Abstract
Exoskeleton robots have been widely applied in military, industrial, and rehabilitation fields, with their practical effectiveness substantially reliant on a comprehensive performance evaluation framework. This paper reviews the prevalent testing methods for exoskeleton robots, including electromyography (EMG), motion capture, human–machine interaction forces, energy consumption monitoring, and both subjective and objective assessments. Through the systematic integration and comparison of these methodologies, this study establishes a methodological foundation for the comprehensive evaluation of performance and provides a theoretical basis for the development of standardized evaluation frameworks in the future. Furthermore, by systematically comparing and integrating these methodologies, this study aims to establish a methodological foundation for the future development of a standardized, multi-dimensional evaluation framework, which is essential for translating exoskeleton technology from laboratory research to practical applications.
1. Introduction
Exoskeleton technology, as an advanced human–robot interaction (HRI) intelligent system, demonstrates considerable potential in military operations, industrial material handling, and medical rehabilitation. Its core functionality primarily involves enhancing, substituting, or restoring the users’ capacity to carry out daily activities via mechanical assistance [1]. However, the ultimate efficacy and user experience of this technology are largely determined by the quality of human-exoskeleton interaction. Therefore, the scientific, systematic, and comprehensive performance testing and evaluation of exoskeleton systems constitutes a crucial step in translating these systems from laboratory research to practical applications.
Currently, a variety of testing methodologies have been developed, including sensing technologies based on electromyography (EMG), kinematics, and pressure distribution, to assess the assistive effects of exoskeletons, such as reducing metabolic cost, decreasing muscle activation, and improving gait patterns [2]. In parallel, an integrated modeling approach that combines human biomechanics with exoskeleton multi-body dynamics has been developed, providing a theoretical framework for system design and optimization [3]. However, existing testing methodologies exhibit several notable limitations: First, many studies focus exclusively on single-modal metrics (e.g., solely examining energy consumption or analyzing only EMG signals), lacking a synergistic analysis of multi-dimensional data (Slade et al.). Second, data acquisition systems are often cumbersome and heavily reliant on laboratory settings, making effective evaluation challenging in real-world, dynamic scenarios (e.g., real-time measurement of interaction forces at the physical human–robot interface remains a significant challenge [4]; Rathore et al.). Third, testing standards have yet to be standardized, hindering the direct comparison and validation of results across different studies (De Bock et al.). Notably, existing studies further indicate that interaction forces demonstrate an uneven distribution under different movement modalities, with peak forces often concentrated in specific physiological regions susceptible to injury. Simultaneously, the increasing complexity of exoskeleton systems imposes higher demands on hardware architectures, necessitating modular, distributed real-time control and communication solutions to ensure interaction safety and system efficacy. Furthermore, at the actuator and control levels, achieving low-impedance, high-precision torque control is critical for enabling smooth and safe human–robot interaction [5,6,7]. Collectively, these issues have left the field of exoskeleton evaluation methodologically isolated, where studies exist in separate “silos,” limiting a holistic understanding and thus impeding comprehensive and accurate characterization of human–robot interaction efficacy and physiological responses.
This review is structured to address the aforementioned challenges through three clearly defined objectives. First, it systematically organizes and critically examines the current landscape of testing methodologies across key dimensions—specifically electromyography (EMG), motion capture, human–robot interface pressure testing, energy consumption monitoring, and subjective assessment—to delineate their complementary strengths and limitations. Second, by placing these diverse methodologies within a unified comparative framework, this study identifies and analyzes the fundamental technical barriers—including data fusion complexities, real-time performance requirements, and system integration challenges—that currently impede the development of a holistic evaluation system [8]. Third, and most significantly, this work synthesizes these insights to construct a foundational methodological framework that not only clarifies the technological pathways toward a comprehensive evaluation system but also provides a principled basis for selecting and integrating appropriate evaluation tools [9]. Consequently, this work paves the way for the future development of intelligent, personalized exoskeletons and helps to bridge the gap between isolated laboratory experiments and holistic real-world performance validation [9]. Furthermore, by contextualizing our analysis with systematic recommendations [10] and innovative component methodologies [11], this review moves beyond merely underscoring potential and instead establishes a concrete methodological foundation for the realization of a future, generalized multi-dimensional evaluation paradigm suitable for complex environments.
Figure 1 illustrates that the research progress of exoskeleton testing technology is closely linked with the evolution of evaluation methodologies. Early studies primarily relied on surface electromyography (sEMG) signal analysis to quantify the extent of muscle activation [12]. Subsequently, motion capture systems were introduced for the analysis of gait. With technological advancement, the evaluation of mechanical properties at the physical human–robot has received increasing attention, and pressure distribution sensing technology has been employed to measure the contact force distribution between exoskeletons and the human body. In recent years, multi-dimensional data fusion approaches have emerged as a research focus. By synchronously acquiring multi-dimensional data, including EMG, kinematics, dynamics, and metabolic energy consumption, a comprehensive performance evaluation system has been established. Recent advancements indicate that artificial intelligence (AI)-based personalized evaluation algorithms and adaptive control strategies are advancing the development of exoskeleton testing toward greater intelligence and standardization [13].
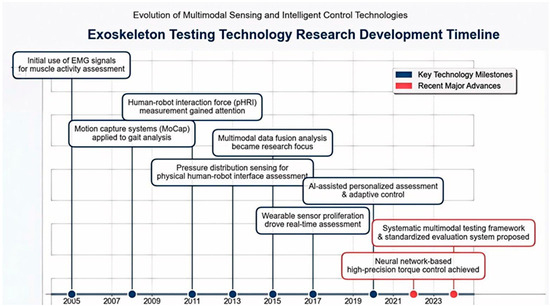
Figure 1.
Evolution of research on exoskeleton testing technologies, adapted from [12].
Literature was retrieved from three major databases: Web of Science (Core Collection), IEEE Xplore, and PubMed, covering the period from 1 January 2010 to 31 March 2025. The search string used was “(exoskeleton OR exosuit) AND (performance evaluation OR testing method OR EMG OR motion capture OR pressure sensing OR energy consumption)”. Studies were included if they explicitly described exoskeleton testing methods and provided quantitative data. Studies were excluded if they were purely theoretical modeling, non-English, or focused solely on structural design. Three researchers independently screened the literature. An initial total of 500 articles was identified, of which 93 were ultimately included. Any disagreements were resolved through discussion.
The purpose of the literature review is to highlight the unique contributions of this study. The core values of this work are summarized as follows:
- Integrate physiological and mechanical sensing data to establish a multi-technology collaborative framework for exoskeleton evaluation;
- Systematically compare the strengths and weaknesses of traditional and innovative testing technologies;
- Identify the key challenges and potential directions for future research.
This study conducted a literature search on exoskeleton testing in Web of Science, IEEE Xplore, and PubMed from 2010 to 2025. The screening criteria encompassed the integrity of experimental design, the novelty of sensor technology, and the breadth of evaluation dimensions.
2. EMG
The core principle of electromyography (EMG) involves recording muscle electrical activity to characterize the functional state of the neuromuscular system. During muscle contraction, motor neurons generate electrical discharges, which induce action potentials in muscle fibers. These electrical signals are acquired and amplified through electrodes to generate EMG signals. Variations in these signals (e.g., amplitude and frequency) are strongly correlated with the degree of muscle activation and the onset of muscle fatigue. EMG signal analysis involves multiple stages, including detection, processing, and classification, utilizing techniques such as time-domain/frequency-domain analysis, wavelet transform, time-frequency distribution, and artificial intelligence-based methods [14]. As a non-invasive technique, surface electromyography (sEMG) has become an essential component in biological control and evaluation for the development of modern exoskeleton technology. It plays a crucial role in optimizing exoskeleton design, investigating the use of muscle signals for controlling exoskeleton operations, and acquiring novel insights into neuromuscular reorganization [15]. Therefore, analyzing EMG signal characteristics can yield critical information about muscle activity, which can be applied to clinical diagnosis, rehabilitation assessment, or human–robot interaction control.
2.1. sEMG for Muscle Fatigue Assessment
Traditional muscle fatigue assessment has predominantly relied on subjective perceptions or invasive measurements, which present limitations such as poor real-time performance, a lack of dynamic monitoring capability, and invasiveness. sEMG technology facilitates the non-invasive acquisition of muscle electrical activity signals. It can extract time-domain features (e.g., root mean square (RMS)) and frequency-domain features (e.g., mean frequency (MF)) in real-time, which are directly correlated with muscle activation and fatigue states, thus offering a standardized method for addressing the aforementioned needs. Recent studies have shown that sEMG, as a laboratory-based quantitative technique, is both safe and feasible for assessing muscle fatigue in acute care settings [16].
Zhou et al. [17] conducted experiments involving subjects who met the anthropometric standards for pilots. They simulated combat operations in a third-generation fighter cockpit, employed sEMG technology, and recorded signals from four bilateral muscle groups for 20 s at both the start and end of the flight using specific sensors. These signals were processed using MATLAB R2022a to extract RMS and MF metrics, with statistical analysis performed using Origin. Additionally, the time taken to hit the target was recorded three times. The results revealed differences in fatigue across muscle groups and a left-right asymmetry, with more pronounced fatigue observed in the right biceps brachii (BB) and tibialis anterior (TA). Furthermore, muscle fatigue was found to potentially impair operational performance (Figure 2). This study preliminarily verified the feasibility of using sEMG to quantitatively assess the correlation between muscle fatigue and operational performance in complex, high-intensity human–robot interaction tasks. However, the simulation tests conducted in a laboratory environment still require further validation in real-world operational contexts. Sylos-Labini et al. identified a strong correlation between muscle fatigue and sEMG RMS in static arm-lifting tasks, whereas Zhou et al. observed only a weak correlation in dynamic flight tasks, attributed to motion artifacts. This suggests the need for optimizing sEMG filtering in dynamic scenarios. Gillette et al. [18] used sEMG technology to conduct on-site measurements with 16 automotive assembly workers (n = 16). The experimental results showed that the exoskeleton significantly reduced the average EMG amplitude (Δ = −3.2%MVC) and fatigue risk score (Δ = −5.1%MVC) of the anterior deltoid, especially in tasks involving prolonged arm elevation. However, sEMG remains dominant in non-invasive assessments, whereas needle EMG continues to hold irreplaceable value in evaluating neuromuscular function in amputees, particularly in analyzing deep muscle activity. Notably, sEMG testing in exoskeleton fatigue assessment has a test–retest reliability of 0.85–0.92, with a measurement error of ±3%. This reliability range ensures that the sEMG-derived metrics (e.g., RMS, MF) can consistently reflect muscle fatigue changes during long-term exoskeleton-assisted tasks.
