Enhanced Removal of Ibuprofen, Paracetamol, and Caffeine in Vertical Constructed Wetlands Using Biochar and Zeolite as Support Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Biochar Preparation
2.3. Influent Characterization and Fortification of the Septic Tank Water with Pharmaceuticals
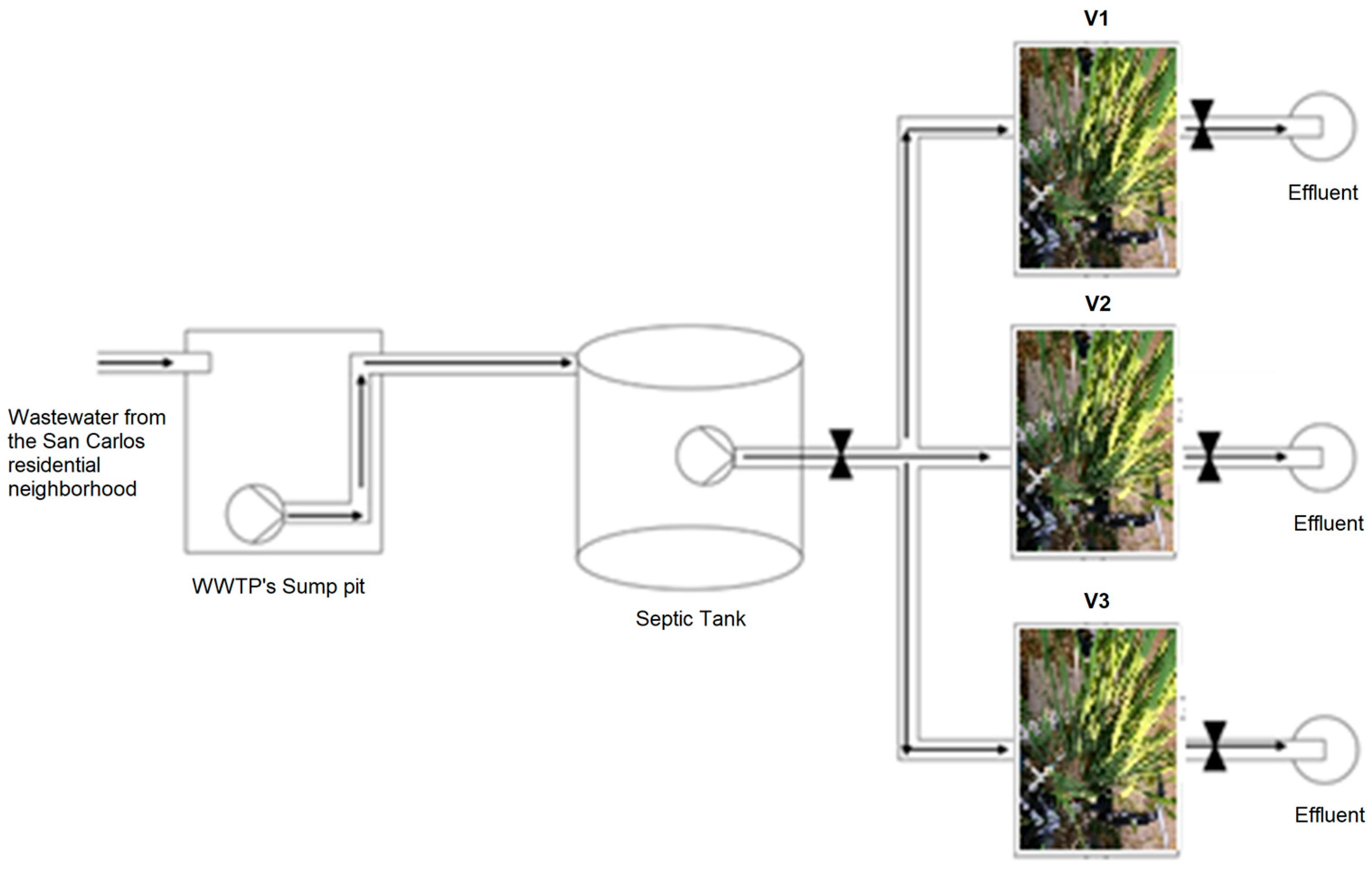
2.4. Experimental Setup and Startup
2.5. System Operation and Monitoring
2.6. Sampling Procedure, Preparation, and Water Analysis
2.7. Data and Statistical Analysis
3. Results
3.1. Influent and Septic Tank Characterization
3.2. Removal of Organic Matter (COD and BOD5) and Suspended Solids (TSS) in V-CW
3.3. Pharmaceutical Removal (Ibuprofen, Paracetamol, Caffeine)
3.4. Statistical Analysis Results
4. Discussion
4.1. Influent Characterization
4.2. Septic Tank Performance
4.3. Removal of Organic Matter (COD and BOD5) and Suspended Solids (TSS)
4.4. Pharmaceutical Removal
4.4.1. Ibuprofen
4.4.2. Paracetamol
4.4.3. Caffeine Removal
4.4.4. Pharmaceutical Removal Inhibition by Other Compounds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APHA | American Public Health Association |
| AOPs | Advanced Oxidation Processes |
| BOD5 | Biochemical Oxygen Demand over 5 days |
| CAF | Caffeine |
| COD | Chemical Oxygen Demand |
| CW | Constructed Wetland |
| d | Days |
| HPLC | High-Performance Liquid Chromatography |
| HLR | Hydraulic Loading Rate |
| IBU | Ibuprofen |
| LOQ | Limit of Quantification |
| LOD | Limit of Detection |
| mg/L | Milligrams per Liter |
| PCM | Paracetamol |
| PPCP | Pharmaceuticals and Personal Care Products |
| Rs | Resolution Factor |
| SPE | Solid Phase Extraction |
| TSS | Total Suspended Solids |
| V1 | Wetland with gravel/sand and vegetation |
| V2 | Wetland with biochar/zeolite and vegetation |
| V3 | Wetland with biochar/zeolite without vegetation |
| VFCW | Vertical Flow Constructed Wetland |
| WWTP | Wastewater Treatment Plant |
| µg/L | Micrograms per Liter |
References
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49. [Google Scholar] [CrossRef]
- Malnes, D.; Ahrens, L.; Köhler, S.; Forsberg, M.; Golovko, O. Occurrence and Mass Flows of Contaminants of Emerging Concern (CECs) in Sweden’s Three Largest Lakes and Associated Rivers. Chemosphere 2022, 294, 133825. [Google Scholar] [CrossRef] [PubMed]
- Ripanda, A.S.; Rwiza, M.J.; Nyanza, E.C.; Njau, K.N.; Vuai, S.A.H.; Machunda, R.L. A Review on Contaminants of Emerging Concern in the Environment: A Focus on Active Chemicals in Sub-Saharan Africa. Appl. Sci. 2021, 12, 56. [Google Scholar] [CrossRef]
- Eapen, J.V.; Thomas, S.; Antony, S.; George, P.; Antony, J. A Review of the Effects of Pharmaceutical Pollutants on Humans and Aquatic Ecosystem. Explor. Drug Sci. 2024, 2, 484–507. [Google Scholar] [CrossRef]
- Ferreira, A.P. Caffeine as an Environmental Indicator for Assessing Urban Aquatic Ecosystems. Cad. Saúde Pública 2005, 21, 1884–1892. [Google Scholar] [CrossRef]
- Moreno-Pérez, P.A.; Hernández-Téllez, M.; Bautista-Gálvez, A. In Danger One of the Largest Aquifers in the World, the Great Mayan Aquifer, Based on Monitoring the Cenotes of the Yucatan Peninsula. Arch. Environ. Contam. Toxicol. 2021, 81, 189–198. [Google Scholar] [CrossRef]
- Matamoros, V.; Caselles-Osorio, A.; García, J.; Bayona, J.M. Behaviour of Pharmaceutical Products and Biodegradation Intermediates in Horizontal Subsurface Flow Constructed Wetland. A Microcosm Experiment. Sci. Total Environ. 2008, 394, 171–176. [Google Scholar] [CrossRef]
- Mumtaj, Z.A.; Khan, A.R.; Alsubih, M.; Aleya, L.; Khan, R.A.; Khan, S. Removal of Pharmaceutical Contaminants from Hospital Wastewater Using Constructed Wetlands: A Review. Environ. Sci. Pollut. Res. 2024, 31, 12856–12870. [Google Scholar] [CrossRef]
- Zou, J.; Guo, X.; Han, Y.; Liu, J.; Liang, H. Study of a Novel Vertical Flow Constructed Wetland System with Drop Aeration for Rural Wastewater Treatment. Water Air Soil Pollut. 2012, 223, 889–900. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, R. Assessment of Pollutant Removal Processes and Kinetic Modelling in Vertical Flow Constructed Wetlands at Elevated Pollutant Loading. Environ. Sci. Pollut. Res. 2019, 26, 18421–18433. [Google Scholar] [CrossRef]
