Effects of Operating Parameters on Combustion Characteristics of Hydrogen-Doped Natural Gas
Abstract
1. Introduction
2. Modeling and Numerical Methods
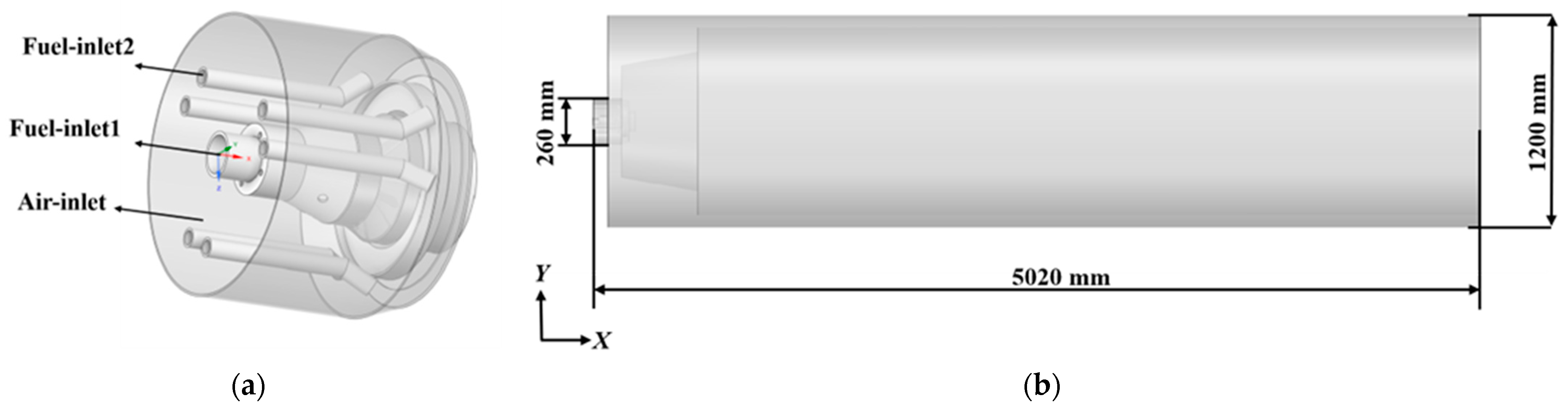
2.1. Physical Model and Computational Domain
2.2. Numerical Methods, Model Validation, and Reliability
2.3. Boundary Conditions and Numerical Setup
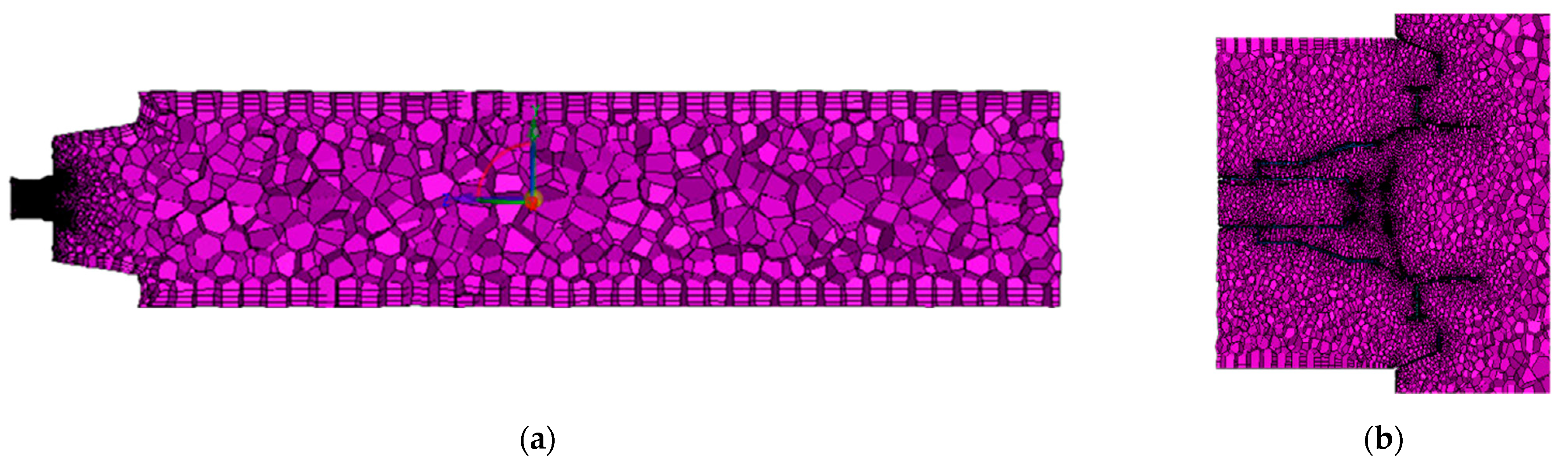
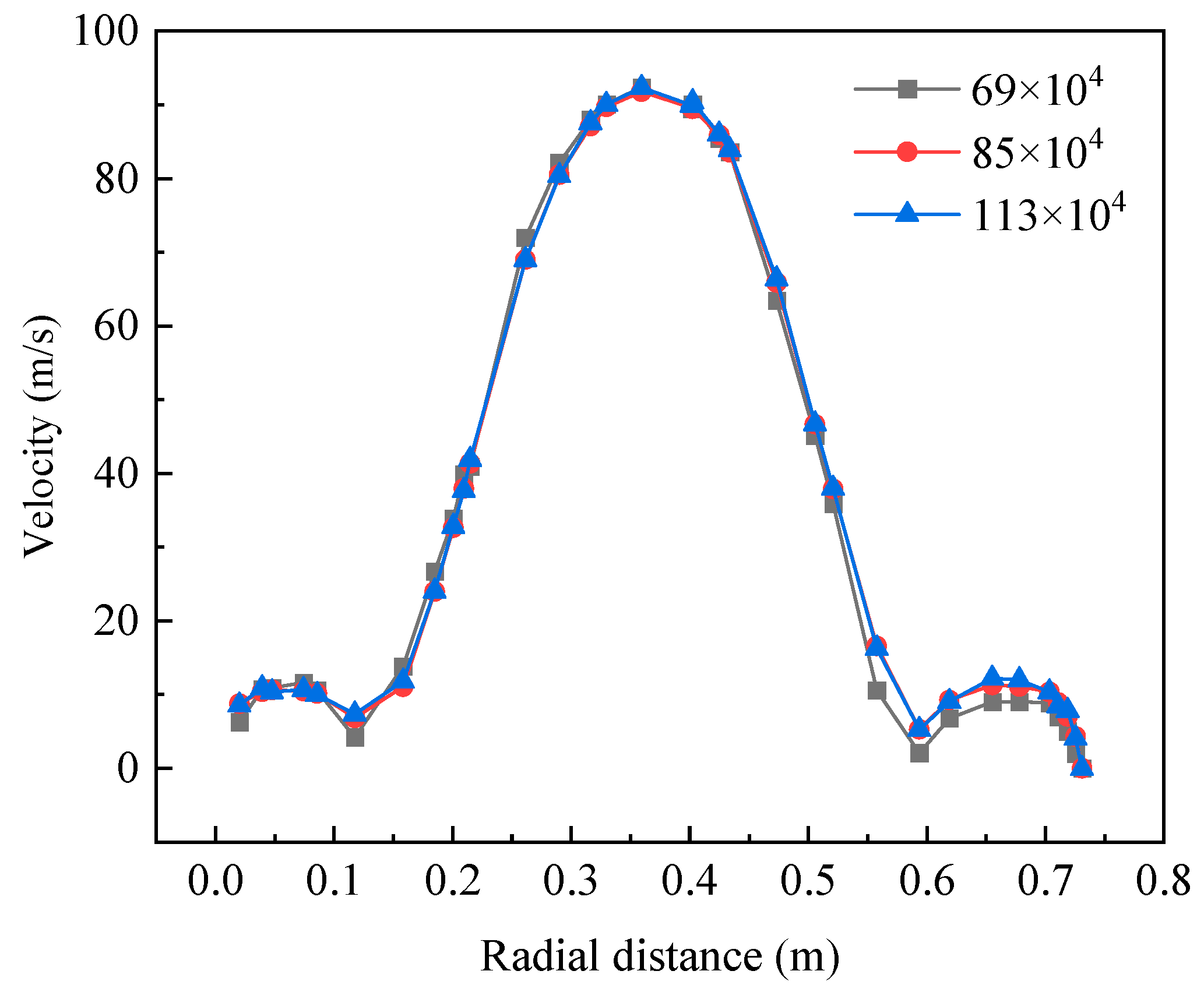
2.4. Meshing and Grid Independence Study
2.5. Data Processing and Analysis
2.5.1. Data Acquisition and Pre-Processing
2.5.2. Calculation of Key Results
2.5.3. Repeatability and Computational Conditions
3. Simulation Results and Analysis
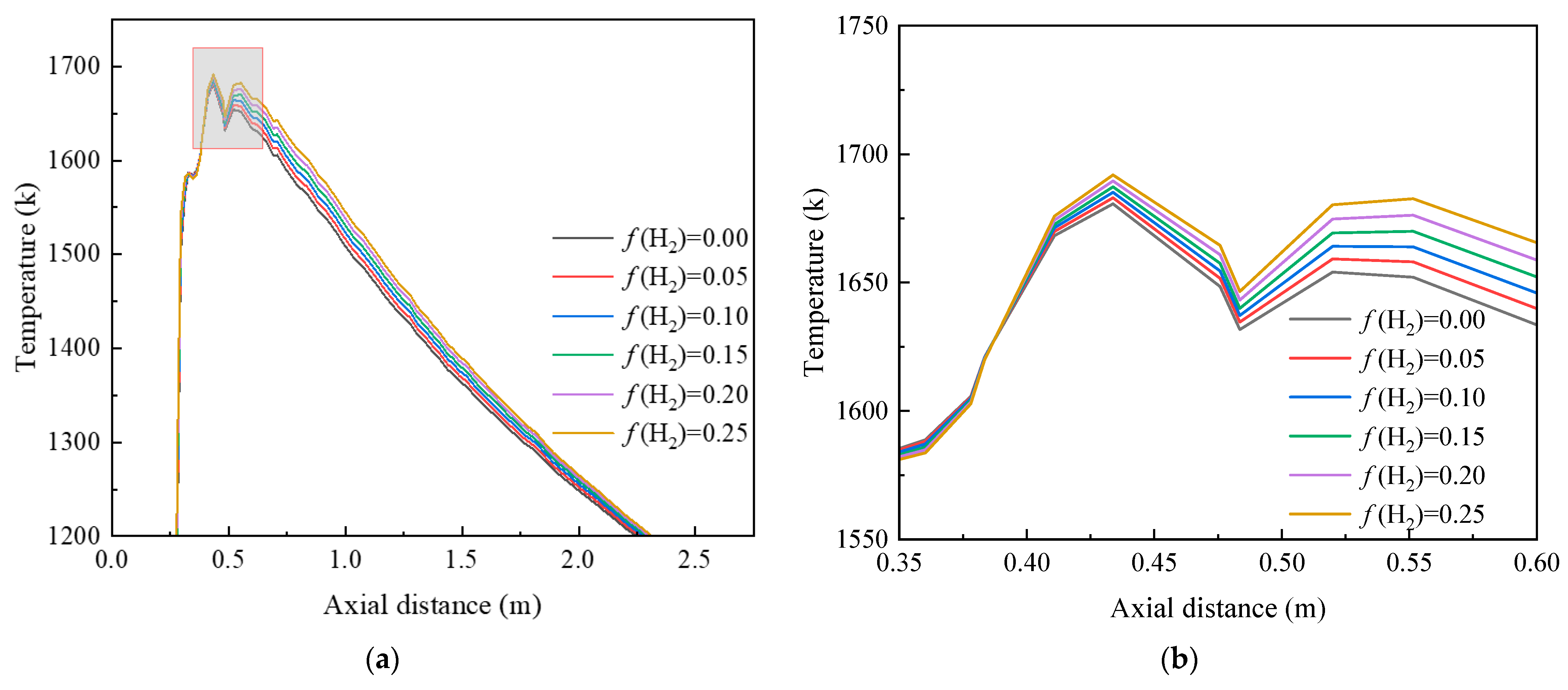
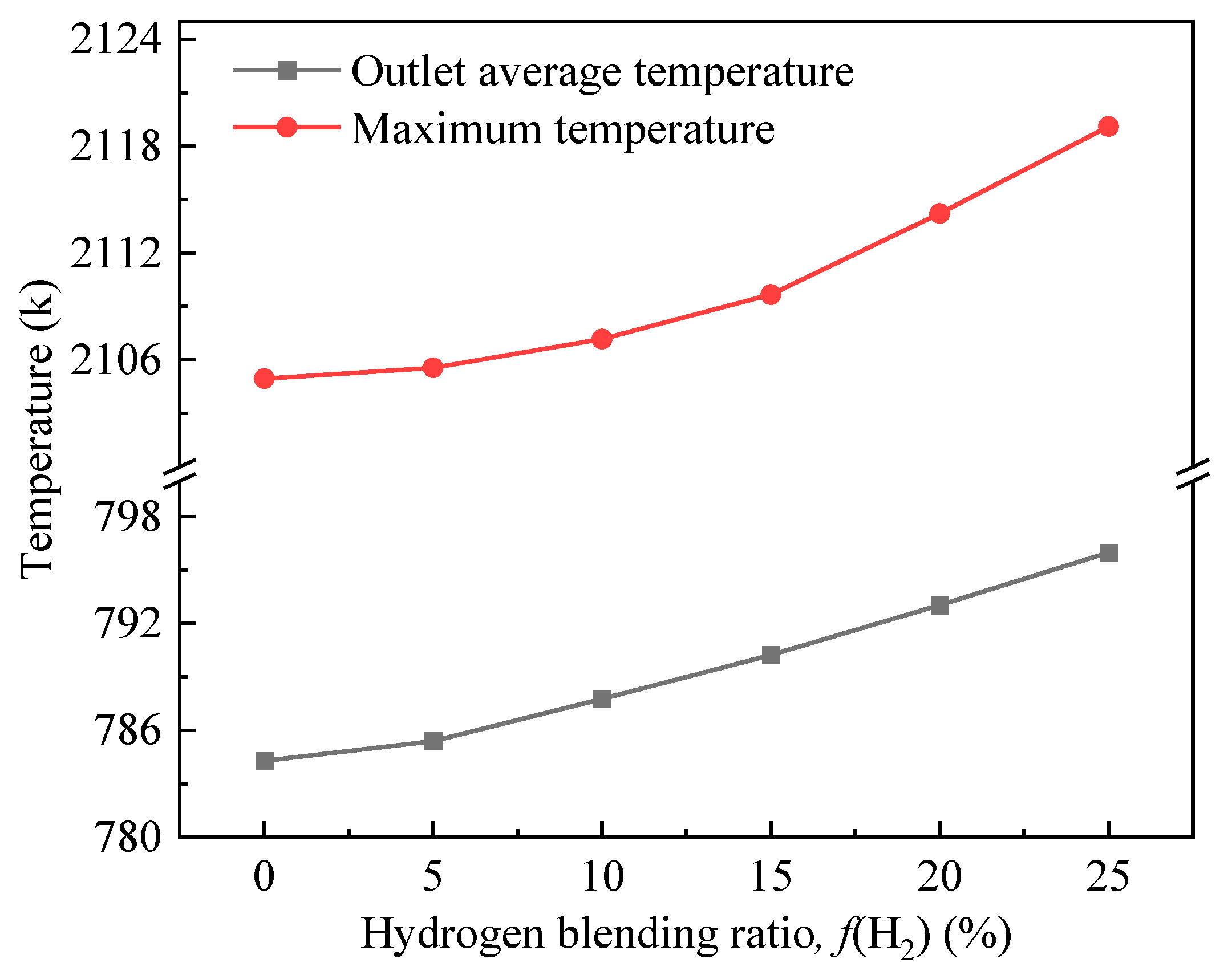
3.1. Effect of the Hydrogen-Doping Ratio on the Combustion Process of the Burner
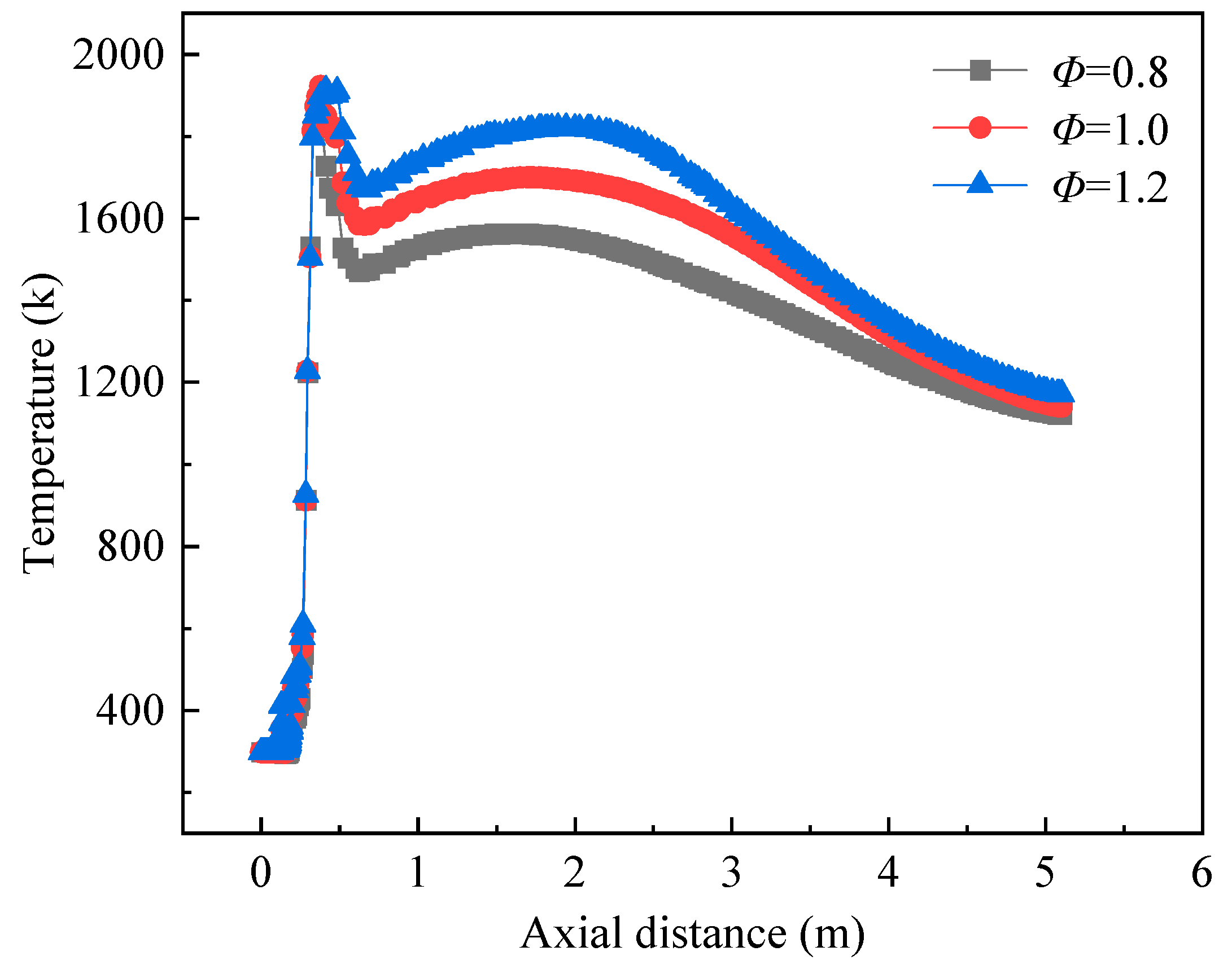
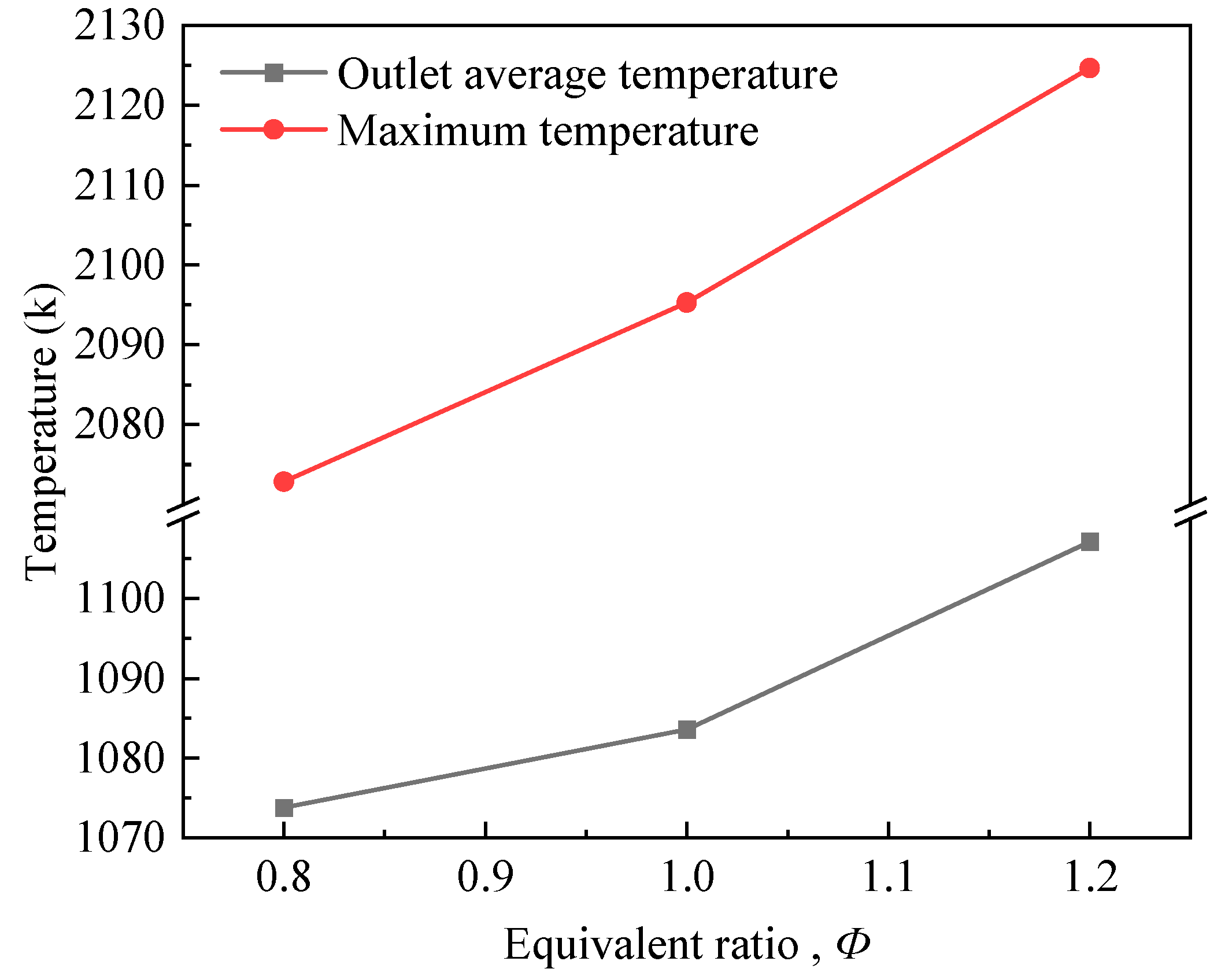
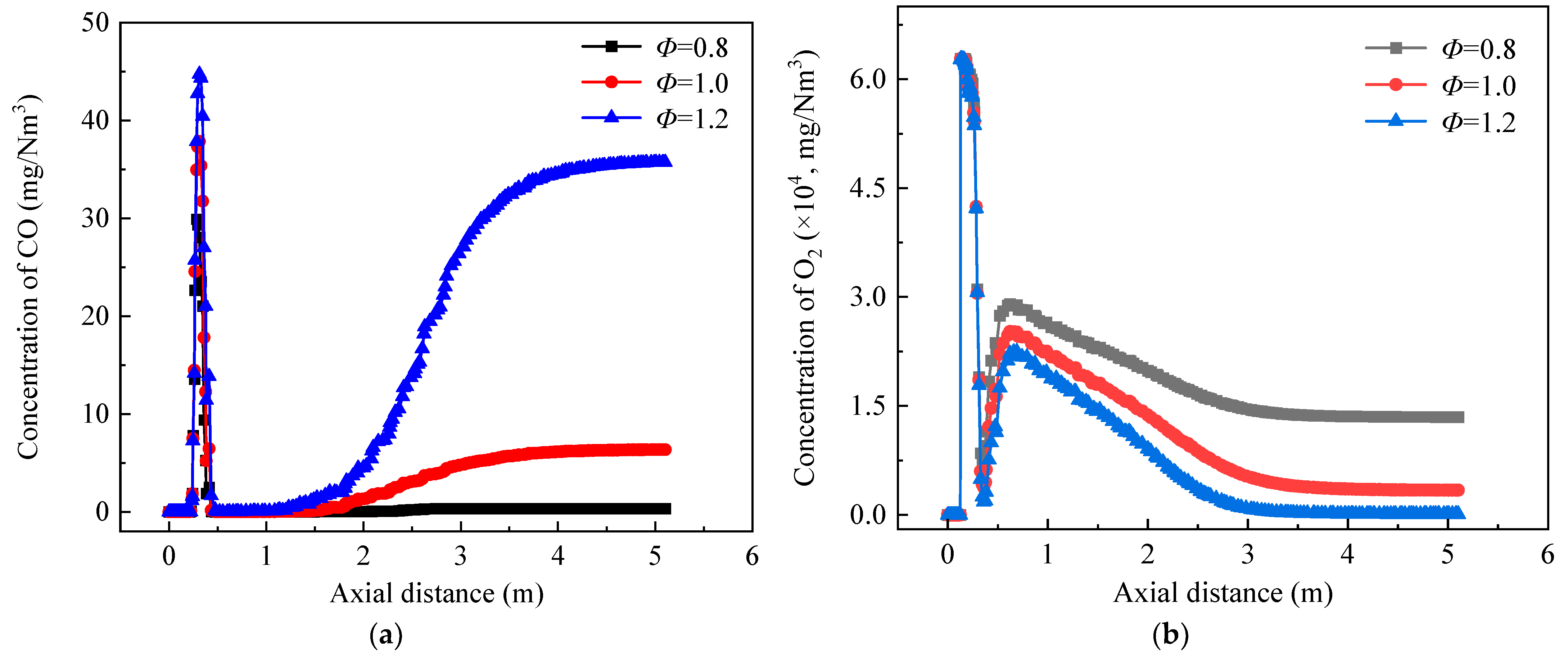
3.2. Effect of Equivalence Ratio on Burner Combustion Processes
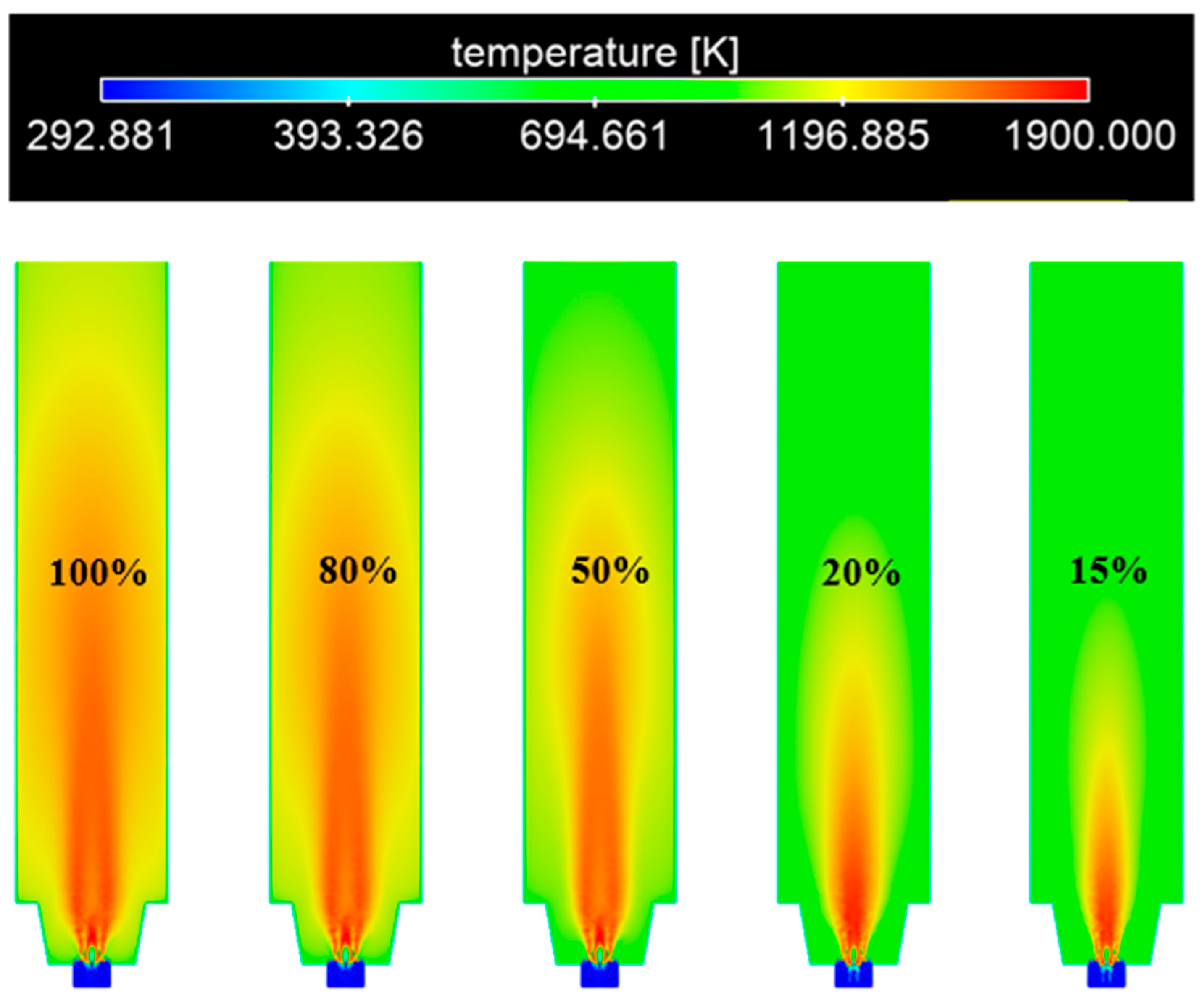
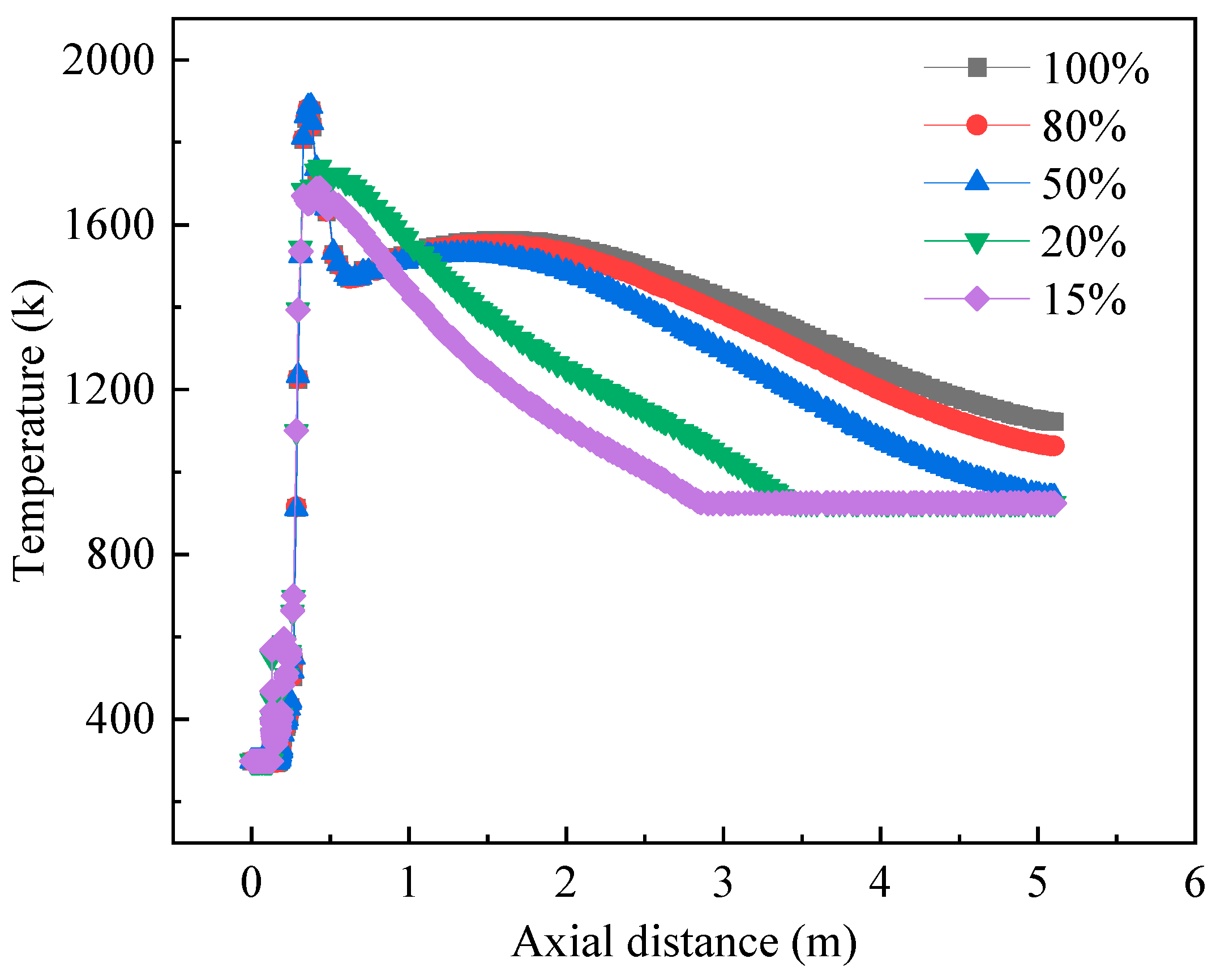
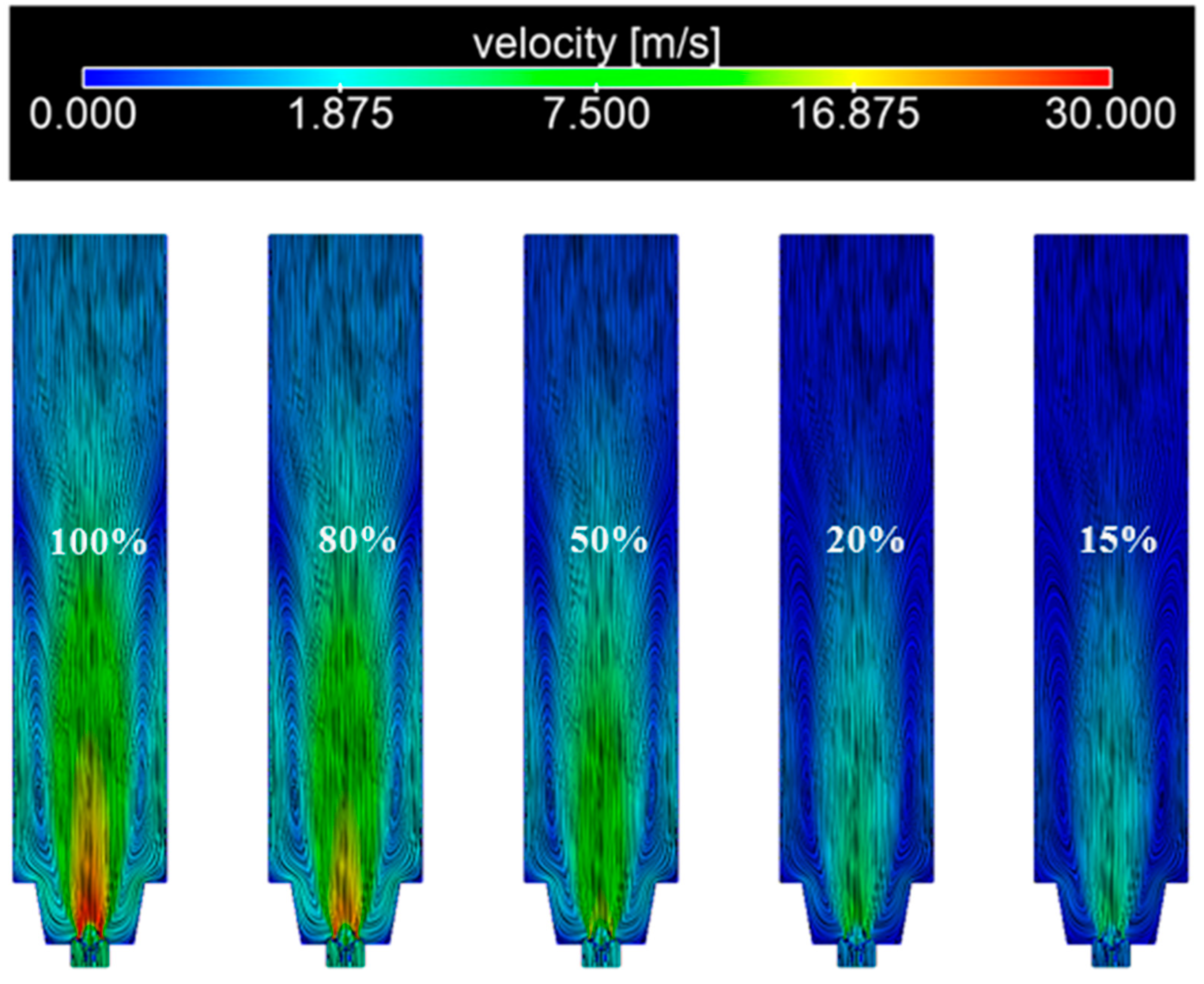
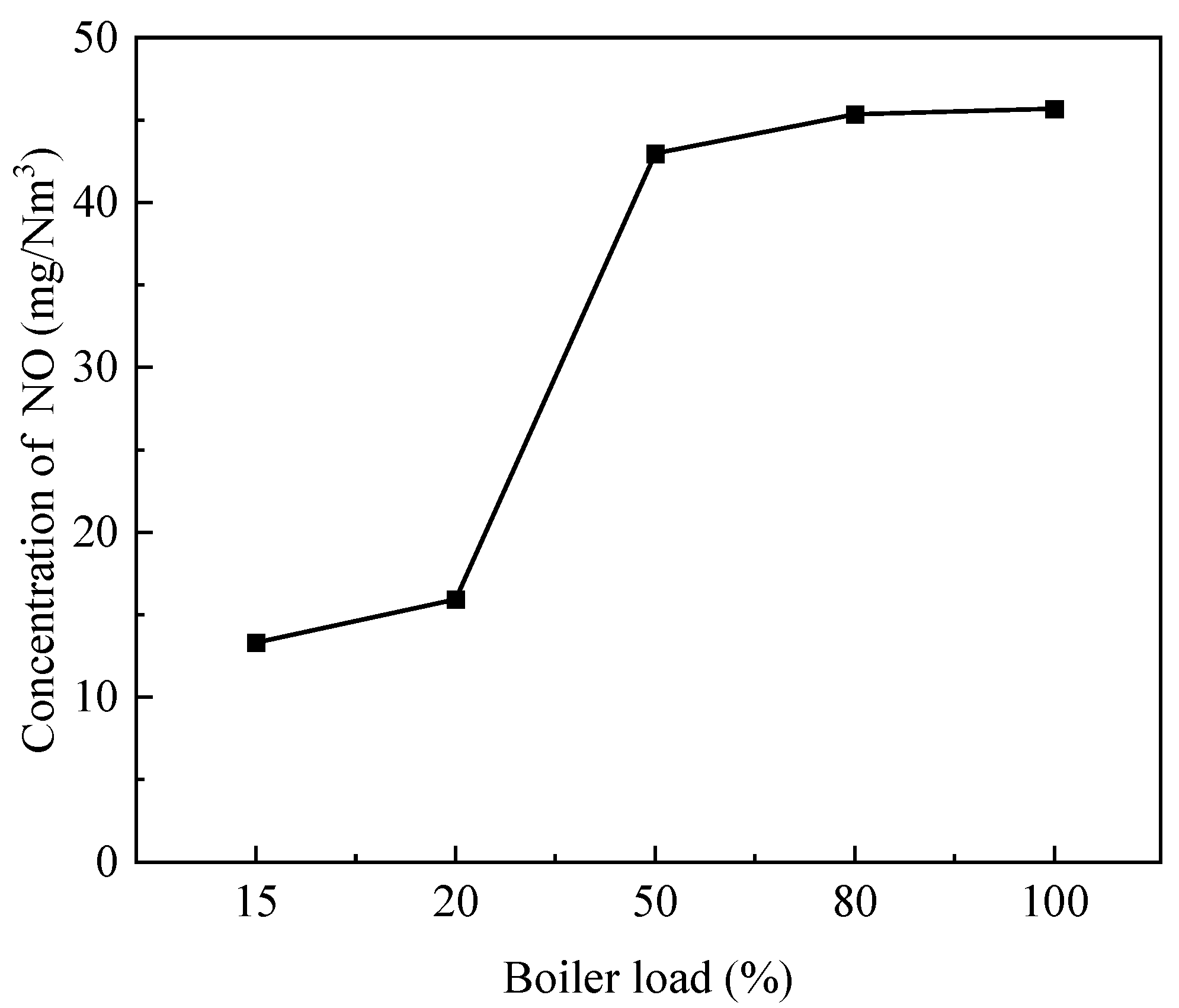
3.3. Influence of Load on the Combustion Process of the Burner
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Boiler Load | Φ | f(H2) | Outlet Concentration (mg/Nm3, 3.5% O2) | ||
|---|---|---|---|---|---|
| Temperature | NO | CO | |||
| 100 | 0.8 | 0 | 1073.81 | 42.7 | 34.4 |
| 5 | 1074.37 | 45.1 | 34.2 | ||
| 10 | 1074.57 | 46.8 | 34.1 | ||
| 15 | 1074.21 | 47.4 | 33.8 | ||
| 20 | 1075.05 | 49.1 | 33.7 | ||
| 25 | 1075.96 | 52.8 | 642 | ||
| 1.0 | 0 | 1083.60 | 199 | 638 | |
| 5 | 1085.45 | 204 | 633 | ||
| 10 | 1087.30 | 217 | 629 | ||
| 15 | 1089.07 | 249 | 624 | ||
| 20 | 1091.05 | 298 | 619 | ||
| 25 | 1094.94 | 463 | 3600 | ||
| 1.2 | 0 | 1107.13 | 490 | 3570 | |
| 5 | 1108.15 | 509 | 3520 | ||
| 10 | 1108.27 | 527 | 3480 | ||
| 15 | 1108.59 | 572 | 3430 | ||
| 20 | 1108.72 | 646 | 3380 | ||
| 25 | 1108.59 | 45.3 | 36.4 | ||
| 80 | 0.8 | 0 | 1013.88 | 45.5 | 36.2 |
| 5 | 1014.07 | 45.9 | 36 | ||
| 10 | 1014.31 | 50.6 | 35.8 | ||
| 15 | 1014.55 | 55.7 | 35.7 | ||