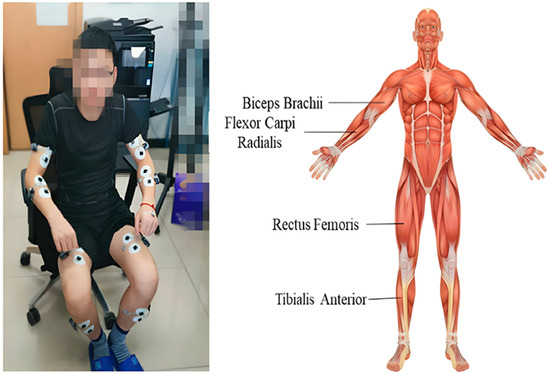
Figure 2.
sEMG electrode placement positions, adapted from [17].
Although the studies mentioned above have confirmed the positive effects of exoskeletons in short-duration tasks, the cumulative fatigue effects associated with long-duration tasks and different movement modalities remain unclear. Mishra et al. [19,20] compared EMG activity during normal and clinical gait and found that the difference in activity between the tibialis anterior and gastrocnemius muscles was most pronounced. This finding provided a crucial basis for optimizing the assistive strategies of lower limb exoskeletons. The experimental results indicated that using a powered lower limb exoskeleton for both overground walking and treadmill walking training significantly induced fatigue in the knee flexor muscles, whereas the sit-to-stand task induced a lower degree of muscle fatigue.
2.2. sEMG Signal Processing and Intelligent Control
sEMG signals are essential bioelectrical signals for enabling human–robot synergistic control in exoskeletons. The technological process begins with reliable signal acquisition and extends through advanced processing algorithms and intelligent control strategies, collectively facilitating the paradigm shift of exoskeletons from “simple traction” to “active collaboration”.
Traditional exoskeletons typically rely on pre-defined motion trajectories or basic force feedback control, which makes it challenging to adjust the assistance force based on real-time muscle activity of the user, leading to poor human–machine collaboration and insufficient wearing comfort. Mainstream exoskeleton drive methods are each tailored to specific applications: Motor drive offers fast response (on the millisecond level) and high precision (torque error <5%), making it ideal for fine upper-limb operations in rehabilitation, but it tends to overheat under heavy loads; Pneumatic drive is cost-effective and impact-resistant, making it ideal for short-term, heavy-load industrial tasks, but its precision is affected by gas compressibility (positional error of 1–2 mm), and it requires an external air supply; Hydraulic drive boasts a high power density (up to 500 W/kg), making it a core choice for military heavy-load exoskeletons, but it has issues such as the risk of oil leakage and high maintenance costs. In practical design, the appropriate drive method should be selected based on requirements such as load and precision. For instance, motor drive is commonly used in rehabilitation upper-limb exoskeletons, while pneumatic or hydraulic drive is often prioritized for industrial handling exoskeletons. The Ag-AgCl electrode, renowned for its high stability, excellent biocompatibility, and strong reproducibility, generates minimal electrical noise during measurements, enabling high-precision biopotential measurements [21]. These advantages have driven its widespread application in fields such as neuroelectrophysiological research, electrocardiography (ECG), and electromyography (EMG). The Yogeswaran team [22] developed a myoelectric feedback exoskeleton system that uses Ag-AgCl electrodes to collect EMG signals, combined with an inertial measurement unit (IMU) sensor (with 97% accuracy) to monitor arm movement. A microcontroller (e.g., Arduino) processes the data to control exoskeleton actions, while a PC-based data acquisition system (e.g., LabVIEW 2015) is used for remote rehabilitation monitoring. This system establishes closed-loop control from muscle signals to exoskeleton movement, demonstrating the potential of multi-sensor fusion in improving human–machine collaboration. Despite a 3-s EMG signal transmission delay, the architecture offers a valuable reference for future research.
In long-term monitoring or rehabilitation scenarios, traditional Ag-AgCl electrodes encounter several challenges, including discomfort during wear, susceptibility to sweat interference, and displacement with prolonged use. Researchers have conducted studies to overcome the limitations of traditional Ag-AgCl electrodes. A study in Sweden [23] reviewed textile-based electrodes (e.g., silver-coated nylon, stainless steel yarn) and identified them as viable alternatives to traditional electrodes, suitable for long-term sEMG monitoring. After resolving signal acquisition issues, improving the accuracy of signal processing and motion recognition became crucial. A research team from the University of Coimbra, Portugal [24], applied machine learning methods (e.g., Linear Discriminant Analysis (LDA), Support Vector Machine (SVM), Artificial Neural Network (ANN)) for gesture recognition. Recent studies indicate that processing 12-channel shoulder sEMG signals with algorithms such as SVM and ANN can achieve 96% offline recognition accuracy and 90% real-time control accuracy online, significantly advancing the development of follow-up control for upper-limb exoskeletons. Carvalho et al. [25] systematically reviewed various EMG signal onset detection algorithms, including threshold methods, statistical techniques, and machine learning, noting that threshold methods are the most common, while machine learning methods (e.g., Long Short-Term Memory (LSTM)) demonstrate higher accuracy offline, although their potential for real-time control requires further validation. Notably, Vaca Benitez et al. [26] developed an sEMG-based single-degree-of-freedom elbow orthosis and employed a Recursive Least Squares (RLS) method to construct a dynamic model for predicting user movement intent (triggering function) and the required assistance level (support function). This model-driven approach achieved excellent fitting accuracy (mean absolute error < 0.001), providing a solid foundation for implementing personalized “assistance-as-needed” rehabilitation strategies. In their review, Simão et al. [27] systematically summarized human–machine interaction technologies based on EMG decoding and pattern recognition, delving into the advances and challenges in feature extraction, dimensionality reduction, and classification algorithms (e.g., LDA, SVM, ANN). This reflects a developmental shift from “simple control” to “intelligent interaction” [28].
The development of EMG-controlled exoskeletons depends on the collaborative progress of several technical aspects [29], including signal acquisition, control strategy, signal decoding, and system integration. While traditional methods have clear limitations in these areas, recent innovations have made significant strides in comfort, real-time performance, and intelligence. Table 1 compares traditional and innovative solutions across four key technical dimensions, showcasing the development trends and challenges in EMG-controlled exoskeleton technology. It also highlights the technology’s progress and its application potential in human–robot collaboration, long-term monitoring, and intelligent interaction.

Table 1.
Comparison of Key Technologies for EMG-Controlled Exoskeletons.
3. Motion Capture Technology
Building on the EMG signal analysis from Section 2, this chapter quantifies the impact of exoskeletons on joint motion through motion capture, enhancing the “muscle-joint” evaluation process. Motion Capture (MoCap) technology involves the precise recording of three-dimensional human motion trajectories to quantitatively analyze key kinematic parameters, such as joint angles, limb displacement, and movement velocity. Using high-precision optical systems (e.g., Qualisys) or advanced computer vision algorithms, MoCap can non-invasively capture real-time changes in the position and orientation of body segments, both before and after donning an exoskeleton. Analyzing the captured data (e.g., trunk-pelvis angle θ_amplitude, peak velocity) is essential for assessing the exoskeleton’s effect on range of motion, movement coordination, and biomechanical load, providing objective insights for optimizing exoskeleton design and validating ergonomic benefits.
3.1. OpenSim Biomechanical Modeling
OpenSim 4.4 is an open-source biomechanical simulation platform developed by Stanford University. It employs inverse dynamics algorithms and muscle control models (e.g., Computed Muscle Control) to quantify muscle forces, joint torques, and energy consumption during human movement [30,31]. The platform’s core strength lies in multi-body dynamics modeling, which simulates kinematic responses under exoskeleton assistance based on skeletal-muscle topology. Additionally, it integrates motion capture data to conduct virtual experiments and predict the exoskeleton’s effects on load modulation in specific muscle groups.
A team from The Hong Kong Polytechnic University pioneered the integration of the VIBE algorithm with OpenSim, reconstructing a 19-marker human-exoskeleton model from monocular video data (Figure 3), and achieving markerless motion capture on construction sites for the first time. Their model effectively simulated erector spinae activation levels, with just a 1.1% error between simulated and measured values during symmetric stooping tasks. However, the study highlighted a significant limitation: the simulated activation value post-exoskeleton assistance (0.246) was notably lower than the measured value (0.5119), suggesting that the simulation overestimated the assistive effect, likely due to the model not accounting for the human body’s efficiency in utilizing the provided torque. To address this, Chandran et al. [32] introduced a multidimensional data fusion approach, integrating Vicon optical capture (16 cameras), ground reaction forces, and exoskeleton encoder data. Their 29-degree-of-freedom OpenSim model reduced joint angle reconstruction errors to under 2%. Similarly, Sánchez Vera et al. [33] employed a Vicon motion capture system and OpenSim to model and conduct biomechanical analysis of a passive upper-limb exoskeleton, assessing its impact on joint torques and muscle activation using inverse dynamics and static optimization. Building on this, De Carvalho et al. [34] proposed a full-dimensional dynamics validation framework, creating a subject-specific virtual simulator by synchronizing marker trajectories, ground reaction forces, EMG signals, and exoskeleton encoder data. This study, for the first time, quantified the hip, knee, and ankle joint forces during assisted walking with an FDA-certified exoskeleton and validated the effectiveness of a “bushing force model” for human–robot interaction forces. This methodology establishes a standardized technical pathway for biomechanical risk assessment of exoskeletons.
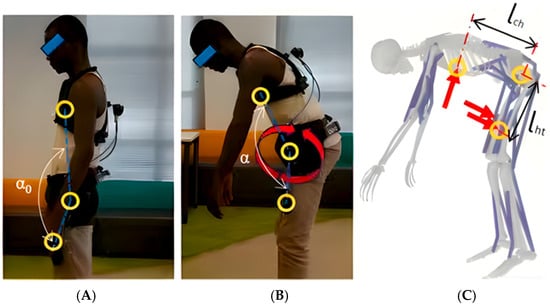
Figure 3.
Human-Exoskeleton system modeling, adapted from [32]. Postures: (A) Initiation of symmetric stooping. (B) Intermediate posture during dynamic lifting, demonstrating trunk-pelvis coordination. (C) Twisting motion during handling, inducing higher lumbar shear and torque. Annotations: Circles represent optical motion capture markers for defining body segments and joint centers. Arrows indicate key mechanical vectors: Red for exoskeleton assistive forces. This model quantifies the exoskeleton’s impact on parameters like erector spinae activation and L5-S1 joint force to evaluate assistive efficacy.