- Porras-Socias, P.; Tomasino, M.P.; Fernandes, J.P.; De Menezes, A.B.; Fernández, B.; Collins, G.; Alves, M.J.; Castro, R.; Gomes, C.R.; Almeida, C.M.R.; et al. Removal of Metals and Emergent Contaminants from Liquid Digestates in Constructed Wetlands for Agricultural Reuse. Front. Microbiol. 2024, 15, 1388895. [Google Scholar] [CrossRef] [PubMed]
- Al Falahi, O.A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ewadh, H.M.; Ismail, N.I.; Imron, M.F.; Kurniawan, S.B. Removal of Ibuprofen and Paracetamol by Rhizobacteria from Roots of Scirpus Grossus Exposed to a Synthetic Mix in Constructed Wetlands. Water 2025, 17, 2396. [Google Scholar] [CrossRef]
- Sánchez, M.; Fernández, M.I.; Ruiz, I.; Canle, M.; Soto, M. Combining Constructed Wetlands and UV Photolysis for the Advanced Removal of Organic Matter, Nitrogen, and Emerging Pollutants from Wastewater. Environments 2023, 10, 35. [Google Scholar] [CrossRef]
- Chand, N.; Suthar, S.; Kumar, K.; Singh, V. Removal of Pharmaceuticals by Vertical Flow Constructed Wetland with Different Configurations: Effect of Inlet Load and Biochar Addition in the Substrate. Chemosphere 2022, 307, 135975. [Google Scholar] [CrossRef]
- Almeida, A.; Oliveira, J.; Matias, F.; Ribeiro, C.; Silveira, D.; Tavares, J. Removal of Emergent Pollutants by a Vertical Flow Constructed Wetland with Vetiveria Zizanioides: A Case Study for Caffeine. KnE Mater. Sci. 2022, 184–192. [Google Scholar] [CrossRef]
- Youssef, Y.A.; Abuarab, M.E.; Mahrous, A.; Mahmoud, M. Enhanced Degradation of Ibuprofen in an Integrated Constructed Wetland-Microbial Fuel Cell: Treatment Efficiency, Electrochemical Characterization, and Microbial Community Dynamics. RSC Adv. 2023, 13, 29809–29818. [Google Scholar] [CrossRef]
- Cobarrubias-Escamilla, D.L.; Saldarriaga-Noreña, H.A.; Vergara-Sánchez, J.; Murillo-Tovar, M.A.; Moeller-Chávez, G.E. Removal of Ibuprofen, Naproxen and 17-β-Estradiol in Water Using L. octovalvis Constructed Wetlands. Int. J. Phytoremediat. 2025, 27, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Castro, E.; Almeida-Naranjo, C.E.; Rivas, A.; Figueroa, N.; Montellano, L.; Villamar-Ayala, C.A. The Removal of Acidic Drugs from Domestic Wastewater Using an Innovative System of Constructed Wetlands/Stabilization Ponds in Series. Water 2025, 17, 1192. [Google Scholar] [CrossRef]
- Kamilya, T.; Majumder, A.; Saidulu, D.; Tripathy, S.; Gupta, A.K. Optimization of a Continuous Hybrid Moving Bed Biofilm Reactor and Constructed Wetland System for the Treatment of Paracetamol-Spiked Domestic Wastewater. Chem. Eng. J. 2023, 477, 147139. [Google Scholar] [CrossRef]
- Lei, Y.; Wagner, T.; Rijnaarts, H.; De Wilde, V.; Langenhoff, A. The Removal of Micropollutants from Treated Effluent by Batch-Operated Pilot-Scale Constructed Wetlands. Water Res. 2023, 230, 119494. [Google Scholar] [CrossRef] [PubMed]
- Munir, R.; Muneer, A.; Sadia, B.; Younas, F.; Zahid, M.; Yaseen, M.; Noreen, S. Biochar Imparted Constructed Wetlands (CWs) for Enhanced Biodegradation of Organic and Inorganic Pollutants along with Its Limitation. Environ. Monit. Assess. 2024, 196, 425. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Panghal, V.; Singh, A.; Arora, D.; Kumar, S. Biochar-Modified Constructed Wetlands Using Eclipta Alba as a Plant for Sustainable Rural Wastewater Treatment. Environ. Sci. Pollut. Res. 2024, 31, 17299–17310. [Google Scholar] [CrossRef]
- Al-Mashaqbeh, O.; Alsalhi, L.; Salaymeh, L.; Dotro, G.; Lyu, T. Treatment of Pharmaceutical Industry Wastewater for Water Reuse in Jordan Using Hybrid Constructed Wetlands. Sci. Total Environ. 2024, 939, 173634. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.G.; Rosales-Loredo, S.; Hernández-Morales, A.; Arvizu-Gómez, J.L.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; Rolón-Cárdenas, G.A.; Pacheco-Aguilar, J.R. Bacterial Communities Associated with the Roots of Typha Spp. and Its Relationship in Phytoremediation Processes. Microorganisms 2023, 11, 1587. [Google Scholar] [CrossRef]
