Engineered Mors1 Enzyme from the Antarctic Bacterium Moraxella TA144 for Enhanced Thermal Stability and Activity for Polyethylene Terephthalate Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strains and Media
2.3. Construction of the pPICZαA-Mors1WT/MUT Plasmid and Its Transformation into E. coli DH5α
2.4. Expression of Recombinant Mors1WT/MUT in P. pastoris
2.5. Purification and Western Blotting
2.6. Mors1WT/MUT Secondary Structure, Kinetics and Stability (pH and Temperature)
2.7. Design and Optimization of the Mors1MUT Reaction for Enhanced PET Degradation
2.8. PET Degradation by Mors1WT/MUT: SEM, AFM, and Hydrolysis Product Analysis
2.9. Statistical Analysis
3. Results
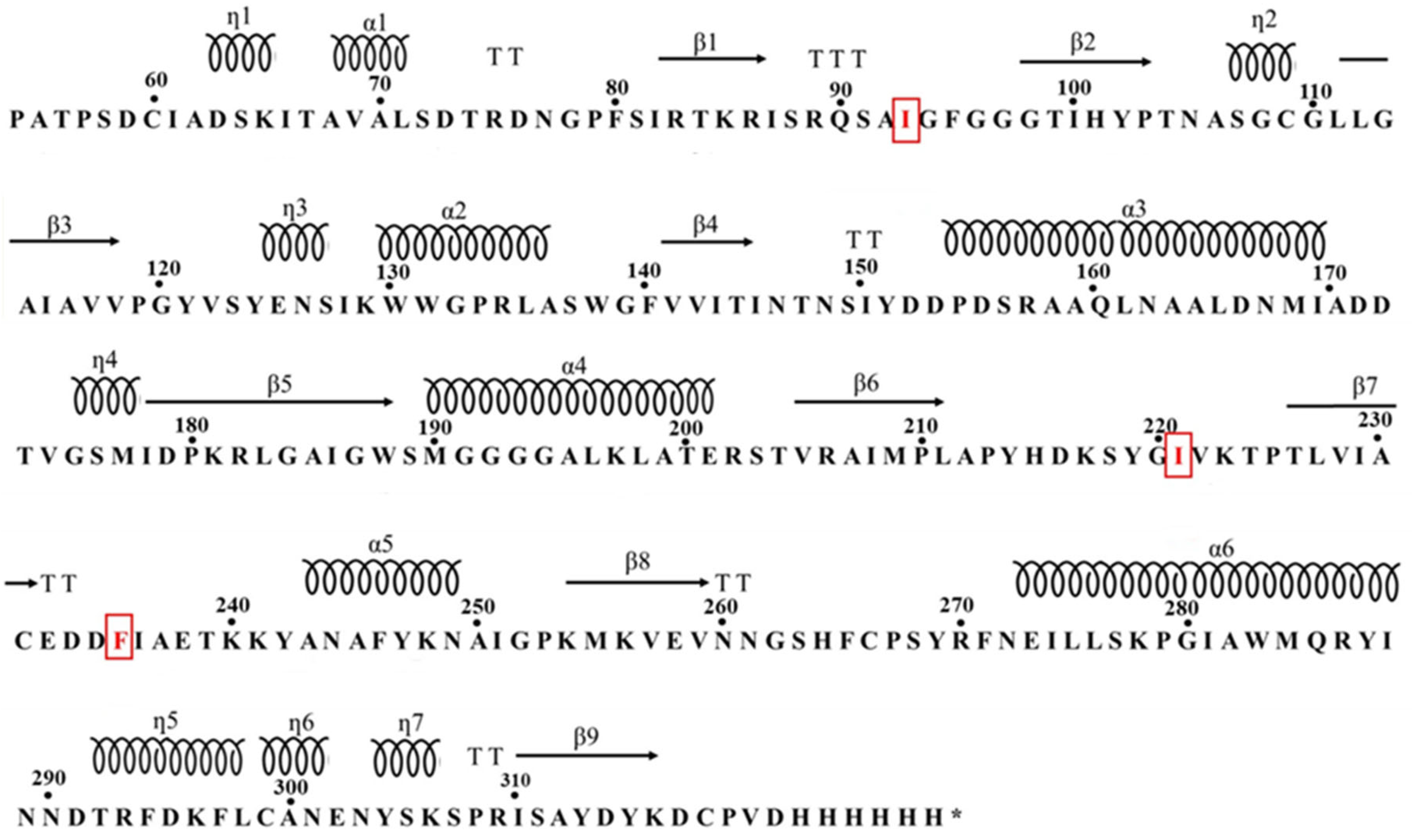
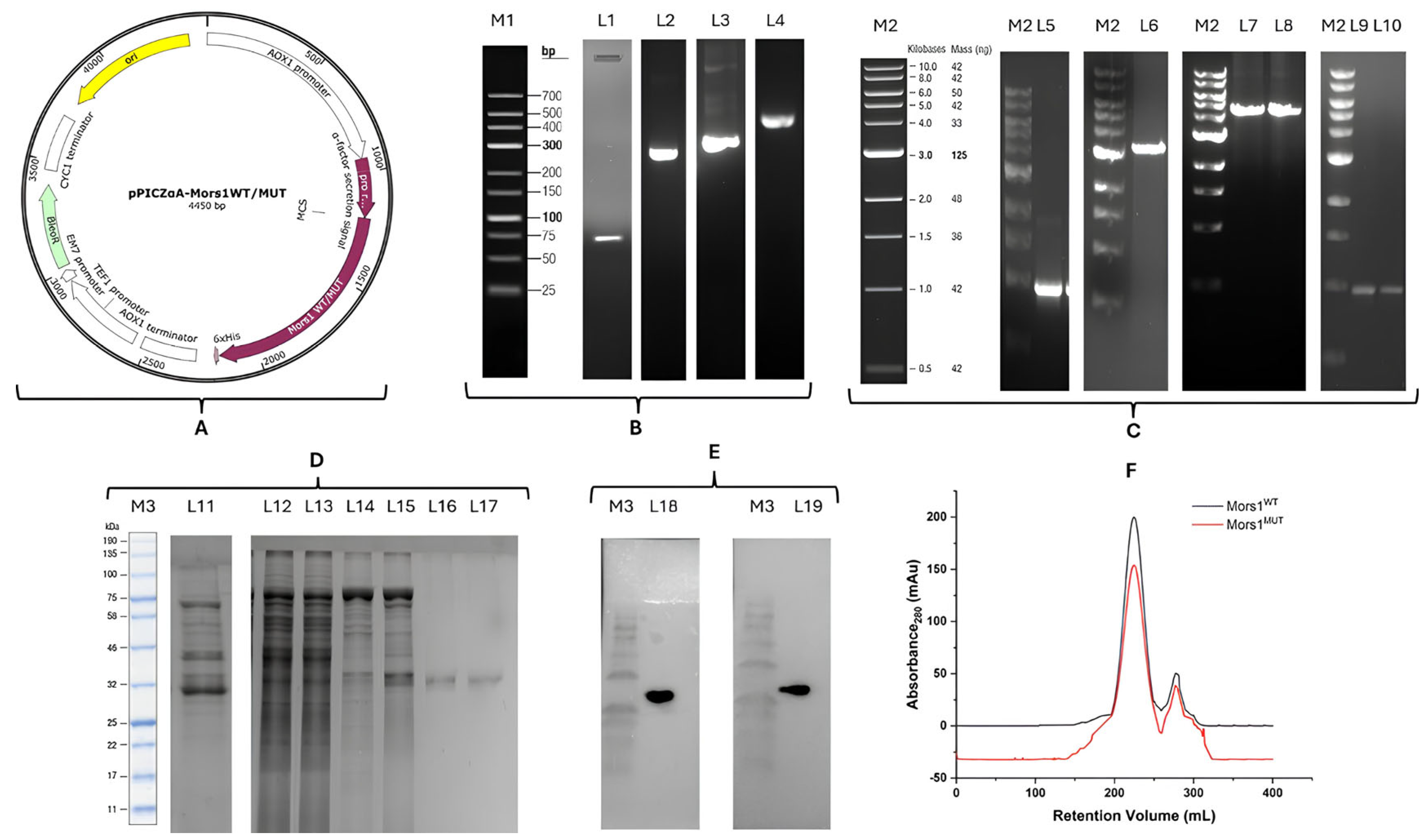
3.1. Cloning, Expression, Purification and Validation of Mors1WT/MUT
3.2. Comparative Analysis of Mors1WT/MUT: Secondary Structure, Kinetics, and Stability
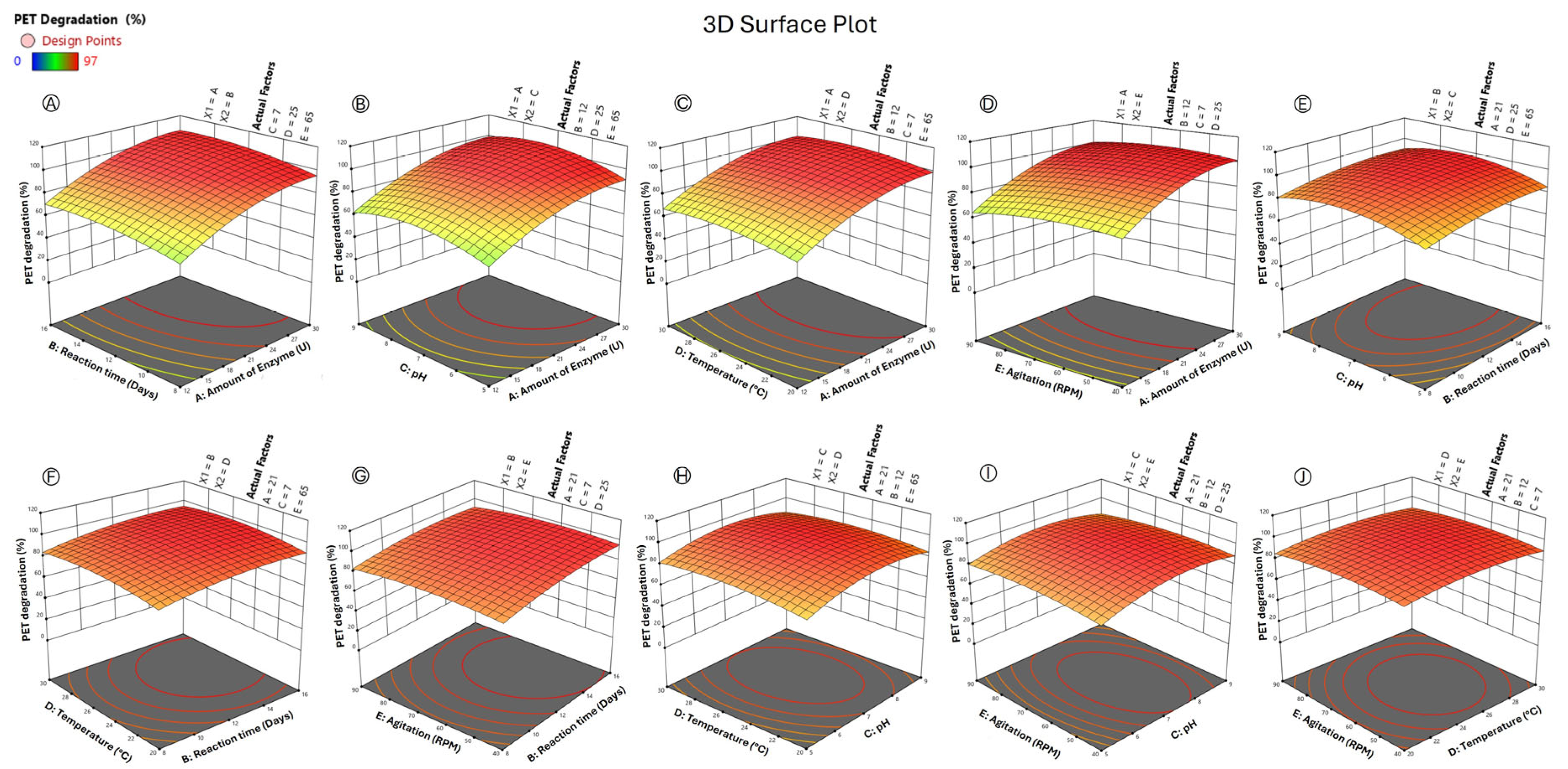
3.3. Optimization of the Mors1MUT Reaction for Enhanced PET Degradation
