389. [Google Scholar] [CrossRef]
- Vieira, A.I.; Guerreiro, A.; Antunes, M.D.; Miguel, M.d.G.; Faleiro, M.L. Edible Coatings Enriched with Essential Oils on Apples Impair the Survival of Bacterial Pathogens through a Simulated Gastrointestinal System. Foods 2019, 8, 57. [Google Scholar] [CrossRef]
- Alegre, I.; Abadias, M.; Anguera, M.; Oliveira, M.; Viñas, I. Factors affecting growth of foodborne pathogens on minimally processed apples. Food Microbiol. 2010, 27, 70–76. [Google Scholar] [CrossRef]
- Ferreira, C.; Ribeiro, C.; Nunes, F.M. Effect of storage conditions on phenolic composition, vitamin C and antioxidant activity of ‘Golden Delicious’ and ‘Red Delicious’ apples. Postharvest Biol. Technol. 2024, 210, 112754. [Google Scholar] [CrossRef]
- Lara, I.; Graell, J.; Ortiz, A. Sugar composition of pectin-containing cell wall fractions is altered in ‘Fuji’ apples in response to calcium dips and controlled atmosphere storage. Postharvest Biol. Technol. 2024, 218, 113147. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Mellidou, I.; Boeckx, J.; Kyomugasho, C.; Bessemans, N.; Verlinden, B.E.; Hertog, M.L.A.T.M.; Hendrickx, M.; Nicolai, B.M.; Geeraerd, A.H. Expression analysis of candidate cell wall-related genes associated with changes in pectin biochemistry during postharvest apple softening. Postharvest Biol. Technol. 2016, 112, 176–185. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A.; Ahsan, H.; Tak, A.; Mir, M.A.; Rather, A.H.; Wani, S.M. Effect of maturity stages and postharvest treatments on physical properties of apple during storage. J. Saudi Soc. Agric. Sci. 2018, 17, 310–316. [Google Scholar] [CrossRef]
- Wijewardana, N.; Guleria, S. Effect of post-harvest coating treatments on apple storage quality. J. Food Sci. Technol. 2009, 46, 549–553. [Google Scholar]
- Shen, X.; Su, Y.; Hua, Z.; Chiu, T.; Wang, Y.; Mendoza, M.; Hanrahan, I.; Zhu, M.J. Evaluating serotype-specific survival of Listeria monocytogenes and Listeria innocua on wax-coated Granny Smith apples during storage. Int. J. Food Microbiol. 2025, 427, 110964. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, D.; Wang, X.; Xu, X.; Yu, W.; Wang, C.; Yuan, Y.; Yang, S.; Cheng, C. Integrated analysis of postharvest storage characteristics of seven apple cultivars and transcriptome data identifies MdBBX25 as a negative regulator of fruit softening during storage in apples. Postharvest Biol. Technol. 2024, 207, 112646. [Google Scholar] [CrossRef]
- Bai, J.; Hagenmaier, R.D.; Baldwin, E.A. Coating selection for ‘Delicious’ and other apples. Postharvest Biol. Technol. 2003, 28, 381–390. [Google Scholar] [CrossRef]