- Duré, G.M.; Medina García, L.; Rodríguez Bonet, S.; Ferrreira, F.; Sezerino, P.H.; López Arias, T. Fitorremediación de Contaminantes Emergentes de Origen Farmacéutico En Humedales Flotantes. Rep. Cient. FACEN 2022, 13, 153–159. [Google Scholar] [CrossRef]
- Díaz Lara, C.O.; Cabañas Vargas, D.; Sacramento Rivero, J.C.; Baz-Rodríguez, S.; Ruiz Espinoza, J.E.; Aguilera-Cauich, E.A.; Baas-López, J.M.; Pacheco-Catalán, D.E. Towards Circularity in Anaerobic Digestion: Methane Yield Enhancement Using Biochar from Co-Pyrolysis of Anaerobic Sludge and Residual Lignocellulosic Biomass. Recycling 2025, 10, 84. [Google Scholar] [CrossRef]
- Vega De Lille, M.I.; Hernández Cardona, M.A.; Tzakum Xicum, Y.A.; Giácoman-Vallejos, G.; Quintal-Franco, C.A. Hybrid Constructed Wetlands System for Domestic Wastewater Treatment under Tropical Climate: Effect of Recirculation Strategies on Nitrogen Removal. Ecol. Eng. 2021, 166, 106243. [Google Scholar] [CrossRef]
- González, F.; Vallejos, G.; Silveira, J.; Franco, C.; García, J.; Puigagut, J. Treatment of Swine Wastewater with Subsurface-Flow Constructed Wetlands in Yucatán, Mexico: Influence of Plant Species and Contact Time. Water Sa 2012, 35. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005; ISBN 0-87553-235-7. [Google Scholar]
- Metcald and Eddy, Inc. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Inc.: Boston, MA, USA, 2003. [Google Scholar]
- Elorriaga, Y.; Marino, D.J.; Carriquiriborde, P.; Ronco, A.E. Screening of Pharmaceuticals in Surface Water Bodies of the Pampas Region of Argentina. Int. J. Environ. Health 2013, 6, 330. [Google Scholar] [CrossRef]
- SanJuan-Reyes, N.; Gómez-Oliván, L.M.; Pérez-Pastén Borja, R.; Luja-Mondragón, M.; Orozco-Hernández, J.M.; Heredia-García, G.; Islas-Flores, H.; Galar-Martínez, M.; Escobar-Huérfano, F. Survival and Malformation Rate in Oocytes and Larvae of Cyprinus Carpio by Exposure to an Industrial Effluent. Environ. Res. 2020, 182, 108992. [Google Scholar] [CrossRef]
- Siemens, J.; Huschek, G.; Siebe, C.; Kaupenjohann, M. Concentrations and Mobility of Human Pharmaceuticals in the World’s Largest Wastewater Irrigation System, Mexico City–Mezquital Valley. Water Res. 2008, 42, 2124–2134. [Google Scholar] [CrossRef]
- Luque-Espinar, J.A.; Navas, N.; Chica-Olmo, M.; Cantarero-Malagón, S.; Chica-Rivas, L. Seasonal Occurrence and Distribution of a Group of ECs in the Water Resources of Granada City Metropolitan Areas (South of Spain): Pollution of Raw Drinking Water. J. Hydrol. 2015, 531, 612–625. [Google Scholar] [CrossRef]
- Martínez-Casales, Y.; León-Aguirre, K.; Lamas-Cosío, E.; Noreña-Barroso, E.; Herrera-Silveira, J.; Arcega-Cabrera, F. Caffeine and Paraxanthine as Tracers of Anthropogenic Wastewater in Coastal Lagoons in Yucatan, Mexico. Bull. Environ. Contam. Toxicol. 2022, 108, 182–189. [Google Scholar] [CrossRef]
- Quadra, G.R.; Paranaíba, J.R.; Vilas-Boas, J.; Roland, F.; Amado, A.M.; Barros, N.; Dias, R.J.P.; Cardoso, S.J. A Global Trend of Caffeine Consumption over Time and Related-Environmental Impacts. Environ. Pollut. 2020, 256, 113343. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, C.; Jia, L.; Feng, L.; Wang, R.; Wu, H. An Innovative Biochar-Amended Substrate Vertical Flow Constructed Wetland for Low C/N Wastewater Treatment: Impact of Influent Strengths. Bioresour. Technol. 2018, 247, 844–850. [Google Scholar] [CrossRef]
- Marchioro, L.G.; Baggiotto, C.; Decezaro, S.T.; Freo, G.R.; Houndedjihou, D.; de Aquino Berriel Arruda, F.; Ramírez, R.J.M.G.; dos Santos, K.S.; Wolff, D.B. Influence of Partial Saturation and Organic Loading on the Performance of Vertical Wetlands for Raw Wastewater Treatment. Int. J. Environ. Sci. Technol. 2025, 22, 6585–6594. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, S.; Huang, Z.; Cui, L.; Wang, X. Evaluation of Organic Matter Removal Efficiency and Microbial Enzyme Activity in Vertical-Flow Constructed Wetland Systems. Environments 2016, 3, 26. [Google Scholar] [CrossRef]