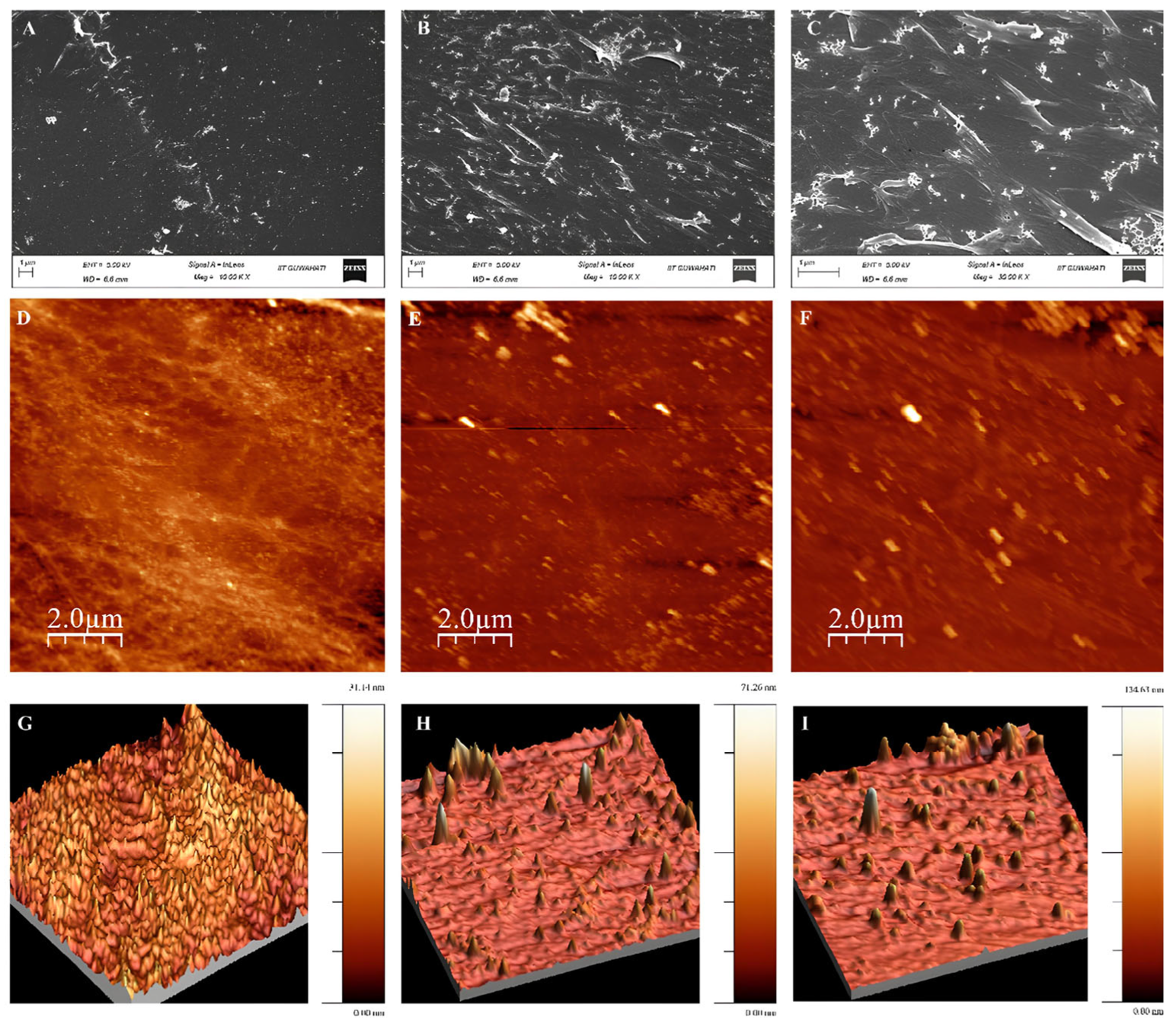
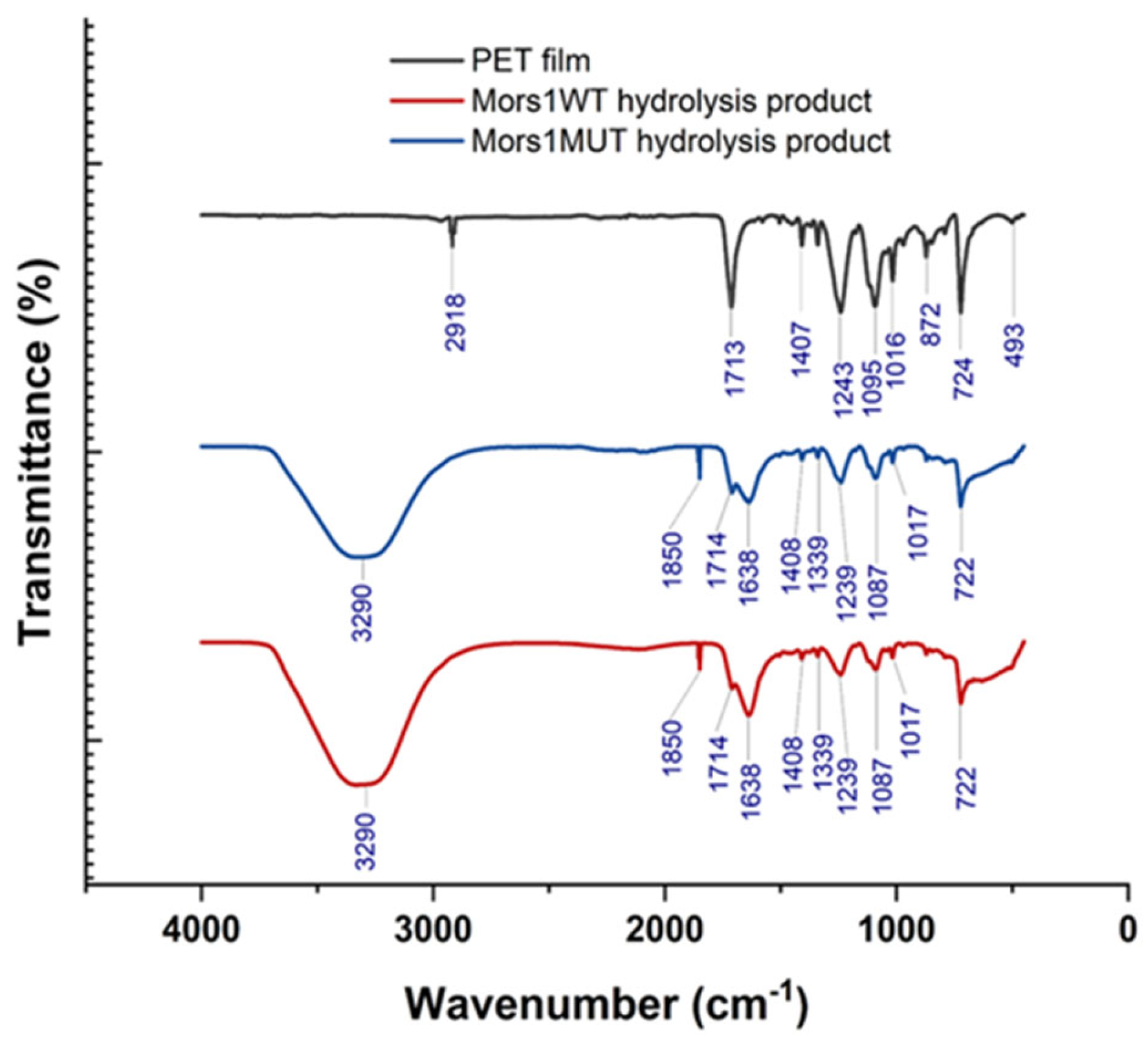
3.4. PET Degrazdation by Mors1WT/MUT: SEM, AFM, and Hydrolysis Product Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horton, A.A. Plastic Pollution: When Do We Know Enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef]
- Bharadwaaj, S.K.; Jaudan, M.; Kushwaha, P.; Saxena, A.; Saha, B. Exploring Cutting-Edge Approaches in Plastic Recycling for a Greener Future. Results Eng. 2024, 23, 102704. [Google Scholar] [CrossRef]
- Al Mamun, A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Lamtai, A.; Elkoun, S.; Robert, M.; Mighri, F.; Diez, C. Mechanical Recycling of Thermoplastics: A Review of Key Issues. Waste 2023, 1, 860–883. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical Recycling of PET: A Stepping-Stone toward Sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Hong, H.; Ki, D.; Seo, H.; Park, J.; Jang, J.; Kim, K.J. Discovery and Rational Engineering of PET Hydrolase with Both Mesophilic and Thermophilic PET Hydrolase Properties. Nat. Commun. 