| 20 | 1014.81 | 56.1 | 34.8 | ||
| 25 | 1015.11 | 191 | 642 | ||
| 1.0 | 0 | 1010.06 | 199 | 638 | |
| 5 | 1011.75 | 204 | 633 | ||
| 10 | 1013.45 | 205 | 629 | ||
| 15 | 1015.04 | 205 | 624 | ||
| 20 | 1016.81 | 231 | 619 | ||
| 25 | 1018.47 | 487 | 3600 | ||
| 1.2 | 0 | 1032.57 | 490 | 3570 | |
| 5 | 1033.50 | 496 | 3520 | ||
| 10 | 1033.55 | 499 | 3480 | ||
| 15 | 1033.80 | 508 | 3430 | ||
| 20 | 1033.86 | 520 | 3380 | ||
| 25 | 1033.81 | 43 | 36.4 | ||
| 50 | 0.8 | 0 | 889.91 | 43.2 | 36.1 |
| 5 | 890.32 | 44 | 36 | ||
| 10 | 890.75 | 45.1 | 35.8 | ||
| 15 | 891.16 | 45.8 | 35.7 | ||
| 20 | 891.57 | 46 | 34.8 | ||
| 25 | 891.97 | 174 | 641 | ||
| 1.0 | 0 | 901.67 | 178 | 637 | |
| 5 | 901.65 | 180 | 633 | ||
| 10 | 902.53 | 181 | 629 | ||
| 15 | 903.50 | 183 | 624 | ||
| 20 | 904.55 | 187 | 619 | ||
| 25 | 906.11 | 516 | 3600 | ||
| 1.2 | 0 | 896.61 | 529 | 3570 | |
| 5 | 897.56 | 534 | 3520 | ||
| 10 | 898.05 | 535 | 3480 | ||
| 15 | 898.96 | 546 | 3430 | ||
| 20 | 899.89 | 549 | 3380 | ||
| 25 | 900.82 | 15.9 | 1460 | ||
| 20 | 0.8 | 0 | 923.75 | 15.9 | 1430 |
| 5 | 926.36 | 16.3 | 1410 | ||
| 10 | 927.31 | 16.8 | 1400 | ||
| 15 | 928.34 | 18.5 | 1390 | ||
| 20 | 929.45 | 19.2 | 1370 | ||
| 25 | 930.66 | 21.3 | 5740 | ||
| 1.0 | 0 | 784.30 | 22.1 | 5680 | |
| 5 | 785.39 | 22.9 | 5610 | ||
| 10 | 787.76 | 23.8 | 5540 | ||
| 15 | 790.24 | 25 | 5460 | ||
| 20 | 793.03 | 26.1 | 5370 | ||
| 25 | 795.98 | 29.8 | 7440 | ||
| 1.2 | 0 | 636.76 | 30.8 | 7380 | |
| 5 | 636.98 | 31.8 | 7320 | ||
| 10 | 637.50 | 32.7 | 7240 | ||
| 15 | 638.20 | 35 | 7160 | ||
| 20 | 639.16 | 39.9 | 7070 | ||
| 25 | 640.41 | 13.3 | 1460 | ||
| 15 | 0.8 | 0 | 923.75 | 14.9 | 1430 |
| 5 | 926.36 | 15.7 | 1410 | ||
| 10 | 927.31 | 19.2 | 1400 | ||
| 15 | 928.34 | 23.5 | 1390 | ||
| 20 | 929.45 | 24 | 1370 | ||
| 25 | 930.66 | 21.8 | 5740 | ||
| 1.0 | 0 | 784.27 | 24.3 | 5680 | |
| 5 | 785.37 | 25.5 | 5610 | ||
| 10 | 787.74 | 27 | 5540 | ||
| 15 | 790.22 | 28.9 | 5460 | ||
| 20 | 793.01 | 30.4 | 5370 | ||
| 25 | 795.95 | 33.8 | 7440 | ||
| 1.2 | 0 | 613.68 | 35.3 | 7380 | |
| 5 | 615.35 | 36.4 | 7320 | ||