- Benvenuti, T.; Hamerski, F.; Giacobbo, A.; Bernardes, A.M.; Zoppas-Ferreira, J.; Rodrigues, M.A.S. Constructed Floating Wetland for the Treatment of Domestic Sewage: A Real-Scale Study. J. Environ. Chem. Eng. 2018, 6, 5706–5711. [Google Scholar] [CrossRef]
- You, Y.; Guo, J. VFCW-MFC for Simultaneous Decontamination and Electricity Production under Sulfadiazine Stress: Effects of Substrates and Analysis of Bacterial Community Structure. Process Saf. Environ. Prot. 2024, 190, 11–21. [Google Scholar] [CrossRef]
- Soundaranayaki, K.; Gandhimathi, R. Enhancing the Nitrogen Removal of Vertical Flow Constructed Wetland by Using Organic Media. Desalination Water Treat. 2020, 175, 125–140. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Yusoff, M.S.; Ahmad Zuki, A.Z. Landfill Leachate Treatment Using Sub-Surface Flow Constructed Wetland by Cyperus Haspan. Waste Manag. 2012, 32, 1387–1393. [Google Scholar] [CrossRef]
- Singh, R.P.; Fu, D.; Fu, D.; Juan, H. Pollutant Removal Efficiency of Vertical Sub-Surface Upward Flow Constructed Wetlands for Highway Runoff Treatment. Arab. J. Sci. Eng. 2014, 39, 3571–3578. [Google Scholar] [CrossRef]
- Aylan, R.A.; Al-Abbawy, D.A.H.; Yaseen, D.A. Development of the Horizontal Flow Wetland Using Palm Waste Biochar for Greywater Reclamation. J. Ecol. Eng. 2023, 24, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Visiy, E.B.; Djousse, B.M.K.; Martin, L.; Zangue, C.N.; Sangodoyin, A.; Gbadegesin, A.S.; Fonkou, T. Effectiveness of Biochar Filters Vegetated with Echinochloa Pyramidalis in Domestic Wastewater Treatment. Water Sci. Technol. 2022, 85, 2613–2624. [Google Scholar] [CrossRef]
- Rabbat, C.; Pinna, A.; Andres, Y.; Villot, A.; Awad, S. Adsorption of Ibuprofen from Aqueous Solution onto a Raw and Steam-Activated Biochar Derived from Recycled Textiles Insulation Panels at End-of-Life: Kinetic, Isotherm and Fixed-Bed Experiments. J. Water Process Eng. 2023, 53, 103830. [Google Scholar] [CrossRef]
- Ocampo-Perez, R.; Padilla-Ortega, E.; Medellin-Castillo, N.A.; Coronado-Oyarvide, P.; Aguilar-Madera, C.G.; Segovia-Sandoval, S.J.; Flores-Ramírez, R.; Parra-Marfil, A. Synthesis of Biochar from Chili Seeds and Its Application to Remove Ibuprofen from Water. Equilibrium and 3D Modeling. Sci. Total Environ. 2019, 655, 1397–1408. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Zhang, S.; Chen, Y.; Wang, R.; Ho, S.-H. Tailoring a Novel Hierarchical Cheese-like Porous Biochar from Algae Residue to Boost Sulfathiazole Removal. Environ. Sci. Ecotechnol. 2022, 10, 100168. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Hassan, M.A.; Omer, K.M.; Hama, S. Biochar as Green Adsorbents for Pharmaceutical Pollution in Aquatic Environments: A Review. Desalination 2024, 583, 117725. [Google Scholar] [CrossRef]
- Chaiwong, C.; Laungphairojana, A.; Artnaseaw, A.; Supanchaiyamat, N.; Hunt, A.J.; Macquarrie, D.J.; Ngernyen, Y. Modified Biochar Derived from Grass Jelly Tree Waste as a Potential Adsorbent for Ibuprofen Adsorption from Water. J. Jpn. Inst. Energy 2025, 104, 54–62. [Google Scholar] [CrossRef]
- Moreno-Pérez, J.; Pauletto, P.S.; Cunha, A.M.; Bonilla-Petriciolet, Á.; Salau, N.P.G.; Dotto, G.L. Three-Dimensional Mass Transport Modeling of Pharmaceuticals Adsorption inside ZnAl/Biochar Composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126170. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic Ozonation for the Removal of Organic Contaminants in Water on ZSM-5 Zeolites. Appl. Catal. B Environ. 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- Dixit, A.; Ahammed, M.M. Use of Modified Biochar for Removal of Endocrine Disrupting Compounds from Water and Wastewater: A Review. Bioresour. Technol. Rep. 2023, 23, 101519. [Google Scholar] [CrossRef]
- Chakraborty, P.; Banerjee, S.; Kumar, S.; Sadhukhan, S.; Halder, G. Elucidation of Ibuprofen Uptake Capability of Raw and Steam Activated Biochar of Aegle Marmelos Shell: Isotherm, Kinetics, Thermodynamics and Cost Estimation. Process Saf. Environ. Prot. 2018, 118, 10–23. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, P. Comparative Study on Pharmaceuticals Adsorption in Reclaimed Water Desalination Concentrate Using Biochar: Impact of Salts and Organic Matter. Sci. Total Environ. 2017, 601–602, 857–864. [Google Scholar] [CrossRef]