- Khera, K.; Büchele, F.; Wood, R.M.; Thewes, F.R.; Wagner, R.; Hagemann, M.H.; Neuwald, D.A. Impact of different storage conditions with combined use of ethylene blocker on ‘Shalimar’ apple variety. Sci. Rep. 2024, 14, 8485. [Google Scholar] [CrossRef]
- Duroňová, K.; Márová, I.; Čertík, M.; Obruča, S. Changes in lipid composition of apple surface layer during long-term storage in controlled atmosphere. Chem. Pap. 2012, 66, 940–948. [Google Scholar] [CrossRef]
- Lee, J.G.; Eum, H.L.; Lee, E.J. Relationship between skin greasiness and cuticular wax in harvested “Hongro” apples. Food Chem. 2024, 450, 139334. [Google Scholar] [CrossRef]
- Lamani, N.A.; Ramaswamy, H.S. Composite Alginate–Ginger Oil Edible Coating for Fresh-Cut Pears. J. Compos. Sci. 2023, 7, 245. [Google Scholar] [CrossRef]
- Alonso-Salinas, R.; López-Miranda, S.; Pérez-López, A.J.; Acosta-Motos, J.R. Strategies to Delay Ethylene-Mediated Ripening in Climacteric Fruits: Implications for Shelf Life Extension and Postharvest Quality. Horticulturae 2024, 10, 840. [Google Scholar] [CrossRef]
- Jung, S.-K.; Choi, H.-S. Fruit Quality and Antioxidant Activities of Yellow-Skinned Apple Cultivars Coated with Natural Sucrose Monoesters. Sustainability 2021, 13, 2423. [Google Scholar] [CrossRef]
- Ou, S.; Wang, Y.; Tang, S.; Huang, C.; Jackson, M.G. Role of ferulic acid in preparing edible films from soy protein isolate. J. Food Eng. 2005, 70, 205–210. [Google Scholar] [CrossRef]
- Fabra, M.; Hambleton, A.; Talens, P.; Debeaufort, F.; Chiralt, A. Effect of ferulic acid and α-tocopherol antioxidants on properties of sodium caseinate edible films. Food Hydrocoll. 2011, 25, 1441–1447. [Google Scholar] [CrossRef]
- Lopez, M.L.; Lavilla, M.T.; Recasens, I.; Graell, J.; Vendrell, M. Changes in aroma quality of ‘Golden Delicious’ apples after storage at different oxygen and carbon dioxide concentrations. J. Sci. Food Agric. 2000, 80, 311–324. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, Y.; Jia, R.; Li, X.; Wang, B.; Zhao, Z. Comparison of Fruit Texture and Storage Quality of Four Apple Varieties. Foods 2024, 13, 1563. [Google Scholar] [CrossRef]
- Rohasmizah, H.; Azizah, M. Pectin-based edible coatings and nanoemulsion for the preservation of fruits and vegetables: A review. Appl. Food Res. 2022, 2, 100221. [Google Scholar] [CrossRef]
- Ali, U.; Basu, S.; Mazumder, K. Improved postharvest quality of apple (Rich Red) by composite coating based on arabinoxylan and β-glucan stearic acid ester. Int. J. Biol. Macromol. 2020, 151, 618–627. [Google Scholar] [CrossRef]