| 10 | 618.42 | 37.4 | 7240 | ||
| 15 | 621.67 | 38.3 | 7160 | ||
| 20 | 625.31 | 39.7 | 7070 | ||
| 25 | 629.20 | 46.1 | 34.4 | ||
References
- Yang, F.Y.; Wang, T.Z.; Deng, X.T.; Dang, J.; Huang, Z.Y.; Hu, S.; Li, Y.Y.; Ouyang, M.G. Review on Hydrogen Safety Issues: Incident Statistics, Hydrogen Diffusion, and Detonation Process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- Yu, J.; Park, J.; Kim, S.; Choi, Y.; Lee, S.H.; Lee, Y. Effect of Operating Conditions on the Characteristics of Flameless CH4 and H2 Combustion for Primary NO Reduction. Fuel 2024, 374, 132507. [Google Scholar] [CrossRef]
- Huang, M.M.; Li, R.C.; Xu, J.K.; Cheng, S.; Deng, H.X.; Rong, Z.Y.; Li, Y.; Zhang, Y.F. Effect of Equivalence Ratio and Staging Ratio on the Methane MILD Combustion in Dual-Stage Combustor. Fuel 2022, 307, 121903. [Google Scholar] [CrossRef]
- Cheng, L.; Li, W.L.; Peng, S.Y.; Chai, C.; Wang, W.B.; Tian, C.; Zhang, J.C.; Liu, L.Q.; Zhang, H.W.; Zhang, Y.X. Study of Combustion Characteristics of Hydrogen-Doped Natural Gas in Industrial Boilers. Int. J. Hydrogen Energy 2024, 92, 590–604. [Google Scholar] [CrossRef]
- Li, R.Z.; Zhang, Y.H.; Jia, Q.S.; Cao, M.T.; Zhang, W.Q.; Si, X.Y.; Shi, C.H. A Study of the Effect of Hydrogen on Methane Deflagration Characteristics in a Square Confined Space. Int. J. Hydrogen Energy 2024, 62, 947–958. [Google Scholar] [CrossRef]
- Zimont, V.L. Conceptual Limitations of the Probability Density Function Method for Modeling Turbulent Premixed Combustion and Closed-Form Description of Chemical Reactions’ Effects. Fluids 2021, 6, 142. [Google Scholar] [CrossRef]
- Lovella, Y.G.; Moya, I.H.; Jayasuriya, J.; Blondeau, J. Reynolds-Average Navier-Stokes Turbulence Models Assessment: A Case Study of CH4/H2/N2-Air Reacting Jet. Heliyon 2024, 10, e26956. [Google Scholar] [CrossRef]
- Hami, K. Turbulence Modeling a Review for Different Used Methods. Int. J. Heat Technol. 2021, 39, 227–234. [Google Scholar] [CrossRef]
- Jung, L.; Mages, A.; Sauer, A. Numerical Investigation and Simulation of Hydrogen Blending into Natural Gas Combustion. Energies 2024, 17, 3819. [Google Scholar] [CrossRef]