- Siara, S.; Elvis, C.; Harishkumar, R.; Velayudhaperumal Chellam, P. ZnAl2O4 Supported on Lychee-Biochar Applied to Ibuprofen Photodegradation. Mater. Res. Bull. 2022, 145, 111530. [Google Scholar] [CrossRef]
- Mondal, S.; Bobde, K.; Aikat, K.; Halder, G. Biosorptive Uptake of Ibuprofen by Steam Activated Biochar Derived from Mung Bean Husk: Equilibrium, Kinetics, Thermodynamics, Modeling and Eco-Toxicological Studies. J. Environ. Manag. 2016, 182, 581–594. [Google Scholar] [CrossRef]
- Sun, L.; Cao, Z.; Jia, S.; Zhang, P.; Zhang, G. Micro-Aeration Strategies: Optimizing Nitrogen and Phosphorus Removal in Vertical Flow Constructed Wetlands for Rural Sewage Treatment. J. Water Process Eng. 2025, 75, 107931. [Google Scholar] [CrossRef]
- Li, Y.; Lian, J.; Wu, B.; Zou, H.; Tan, S.K. Phytoremediation of Pharmaceutical-Contaminated Wastewater: Insights into Rhizobacterial Dynamics Related to Pollutant Degradation Mechanisms during Plant Life Cycle. Chemosphere 2020, 253, 126681. [Google Scholar] [CrossRef]
- Matamoros, V.; Hijosa, M.; Bayona, J.M. Assessment of the Pharmaceutical Active Compounds Removal in Wastewater Treatment Systems at Enantiomeric Level. Ibuprofen and Naproxen. Chemosphere 2009, 75, 200–205. [Google Scholar] [CrossRef]
- Mohapatra, S.; Tong, X.; Mukherjee, S.; Dubey, M.; Subhash, S.; Luhua, Y.; Van Der Hoek, J.P.; Gin, K.Y.-H. Comprehensive Insights on the Detection, Occurrence and Modelling of Pharmaceuticals in Surface Water, Groundwater, and Drinking Water Treatment Plants. J. Hazard. Mater. Adv. 2025, 18, 100707. [Google Scholar] [CrossRef]
- Leiva, A.M.; Gutierrez, E.; Arias, C.A.; Vidal, G. Influence of Water Quality Parameters on the Removal of Triclosan and Ibuprofen in Vertical Subsurface Flow Constructed Wetlands Using Multivariate Analysis. Environ. Technol. Innov. 2021, 24, 101846. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, J.; Lee, Z.M.P.; Maspolim, Y.; Gersberg, R.M.; Liu, Y.; Tan, S.K.; Ng, W.J. Characterization of Bacterial Communities in Wetland Mesocosms Receiving Pharmaceutical-Enriched Wastewater. Ecol. Eng. 2016, 90, 215–224. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A Review on Removing Pharmaceutical Contaminants from Wastewater by Constructed Wetlands: Design, Performance and Mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef]
- Ávila, C.; Nivala, J.; Olsson, L.; Kassa, K.; Headley, T.; Mueller, R.A.; Bayona, J.M.; García, J. Emerging Organic Contaminants in Vertical Subsurface Flow Constructed Wetlands: Influence of Media Size, Loading Frequency and Use of Active Aeration. Sci. Total Environ. 2014, 494–495, 211–217. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, T.; Zhang, Y.; Stein, O.R.; Arias, C.A.; Brix, H.; Carvalho, P.N. Effects of Constructed Wetland Design on Ibuprofen Removal–A Mesocosm Scale Study. Sci. Total Environ. 2017, 609, 38–45. [Google Scholar] [CrossRef]
- Bandura, L.; Białoszewska, M.; Malinowski, S.; Franus, W. Adsorptive Performance of Fly Ash-Derived Zeolite Modified by β-Cyclodextrin for Ibuprofen, Bisphenol A and Caffeine Removal from Aqueous Solutions–Equilibrium and Kinetic Study. Appl. Surf. Sci. 2021, 562, 150160. [Google Scholar] [CrossRef]
- Smiljanić, D.; De Gennaro, B.; Daković, A.; Galzerano, B.; Germinario, C.; Izzo, F.; Rottinghaus, G.E.; Langella, A. Removal of Non-Steroidal Anti-Inflammatory Drugs from Water by Zeolite-Rich Composites: The Interference of Inorganic Anions on the Ibuprofen and Naproxen Adsorption. J. Environ. Manag. 2021, 286, 112168. [Google Scholar] [CrossRef]
- Gezahegn, A.; Selassie, Y.G.; Agegnehu, G.; Addisu, S.; Asargew Mihretie, F.; Kohira, Y.; Sato, S. Pyrolysis Temperature Changes the Physicochemical Characteristics of Water Hyacinth-Based Biochar as a Potential Soil Amendment. Biomass Convers. Biorefin. 2025, 15, 3737–3752. [Google Scholar] [CrossRef]