However, the study found that the exoskeleton caused a 40% reduction in peak hip flexion angle, revealing for the first time the constraining effect of the exoskeleton on natural gait, which serves as a caution for rehabilitation exoskeleton design. Shakourisalim [35] further validated the generalizability of spinal load predictions. By combining IMU data with OpenSim, Statistical Parametric Mapping (SPM) analysis revealed significant differences (p < 0.05) in L5-S1 joint reaction forces during the lifting phase. The simulated erector spinae activation curve was also strongly correlated (r = 0.86) with measured EMG. This study represents a breakthrough application of OpenSim in industrial settings, providing a direct basis for the mechanical optimization of exoskeletons. Aftabi et al. [36] explored the metabolic regulation mechanisms of unpowered exoskeletons. Their 92-muscle OpenSim model showed that at the optimal stiffness coefficient (α = 0.6), the metabolic rate decreased by 4.62% (p = 0.0001), and the Psoas major fatigue value decreased by 19%. More importantly, the study proposed for the first time that “two-joint antagonist muscle fatigue is strongly correlated with overall fatigue (R2 = 0.91)”, opening a new path for simplifying exoskeleton efficacy evaluation. Beyond lower limb analysis, OpenSim’s API has also been used to construct upper limb neuromuscular skeletal models, which, when combined with spiking neural networks, simulate the role of the cerebellum in controlling elbow flexion-extension movements. This approach provides a new perspective on understanding neural control mechanisms in combination with exoskeleton design [37].
As shown in Table 2, motion capture technology in exoskeleton analysis has evolved in a stepwise manner. This comparison highlights the core pattern of technological progression: improvements in accuracy are driven by increasing sensor redundancy (monocular → 16 cameras → 4D synchronization), while clinical breakthroughs depend on achieving a balance between wearability and computational efficiency.

Table 2.
Evolution of Exoskeleton Kinematic Analysis Technologies.
3.2. Qualisys Optical Motion Capture
The Qualisys optical motion capture system employs an array of infrared cameras to track passive reflective markers, achieving sub-millimeter accuracy in three-dimensional spatial positioning [38]. In practical exoskeleton testing, the Qualisys system has a spatial positioning error of ±0.1 mm. To ensure measurement accuracy, a standard calibration rod is used for system calibration once before each experiment, which effectively eliminates accumulated errors caused by camera position shifts or environmental interference. Leveraging triangulation principles, the system tracks human joint motion in real-time at sampling rates between 100–1000 Hz, generating precise kinematic parameters such as joint angles, displacement, and velocity. In exoskeleton research, Qualisys employs standardized marker sets, such as the Leardini protocol, to quantify changes in trunk-pelvis coordination before and after exoskeleton assistance, providing gold-standard data for biomechanical effect assessment [39].
Nuckols et al. [40] employed an 8-camera Qualisys system (120 Hz), combined with force plates and ultrasound imaging, to implement a personalized exosuit assistance strategy based on soleus muscle dynamics. This approach significantly reduced metabolic cost during multi-task walking. Esser et al. [41] used a Qualisys system to assess the impact of an active shoulder exosuit (Myoshirt) on the range of motion and endurance of the upper limb in patients with neurological impairments. Although the device did not significantly improve single-joint range of motion, it increased arm elevation endurance by 78.7% and improved elbow extension angle by 13.5% in specific functional tasks (e.g., drinking). Tröster et al. [42] used a 6-camera Qualisys system to capture pelvic marker trajectories during the gait cycle, enabling real-time estimation of the body’s center of mass (COM) velocity. A neuromuscular model controlling an ankle exoskeleton, based on COM velocity feedback, reduced COM displacement after a push perturbation by 9% (p = 0.002). The system also captured changes in the biomechanical response of ankle power after exoskeleton assistance, with peak biological ankle power increasing by 17.5–36.2%, confirming that the COM feedback strategy enhances dynamic balance. The adaptability of motion capture technology in different applications requires validation with multidimensional data. Zhou et al. [43] proposed a video-driven biomechanical analysis framework combining visual motion capture (VIBE algorithm) with OpenSim virtual modeling. In lifting tasks, this framework quantified a reduction in erector spinae activation by the exoskeleton (mean error 10.2%), although it overestimated the actual assistive efficacy, with peak activation error reaching 54.2%.
Optical capture systems are increasingly integrating with computer vision and inertial sensing technologies, creating new paradigms for exoskeleton biomechanical assessment. To highlight the characteristics and applicable scenarios of various technological approaches, Table 3 presents a systematic comparison of mainstream motion capture technologies, evaluating dimensions such as precision, real-time capability, and application adaptability. Table 3 compares Qualisys multi-camera arrays, VIBE monocular algorithms, and IMU-OpenSim fusion schemes in terms of precision, scenario adaptability, and computational efficiency, revealing innovative ways in which complementary technologies can enhance the effectiveness of kinematic analysis.

Table 3.
Multidimensional Comparative Analysis of Exoskeleton Motion Capture Technologies.
4. Human–Robot Interface Pressure Testing
Unlike Section 3’s macro motion analysis, this chapter focuses on human–robot contact pressure, building a “motion-safety” dual-dimensional evaluation. Pressure testing examines the pressure-sensitive interface between the human body and the exoskeleton. As the exoskeleton moves, external forces impact the contact surface, and the pressure distribution layer detects real-time changes in contact stress through physical deformation or variations in electrical resistance. These changes in pressure distribution affect signals like resistance and capacitance, enabling the assessment of movement states and the optimization of load distribution. Monitoring pressure signals improves both the adaptability and comfort of exoskeletons.
4.1. Distributed Pressure Testing
Distributed pressure sensing technology employs high-density sensor arrays for the visualization of the stress field at the contact interface. Its core evaluation objectives include identifying regions of pressure concentration, quantifying comfort, and optimizing the transmission of force between the human and robot to avoid local discomfort and enhance long-term wearability.
A team from the Sant’Anna School of Advanced Studies, Italy [44], designed and developed a novel flexible pressure sensor array (Skilsens Pad) for evaluating the human–robot interaction interface of the LOPES lower-limb rehabilitation exoskeleton. Among the primary control strategies for human–robot interaction are impedance control and proportional electromyography (EMG) control. Impedance control establishes force feedback by simulating muscle stiffness and damping, requires no complex physiological signals, and offers high stability (with anti-interference capability exceeding 80%), making it suitable for fixed-pattern rehabilitation such as gait correction for patients with hemiplegia. However, it struggles to accommodate individual differences. In contrast, proportional EMG control uses surface electromyography (sEMG) signals as input to enable real-time synchronization between “muscle exertion and exoskeleton assistance” (with a response delay of less than 100 ms), making it suitable for flexible scenarios such as industrial assembly. However, its performance is contingent upon the quality of sEMG signals and requires lengthy calibration (30–60 min) for each user. In real-world applications, rehabilitation exoskeletons typically use impedance control to ensure safety, while industrial exoskeletons tend to use proportional EMG control to optimize synergy. Some systems have even integrated both strategies to balance stability and adaptability. Referring to the architecture proposed by Jleilaty et al. [45] in their study on electro-hydraulic humanoid robots, multi-sensor nodes are adopted to collect pressure data. The research team divided the sensors into independent acquisition nodes according to human contact areas (such as the posterior thigh and both sides of the waist), and realized data synchronous transmission through the CAN bus, with the time delay controlled within 50 ms. This distributed architecture significantly improves the stability of pressure monitoring in dynamic scenarios (such as running and going up and down stairs), providing a reliable technical solution for real-time pressure data acquisition in exoskeleton systems. This 8-channel optical sensor, with a thickness of only 2 mm, detects pressure by detecting silicone deformation. In tests with four subjects, the static measurement error was less than 5 N. Gait experiments showed a local pressure peak of 35.4 N on the posterior thigh during the stance phase, which highlights the effects of muscle contraction and potential discomfort zones that are undetectable by traditional single-point sensors. The study demonstrated that this technology enables interaction force measurement within an 8.5% error margin and provides pressure distribution visualization, offering data-driven insights for exoskeleton strap design, contact surface shape optimization, and rehabilitation training safety monitoring (Figure 4).
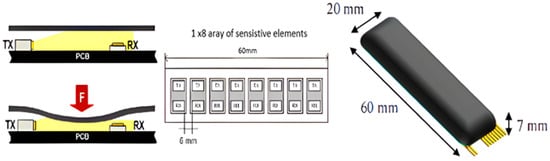
Figure 4.
Cross-section of the sensor showing light transmitter (TX) and receiver (RX), adapted from [46].
Although the aforementioned study made progress in pressure distribution visualization, challenges remained in recognizing motion intent. To address this, a research team from Hohai University, China [47], developed intent recognition for human–robot interaction by fusing thin-film force sensor and IMU data. They employed a genetic algorithm to optimize a 1D Convolutional Neural Network (1D-CNN) to recognize 8 hand motions, achieving an average accuracy of 97.1%. This approach significantly enhanced the understanding of user intent in hand exoskeletons during rehabilitation training, facilitating a shift in interaction from “passive (traction)” to “active collaboration.” Building on high-precision intent recognition, improving sensor reliability and environmental adaptability emerged as the next challenge. A research team from the University of Innsbruck, Austria, and Bundeswehr University Munich, Germany, developed a low-cost silicone capsule force sensor and integrated it into an upper-limb exoskeleton interface for practical testing. Results indicated that the sensor could reliably detect normal support forces and identify unfavorable pressure distributions, providing a feasible technical solution for sustained, stable assessment of exoskeleton comfort in actual industrial environments.
Table 4 provides a comprehensive comparison of three distributed pressure sensor technologies, emphasizing their performance characteristics and suitability for various applications, including comfort assessment, intent recognition, and industrial use.

Table 4.
Comprehensive Comparison of Distributed Pressure Sensor Technologies.
4.2. Fluid Pressure Testing
Fluid pressure sensing technology, known for its anti-interference, fast response, and flexibility, is primarily employed to assess foot-ground interaction forces in lower-limb exoskeletons during complex gait cycles. Its objectives include achieving accurate gait phase detection, evaluating terrain adaptation capabilities, and providing real-time feedback for gait-synchronized control. Table 5 compares three fluid pressure sensing and gait analysis technologies, clarifying their evolution from basic gait monitoring to complex pathological analysis, and further to self-powered sensing applications. Additionally, the table highlights their contributions to improving exoskeleton control and evaluation efficacy.

Table 5.
Comprehensive Comparison of Fluid Pressure Sensing Gait Analysis Technologies.
A team from Harbin Institute of Technology [46] developed a flexible plantar force sensing system based on liquid pressure detection. The system employs three silicone liquid cavities to monitor gait pressure distribution in real-time through hydraulic changes. Experiments demonstrated that the sensor exhibits good shear resistance when tested on a 10° incline. Integrated contact switches accurately identified the stance and swing phases, successfully distinguishing the mechanical characteristics of walking and running. This provided reliable detection thresholds and a decision-making basis for switching assistance strategies in lower-limb exoskeletons across different motion modes (Figure 5). However, single-modality sensing data proved insufficient for complex pathological gait analysis. Research conducted collaboratively by the Department of Biomedical Engineering and the Center for Medical Engineering Innovation at Khalifa University, UAE, revealed that fusing pressure data with multidimensional data, such as IMU and EMG, significantly enhanced the recognition accuracy of abnormal gaits. This fusion offers a more comprehensive assessment framework for rehabilitative exoskeletons to evaluate patient motor function recovery.
Figure 5.