- Manatunga, M.; Christo, F.C.; Schlüter, J.; Shelyag, S. Evaluating the Steady Flamelet Assumption: Impacts on Flame Structure and NO Emissions in Hydrogen/Air Turbulent Jet Diffusion Flames. Combust. Sci. Technol. 2025, 1–23. [Google Scholar] [CrossRef]
- Wang, K.; Xu, R.; Parise, T.; Shao, J.; Movaghar, A.; Lee, D.J.; Park, J.W.; Gao, Y.; Lu, T.F.; Egolfopoulos, F.N.; et al. A Physics-Based Approach to Modeling Real-Fuel Combustion Chemistry-IV. HyChem Modeling of Combustion Kinetics of a Bio-Derived Jet Fuel and Its Blends with a Conventional Jet A. Combust. Flame 2018, 198, 477–489. [Google Scholar] [CrossRef]
- Gerasimov, I.E.; Bolshova, T.A.; Osipova, K.N.; Dmitriev, A.M.; Knyazkov, D.A.; Shmakov, A.G. Flame Structure at Elevated Pressure Values and Reduced Reaction Mechanisms for the Combustion of CH4/H2 Mixtures. Energies 2023, 16, 7489. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.M.; He, S.; Wang, S.M.; Zhang, Z.X. Numerical Simulation on Combustion Characteristics of Methane/Hydrogen Blended Fuel for Non-Premixed Conical Bluff Body Burner. Int. J. Hydrogen Energy 2024, 65, 50–60. [Google Scholar] [CrossRef]
- Panesar, A.; Allan, J.; Wylie, E. Medium Speed Lean Hydrogen Engine Modelling and Validation. Int. J. Hydrogen Energy 2025, 171, 151268. [Google Scholar] [CrossRef]
- Pan, H.J.T.; Geng, S.J.; Yang, H.; Zhang, G.H.; Bian, H.; Liu, Y.H. Influence of H2 Blending on NOx Production in Natural Gas Combustion: Mechanism Comparison and Reaction Routes. Int. J. Hydrogen Energy 2023, 48, 784–797. [Google Scholar] [CrossRef]
- Glanville, P.; Fridlyand, A.; Sutherland, B.; Liszka, M.; Zhao, Y.; Bingham, L.; Jorgensen, K. Impact of Hydrogen/Natural Gas Blends on Partially Premixed Combustion Equipment: NOx Emission and Operational Performance. Energies 2022, 15, 1706. [Google Scholar] [CrossRef]
- Yu, H.Y.; Díaz, A.; Lu, X.; Sun, B.H.; Ding, Y.; Koyama, M.; He, J.Y.; Zhou, X.; Oudriss, A.; Feaugas, X.; et al. Hydrogen Embrittlement as a Conspicuous Material Challenge-Comprehensive Review and Future Directions. Chem. Rev. 2024, 124, 6271–6392. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.J.; Wang, J.H.; Zhang, W.J.; Wang, Y.C.; Huang, Z.H.; Bai, X.S. Structure and Thermoacoustic Instability of Turbulent Swirling Lean Premixed Methane/Hydrogen/Air Flames in a Model Combustor. Int. J. Hydrogen Energy 2024, 60, 890–901. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Su, Y.; Liu, T.; Huang, H.T.; Guo, Y.J.; Li, Y.X. Application and Progress of Low-NOx Emission Technology for Gas Boilers. Chem. Eng. J. 2024, 52, 18–24. [Google Scholar] [CrossRef]
- Seddik, H.; Braiek, E.B. Efficient Noise Removing Based Optimized Smart Dynamic Gaussian Filter. Int. J. Comput. Appl. 2012, 51, 1–13. [Google Scholar] [CrossRef]
- Zhao, Q.N.; Liu, X.M.; Liu, F.; Xu, H.T.; Liu, Z.H.; Liao, X.W. Numerical Simulation on Emissions Mechanism of Nitrogen Oxides in Gas-fired Boilers Blended with Hydrogen. Chem. Ind. Eng. Prog. 2023, 42, 5637–5647. [Google Scholar] [CrossRef]
- NIST/SEMATECH. e-Handbook of Statistical Methods: 1.3.5.17.1. Grubbs’ Test for Outliers; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2012. Available online: https://www.itl.nist.gov/div898/handbook/eda/section3/eda35h1.htm (accessed on 20 October 2025).
- Liu, L.M.; Xie, J.F.; Zhang, C.Z. Application and Enlightenment of Reference Oxygen Content in Emission Standards of Atmospheric Stationary Source Pollutants. Environ. Monit. China 2022, 38, 248–254. [Google Scholar] [CrossRef]
- Ziani, L.; Chaker, A.; Chetehouna, K.; Malek, A.; Mahmah, B. Numerical Simulations of Non-Premixed Turbulent Combustion of CH4-H2 Mixtures Using the PDF Approach. Int. J. Hydrogen Energy 2013, 38, 8597–8603. [Google Scholar] [CrossRef]
- Duan, H.; Huang, Y.; Mehra, R.K.; Song, P.; Ma, F. Study on Influencing Factors of Prediction Accuracy of Support Vector Machine (SVM) Model for NOx Emission of a Hydrogen Enriched Compressed Natural Gas Engine. Fuel 2018, 234, 954–964. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Q.L.; Zhang, X.G.; Zhao, Q.X.; Xu, T.M.; Hui, S.E. Experimental Study on the Laminar Flame Speed of Hydrogen/Natural Gas/Air Mixtures. Front. Chem. Sci. Eng. 2010, 4, 417–422. [Google Scholar] [CrossRef]



| Areas/mm2 | Hydraulic Diameters/m | |
|---|---|---|