- Madariaga-Segovia, P.; Villamar-Ayala, C.A.; Ramos, N.; Sánchez-Domínguez, M.; Lavín, R. Influence of Pyrolysis Conditions on the Adsorbent Properties of Hazelnut Shell Biochar to Remove Paracetamol, Amoxicillin, and Triclosan. Case Stud. Chem. Environ. Eng. 2025, 12, 101251. [Google Scholar] [CrossRef]
- Tortet, L.; Ligner, E.; Blanluet, W.; Noguez, P.; Marichal, C.; Schäf, O.; Paillaud, J.-L. Adsorptive Elimination of Paracetamol from Physiological Solutions: Interaction with MFI-Type Zeolite. Microporous Mesoporous Mater. 2017, 252, 188–196. [Google Scholar] [CrossRef]
- Matějová, L.; Bednárek, J.; Tokarský, J.; Koutník, I.; Sokolová, B.; Cruz, G.J.F. Adsorption of the Most Common Non-Steroidal Analgesics from Aquatic Environment on Agricultural Wastes-Based Activated Carbons; Experimental Adsorption Study Supported by Molecular Modeling. Appl. Surf. Sci. 2022, 605, 154607. [Google Scholar] [CrossRef]
- Palma, T.L.; Donaldben, M.N.; Costa, M.C.; Carlier, J.D. Putative Role of Flavobacterium, Dokdonella and Methylophilus Strains in Paracetamol Biodegradation. Water Air Soil Pollut. 2018, 229, 200. [Google Scholar] [CrossRef]
- Park, S.; Oh, S. Activated Sludge-Degrading Analgesic Drug Acetaminophen: Acclimation, Microbial Community Dynamics, Degradation Characteristics, and Bioaugmentation Potential. Water Res. 2020, 182, 115957. [Google Scholar] [CrossRef]
- Sosa Alderete, L.G.; Vezza, M.; Ibañez, S.G.; Schroeder, P.; Agostini, E.; Talano, M.A. Unraveling Paracetamol Metabolism and Its Circadian Regulation: Insights from Tobacco Hairy Roots as a Model System. Plants 2025, 14, 2812. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, E.; Verlicchi, P.; Young, T.M. Paracetamol Removal in Subsurface Flow Constructed Wetlands. J. Hydrol. 2011, 404, 130–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, S.; Li, H.; Wang, D. Adsorption Behaviors of Acetaminophen onto Sediment in the Weihe River, Shaanxi, China. Int. J. Sediment Res. 2015, 30, 263–271. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J. Effect of Solution pH on the Adsorption of Paracetamol on Chemically Modified Activated Carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Lütke, S.F.; Dotto, G.L.; Salau, N.P.G. Exploring the Simultaneous Mass Transport of Nimesulide and Paracetamol Adsorption on Activated Carbon: A PVSDM Approach. Sep. Purif. Technol. 2024, 329, 125148. [Google Scholar] [CrossRef]
- Matamoros, V.; Arias, C.; Brix, H.; Bayona, J.M. Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Urban Wastewater in a Pilot Vertical Flow Constructed Wetland and a Sand Filter. Environ. Sci. Technol. 2007, 41, 8171–8177. [Google Scholar] [CrossRef]
- de Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Filho, F.J.C.M. Ibuprofen and Caffeine Removal in Vertical Flow and Free-Floating Macrophyte Constructed Wetlands with Heliconia Rostrata and Eichornia Crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Zhang, J.; Chen, A.; He, X. Adsorption/Desorption of Ammonium and Phosphorus on Four Substrates in Constructed Wetland. Asian J. Chem. 2015, 27, 2477–2481. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Oesterle, P.; Wurzer, C.; Mašek, O.; Jansson, S. Removal of Contaminants of Emerging Concern from Multicomponent Systems Using Carbon Dioxide Activated Biochar from Lignocellulosic Feedstocks. Bioresour. Technol. 2021, 340, 125561. [Google Scholar] [CrossRef]
- Mengesha, D.N.; Abebe, M.W.; Appiah-Ntiamoah, R.; Kim, H. Ground Coffee Waste-Derived Carbon for Adsorptive Removal of Caffeine: Effect of Surface Chemistry and Porous Structure. Sci. Total Environ. 2022, 818, 151669. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.G.; Magalhães, T.F.D.S.; Golin, R.; Sousa, H.M.; Fukumoto, A.A.F.; Vasconcelos, L.G.D.; Morais, E.B.D. Adsorption of Caffeine in Aqueous Solution by Biochar from Cotton Processing Residues. NAT 2024, 12, 474–481. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized Biochar Obtained from Pine Needles as a Novel Adsorbent to Remove Caffeine from Aqueous Solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I. Τhe Application of Oxidized Carbon Derived from Luffa Cylindrica for Caffeine Removal. Equilibrium, Thermodynamic, Kinetic and Mechanistic Analysis. J. Mol. Liq. 2019, 296, 112078. [Google Scholar] [CrossRef]