| Substance | Quantity (g) |
|---|---|
| Apple pectin (AP) | 5 |
| Caffeic acid (CFA) | 0.25 |
| Protocatechuic acid (PCA) | 0.25 |
| Glycerol (GLY) | 2.5 |
| Water | 92.25 |
| Time (Days) | Sample | |||
|---|---|---|---|---|
| Control | AP | AP_CFA | AP_PCA | |
| L* | ||||
| 0 | 75.77 ± 1.07 a,A | |||
| 7 | 75.40 ± 2.45 a,A | 72.03 ± 5.56 a,A | 72.05 ± 1.26 b,A | 72.21 ± 1.76 a,A |
| 14 | 75.01 ± 1.98 a,B | 70.45 ± 1.50 a,A | 69.49 ± 1.40 a,A | 68.64 ± 2.12 a,A |
| 21 | 74.70 ± 1.59 c,B | 70.30 ± 1.50 a,A | 68.79 ± 2.44 a,A | 68.01 ± 1.87 a,A |
| 28 | 74.67 ± 1.79 a,B | 64.64 ± 7.31 a,A | 67.37 ± 2.46 a,A | 64.66 ± 7.17 a,A |
| a* | ||||
| 0 | −11.29 ± 0.68 a,A | |||
| 7 | −7.81 ± 2.32 b,A | −9.18 ± 0.94 a,A | −9.26 ± 1.20 a,A | −8.11 ± 1.49 a,A |
| 14 | −4.64 ± 2.00 b,B | −8.57 ± 1.38 a,A | −8.51 ± 1.35 a,A | −8.04 ± 1.54 a,A |
| 21 | −2.75 ± 2.16 b,B | −6.12 ± 1.60 b,A | −5.96 ± 2.09 b,A | −5.58 ± 1.91 b,A |
| 28 | −2.16 ± 1.28 b,A | −4.35 ± 1.38 b,A | −3.72 ± 1.64 b,A | −3.96 ± 1.65 b,A |
| b* | ||||
| 0 | 46.83 ± 0.63 a,A | |||
| 7 | 55.21 ± 2.33 b,B | 46.29 ± 2.74 a,A | 46.28 ± 1.73 a,A | 46.13 ± 1.93 a,A |
| 14 | 56.18 ± 1.47 b,B | 44.51 ± 1.68 a,A | 43.68 ± 1.41 a,A | 44.15 ± 2.14 a,A |
| 21 | 57.22 ± 2.43 b,B | 44.39 ± 2.26 a,A | 43.39 ± 2.04 a,A | 43.93 ± 2.03 a,A |
| 28 | 57.76 ± 3.08 b,B | 42.97 ± 4.25 a,A | 42.69 ± 2.64 a,A | 42.59 ± 1.43 a,A |
| Time (Days) | Sample | |||
|---|---|---|---|---|
| Control | AP | AP_CFA | AP_PCA | |
| TSS (°Brix) | ||||
| 0 | 13.47 ± 0.37 a,A | |||
| 7 | 13.77 ± 0.11 a,A | 13.50 ± 0.10 a,A | 13.48 ± 0.23 a,A | 13.53 ± 0.40 a,A |
| 14 | 14.03 ± 0.39 a,A | 13.50 ± 0.24 a,A | 13.52 ± 0.21 a,A | 13.50 ± 0.37 a,A |
| 21 | 14.35 ± 0.19 b,B | 13.53 ± 0.07 a,A | 13.52 ± 0.18 a,A | 13.55 ± 0.36 a,A |
| 28 | 14.95 ± 0.48 b,B | 13.53 ± 0.19 a,A | 13.52 ± 0.32 a,A | 13.57 ± 0.27 a,A |
| Titratable acidity (% of malic acid) | ||||
| 0 | 1.37 ± 0.02 b,A | |||
| 7 | 0.82 ± 0.05 a,A | 0.93 ± 0.05 a,A | 0.91 ± 0.02 a,A | 0.89 ± 0.11 a,A |
| 14 | 0.90 ± 0.07 a,B | 0.79 ± 0.08 a,AB | 0.77 ± 0.03 a,A | 0.77 ± 0.09 a,A |
| 21 | 0.88 ± 0.04 a,B | 0.75 ± 0.05 a,A | 0.76 ± 0.08 a,A | 0.77 ± 0.05 a,A |
| 28 | 0.80 ± 0.05 a,A | 0.79 ± 0.05 a,A | 0.76 ± 0.05 a,A | 0.73 ± 0.02 a,A |
| pH | ||||
| 0 | 3.59 ± 0.04 a,A | |||
| 7 | 3.84 ± 0.02 b,A | 3.84 ± 0.01 b,A | 3.83 ± 0.03 a,A | 3.79 ± 0.05 a,A |
| 14 | 3.90 ± 0.03 b,A | 3.98 ± 0.09 b,A | 3.98 ± 0.06 b,A | 4.04 ± 0.01 b,A |