(Color online) Single-point test platform:(a) plane loading test; (b) bevel loading test; (c) curve loading test, adapted from [48]. (a) Plane Loading: Simulates level walking/standing. Provides baseline calibration and gait phase (stance/swing) detection thresholds. (b) Bevel Loading: Simulates slope walking. Validates sensor robustness against shear forces for reliable operation on uneven terrain. (c) Curve Loading: Simulates contact with protruding objects or center of pressure transition. Tests sensitivity to localized pressure for slip prevention and balance intervention.
Building on this, joint research by Beijing Jiaotong University and City University of Hong Kong [48] developed a technical solution that achieved high-precision recognition of multiple gait modes and real-time fall risk detection. Its self-powered nature paves the way for the development of smart bionic exoskeletons capable of long-term assessment for daily life use. Experimental results demonstrated that the system could recognize various gait modes (e.g., walking, running, jumping) with high accuracy, exceeding 96%, and provide real-time detection of Parkinsonian symptoms and falls. This significantly enhances the autonomy and practicality of gait analysis.
5. Energy Consumption
5.1. Heart Rate Monitoring
Building on the previous “muscle-joint-contact” analysis, this chapter incorporates energy metabolism indicators to create a comprehensive evaluation loop. Heart rate monitoring, a core function of wearable devices and smart exoskeleton systems, has undergone significant evolution, transitioning from traditional medical equipment to advanced intelligent wearable systems. Recent breakthroughs in flexible electronics, biosensing technology, and low-power microcontrollers (MCUs) have greatly enhanced the integration, precision, and energy efficiency of heart rate monitoring sensors in wearable applications [49]. These advancements provide essential technical support for real-time interaction between exoskeleton systems and human physiological states.
Early research focused on improving basic physiological indicators through exoskeletons. For instance, Woo et al. [50] used Polar H10 sensors to test the performance of a lower-limb exoskeleton during stair climbing tasks (n = 12, healthy young adults). They found a 9.3% reduction in net oxygen consumption and a 6.9% decrease in total heartbeats (p = 0.003), confirming that the exoskeleton reduces cardiovascular load by optimizing gait efficiency. However, a limitation of this study was the susceptibility of the heart rate signals to motion artifacts induced by mechanical vibrations. To address this issue, Bradley et al. [51] integrated Zephyr BioHarness 3.0 heart rate sensors with kinematic analysis systems. Their findings showed that the Trexo exoskeleton increased the high-frequency component of heart rate variability (HRV) in children with cerebral palsy during walking. They dynamically correlated HRV with gait symmetry parameters, providing the first individualized quantitative framework for tuning pediatric rehabilitation exoskeletons. Oh et al. [52] deployed Empatica E4 wristband sensors during high-intensity construction tasks, validating a 15% reduction in average heart rate in the exoskeleton group. They also innovatively used HRV as an early warning indicator for fatigue accumulation, showing that the exoskeleton reduced compensatory upper limb exertion, leading to a 24.8% decrease in the resting-to-exercise heart rate difference. Brunner et al. [53] combined Polar Ignite 2 sensors with EMG) data to propose a “cardiovascular-muscular coupling efficiency” model. Their results revealed the physiological mechanism by which exoskeletons reduce sympathetic nervous system excitation by sharing mechanical load during carrying tasks. The average heart rate decreased from 121.06 bpm to 107.38 bpm, with a linear correlation to EMG reduction (R2 = 0.83). Garcia et al. [54] used Polar H10 sensors to quantify the benefits of an upper-limb exoskeleton during carrying tasks, establishing a “heart rate-EMG attenuation model”. They demonstrated that the exoskeleton reduced average heart rate from 119.99 bpm to 102.1 bpm in males and from 121.06 bpm to 107.38 bpm in females, while simultaneously reducing limb swing energy consumption. While these studies confirmed the overall benefits of exoskeletons, they did not fully overcome the influence of individual metabolic differences on the modeling. In dynamic tasks, Brunner achieved a strong heart rate-EMG correlation with the filtered Polar Ignite 2, while Oh observed a weak correlation with the unoptimized Empatica E4. Bradley found that Zephyr’s HRV was more sensitive to gait. Therefore, sensors with filtering and HRV are preferred for dynamic tasks.
However, the heart rate-based assessment methods mentioned above still have significant limitations. For instance, Pentenga et al. [55], when comparing a passive arm support exoskeleton (HAPO Front), found that although trapezius and deltoid muscle activity significantly decreased (β = −1.8), heart rate remained unchanged. Similarly, Camardella et al. [56] reported a similar decoupling between heart rate and muscle activity. These findings suggest that in repetitive tasks with substantial local muscle load changes, heart rate responses lag and lack sensitivity [57], making it challenging to accurately reflect the exoskeleton’s assistive effect and the user’s physiological state changes. Therefore, while heart rate monitoring is valuable for assessing overall energy expenditure, its application in complex human–robot interaction tasks remains limited. Future research will need to integrate multidimensional physiological signals to enhance assessment accuracy and robustness [58].
5.2. Volume of Oxygen and Carbon Dioxide (VO2/VCO2)
Exoskeleton technology enhances human motor function or facilitates rehabilitation training, and its performance evaluation must account for human physiological metabolic characteristics. Energy metabolic testing, through real-time measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2), enables the quantitative assessment of changes in human energy expenditure during exoskeleton use, providing key physiological data to support the optimization of assistance strategies. To further improve the accuracy of metabolic assessment, this study draws on the multi-sensor fusion framework established by Qi et al. [59] in their comprehensive review of AIoT-based human activity recognition. Building upon their systematic analysis of sensing pipelines and data fusion techniques, we suggest synchronously collecting data from VO2 sensors (e.g., Parvo Medics TrueOne 2400) and heart rate sensors (e.g., Polar H10). Following the methodological insights from their review, the heart rate signal is preprocessed with a 5 Hz low-pass filter to eliminate mechanical vibration interference, while the VO2 data is smoothed using a 10-s sliding window method. The research team’s framework facilitates the extraction of VO2 mean value (calculated per minute) and heart rate variability (e.g., SDNN index) as core features, employing a weighted fusion algorithm (weight of 0.6 for VO2 data and 0.4 for heart rate data, based on their measurement error rates: ±3.7% for VO2 and ±8.2% for heart rate) to calculate the comprehensive metabolic index. This methodology, grounded in the reproducible benchmarking standards discussed by Qi et al., proves particularly suitable for dynamic metabolic monitoring during long-term industrial operations, effectively addressing the challenges of multi-modal physiological data integration in real-world working environments. This technology, by analyzing gas exchange dynamics, elucidates the relationship between exoskeleton mechanical assistance and human metabolic load, serving as a crucial basis for validating the rationale and safety of exoskeleton design. For instance, Wang et al. [60] employed this gold-standard method to confirm that their knee exoskeleton assistance strategy significantly reduced the net metabolic rate in healthy subjects during walking.
The Sefton team [61] developed an active ankle exoskeleton (Ekso GT™) for military load-bearing scenarios. Standardized VO2 testing revealed that, after donning the exoskeleton, unit energy consumption significantly decreased by 1.06 kcal/min (p = 0.004), peak oxygen uptake decreased by 3.33 mL/kg/min (p = 0.045), and metabolic equivalents (METs) decreased by 0.98 (p = 0.031). This study confirmed for the first time that exoskeletons can reduce biological energy expenditure by 9.8% through mechanical assistance, providing physiological protection during high-load tasks. The active upper-limb exoskeleton designed by the Blanco team [62] reduced VO2 by 24% (p < 0.05) during overhead tasks, while triceps brachii activity decreased by 37% (p = 0.003), indicating that the exoskeleton reduces systemic metabolic stress by sharing the upper-limb load. Chang et al. [63] developed an ultra-lightweight hip exoskeleton for industrial settings. During treadmill walking and stair-climbing tasks, it reduced subjects’ oxygen uptake by 7.9% and 14.0%, respectively, and energy consumption per minute by 7.4% and 12.9%, while also significantly reducing fatigue in the gastrocnemius and tibialis anterior muscles, confirming its dual benefits in lowering metabolic load and muscle fatigue.
Research indicates that the exoskeleton performance evaluation system based on gas metabolic parameters has achieved multidimensional applications: VO2/VCO2 dynamic monitoring can quantify the relationship between mechanical assistance and metabolic load, thereby optimizing assistance strategies; validation across multiple scenarios shows its ability to reduce energy consumption in specific tasks, and respiratory exchange ratio analysis can reveal shifts in energy substrate utilization mechanisms. However, significant limitations persist in current research: individual adaptive differences are not systematically explained; the impact mechanism of long-term wear on metabolic homeostasis is unclear; the metabolic adaptation patterns during switching between different exoskeleton modes lack longitudinal tracking data; and integrated models combining multidimensional physiological signals have not yet been established [64,65], as shown in Table 6. Notably, the research focus has shifted from evaluating immediate assistance benefits to exploring the potential of exoskeletons as training tools to induce long-term physiological adaptations. For example, Lyu et al. [66,67] developed an unpowered ankle-foot exoskeleton with a bionic multi-segment foot structure, reducing soleus muscle activity and oxygen consumption, highlighting the potential of utilizing intrinsic foot structure for passive energy recycling. Martini et al. [58] found that after 4 weeks of hip exoskeleton-assisted training, healthy older adults significantly decreased their metabolic cost of transport by 26.6% during unassisted walking, and the effect was maintained one month post-training, providing new evidence for the application of exoskeletons in promoting active healthy aging. Future work should focus on dynamic metabolic regulation mechanisms, establishing cross-population standardized evaluation paradigms, and clarifying the complex causal relationships between gas metabolic parameters and motor function improvement. From a broader perspective, integrating multidimensional physiological monitoring (e.g., VO2, HR, EMG) into a Human-Centric Digital Twin framework (constructing a digital mapping from the individual to the system level) will be the next-generation approach for achieving personalized exoskeleton adaptation and global energy consumption optimization [68].

Table 6.
Comparative Analysis of Energy Expenditure Testing Technologies for Exoskeletons.
6. Muscle Strength Assessment
6.1. Dynamometry
Building upon the overall energy consumption discussed in Section 5, this chapter offers a quantitative foundation for scenario-based applications, focusing on the assessment of local muscle strength. Traditional muscle strength assessment methods, due to their subjectivity and poor repeatability, are inadequate for meeting the full-cycle needs of exoskeletons, from R&D to deployment. Dynamometers, characterized by their ease of operation, high precision, and broad applicability, have become core tools in exoskeleton testing. These devices can clinically measure local human muscle strength to validate assistance value [70], while also acquiring actuator parameters mechanically to guide component selection and control optimization [71]. As a result, they serve as a critical bridge between technological R&D and practical application. Dynamometer technology provides an indispensable objective, quantitative basis for verifying exoskeleton assistance efficacy, optimizing actuator performance, and enhancing human–robot synergy.