- Samara, F.; Al Abdel Hamid, A.A.; Gopal, V.; Dronjak, L.; Feghaly, F.; Kanan, S. Modified Zeolites for the Removal of Emerging Bio-Resistive Pollutants in Water Resources. Catalysts 2025, 15, 138. [Google Scholar] [CrossRef]
- Keerthanan, S.; Rajapaksha, S.M.; Trakal, L.; Vithanage, M. Caffeine Removal by Gliricidia Sepium Biochar: Influence of Pyrolysis Temperature and Physicochemical Properties. Environ. Res. 2020, 189, 109865. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Jaramillo, J.A.; Carrero-Mantilla, J.I.; Sanabria-González, N.R. A Review of Caffeine Adsorption Studies onto Various Types of Adsorbents. Sci. World J. 2021, 2021, 9998924. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.S.; Gummadi, S.N. Catabolic Pathways and Biotechnological Applications of Microbial Caffeine Degradation. Biotechnol. Lett. 2006, 28, 1993–2002. [Google Scholar] [CrossRef]
- Summers, R.M.; Mohanty, S.K.; Gopishetty, S.; Subramanian, M. Genetic Characterization of Caffeine Degradation by Bacteria and Its Potential Applications. Microb. Biotechnol. 2015, 8, 369–378. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Leiva, A.M.; Gómez, G.; Vidal, G. Performance of Vertical Subsurface Flow Constructed Wetlands for Commercial Laundry Greywater Treatment: Effects of Design and Operational Parameters. J. Water Process Eng. 2025, 75, 107951. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhu, C.-Y.; Weng, B.-S.; Mo, P.-W.; Xu, Z.-J.; Tian, P.; Cui, B.-S.; Bai, J.-H. Regulation of Heavy Metals Accumulated by Acorus Calamus L. in Constructed Wetland through Different Nitrogen Forms. Chemosphere 2021, 281, 130773. [Google Scholar] [CrossRef] [PubMed]
- Batkhuyag, N.; Matyakubov, B.; Mang, N.Z.L.; Lee, T. Additive Inhibitory Effects of Heavy Metals on Phenol-Utilizing Microorganism. Environ. Eng. Res. 2021, 27, 210342. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, J.; Li, R.; Chen, X.; Cao, M. Dissecting the Negative Influence Mechanisms of Microplastics on Plant and Microbial Mediated Nitrogen Removal in Constructed Wetlands. Chem. Eng. J. 2024, 491, 151910. [Google Scholar] [CrossRef]
- Escolà Casas, M.; Matamoros, V. Novel Constructed Wetland Configurations for the Removal of Pharmaceuticals in Wastewater. In Handbook of Environmental Chemistry; Springer Science and Business Media Deutschland GmbH: Cham, Switzerland, 2021; Volume 108, pp. 163–190. [Google Scholar]

| Treatment System | Support Medium Used | Reported Pharmaceutical Removal |
|---|---|---|
| V-CWs [12] | Mixture of gravel and sand | >75% for Ibuprofen and Paracetamol |
| Combined Anaerobic Digester, V-CWs and UV Photolysis [13] | Mixture of gravel and sand | 100% removal of Ibuprofen, 98% for Paracetamol, and 87% for Caffeine |
| V-CWs [14] | Mixture of gravel, sand and biochar | 79.93% removal of Ibuprofen, and 87.53% of caffeine |
| V-CWs [15] | Light expanded clay aggregates (LECA) | 87–93% removal of Caffeine |
| V-CW with Microbial Fuel Cell (MFC) [16] | Gravel and granular activated carbon (GAC) | 49–62% removal of Ibuprofen |
| Horizontal-CWs (HCWs) [17] | Gravel | 94.03 average removal of Ibuprofen |
| HCWs and Stabilization Ponds [18] | Tezontle | Increased concentrations of Ibuprofen due to anoxic conditions. |
| Aerobic reactor and H-CWs [19] | Gravel, GAC, river sand, and potting soil | 94% removal of paracetamol |
| V-CWs [20] | Biochar | Up to 99% removal of Ibuprofen |
| Parameter | Raw Domestic Water | Typical and Reported Concentrations in Domestic Wastewater [31] |
|---|---|---|
| COD (mg/L) | 817.70 ± 71.83 | 250–1000 |
| BOD5 (mg/L) | 365.76 ± 75.79 | 230–560 |
| TSS (mg/L) | 644.57 ± 118.19 | 150–350 |
| Ibuprofen (µg/L) | 0.97 ± 0.12 | 1.05–1096 |
| Paracetamol (µg/L) | 4.21 ± 0.39 | 0.04–41.7 |
| Caffeine (µg/L) | 11.90 ± 0.44 | 9.05–89.5 |
| Parameter | Septic Tank | Average Removal Efficiency | |
|---|---|---|---|