| Fuel-inlet1 | 923.628 | 0.014 |
| Fuel-inlet 2 | 1017.876 | 0.036 |
| Air-inlet | 48,559.598 | 0.152 |
| Outlet | 890,818.232 | 1.065 |
| Model | Velocity Prediction Error | Temperature Prediction | Computational Time (Core-Hours/Case) |
|---|---|---|---|
| Modified k-ε | 6–8% | 6–8% | 1200 |
| RNG k-ε | 9–11% | 8–10% | 1300 |
| RSM | 10–13% | 9–12% | 2000 |
| LES | 4–6% | 4–6% | 2400 |
| Boiler Load | Φ | f(H2) | Air Flow (m3/h) | Air-Inlet Flow Rate (m/s) | Flue-Inlet1 Flow Rate (m/s) | Flue-Inlet2 Flow Rate (m/s) |
|---|---|---|---|---|---|---|
| 100 | 0.8 | 0 | 2489.00 | 14.24 | 51.16 | 10.89 |
| 5 | 2482.36 | 14.20 | 53.01 | 11.28 | ||
| 10 | 2475.22 | 14.16 | 54.99 | 11.70 | ||
| 15 | 2467.52 | 14.12 | 57.13 | 12.16 | ||
| 20 | 2459.20 | 14.07 | 59.45 | 12.65 | ||
| 25 | 2450.18 | 14.02 | 61.96 | 13.19 | ||
| 1.0 | 0 | 1991.20 | 11.39 | 51.16 | 10.89 | |
| 5 | 1985.89 | 11.36 | 53.01 | 11.28 | ||
| 10 | 1980.17 | 11.33 | 54.99 | 11.70 | ||
| 15 | 1974.02 | 11.29 | 57.13 | 12.16 | ||
| 20 | 1967.36 | 11.25 | 59.45 | 12.65 | ||
| 25 | 1960.14 | 11.21 | 61.96 | 13.19 | ||
| 1.2 | 0 | 1659.33 | 9.49 | 51.16 | 10.89 | |
| 5 | 1654.91 | 9.47 | 53.01 | 11.28 | ||
| 10 | 1650.15 | 9.44 | 54.99 | 11.70 | ||
| 15 | 1645.01 | 9.41 | 57.13 | 12.16 | ||
| 20 | 1639.47 | 9.38 | 59.45 | 12.65 | ||
| 25 | 1633.45 | 9.34 | 61.96 | 13.19 | ||
| 80 | 0.8 | 0 | 1991.20 | 11.39 | 40.93 | 8.71 |
| 5 | 1985.89 | 11.36 | 42.40 | 9.03 | ||
| 10 | 1980.17 | 11.33 | 43.99 | 9.36 | ||
| 15 | 1974.02 | 11.29 | 45.71 | 9.73 | ||
| 20 | 1967.36 | 11.25 | 47.56 | 10.12 | ||
| 25 | 1960.14 | 11.21 | 49.57 | 10.55 | ||
| 1.0 | 0 | 1592.96 | 9.11 | 40.93 | 8.71 | |
| 5 | 1588.71 | 9.09 | 42.40 | 9.03 | ||
| 10 | 1584.14 | 9.06 | 43.99 | 9.36 | ||
| 15 | 1579.21 | 9.03 | 45.71 | 9.73 | ||
| 20 | 1573.89 | 9.00 | 47.56 | 10.12 | ||
| 25 | 1568.11 | 8.97 | 49.57 | 10.55 | ||
| 1.2 | 0 | 1327.47 | 7.59 | 40.93 | 8.71 | |
| 5 | 1323.92 | 7.57 | 42.40 | 9.03 | ||
| 10 | 1320.12 | 7.55 | 43.99 | 9.36 | ||
| 15 | 1316.01 | 7.53 | 45.71 | 9.73 | ||
| 20 | 1311.57 | 7.50 | 47.56 | 10.12 | ||
| 25 | 1306.76 | 7.48 | 49.57 | 10.55 | ||