| 21 | 4.00 ± 0.02 b,B | 4.09 ± 0.06 b,A | 4.12 ± 0.08 b,A | 4.16 ± 0.01 b,A |
| 28 | 4.05 ± 0.06 b,A | 4.11 ± 0.07 b,A | 4.12 ± 0.03 b,A | 4.26 ± 0.04 b,A |
| Time (Days) | Sample | |||
|---|---|---|---|---|
| Control | AP | AP_CFA | AP_PCA | |
| Firmness (N) | ||||
| 0 | 58.03 ± 6.50 c,A | |||
| 7 | 50.77 ± 2.40 b,B | 49.01 ± 1.60 b,AB | 48.32 ± 2.60 a,A | 48.03 ± 1.60 a,A |
| 14 | 42.80 ± 3.70 a,A | 45.86 ± 2.70 b,AB | 47.40 ± 2.60 a,B | 46.45 ± 2.80 a,B |
| 21 | 39.73 ± 2.50 a,A | 43.94 ± 3.70 a,B | 44.70 ± 3.30 a,B | 45.33 ± 2.90 a,B |
| 28 | 36.84 ± 1.30 a,A | 43.64 ± 3.50 a,B | 44.20 ± 4.10 a,B | 44.61 ± 4.80 a,B |
| Time (Days) | Sample | |||
|---|---|---|---|---|
| Control | AP | AP_CFA | AP_PCA | |
| Ethylene (ppm C2H4/kg/h) | ||||
| 0 | 60.40 ± 3.00 b,A | |||
| 7 | 63.80 ± 7.10 b,B | 18.25 ± 0.65 b,A | 8.85 ± 1.15 a,A | 6.80 ± 0.10 a,A |
| 14 | 67.00 ± 1.70 b,B | 15.50 ± 0.55 a,A | 13.70 ± 2.30 b,A | 11.85 ± 1.00 a,A |
| 21 | 67.65 ± 7.45 b,B | 28.75 ± 1.05 d,A | 25.25 ± 3.45 c,A | 23.25 ± 1.85 a,A |
| 28 | 52.75 ± 3.05 a,B | 25.70 ± 0.70 c,A | 23.90 ± 0.50 c,A | 23.55 ± 1.65 a,A |
| Carbon dioxide (mg/kg/h) | ||||
| 0 | 0.23 ± 0.13 c,A | |||
| 7 | 0.27 ± 0.05 d,B | 0.21 ± 0.01 d,A | 0.16 ± 0.01 c,A | 0.17 ± 0.04 c,A |
| 14 | 0.14 ± 0.00 a,A | 0.15 ± 0.02 b,A | 0.15 ± 0.02 b,A | 0.18 ± 0.02 d,A |
| 21 | 0.16 ± 0.02 b,A | 0.18 ± 0.04 c,A | 0.14 ± 0.01 a,A | 0.14 ± 0.03 b,A |
| 28 | 0.14 ± 0.02 a,A | 0.13 ± 0.02 a,A | 0.15 ± 0.01 b,A | 0.13 ± 0.01 a,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikus, M.; Szulc, K.; Galus, S. The Use of Bioactive Edible Coatings Based on Pectin and Phenolic Acids for Enhancing Quality Attributes of Golden Delicious Apples During Storage. Processes 2025, 13, 3821. https://doi.org/10.3390/pr13123821
Mikus M, Szulc K, Galus S. The Use of Bioactive Edible Coatings Based on Pectin and Phenolic Acids for Enhancing Quality Attributes of Golden Delicious Apples During Storage. Processes. 2025; 13(12):3821. https://doi.org/10.3390/pr13123821
Chicago/Turabian StyleMikus, Magdalena, Karolina Szulc, and Sabina Galus. 2025. "The Use of Bioactive Edible Coatings Based on Pectin and Phenolic Acids for Enhancing Quality Attributes of Golden Delicious Apples During Storage" Processes 13, no. 12: 3821. https://doi.org/10.3390/pr13123821
APA StyleMikus, M., Szulc, K., & Galus, S. (2025). The Use of Bioactive Edible Coatings Based on Pectin and Phenolic Acids for Enhancing Quality Attributes of Golden Delicious Apples During Storage. Processes, 13(12), 3821. https://doi.org/10.3390/pr13123821