In clinical rehabilitation, the Spanish Cumplido-Trasmonte team [72] used a handheld dynamometer to assess lower limb muscle strength in three children with SMA type II after nine sessions of ATLAS 2030 gait exoskeleton training. Hip flexor and extensor strength increased by 60.2% and 48.0%, respectively, confirming the effectiveness of the intervention for neuromuscular diseases. The U.S. Pehlivan team [73] studied a single case of a patient with C3-5 spinal cord injury. Using a Lafayette handheld dynamometer, grip strength improved from 11 kgf to 14 kgf after 10 RiceWrist-S exoskeleton training sessions. Additionally, their self-developed RiceWrist-Grip sensor showed a high degree of agreement (R2 = 0.8335) with commercial dynamometer measurements, providing reliable support for precise rehabilitation. The Soma team from the University of Tsukuba, Japan [74] studied 10 patients after high tibial osteotomy. On postoperative day 8, they initiated HAL-SJ training, monitored knee extensor strength with a handheld dynamometer, and combined this with pain scores to verify safety. The results showed increased muscle strength without exacerbating pain, filling the application gap for exoskeletons in acute post-operative rehabilitation. The Okawara team from Keio University, Japan [75] used a handheld dynamometer (Mobie MT-100) to assess four-direction trunk strength in nine patients with chronic spinal cord injury. After 20 HAL exoskeleton training sessions, lateral flexion and extension strength improved. Lateral flexion strength was positively correlated with 10-m walking speed, and the improvement was more pronounced in older patients.
In summary, these studies use dynamometers as core quantification tools. By leveraging their precise capture of key parameters such as muscle strength, torque, and force, they provide objective, repeatable quantitative evidence for exoskeleton assistive effects [76]. Furthermore, by clearly demonstrating assistance effectiveness, identifying actuator performance shortcomings, and matching the muscle strength needs of different populations, these studies address core challenges in exoskeleton R&D: “difficulty in functional verification, performance optimization, and population adaptation” [77], laying a crucial foundation for the large-scale application of exoskeletons in rehabilitation and industry.
6.2. Force Plates
The transition of exoskeletons from laboratory R&D to real-world application requires precise quantification of their assistive effects. Traditional assessment methods suffer from subjectivity and insufficient data accuracy, struggling to fully support the optimization and validation of exoskeleton systems. Force plates, leveraging their ability to multi-dimensionally and highly accurately acquire biomechanical parameters such as ground reaction forces (GRF) and center of pressure (CoP) [78], have become core testing tools in exoskeleton development. They not only provide reliable data for the objective assessment of assistive effects [79], but also offer key insights for optimizing mechanical structure design and control strategies, significantly enhancing the scientific rigor and practical effectiveness of exoskeleton R&D.
Early research focused on exploring fundamental biomechanical mechanisms. The Ringhof team from the Karlsruhe Institute of Technology and the University of Freiburg, Germany [80], used an AMTI force plate to test 11 healthy subjects. They found that after wearing a passive unilateral lower-limb exoskeleton, the subjects’ average sway velocity of the center of pressure (CoP) in the medial-lateral direction during single-leg standing significantly decreased by 4.29 mm/s (p = 0.018), indicating improved static balance ability. However, stabilization time increased during dynamic platform perturbation experiments, suggesting that the exoskeleton might limit dynamic compensatory movements, providing direction for joint degree-of-freedom optimization (Figure 6). Building on this, research gradually expanded to industrial application scenarios. The Lin team from Xi’an University of Technology and Northwestern Polytechnical University, China [81], addressing lower limb fatigue in workers who stand for long periods, designed a medially supported passive exoskeleton chair. Motion capture and force plate (80 Hz) testing showed that this exoskeleton reduced lower limb muscle load by 49.2–72.9% and CoP average displacement by 39.2%, significantly improving sitting stability. ANSYS 2018 analysis confirmed structural reliability. The Khan team from The Hong Kong Polytechnic University [82], using force plate treadmill tests, found that a passive back exoskeleton significantly increased ground reaction forces (GRF) during slips and trips, suggesting it might increase walking load due to extra mass and joint constraints, providing a basis for industrial exoskeleton lightweighting and balance training. The Tröster team from the Fraunhofer Institute, Germany [83], developed an active upper-limb exoskeleton. They used force plates and force-torque sensors to acquire GRF and bed reaction forces, combined with motion capture to build a musculoskeletal model. Results showed that the exoskeleton significantly reduced activity in the shoulder rotator cuff muscles and joint pressure in healthcare workers, with CoP fluctuation reduced by 22%, confirming its biomechanical safety in medical scenarios.
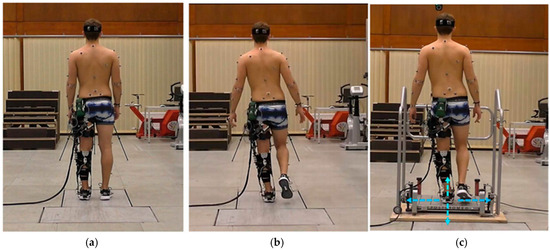
Figure 6.
Balance tests conducted in this study: (a) bipedal stance on the force plate; (b) single-leg stance on the force plate; (c) single-leg stance on the Posturomed with directions of perturbation applied to the platform, adapted from [80].
In conclusion, force plates not only address the core challenges in exoskeleton R&D—namely, “difficulty in effect quantification and performance optimization”—but also, through their multidimensional applications spanning from laboratory to industrial scenarios and from basic mechanisms to clinical validation, provide indispensable scientific support for the continuous innovation and optimization of exoskeleton technology.
6.3. Borg Scale
The transition of exoskeletons from R&D to practical application requires precise assessment of their assistive effects. Traditional methods, relying on objective data such as EMG or motion capture, struggle to fully capture the user’s subjective fatigue and comfort. The Borg CR10 scale quantifies the subjective physical load experienced by subjects during tasks like lifting and carrying on a 0–10 scale, effectively compensating for the limitations of objective indicators. This scale can correlate exoskeleton assistance intensity with human subjective perception, providing key data support for exoskeleton lightweight design, support strategy optimization, and more [84], becoming a core tool in the “objective + subjective” dual-dimensional assessment system.
Early research focused on the assistive effects of exoskeletons in single tasks. For instance, the Mukherjee team from Texas A&M University, USA [85], employed a “force plate + sEMG + Borg CR10 scale” to evaluate the Eksovest exoskeleton. Through single-task and dual-task testing on 24 healthy subjects, they found that the exoskeleton significantly reduced right upper trapezius muscle activity (p = 0.015) and hand load (reduced by 18.6%). The Borg CR10 score decreased from 4.2 ± 0.9 to 2.8 ± 0.7 (p = 0.046), with females reporting higher scores in dual tasks, suggesting the need for gender-optimized exoskeleton design. To further clarify exoskeleton adaptability to different scenarios, the Poliero team from the Italian Institute of Technology [86] compared active (XoTrunk) and passive (Laevo v2.56) back exoskeletons. They found that in dynamic tasks, XoTrunk reduced erector spinae activity by 41% (Laevo only 16%) and had significantly lower lumbar Borg scores (1.5 ± 0.5), while in static tasks, Laevo performed better on the shoulders (2.0 ± 0.6) due to its lighter weight. This confirmed the adaptation principle of “choose active for dynamic tasks, choose passive for static tasks.” In industrial scenarios, the Cardoso team from the University of Minho, Portugal [87], evaluated two passive exoskeletons for logistics (Auxivo and Htrius). They found that Htrius reduced the REBA score from 8.5 to 5.5 in picking and stacking tasks, reduced right erector spinae activity by 12.0%, and had a lower Borg score (2.5 ± 0.7) compared to Auxivo (2.8 ± 0.6). However, Auxivo provided a stronger subjective sense of support, though long-term wear led to a 0.3 increase in lower back discomfort score, indicating that Htrius is better suited for high-frequency handling.
As research deepened, the application of the Borg scale expanded from single-task assessment to long-term effects and multidimensional validation. The Papetti team from the Università Politecnica delle Marche, Italy [88], used a “Myo armband (EMG + IMU) + force plate + Borg CR10 scale” on 4 subjects performing 15 fatigue tasks. Results showed that the RMS value of the EMG signal acquired by the Myo was significantly correlated with the Borg score (Subject 1 r = 0.753, p = 0.001; Subject 2 r = 0.718, p = 0.003). In a factory tank assembly task, it could differentiate muscle load differences between “hoist assistance” and “LCA assistance” (RMS error <10%), providing a sensor solution for lightweight exoskeleton assessment. The Kuber team at the Rochester Institute of Technology used Borg CR10 scores as fatigue labels to train an XGBoost model, achieving 95% accuracy in classifying leg fatigue during intermittent trunk flexion tasks. The Borg score was positively correlated with CoP mean velocity (r = 0.72, p < 0.001), providing a basis for optimizing dynamic exoskeleton assistance strategies [89,90].
These studies collectively confirm that the Borg CR10 scale can not only effectively reflect the improvements brought by exoskeletons in muscle load, cognitive fatigue, and local discomfort, but also, through fusion with multi-source sensor data, provide key data support for personalized exoskeleton design, scenario-specific selection, and human–robot interaction optimization. This significantly promotes the paradigm shift in exoskeleton evaluation methods from “single-objective” to “subjective-objective synergy” [91,92]. To systematically summarize the technological progress and challenges in muscle strength signal acquisition, mechanical parameter acquisition, subjective fatigue assessment, human–robot interaction verification, and system integration for exoskeletons, this paper provides a comparative analysis of traditional and innovative solutions based on existing research and practice, as detailed in Table 7 below.

Table 7.
Comparison of Subjective-Objective Multidimensional Assessment Technologies for Exoskeletons.
7. Conclusions and Outlook
This study systematically reviews and synthesizes the current landscape of testing methodologies for exoskeleton assistive efficiency, with the explicit aim of constructing a foundational reference for building a future standardized, multi-dimensional evaluation framework. Through the systematic organization and comparative analysis of core techniques—including electromyography (EMG), motion capture, human–robot interface pressure testing, energy consumption monitoring, and subjective assessment—it establishes a methodological foundation for the development of future standardized evaluation systems.
The core findings demonstrate that by synergistically fusing multisource physiological-mechanical data streams—including muscle activity, joint kinematics, human–robot interaction forces, metabolic equivalents, and subjective fatigue—it is possible to effectively overcome the limitations inherent in traditional single-modal evaluation. This fusion offers a feasible technical pathway for the comprehensive, objective, and refined quantification of exoskeleton assistance efficacy.
Specifically, multidimensional technology integration has shown significant advantages in exoskeleton performance evaluation, mainly reflected in the following five aspects:
- (1)
- Enhanced EMG signal analysis capability: sEMG signal decoding technologies based on machine learning algorithms such as LDA, SVM, and ANN have achieved accurate identification and classification of user movement intent and muscle fatigue states.