| Inlet Concentration | Outlet Concentration | ||
| COD (mg/L) | 817.70 ± 71.83 | 462.19 ± 57.66 | 43.47% |
| BOD5 (mg/L) | 365.76 ± 75.79 | 286.39 ± 52.49 | 20.87% |
| TSS (mg/L) | 644.57 ± 118.19 | 304.99 ± 68.66 | 52.68% |
| Ibuprofen (µg/L) | 0.97 ± 0.12 | 0.89 ± 0.02 | 8.24% |
| Paracetamol (µg/L) | 4.21 ± 0.39 | 4.06 ± 0.08 | 3.56% |
| Caffeine (µg/L) | 11.90 ± 0.44 | 10.92 ± 0.34 | 8.23% |
| Parameters | Concentration in CW Units Effluent | |||
|---|---|---|---|---|
| Septic Tank | V1 | V2 | V3 | |
| COD (mg/L) | 462.19 ± 57.66 | 49.38 ± 11.06 | 38.78 ± 7.33 | 40.69 ± 12.73 |
| BOD5 (mg/L) | 286.39 ± 52.49 | 19.71 ± 9.55 | 18.09 ± 3.65 | 18.39 ± 6.92 |
| TSS (mg/L) | 304.99 ± 68.66 | 18.14 ± 11.39 | 13.06 ± 7.67 | 11.16 ± 6.54 |
| Parameters | Removal (%) in CW Units | ||
|---|---|---|---|
| V1 | V2 | V3 | |
| COD | 89.4 ± 2.7 | 91.7 ± 1.4 | 91.4 ± 2.3 |
| BOD5 | 93.3 ± 2.9 | 93.8 ± 1.1 | 93.7 ± 1.9 |
| TSS | 94.5 ± 3.5 | 96.0 ± 2.3 | 96.6 ± 2.0 |
| Parameters | Concentrations in CW Units Effluent | |||
|---|---|---|---|---|
| Septic Tank | V1 | V2 | V3 | |
| Ibuprofen (µg/L) | 9.54 ± 0.61 | 1.64 ± 0.73 | 0.77 ± 0.42 | 0.86 ± 0.44 |
| Paracetamol (µg/L) | 9.78 ± 0.34 | 0.97 ± 0.46 | 0.56 ± 0.36 | 0.55 ± 0.34 |
| Caffeine (µg/L) | 9.78 ± 0.29 | 0.66 ± 0.21 | 0.26 ± 0.19 | 0.33 ± 0.26 |
| Parameters | Removal (%) in CW Units | ||
|---|---|---|---|
| V1 | V2 | V3 | |
| Ibuprofen | 81.8 ± 8.2 | 91.5 ± 4.7 | 90.4 ± 4.9 |
| Paracetamol | 90.0 ± 4.6 | 94.2 ± 3.7 | 94.3 ± 3.5 |
| Caffeine | 93.1 ± 2.3 | 97.2 ± 2.1 | 96.5 ± 2.8 |
| Parameter | Planted vs. Unplanted | Gravel/Sand vs. Biochar/Zeolite | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| COD | 0.024 | 0.878 | 0.193 | 0.664 |
| BOD5 | 0.001 | 0.973 | 0.009 | 0.924 |
| TSS | 0.433 | 0.517 | 0.813 | 0.377 |
| Ibuprofen | 1.310 | 0.266 | 10.720 | 0.003 |
| Paracetamol | 0.844 | 0.368 | 3.475 | 0.075 |
| Caffeine | 0.524 | 0.476 | 4.585 | 0.043 |
| Factor | n | Mean Removal (mg/L) | Grouping |
|---|---|---|---|
| Biochar/Zeolite | 16 | 8.222 | A |
| Gravel/Sand | 8 | 7.397 | B |
| Factor | n | Mean Removal (mg/L) | Grouping |
|---|---|---|---|
| Biochar/Zeolite | 16 | 9.241 | A |
| Gravel/Sand | 8 | 8.878 | B |
| Parameter | Planted vs. Unplanted | |
|---|---|---|
| F-Value | p-Value | |
| COD | 0.010 | 0.941 |
| BOD5 | 0.000 | 0.987 |
| TSS | 0.070 | 0.800 |
| Ibuprofen | 0.160 | 0.698 |
| Paracetamol | 0.000 | 0.964 |
| Caffeine | 0.500 | 0.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cardona, M.A.; Giácoman-Vallejos, G.; Vega-De-Lille, M.I.; Méndez-Novelo, R.I.; González-Sánchez, A.A.; Hernández-Núñez, E.; Ponce-Caballero, C.; Góngora-Echeverría, V.R. Enhanced Removal of Ibuprofen, Paracetamol, and Caffeine in Vertical Constructed Wetlands Using Biochar and Zeolite as Support Media. Processes 2025, 13, 3679. https://doi.org/10.3390/pr13113679
Hernández-Cardona MA, Giácoman-Vallejos G, Vega-De-Lille MI, Méndez-Novelo RI, González-Sánchez AA, Hernández-Núñez E, Ponce-Caballero C, Góngora-Echeverría VR. Enhanced Removal of Ibuprofen, Paracetamol, and Caffeine in Vertical Constructed Wetlands Using Biochar and Zeolite as Support Media. Processes. 2025; 13(11):3679. https://doi.org/10.3390/pr13113679
Chicago/Turabian StyleHernández-Cardona, Marco A., Germán Giácoman-Vallejos, Marisela I. Vega-De-Lille, Roger I. Méndez-Novelo, Avel A. González-Sánchez, Emanuel Hernández-Núñez, Carmen Ponce-Caballero, and Virgilio R. Góngora-Echeverría. 2025. "Enhanced Removal of Ibuprofen, Paracetamol, and Caffeine in Vertical Constructed Wetlands Using Biochar and Zeolite as Support Media" Processes 13, no. 11: 3679. https://doi.org/10.3390/pr13113679
APA StyleHernández-Cardona, M. A., Giácoman-Vallejos, G., Vega-De-Lille, M. I., Méndez-Novelo, R. I., González-Sánchez, A. A., Hernández-Núñez, E., Ponce-Caballero, C., & Góngora-Echeverría, V. R. (2025). Enhanced Removal of Ibuprofen, Paracetamol, and Caffeine in Vertical Constructed Wetlands Using Biochar and Zeolite as Support Media. Processes, 13(11), 3679. https://doi.org/10.3390/pr13113679