| 50 | 0.8 | 0 | 1244.50 | 7.12 | 25.58 | 5.44 |
| 5 | 1241.18 | 7.10 | 26.50 | 5.64 | ||
| 10 | 1237.61 | 7.08 | 27.50 | 5.85 | ||
| 15 | 1233.76 | 7.06 | 28.57 | 6.08 | ||
| 20 | 1229.60 | 7.03 | 29.72 | 6.33 | ||
| 25 | 1225.09 | 7.01 | 30.98 | 6.59 | ||
| 1.0 | 0 | 995.60 | 5.70 | 25.58 | 5.44 | |
| 5 | 992.94 | 5.68 | 26.50 | 5.64 | ||
| 10 | 990.09 | 5.66 | 27.50 | 5.85 | ||
| 15 | 987.01 | 5.65 | 28.57 | 6.08 | ||
| 20 | 983.68 | 5.63 | 29.72 | 6.33 | ||
| 25 | 980.07 | 5.61 | 30.98 | 6.59 | ||
| 1.2 | 0 | 829.67 | 4.75 | 25.58 | 5.44 | |
| 5 | 827.45 | 4.73 | 26.50 | 5.64 | ||
| 10 | 825.07 | 4.72 | 27.50 | 5.85 | ||
| 15 | 822.51 | 4.71 | 28.57 | 6.08 | ||
| 20 | 819.73 | 4.69 | 29.72 | 6.33 | ||
| 25 | 816.73 | 4.67 | 30.98 | 6.59 | ||
| 20 | 0.8 | 0 | 497.80 | 2.85 | 10.23 | 6.01 |
| 5 | 496.47 | 2.84 | 10.60 | 6.23 | ||
| 10 | 495.04 | 2.83 | 11.00 | 6.46 | ||
| 15 | 493.50 | 2.82 | 11.43 | 6.71 | ||
| 20 | 491.84 | 2.81 | 11.89 | 6.98 | ||
| 25 | 490.04 | 2.80 | 12.39 | 7.28 | ||
| 1.0 | 0 | 398.24 | 2.28 | 10.23 | 6.01 | |
| 5 | 397.18 | 2.27 | 10.60 | 6.23 | ||
| 10 | 396.03 | 2.27 | 11.00 | 6.46 | ||
| 15 | 394.80 | 2.26 | 11.43 | 6.71 | ||
| 20 | 393.47 | 2.25 | 11.89 | 6.98 | ||
| 25 | 392.03 | 2.24 | 12.39 | 7.28 | ||
| 1.2 | 0 | 331.87 | 1.90 | 10.23 | 6.01 | |
| 5 | 330.98 | 1.89 | 10.60 | 6.23 | ||
| 10 | 330.03 | 1.89 | 11.00 | 6.46 | ||
| 15 | 329.00 | 1.88 | 11.43 | 6.71 | ||
| 20 | 327.89 | 1.88 | 11.89 | 6.98 | ||
| 25 | 326.69 | 1.87 | 12.39 | 7.28 | ||
| 15 | 0.8 | 0 | 373.35 | 2.14 | 7.67 | 4.51 |
| 5 | 372.35 | 2.13 | 7.95 | 4.67 | ||
| 10 | 371.28 | 2.12 | 8.25 | 4.84 | ||
| 15 | 370.13 | 2.12 | 8.57 | 5.03 | ||
| 20 | 368.88 | 2.11 | 8.92 | 5.24 | ||
| 25 | 367.53 | 2.10 | 9.29 | 5.46 | ||
| 1.0 | 0 | 298.68 | 1.71 | 7.67 | 4.51 | |
| 5 | 297.88 | 1.70 | 7.95 | 4.67 | ||
| 10 | 297.03 | 1.70 | 8.25 | 4.84 | ||
| 15 | 296.10 | 1.69 | 8.57 | 5.03 | ||
| 20 | 295.10 | 1.69 | 8.92 | 5.24 | ||
| 25 | 294.02 | 1.68 | 9.29 | 5.46 | ||
| 1.2 | 0 | 248.90 | 1.42 | 7.67 | 4.51 | |
| 5 | 248.24 | 1.42 | 7.95 | 4.67 | ||
| 10 | 247.52 | 1.42 | 8.25 | 4.84 | ||
| 15 | 246.75 | 1.41 | 8.57 | 5.03 | ||