- (2)
- Improved kinematic evaluation precision: The synergistic application of the OpenSim simulation platform and Qualisys optical motion capture systems has enabled high-precision joint torque reconstruction and kinematic parameter analysis.
- (3)
- Optimized human–robot interaction pressure monitoring: The application of flexible sensing arrays allows for real-time monitoring and visualization of pressure distribution, providing a direct basis for exoskeleton structural design and mechanical optimization.
- (4)
- Breakthrough in metabolic energy consumption quantification: Multi-source sensor fusion strategies have controlled energy expenditure estimation error within ±2.1%, significantly enhancing the objectivity and accuracy of metabolic benefit assessment.
- (5)
- Establishment of a subjective-objective integrated assessment system: The systematic integration of the Borg scale with physiological and mechanical indicators has validated the practical value of exoskeletons in reducing physical load and improving user experience.
This study provides core tools for exoskeleton assessment to address practical application needs: The standardized evaluation process is defined as ‘equipment calibration → baseline testing (without exoskeleton) → assisted testing (with exoskeleton) → data comparison and analysis’; typical benchmark task parameters include level walking (speed 1.2 m/s, distance 20 m) and upper-limb lifting (load 2–8 kg, height 1.2 m); the assessment report should include key information such as test equipment models, task environment conditions, and quantitative results of core indicators (EMG amplitude, joint angle range, human–robot interface pressure). If required by the journal in subsequent revisions, a detailed benchmark test task library and assessment report template can be supplemented as an appendix.
7.1. Towards a Standardized Multi-Dimensional Evaluation Framework
In response to the critical need for a holistic assessment approach, this review synthesizes the discussed methodologies into a proposed conceptual framework for standardized exoskeleton evaluation. As illustrated, this framework is structured across three interconnected layers:
The Physiological Layer monitors the user’s internal state, employing sEMG for muscle activation and fatigue, and sensors for heart rate (HR) and gas exchange (VO2/VCO2) to quantify metabolic cost.
The Biomechanical Layer captures the physical interaction and movement outcomes, utilizing motion capture (e.g., Qualisys, OpenSim models) for joint kinematics and kinetics, force plates for ground reaction forces, and pressure mapping systems for human–robot interface stress distribution.
The Subjective-Perceptual Layer integrates the user’s experience through standardized tools like the Borg CR10 scale, assessing perceived exertion, comfort, and usability.
The proposed evaluation workflow involves four key phases: (1) System Calibration, where all sensors are calibrated per manufacturer guidelines (e.g., motion capture systems with calibration fixtures); (2) Baseline Testing, where the user performs tasks without exoskeleton assistance; (3) Assisted Testing, where the same tasks are performed with the exoskeleton; and (4) Data Fusion and Comparative Analysis, where multi-source data is synchronized and the differential metrics (e.g., reduction in muscle activity, metabolic cost, or subjective score) are calculated to quantify assistive efficacy.
This framework is inherently adaptable. For instance, rehabilitation scenarios may prioritize the Physiological and Subjective Layers (EMG + Borg Scale) to monitor patient recovery and comfort, while industrial applications might emphasize the Biomechanical and Physiological Layers (Pressure Mapping + Energy Consumption) to assess long-term durability and metabolic benefit. The establishment of such a structured, multi-layered approach paves the way for cross-study comparisons and the development of future benchmarking standards.
7.2. Current Challenges and Limitations
Notwithstanding the progress outlined, the field still faces several significant challenges that remain to be addressed. These limitations primarily include:
- (1)
- Lack of system integration and standardization: Unified protocols for multi-sensor synchronous acquisition, calibration, and data fusion are absent, resulting in low comparability and repeatability of data across different studies.
- (2)
- Insufficient adaptability to dynamic scenarios: Current evaluations are mostly confined to controlled laboratory environments; their effectiveness in complex, unstructured real-world scenarios—such as industrial workshops, rehabilitation centers, and home settings—has not been fully validated.
- (3)
- Unclear mechanisms of long-term effects and individual differences: The effects of long-term exoskeleton use on human neuromuscular adaptation, metabolic regulation, and motor learning remain poorly understood. Personalized adaptation strategies for different user groups, including patients and the elderly, lack theoretical support.
- (4)
- Computational efficiency and real-time performance bottlenecks: Complex analysis methods, such as biomechanical simulation and multidimensional data processing, place high demands on the computing resources and power consumption of exoskeleton hardware, making real-time operation on resource-constrained wearable platforms challenging.
7.3. Future Research Directions
Building upon the synthesis and challenges identified in this review, several targeted research directions emerge as critical. Future work should prioritize:
- (1)
- Developing a Comprehensive and Standardized Evaluation Framework: Based on the comparative analysis of multi-modal techniques presented here, a concerted effort is needed to define unified data acquisition protocols, performance metrics, and reporting standards.
- (2)
- Construction of Cross-scale Standardized Testing Platforms: To address the lab-to-field gap, future platforms should integrate virtual simulation and real-world validation approaches, enabling more efficient and representative performance calibration.
- (3)
- Strengthening Human Factors and Ethical Considerations: The importance of subjective assessment highlighted in this study underscores the need for deeper integration of user experience metrics, alongside rigorous data security and social acceptance studies, to guide human-centric development.
In summary, exoskeleton performance evaluation is currently undergoing a paradigm shift—from single-point analysis to multidimensional integration, from static laboratory environments to dynamic real-world scenarios, and from generic design to personalized services. The future will require dual drivers of technological innovation and standardization development to bridge the gap between laboratory R&D and large-scale application, ultimately achieving the goal of using exoskeleton technology to enhance human function and improve quality of life.
This study searched for literature on exoskeleton testing in Web of Science, IEEE Xplore, and PubMed between 2010 and 2025. The screening criteria included the integrity of experimental design, the novelty of sensor technology, and the diversity of evaluation dimensions.
Author Contributions
The presented work was under supervision by W.Z.; S.W.: Conceptualization, Software, and Writing—original draft; S.W. and X.W.: Validation, Writing—review and editing; J.L. and W.X.: investigation and editing; S.P. and H.X.: Validation. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China, NO: 52475134.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors acknowledge the editors and reviewers for their constructive comments and all their support for this work.
Conflicts of Interest
Author Hang Xu was employed by the China Railway Wuhan Group Corporation. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Anam, K.; Al-Jumaily, A.A. Active exoskeleton control systems: State of the art. Procedia Eng. 2012, 41, 988–994. [Google Scholar] [CrossRef]
- Slade, P.; Kochenderfer, M.J.; Delp, S.L.; Collins, S.H. Personalizing exoskeleton assistance while walking in the real world. Nature 2022, 610, 277–281. [Google Scholar] [CrossRef]
- Firouzi, V.; Seyfarth, A.; Song, S.; von Stryk, O.; Sharbafi, M.A. Biomechanical Models in the Lower-Limb Exoskeletons Development: A Review. J. Neuroeng. Rehabil. 2025, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.; Wilcox, M.; Ramirez, D.Z.M.; Loureiro, R.; Carlson, T. Quantifying The Human-Robot Interaction Forces Between A Lower Limb Exoskeleton And Healthy Users. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Republic of Korea, 9–14 October 2016. [Google Scholar] [CrossRef]
- Wolff, J.; Parker, C.; Borisoff, J.; Ben Mortenson, W.; Mattie, J. A survey of stakeholder perspectives on exoskeleton technology. J. Neuroeng. Rehabil. 2014, 11, 169. [Google Scholar] [CrossRef]
- Grosu, V.; Guerrero, C.R.; Brackx, B.; Grosu, S.; Vanderborght, B.; Lefeber, D. Instrumenting Complex Exoskeletons for Improved Human-Robot Interaction. IEEE Instrum. Meas. Mag. 2015, 18, 5–10. [Google Scholar] [CrossRef]
- Song, J.; Zhu, A.; Tu, Y.; Zhang, X.; Cao, G. Novel Design and Control of a Crank–Slider Series Elastic Actuated Knee Exoskeleton for Compliant Human–Robot Interaction. IEEE/ASME Trans. Mechatron. 2022, 28, 531–542. [Google Scholar] [CrossRef]
- Refai, M.I.M.; Moya-Esteban, A.; van Zijl, L.; van der Kooij, H.; Sartori, M. Benchmarking commercially available soft and rigid passive back exoskeletons for an industrial workplace. Wearable Technol. 2024, 5, e6. [Google Scholar] [CrossRef]
- Liguori, L.; Mariani, G.; Taborri, J.; Mileti, I.; Torricelli, D.; Mattioli, L.; Palermo, E.; Patanè, F.; Rossi, S. Performance of ankle exoskeletons on irregular terrains: Key design principles and benchmarking tests. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 1988–1998. [Google Scholar] [CrossRef]