2023, 14, 4556. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, G.; Miao, R.; Wu, N.; Zhang, W.; Yao, B.; Guan, F.; Huang, H.; Tian, J. Rational Redesign of Thermophilic PET Hydrolase LCCICCG to Enhance Hydrolysis of High Crystallinity Polyethylene Terephthalates. J. Hazard. Mater. 2023, 453, 131386. [Google Scholar] [CrossRef]
- Meyer Cifuentes, I.E.; Wu, P.; Zhao, Y.; Liu, W.; Neumann-Schaal, M.; Pfaff, L.; Barys, J.; Li, Z.; Gao, J.; Han, X.; et al. Molecular and Biochemical Differences of the Tandem and Cold-Adapted PET Hydrolases Ple628 and Ple629, Isolated from a Marine Microbial Consortium. Front. Bioeng. Biotechnol. 2022, 10, 930140. [Google Scholar] [CrossRef]
- Liu, C.; Shi, C.; Zhu, S.; Wei, R.; Yin, C.C. Structural and Functional Characterization of Polyethylene Terephthalate Hydrolase from Ideonella Sakaiensis. Biochem. Biophys. Res. Commun. 2019, 508, 289–294. [Google Scholar] [CrossRef]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Rational Protein Engineering of Thermo-Stable PETase from Ideonella Sakaiensis for Highly Efficient PET Degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Sun, J.; Cui, Y.; Wu, B. GRAPE, a Greedy Accumulated Strategy for Computational Protein Engineering. Methods Enzymol. 2021, 648, 207–230. [Google Scholar] [CrossRef]
- Shroff, R.; Cole, A.W.; Diaz, D.J.; Morrow, B.R.; Donnell, I.; Annapareddy, A.; Gollihar, J.; Ellington, A.D.; Thyer, R. Discovery of Novel Gain-of-Function Mutations Guided by Structure-Based Deep Learning. ACS Synth. Biol. 2020, 9, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine Learning-Aided Engineering of Hydrolases for PET Depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.M.; DeVido, D.R.; Dorsey, J.G. Evaluation of Methods for Measuring Amino Acid Hydrophobicities and Interactions. J. Chromatogr. A 2003, 1000, 637–655. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, Q.; Pires, D.E.V.; Rodrigues, C.H.M.; Ascher, D.B. DDMut: Predicting Effects of Mutations on Protein Stability Using Deep Learning. Nucleic Acids Res. 2023, 51, W122–W128. [Google Scholar] [CrossRef]
- Vila, J.A. Proteins’ Evolution upon Point Mutations. ACS Omega 2022, 7, 14371–14376. [Google Scholar] [CrossRef]
- Invitrogen life technologies. User Manual EasySelect TM Pichia Expression Kit for Expression of Recombinant Proteins Using PPICZ and PPICZα in Pichia Pastoris. Available online: https://documents.thermofisher.com/TFS-Assets/LSG/manuals/easyselect_man.pdf (accessed on 18 August 2024).
- Offei, B.; Braun-Galleani, S.; Venkatesh, A.; Casey, W.T.; O’Connor, K.E.; Byrne, K.P.; Wolfe, K.H. Identification of Genetic Variants of the Industrial Yeast Komagataella Phaffii (Pichia pastoris) That Contribute to Increased Yields of Secreted Heterologous Proteins. PLoS Biol. 2022, 20, e3001877. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Xanthine Oxidase Driven Bio-Fenton System for Advanced Pollutant Degradation in Sustainable Wastewater Treatment. Int. J. Biol. Macromol. 2025, 313, 144323. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Kernya, L.; Lee, Y.H.; Goto, Y.; Réfrégiers, M.; Kardos, J. Accurate Secondary Structure Prediction and Fold Recognition for Circular Dichroism Spectroscopy. Proc. Natl. Acad. Sci. USA 2015, 112, E3095–E3103. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blázquez-Sánchez, P.; Engelberger, F.; Cifuentes-Anticevic, J.; Sonnendecker, C.; Griñén, A.; Reyes, J.; Díez, B.; Guixé, V.; Richter, P.K.; Zimmermann, W.; et al. Antarctic Polyester Hydrolases Degrade Aliphatic and Aromatic Polyesters at Moderate Temperatures. Appl. Environ. Microbiol. 2022, 88, e01842-21. [Google Scholar] [CrossRef]
- Furtado, A.A.; Blazquez-Sanchez, P.; Grinen, A.; Vargas, J.A.; Leonardo, D.A.; Sculaccio, S.A.; Pereira, H.M.; Diez, B.; Garratt, R.C.; Ramirez-Sarmiento, C.A. Crystal Structure of Antarctic PET-Degrading Enzyme. Available online: https://www.wwpdb.org/pdb?id=pdb_00008spk (accessed on 23 December 2024).
