Tuning Whey Protein Properties: Ohmic Heating Effects on Interfacial Properties and Hydrophobic and Hydrophilic Interactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Ohmic Heating
2.2. Free Sulfhydryls
2.3. Surface Hydrophobicity
2.4. Zeta Potential
2.5. Solubility
2.6. Particle Size
2.7. Intrinsic Fluorescence
2.8. Formation of Modified WPC/β-Carotene and Modified WPC/Caffeic Acid Complexes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Thermal and Electrical Parameters
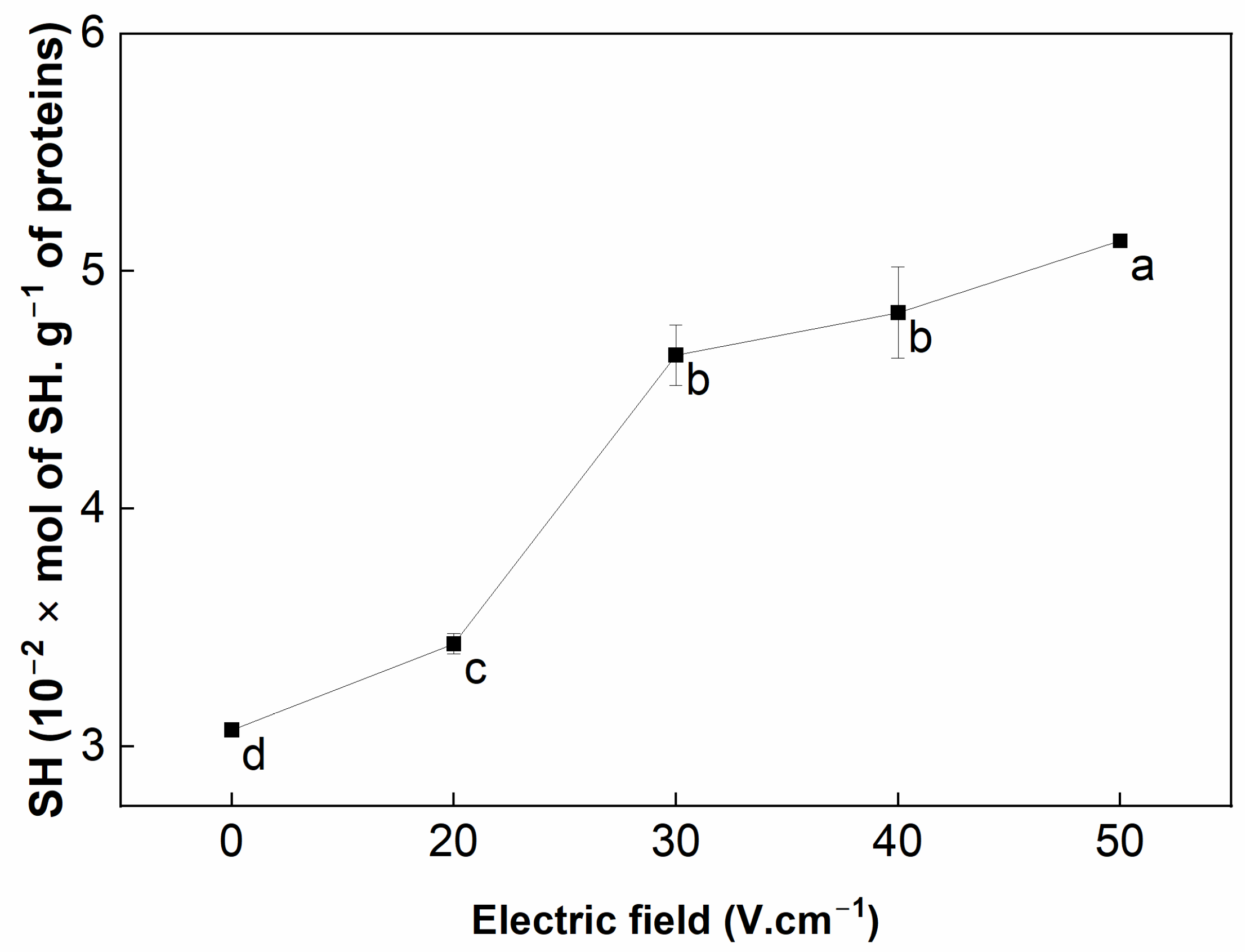
3.2. Free Sulfhydryl
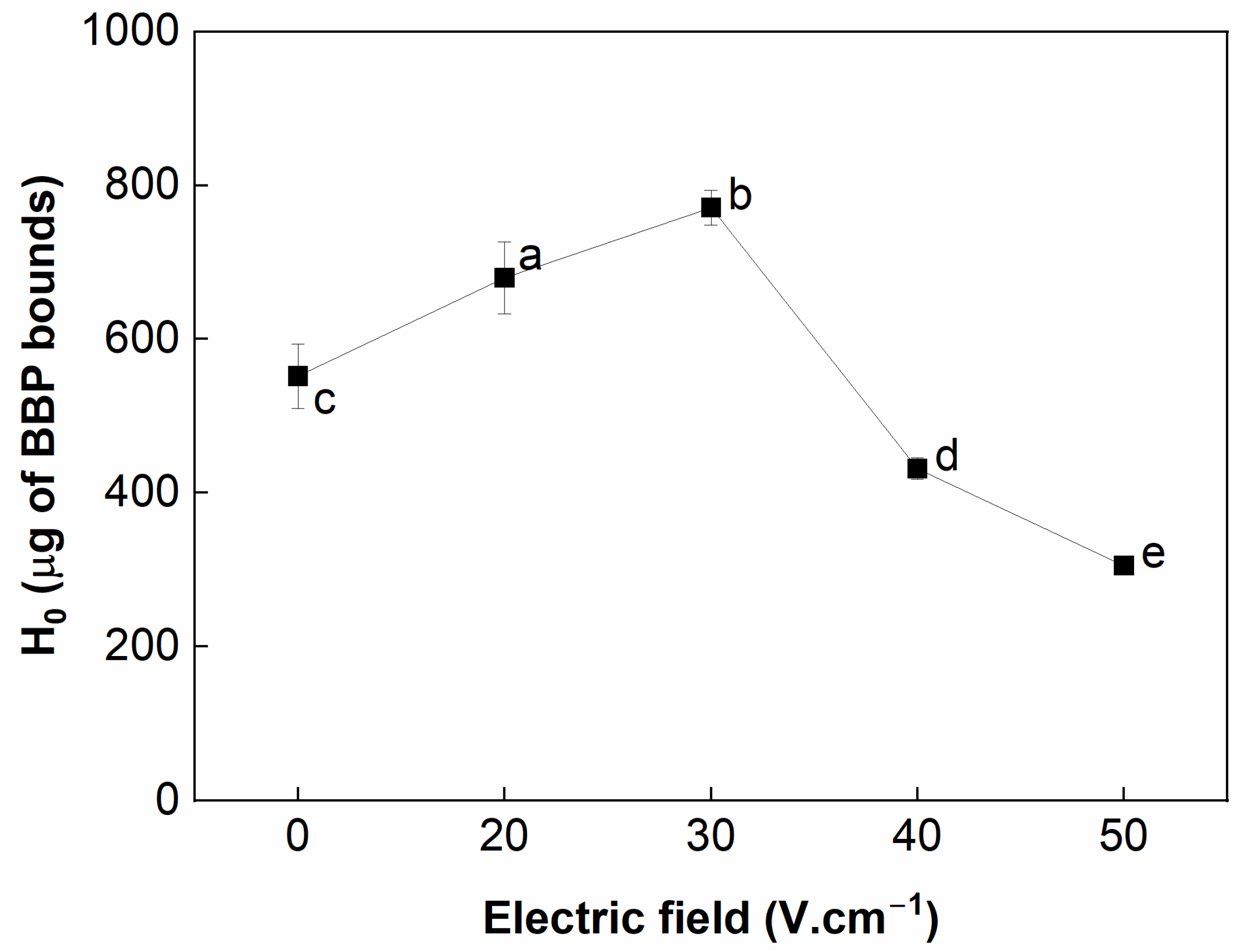