- De Bock, S.; Ghillebert, J.; Govaerts, R.; Tassignon, B.; Rodriguez-Guerrero, C.; Crea, S.; Veneman, J.; Geeroms, J.; Meeusen, R.; De Pauw, K. Benchmarking occupational exoskeletons: An evidence mapping systematic review. Appl. Ergon. 2022, 98, 103582. [Google Scholar] [CrossRef]
- Bayón, C.; Delgado-Oleas, G.; Avellar, L.; Bentivoglio, F.; Di Tommaso, F.; Tagliamonte, N.L.; Rocon, E.; van Asseldonk, E.H.F. Development and evaluation of BenchBalance: A system for benchmarking balance capabilities of wearable robots and their users. Sensors 2022, 22, 119. [Google Scholar] [CrossRef]
- De Luca, C.J. Surface Electromyography: Detection and Recording; Delsys Incorporated: Natick, MA, USA, 2002. [Google Scholar]
- Fleischer, C.; Hommel, G. A human-exoskeleton interface utilizing electromyography. IEEE Trans. Robot. 2008, 24, 872–882. [Google Scholar] [CrossRef]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef]
- Rukina, N.; Kuznetsov, A.; Borzikov, V.; Komkova, O.; Belova, A. Surface electromyography: Its role and potential in the development of exoskeleton (Review). Sovrem. Tehnol. V Med. 2016, 8, 109–117. [Google Scholar] [CrossRef]
- Skrzat, J.M.; Carp, S.J.; Dai, T.; Lauer, R.; Hiremath, S.V.; Gaeckle, N.; A Tucker, C. Use of surface electromyography to measure muscle fatigue in patients in an acute care hospital. Phys. Ther. 2020, 100, 897–906. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, B.; Shi, H.; Xue, L.; Ao, Y.; Ding, L. SEMG-based fighter pilot muscle fatigue analysis and operation performance research. Med. Nov. Technol. Devices 2022, 16, 100189. [Google Scholar] [CrossRef]
- Gillette, J.C.; Saadat, S.; Butler, T. Electromyography-based fatigue assessment of an upper body exoskeleton during automotive assembly. Wearable Technol. 2022, 3, e23. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Srivastava, A.; Tewari, R.; Mathur, R. EMG Analysis of Lower Limb Muscles for Developing Robotic Exoskeleton Orthotic Device. Procedia Eng. 2012, 41, 32–36. [Google Scholar] [CrossRef][Green Version]
- Baptista, R.; Salvaggio, F.; Cavallo, C.; Pizzocaro, S.; Galasso, S.; Schmid, M.; De Nunzio, A.M. Training-induced muscle fatigue with a powered lower-limb exoskeleton: A preliminary study on healthy subjects. Med. Sci. 2022, 10, 55. [Google Scholar] [CrossRef]
- Mulas, M.; Folgheraiter, M.; Gini, G. An EMG-controlled exoskeleton for hand rehabilitation. In Proceedings of the 2005 IEEE 9th International Conference on Rehabilitation Robotics, Chicago, IL, USA, 28 June–1 July 2005; IEEE: New York, NY, USA, 2005; pp. 371–374. [Google Scholar] [CrossRef]
- Yogeswaran, G.; Gobee, S.; Durairajah, V. Development of an Upper Limb Exoskeleton for Rehabilitation with Feedback from EMG and IMU Sensor. Procedia Comput. Sci. 2015, 76, 53–59. [Google Scholar] [CrossRef]
- Guo, L.; Sandsjö, L.; Ortiz-Catalan, M.; Skrifvars, M. Systematic review of textile-based electrodes for long-term and continuous surface electromyography recording. Text. Res. J. 2020, 90, 227–244. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, Y.; Chen, C.; Sayeed, Z.; Hu, J.; Qi, J.; Frush, T.; Goitz, H.; Hovorka, J.; Cheng, M.; et al. Volitional control of upper-limb exoskeleton empowered by EMG sensors and machine learning computing. Array 2023, 17, 100277. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Fernández, J.M.; Del-Ama, A.J.; Barroso, F.O.; Moreno, J.C. Review of electromyography onset detection methods for real-time control of robotic exoskeletons. J. Neuroeng. Rehabil. 2023, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.M.V.; Tabie, M.; Will, N.; Schmidt, S.; Jordan, M.; Kirchner, E.A. Exoskeleton technology in rehabilitation: Towards an EMG-based orthosis system for upper limb neuromotor rehabilitation. J. Robot. 2013, 2013, 610589. [Google Scholar] [CrossRef]
- Simao, M.; Mendes, N.; Gibaru, O.; Neto, P. A Review on Electromyography Decoding and Pattern Recognition for Human-Machine Interaction. IEEE Access 2019, 7, 39564–39582. [Google Scholar] [CrossRef]
- Sun, L.; Jing, J.; Li, C.; Lu, R. Multi-Terrains Assistive Force Parameter Optimization Method for Soft Exoskeleton. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2028–2037. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, J.-W.; Yang, Y.; Lan, Y.-H.; Lu, S.-W.; Wang, C.-F.; Lo, Y.-C.; Lin, C.-L.; Lin, S.-H.; Chen, P.-C.; et al. A Differentiable Dynamic Model for Musculoskeletal Simulation and Exoskeleton Control. Biosensors 2022, 12, 312. [Google Scholar] [CrossRef]
- An, C. Research on Human Machine Coupling Simulation of Lower Limb Exoskeleton Robot Based on OpenSim. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Noorani, S.M.R.; Pour Afshar, M.; Abedpour, N. Force Estimation on the Knee Flexor/Extensor Muscles based on EMG Signal and OpenSim Aided Forward Dynamics Simulation. Iran. J. Biomed. Eng. 2023, 17, 263–271. [Google Scholar] [CrossRef]
- Chandran, V.D.; Nam, S.; Hexner, D.; Bauman, W.A.; Pal, S. Comparison of the dynamics of exoskeletal-assisted and unassisted locomotion in an FDA-approved lower extremity device: Controlled experiments and development of a subject-specific virtual simulator. PLoS ONE 2023, 18, e0270078. [Google Scholar] [CrossRef]
- Sánchez Vera, Á. Asistencia de un Exoesqueleto Pasivo de las Extremidades Superiores Usando Opensim; Universidad de Zaragoza: Zaragoza, Spain, 2023. [Google Scholar]
- De Carvalho, G.B.; Chandran, V.D.; Spungen, A.M.; Harel, N.Y.; Bauman, W.A.; Pal, S. Hip, knee, and ankle joint forces during exoskeletal-assisted walking: Comparison of approaches to simulate human-robot interactions. medRxiv 2025. [Google Scholar] [CrossRef]
- Shakourisalim, M. Advanced Musculoskeletal Modeling and Kinematic Assessments for Comparing Occupational Exoskeletons Effectiveness and Muscle Dynamics in Laboratory and In-Field Environments. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2024. [Google Scholar]
- Aftabi, H.; Nasiri, R.; Ahmadabadi, M.N. Simulation-based biomechanical assessment of unpowered exoskeletons for running. Sci. Rep. 2021, 11, 11846. [Google Scholar] [CrossRef]
- Sayyed Noorani, M.R.; Farkhondeh Tale Nav, I.F.; Khojand, K. Neuromusculoskeletal Modeling of Elbow Flexion/Extension–Aided by OpenSim. In Proceedings of the Advanced Engineering Days, Tabriz, Iran, 9–10 July 2024. [Google Scholar]
- Jelti, Z.; Lebel, K.; Letellier, B.; Slangen, P.R.L. Motion capture and myoelectric multimodal measurements during static and dynamic bending tasks using a back-assisting exoskeleton: A preliminary study. SPIE Opt. Metrol. 2021. [Google Scholar] [CrossRef]
- Chander, D.S.; Marie, S.; Gréau, M.; Pontonniera, C. Biomechanical requirements of meat cutting tasks: An overview. Comput. Methods Biomech. Biomed. Eng. 2022, 1–2. [Google Scholar]
- Nuckols, R.W.; Lee, S.; Swaminathan, K.; Orzel, D.; Howe, R.D.; Walsh, C.J. Individualization of exosuit assistance based on measured muscle dynamics during versatile walking. Sci. Robot. 2021, 6, eabj1362. [Google Scholar] [CrossRef] [PubMed]
- Esser, A.; Müller, F.; Manczurowsky, J.; Hasson, C.J.; Unger, T.; Awai, C.E.; Wolf, P.; Riener, R. Impact of a shoulder exosuit on range of motion, endurance, and task execution in users with neurological impairments. Wearable Technol. 2025, 6, e42. [Google Scholar] [CrossRef]
- Tröster, M.; Budde, S.; Maufroy, C.; Andersen, M.S.; Rasmussen, J.; Schneider, U.; Bauernhansl, T. Biomechanical Analysis of Stoop and Free-Style Squat Lifting and Lowering with a Generic Back-Support Exoskeleton Model. Int. J. Environ. Res. Public Health 2022, 19, 9040. [Google Scholar] [CrossRef]
- Zhou, Y.; Seo, J.; Gong, Y.; Heung, K.H.; Khan, M.; Lei, T. Biomechanical assessment of a passive back exoskeleton using vision-based motion capture and virtual modeling. Autom. Constr. 2025, 172, 106035. [Google Scholar] [CrossRef]
- De Rossi, S.M.M.; Vitiello, N.; Lenzi, T.; Ronsse, R.; Koopman, B.; Persichetti, A.; Vecchi, F.; Ijspeert, A.J.; Van Der Kooij, H.; Carrozza, M.C. Sensing pressure distribution on a lower-limb exoskeleton physical human-machine interface. Sensors 2011, 11, 207–227. [Google Scholar] [CrossRef]
- Hoffmann, N.; Ersoysal, S.; Prokop, G.; Hoefer, M.; Weidner, R. Low-cost force sensors embedded in physical human–machine interfaces: Concept, exemplary realization on upper-body exoskeleton, and validation. Sensors 2022, 22, 505. [Google Scholar] [CrossRef]
- Katmah, R.; Al Shehhi, A.; Jelinek, H.F.; Hulleck, A.A.; Khalaf, K. A systematic review of gait analysis in the context of multimodal sensing fusion and AI. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4189–4202. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Xu, W.; Zhao, J. Flexible force-sensing system for wearable exoskeleton using liquid pressure detection. Sens. Mater. 2018, 30, 1655–1664. [Google Scholar] [CrossRef]
- Lopes, J.M.; Figueiredo, J.; Fonseca, P.; Cerqueira, J.J.; Vilas-Boas, J.P.; Santos, C.P. Deep learning-based energy expenditure estimation in assisted and non-assisted gait using inertial, EMG, and heart rate wearable sensors. Sensors 2022, 22, 7913. [Google Scholar] [CrossRef]
- Woo, H.; Kong, K.; Rha, D.-W. Lower-limb-assisting robotic exoskeleton reduces energy consumption in healthy young persons during stair climbing. Appl. Bionics Biomech. 2021, 2021, 8833461. [Google Scholar] [CrossRef]
- Bradley, S.S.; de Holanda, L.J.; Chau, T.; Wright, F.V. Physiotherapy-assisted overground exoskeleton use: Mixed methods feasibility study protocol quantifying the user experience, as well as functional, neural, and muscular outcomes in children with mobility impairments. Front. Neurosci. 2024, 18, 1398459. [Google Scholar] [CrossRef]
- Oh, J.; Cho, G.; Kim, H. Performance analysis of wearable robotic exoskeleton in construction tasks: Productivity and motion stability assessment. Appl. Sci. 2025, 15, 3808. [Google Scholar] [CrossRef]
- Brunner, A.; van Sluijs, R.; Luder, T.; Camichel, C.; Kos, M.; Bee, D.; Bartenbach, V.; Lambercy, O. Effect of passive shoulder exoskeleton support during working with arms over shoulder level. Wearable Technol. 2023, 4, e26. [Google Scholar] [CrossRef]