- Khairul Anuar, N.F.S.; Abdul Wahab, R.; Huyop, F.; Normi, Y.M.; Oyewusi, H.A.; Susanti, E. In Silico Mutagenesis on Active Site Residues of Acinetobacter haemolyticus Lipase KV1 for Improved Binding to Polyethylene Terephthalate (PET). J. Biomol. Struct. Dyn. 2024, 28, 1–23. [Google Scholar] [CrossRef]
- Tiong, E.; Koo, Y.S.; Bi, J.; Koduru, L.; Koh, W.; Lim, Y.H.; Wong, F.T. Expression and Engineering of PET-Degrading Enzymes from Microbispora, Nonomuraea, and Micromonospora. Appl. Environ. Microbiol. 2023, 89, e00632-23. [Google Scholar] [CrossRef]
- Guo, R.T.; Li, X.; Yang, Y.; Huang, J.W.; Shen, P.; Liew, R.K.; Chen, C.C. Natural and Engineered Enzymes for Polyester Degradation: A Review. Environ. Chem. Lett. 2024, 22, 1275–1296. [Google Scholar] [CrossRef]
- Jaiswal, N.; Jaiswal, P. Thermostable α-Amylases and Laccases: Paving the Way for Sustainable Industrial Applications. Processes 2024, 12, 1341. [Google Scholar] [CrossRef]
- Kauts, S.; Mishra, Y.; Yousuf, S.; Bhardwaj, R.; Singh, S.K.; Alshabrmi, F.M.; Abdurahman, M.; Vamanu, E.; Singh, M.P. Toxicological Profile of Polyethylene Terephthalate (PET) Microplastic in Ingested Drosophila melanogaster (Oregon R+) and Its Adverse Effect on Behavior and Development. Toxics 2023, 11, 782. [Google Scholar] [CrossRef] [PubMed]
- Arnal, G.; Anglade, J.; Gavalda, S.; Tournier, V.; Chabot, N.; Bornscheuer, U.T.; Weber, G.; Marty, A. Assessment of Four Engineered PET Degrading Enzymes Considering Large-Scale Industrial Applications. ACS Catal. 2023, 13, 13156–13166. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, L.; Gao, J.; Li, Z.; Jäckering, A.; Weber, G.; Mican, J.; Chen, Y.; Dong, W.; Han, X.; Feiler, C.G.; et al. Multiple Substrate Binding Mode-Guided Engineering of a Thermophilic PET Hydrolase. ACS Catal. 2022, 12, 9790–9800. [Google Scholar] [CrossRef]
- Jayasekara, S.K.; Joni, H.D.; Jayantha, B.; Dissanayake, L.; Mandrell, C.; Sinharage, M.M.S.; Molitor, R.; Jayasekara, T.; Sivakumar, P.; Jayakody, L.N. Trends in In-Silico Guided Engineering of Efficient Polyethylene Terephthalate (PET) Hydrolyzing Enzymes to Enable Bio-Recycling and Upcycling of PET. Comput. Struct. Biotechnol. J. 2023, 21, 3513–3521. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; You, S.; Lin, W.; Zhang, B.; Wang, M.; Su, R.; Qi, W. High Terephthalic Acid Purity: Effective Polyethylene Terephthalate Degradation Process Based on PH Regulation with Dual-Function Hydrolase. Bioresour. Technol. 2024, 413, 131461. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Liu, X.H.; Xu, N.; Pan, X.L. Efficient Biodegradation and Upcycling of Polyethylene Terephthalate Mediated by Cell-Factories. Front. Microbiol. 2025, 16, 1599470. [Google Scholar] [CrossRef]
- Knott, B.C.; Erickson, E.; Allen, M.D.; Gado, J.E.; Graham, R.; Kearns, F.L.; Pardo, I.; Topuzlu, E.; Anderson, J.J.; Austin, H.P.; et al. Characterization and Engineering of a Two-Enzyme System for Plastics Depolymerization. Proc. Natl. Acad. Sci. USA 2020, 117, 25476–25485. [Google Scholar] [CrossRef]
- Das, B.; Satyam; Patra, S. Some Recent Innovations Related to Enzyme Immobilization. Biocatalyst Immobilization Foundations and Applications. In Biocatalyst immobilization; Academic Press: Cambridge, MA, USA, 2023; pp. 149–163. [Google Scholar] [CrossRef]