| 20 | 245.92 | 1.41 | 8.92 | 5.24 | ||
| 25 | 245.02 | 1.40 | 9.29 | 5.46 |
| Hydrogen-Enriched Natural Gas Components | f(H2) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | |
| CH4 (%) | 96.00 | 91.20 | 86.40 | 81.60 | 76.80 | 72.00 |
| N2 (%) | 2.00 | 1.90 | 1.80 | 1.70 | 1.60 | 1.50 |
| C2H6 (%) | 1.92 | 1.82 | 1.73 | 1.63 | 1.54 | 1.44 |
| C3H8 (%) | 0.08 | 0.08 | 0.07 | 0.07 | 0.06 | 0.06 |
| H2 (%) | 0.00 | 5.00 | 10.00 | 15.00 | 20.00 | 25.00 |
| Grid Density (Number of Cells) | Peak Temperature Error (%) (Relative to Fine Grid) | Radial Velocity Error (%) (Relative to Fine Grid) | NO Mole Fraction Error (%) (Relative to Fine Grid) | CO Mole Fraction Error (%) (Relative to Fine Grid) | Grid Density (Number of Cells) | Peak Temperature Error (%) (Relative to Fine Grid) |
|---|---|---|---|---|---|---|
| 0.69 × 106 | 3.8 | 4.5 | 5.2 | 4.9 | 0.69 × 106 | 3.8 |
| 0.85 × 106 | 0.9 | 1.8 | 1.5 | 1.2 | 0.85 × 106 | 0.9 |
| 1.13 × 106 (Fine Grid) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 1.13 × 106 (Fine Grid) | 0 (Reference) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Feng, N.; Zheng, W.; Li, W.; Lu, Y.; Wang, Z.; Sun, C.; Zhang, Y.; Lv, L.; Xu, M. Effects of Operating Parameters on Combustion Characteristics of Hydrogen-Doped Natural Gas. Processes 2025, 13, 3477. https://doi.org/10.3390/pr13113477
Wang P, Feng N, Zheng W, Li W, Lu Y, Wang Z, Sun C, Zhang Y, Lv L, Xu M. Effects of Operating Parameters on Combustion Characteristics of Hydrogen-Doped Natural Gas. Processes. 2025; 13(11):3477. https://doi.org/10.3390/pr13113477
Chicago/Turabian StyleWang, Pengtao, Nana Feng, Wei Zheng, Wenlin Li, Yanghui Lu, Zhining Wang, Chen Sun, Yangxin Zhang, Liangliang Lv, and Meng Xu. 2025. "Effects of Operating Parameters on Combustion Characteristics of Hydrogen-Doped Natural Gas" Processes 13, no. 11: 3477. https://doi.org/10.3390/pr13113477
APA StyleWang, P., Feng, N., Zheng, W., Li, W., Lu, Y., Wang, Z., Sun, C., Zhang, Y., Lv, L., & Xu, M. (2025). Effects of Operating Parameters on Combustion Characteristics of Hydrogen-Doped Natural Gas. Processes, 13(11), 3477. https://doi.org/10.3390/pr13113477