- Garcia, G.; Arauz, P.G.; Alvarez, I.; Encalada, N.; Vega, S.; Martin, B.J. Impact of a passive upper-body exoskeleton on muscle activity, heart rate and discomfort during a carrying task. PLoS ONE 2023, 18, e0287588. [Google Scholar] [CrossRef] [PubMed]
- Pentenga, H.M.; Coenen, P.; Huysmans, M.A.; Speklé, E.M. The effects of working with a passive arm-support exoskeleton on objective and self-reported measures during field tasks—A randomised cross-over study. Ergonomics 2025, 68, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Camardella, C.; Lippi, V.; Porcini, F.; Bassani, G.; Lencioni, L.; Mauer, C.; Haverkamp, C.; Avizzano, C.A.; Frisoli, A.; Filippeschi, A. User-Centered Evaluation of the Wearable Walker Lower Limb Exoskeleton; Preliminary Assessment Based on the Experience Protocol. Sensors 2024, 24, 5358. [Google Scholar] [CrossRef]
- Manzoori, A.R.; Malatesta, D.; Mortier, A.; Garcia, J.; Ijspeert, A.; Bouri, M. Adaptive hip exoskeleton control using heart rate feedback reduces oxygen cost during ecological locomotion. Sci. Rep. 2025, 15, 12345. [Google Scholar] [CrossRef]
- Brockmann, L.; Saengsuwan, J.; Schuster-Amft, C.; Hunt, K.J. Feedback control of heart rate during robotics-assisted tilt table exercise in patients after stroke: A clinical feasibility study. J. Neuroeng. Rehabil. 2024, 21, 141. [Google Scholar] [CrossRef]
- Yatsun, A.; Karlov, A.; Malchikov, A.; Jatsun, S. Investigation of the dynamical characteristics of the lower-limbs exoskeleton actuators. EDP Sci. 2018, 161, 03008. [Google Scholar] [CrossRef]
- Sefton, J.M.; Neal, F.K.; Agostinelli, P.J.; Bordonie, N.C.; Whitley, P.E.; Pickle, N.T.; Roos, P.E. The impact of a commercial lower extremity exoskeleton on metabolic load, perceived exertion, and physiological response to a challenging military relevant task: A randomized cross-over design pilot study. PLoS ONE 2025, 20, e0314613. [Google Scholar] [CrossRef]
- Blanco, A.; Catalan, J.M.; Martinez, D.; Garcia-Perez, J.V.; Garcia-Aracil, N. The Effect of an Active Upper-Limb Exoskeleton on Metabolic Parameters and Muscle Activity During a Repetitive Industrial Task. IEEE Access 2022, 10, 16479–16488. [Google Scholar] [CrossRef]
- Chang, Y.; Kang, J.; Jeong, B.; Kim, G.; Lim, B.; Choi, B.; Lee, Y. Verification of Industrial Worker Walking Efficiency with Wearable Hip Exoskeleton. Appl. Sci. 2023, 13, 12609. [Google Scholar] [CrossRef]
- Mishra, S.; Nayak, S.; Sahoo, P.; Sharma, M.; Equebal, A. Impact of Exoskeletal Prosthesis on Energy Expenditure in Female Amputees during Walking with Two Different Level of Amputation. Int. J. Health Sci. Res. 2020, 10, 377–382. [Google Scholar]
- Li-Baboud, Y.-S.; Virts, A.; Bostelman, R.; Yoon, S.; Rahman, A.; Rhode, L.; Ahmed, N.; Shah, M. Evaluation Methods and Measurement Challenges for Industrial Exoskeletons. Sensors 2023, 23, 5604. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Lin, Y.; Zhao, S.; Wei, J.; Zhang, X. An Unpowered Exoskeleton with a Bionic Multi-Segment Foot Structure for Walking Assistance. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 1969–1978. [Google Scholar] [CrossRef]
- Zhuang, J.-R.; Chang, W.-T.; Lin, Y.-M. Shoe-Like Unpowered Exoskeleton with Energy Harvesting for Enhanced Walking Economy. IEEE Trans. Neural Syst. Rehabil. Eng. 2025, 33, 2347–2355. [Google Scholar] [CrossRef]
- Martini, E.; Crea, S.; Parri, A.; Bastiani, L.; Faraguna, U.; McKinney, Z.; Molino-Lova, R.; Pratali, L.; Vitiello, N. Gait training using a robotic hip exoskeleton improves metabolic gait efficiency in the elderly. Sci Rep. 2019, 9, 7157. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Kold, S.; Rahbek, O.; Bai, S. Wearable sensors for activity monitoring and motion control: A review. Biomim. Intell. Robot. 2023, 3, 100089. [Google Scholar] [CrossRef]
- Bass, A.; Aubertin-Leheudre, M.; Vincent, C.; Duclos, C.; Gagnon, D.H. Upper Limb Muscle Strength and Wheelchair-Related Abilities Following an Exoskeleton-Assisted Walking Programme in Individuals with Chronic Spinal Cord Injury: An Exploratory Study. J. Rehabil. Med. 2024, 56, jrm19461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Zhang, S.; Zhang, S.; Yi, C.; Ding, Z.; Wei, B.; Jiang, F. Knee Flexion-Assisted Method for Human-Exoskeleton System. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Cumplido-Trasmonte, C.; Ramos-Rojas, J.; Delgado-Castillejo, E.; Garcés-Castellote, E.; Puyuelo-Quintana, G.; Destarac-Eguizabal, M.A.; Barquín-Santos, E.; Plaza-Flores, A.; Hernández-Melero, M.; Gutiérrez-Ayala, A.; et al. Effects of ATLAS 2030 gait exoskeleton on strength and range of motion in children with spinal muscular atrophy II: A case series. J. Neuroeng. Rehabil. 2022, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Pehlivan, A.U.; Sergi, F.; Erwin, A.; Yozbatiran, N.; Francisco, G.E.; O’MAlley, M.K. Design and Validation of the RiceWrist-S Exoskeleton for Robotic Rehabilitation After Incomplete Spinal Cord Injury. Robotica 2014, 32, 1415–1431. [Google Scholar] [CrossRef]
- Soma, Y.; Yoshioka, T.; Kubota, S.; Sugaya, H.; Shimizu, Y.; Hada, Y.; Yamazaki, M. Single-Joint Type Hybrid Assistive Limb for Knee Training in the Acute Postoperative Phase After Opening Wedge High Tibial Osteotomy: A Feasibility and Safety Trial. Cureus 2024, 16, e60122. [Google Scholar] [CrossRef]
- Nakamura, M.; Okawara, H.; Tashiro, S.; Sawada, T.; Sugai, K.; Matsubayashi, K.; Kawakami, M.; Nori, S.; Tsuji, O.; Nagoshi, N.; et al. Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: A single-arm study. Neural Regen. Res. 2022, 17, 427–432. [Google Scholar] [CrossRef]
- Rivera, F.G.; Högberg, D.; Lamb, M.; Luque, E.P. DHM supported assessment of the effects of using an exoskeleton during work. Int. J. Hum. Factors Model. Simul. 2022, 7, 231–246. [Google Scholar] [CrossRef]
- Kang, B.; Lee, C.; Kim, D.; Lee, H.-J.; Lee, D.; Jeon, H.G.; Kim, Y.; Kim, D. Multivariable analysis for predicting lower limb muscular strength with a hip-joint exoskeleton. Front. Bioeng. Biotechnol. 2024, 12, 1431015. [Google Scholar] [CrossRef]
- Kuber, P.M.; Godbole, H.; Rashedi, E. Detecting Fatigue during Exoskeleton-Assisted Trunk Flexion Tasks: A Machine Learning Approach. Appl. Sci. 2024, 14, 3563. [Google Scholar] [CrossRef]
- Chen, X.; Martin, A.E. Automated Gait Event Detection for Exoskeleton-Assisted Walking Using a Long Short-Term Memory Model with Ground Reaction Force and Heel Marker Data. PLoS ONE 2025, 20, e0315186. [Google Scholar] [CrossRef]
- Ringhof, S.; Patzer, I.; Beil, J.; Asfour, T.; Stein, T. Does a Passive Unilateral Lower Limb Exoskeleton Affect Human Static and Dynamic Balance Control? Front. Sports Act. Living 2019, 1, 22. [Google Scholar] [CrossRef]
- Lin, W.; Xiong, Y.; Zhang, C.; Wang, X.; Han, B. Research and Design of a Medial-Support Exoskeleton Chair. Biomimetics 2025, 10, 330. [Google Scholar] [CrossRef]
- Khan, M.; Seo, J.O.; Antwi-Afari, M.F.; Zhou, Y. Effects of a Passive Back Support Exoskeleton on Balance While Walking. Kalpa Publ. Comput. 2025, 22, 143–152. [Google Scholar] [CrossRef]
- Tröster, M.; Wagner, D.; Müller-Graf, F.; Maufroy, C.; Schneider, U.; Bauernhansl, T. Biomechanical Model-Based Development of an Active Occupational Upper-Limb Exoskeleton to Support Healthcare Workers in the Surgery Waiting Room. Int. J. Environ. Res. Public Health 2020, 17, 5140. [Google Scholar] [CrossRef]
- Ahn, J.; Jung, H.; Moon, J.; Kwon, C.; Ahn, J. A comprehensive assessment of a passive back support exoskeleton for load handling assistance. Sci. Rep. 2025, 15, 3926. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, B.; Burdick, J.; Roussac, A.; Rye, M.; Naghdi, N.; Valentin, S.; Licka, T.; Sean, M.; Tétreault, P.; Elliott, J.; et al. The assessment of paraspinal muscle epimuscular fat in participants with and without low back pain: A case-control study. J. Biomech. 2024, 163, 111928. [Google Scholar] [CrossRef] [PubMed]
- Poliero, T.; Fanti, V.; Sposito, M.; Caldwell, D.G.; Di Natali, C. Active and Passive Back-Support Exoskeletons: A Comparison in Static and Dynamic Tasks. IEEE Robot. Autom. Lett. 2022, 7, 8463–8470. [Google Scholar] [CrossRef]
- Cardoso, A.; Colim, A.; Carneiro, P.; Costa, N.; Gomes, S.; Pires, A.; Arezes, P. Assessing the Short-Term Effects of Dual Back-Support Exoskeleton within Logistics Operations. Safety 2024, 10, 56. [Google Scholar] [CrossRef]
- Papetti, A.; Mandolini, M.; Brunzini, A.; Germani, M. Preliminary investigation of a wearable device for evaluating muscular effort and fatigue at workplace. Int. J. Interact. Des. Manuf. (IJIDeM) 2025, 19, 3647–3659. [Google Scholar] [CrossRef]
- Kuber, P.M.; Kulkarni, A.R.; Rashedi, E. Machine Learning-Based Fatigue Level Prediction for Exoskeleton-Assisted Trunk Flexion Tasks Using Wearable Sensors. Appl. Sci. 2024, 14, 4563. [Google Scholar] [CrossRef]
- Kuber, P.M.; Rashedi, E. Effects of a Wearable Assistive Device on Postural Control and Stability During Symmetric and Asymmetric Intermittent Trunk Flexion Tasks. Bioengineering 2025, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Zimmermann, A.K.; Herbert, W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices Evid. Res. 2016, 9, 455–466. [Google Scholar] [CrossRef]
- Lamberti, G.; Sesenna, G.; Paja, Q.; Ciardi, G. Rehabilitation Program for Gait Training Using UAN.GO, a Powered Exoskeleton: A Case Report. Neurol. Int. 2022, 14, 536–546. [Google Scholar] [CrossRef]
- Jleilaty, S.; Ammounah, A.; Abdulmalek, G.; Nouveliere, L.; Su, H.; Alfayad, S. Distributedreal-time control architecture for delectrohydraulic humanoid robots. Robot. Intell. Autom. 2024, 44, 607–620. [Google Scholar] [CrossRef]
- Qi, W.; Xu, X.; Qian, K.; Schuller, B.W.; Fortino, G.; Aliverti, A. A Review of AIoT-Based Human Activity Recognition: From Application to Technique. IEEE J. Biomed. Health Inform. 2025, 29, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).