- Pasula, R.R.; Lim, S.; Ghadessy, F.J.; Sana, B. The Influences of Substrates’ Physical Properties on Enzymatic PET Hydrolysis: Implications for PET Hydrolase Engineering. Eng. Biol. 2022, 6, 17–22. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Almdal, K.; Meyer, A.S. Significance of Poly(Ethylene Terephthalate) (PET) Substrate Crystallinity on Enzymatic Degradation. New Biotechnol. 2023, 78, 162–172. [Google Scholar] [CrossRef]

| Clone | Type | Primer (5′→3′) |
|---|---|---|
| Wild | Forward | AGAGAGGCTGAAGCTGAATTCATGTTCATCATGATTAAGAAATCTGAATTGGC |
| Reverse | CAATGATGATGATGATGATGGTCGACAGGACAATCCTTATAATCGTAAGCAGAGATTCT | |
| Mutant | Forward | AGAGAGGCTGAAGCTGAATTCATGTTCATCATGATTAAGAAATCTGAATTGGC |
| Reverse | ACCGAAACCAATAGCAGATTGTCTAGAG | |
| Forward | CTGCTATTGGTTTCGGTGGTG | |
| Reverse | GGAGTTTTAACAATTCCATAAGACTTATCAT | |
| Forward | TTATGGAATTGTTAAAACTCCAACTTTGGT | |
| Reverse | GTATTTCTTAGTCTCAGCAATGAAATCATCTTCACAAG | |
| Forward | TTCATTGCTGAGACTAAGAAATACGCTAACG | |
| Reverse | CAATGATGATGATGATGATGGTCGACAGGACAATCCTTATAATCGTAAGCAGAGATTCT |
| Independent Variable | Symbol | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | ||
| Amount of enzyme (U) | X1 | 0 | 12 | 21 | 30 | 42 |
| Reaction time (Days) | X2 | 2.5 | 8 | 12 | 16 | 21.5 |
| pH | X3 | 2.2 | 5 | 7 | 9 | 11 |
| Temperature (°C) | X4 | 13 | 20 | 25 | 30 | 37 |
| Agitation (RPM) | X5 | 5.5 | 40 | 65 | 90 | 124 |
| Variable | PET Degradation | |
|---|---|---|
| Regression Coefficients | p Value * | |
| A-Amount of Enzyme | 17.36 | 0.0001 |
| B-Reaction time | 4.96 | 0.0001 |
| C-pH | 3.00 | 0.0001 |
| D-Temperature | 1.46 | 0.0007 |
| E-Agitation | −1.16 | 0.0055 |
| AB | 0.37 | 0.4117 |
| AC | 0.94 | 0.0463 |
| AD | −0.37 | 0.4117 |
| AE | 0.81 | 0.0816 |
| BC | −0.19 | 0.6802 |
| BD | 0.37 | 0.4117 |
| BE | −0.81 | 0.0816 |
| CD | −1.06 | 0.0252 |
| CE | −1.37 | 0.0048 |
| DE | 0.56 | 0.2216 |
| A2 | −9.40 | 0.0001 |
| B2 | −4.28 | 0.0001 |
| C2 | −9.85 | 0.0001 |
| D2 | −4.80 | 0.0001 |
| E2 | −4.03 | 0.0001 |
| Lack of fit and R2 | ||
| Lack of Fit | Non-significant | |
| R2 | 0.9922 | |
| Adjusted R2 | 0.9868 | |
| Predicted R2 | 0.9641 | |
| Optimum Conditions | Coded Levels | Actual Levels |
|---|---|---|
| Amount of enzyme (U) | 0.66 | 27 |
| Reaction time (Days) | −0.1 | 11.60 |
| pH | 0.17 | 8.75 |
| Temperature (°C) | 0.12 | 25.60 |
| Agitation (RPM) | 0.84 | 86 |
| Response | Predicted Value | Experimental Value |
| Optimized PET degradation efficiency (%) | 100 | 98 ± 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satyam, S.; Patra, S. Engineered Mors1 Enzyme from the Antarctic Bacterium Moraxella TA144 for Enhanced Thermal Stability and Activity for Polyethylene Terephthalate Degradation. Processes 2025, 13, 3320. https://doi.org/10.3390/pr13103320
Satyam S, Patra S. Engineered Mors1 Enzyme from the Antarctic Bacterium Moraxella TA144 for Enhanced Thermal Stability and Activity for Polyethylene Terephthalate Degradation. Processes. 2025; 13(10):3320. https://doi.org/10.3390/pr13103320
Chicago/Turabian StyleSatyam, Satyam, and Sanjukta Patra. 2025. "Engineered Mors1 Enzyme from the Antarctic Bacterium Moraxella TA144 for Enhanced Thermal Stability and Activity for Polyethylene Terephthalate Degradation" Processes 13, no. 10: 3320. https://doi.org/10.3390/pr13103320
APA StyleSatyam, S., & Patra, S. (2025). Engineered Mors1 Enzyme from the Antarctic Bacterium Moraxella TA144 for Enhanced Thermal Stability and Activity for Polyethylene Terephthalate Degradation. Processes, 13(10), 3320. https://doi.org/10.3390/pr13103320