3.3. Surface Hydrophobicity
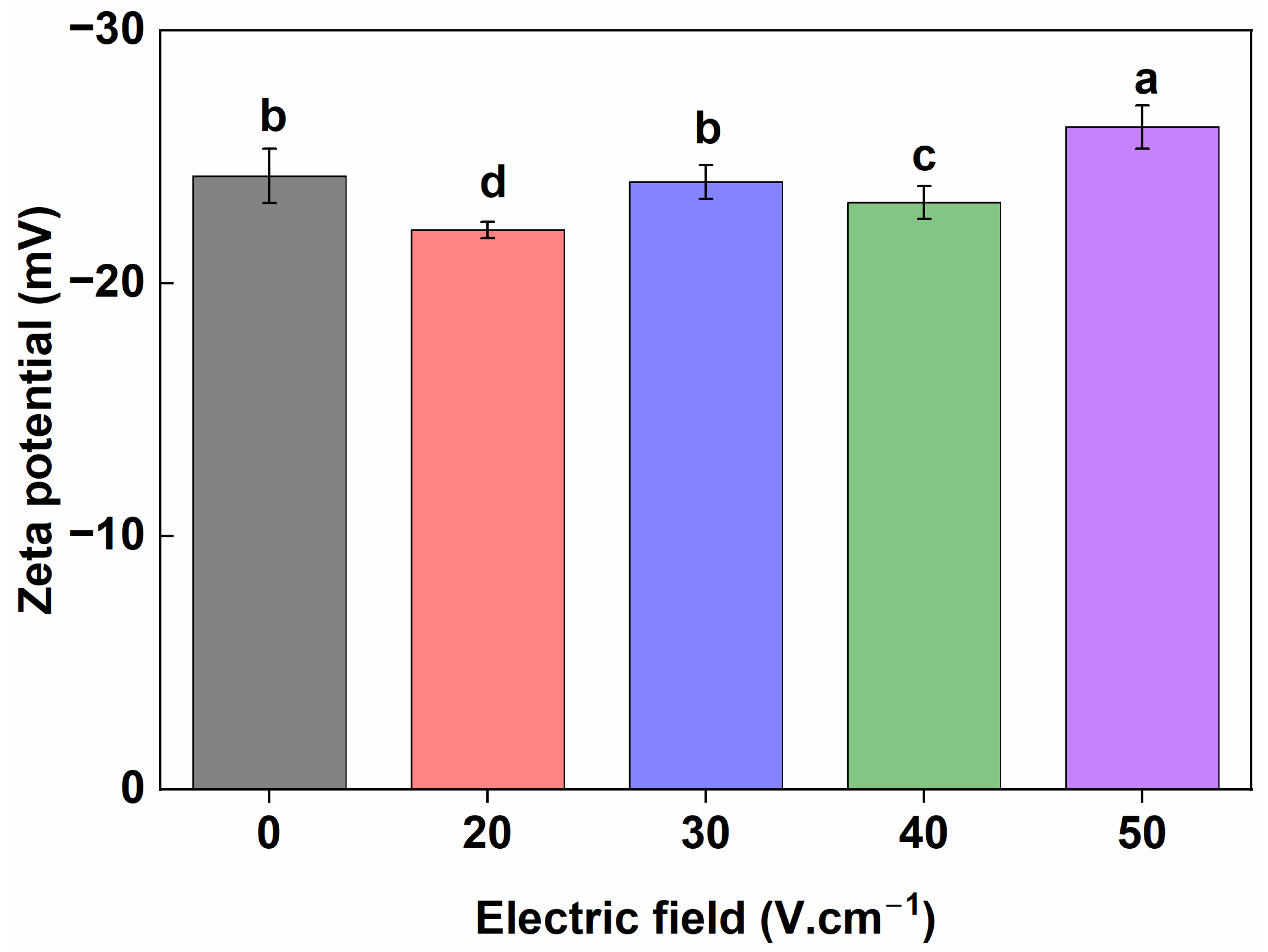
3.4. Zeta Potential
3.5. Intrinsic Fluorescence
3.6. Solubility
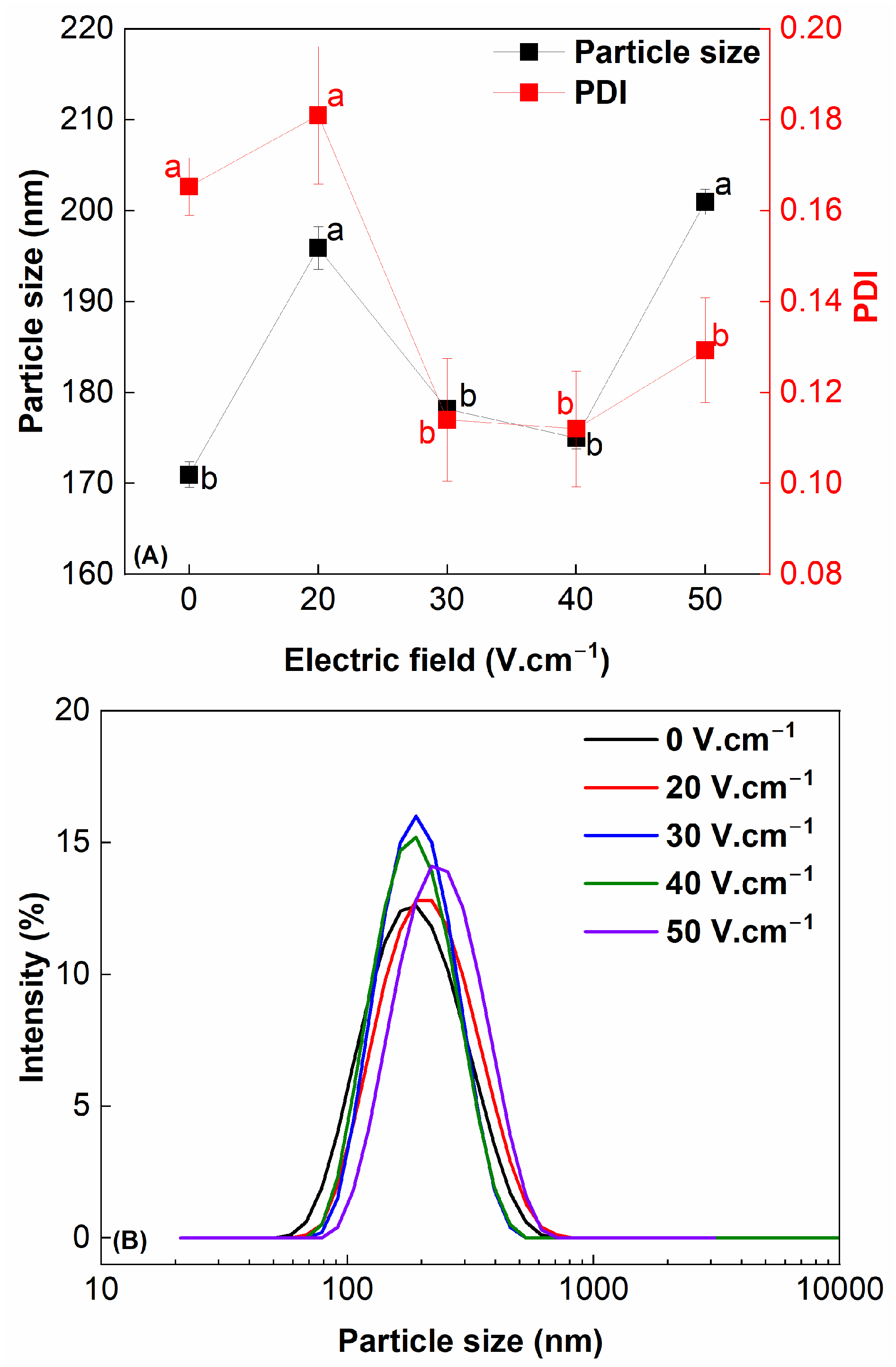
3.7. Particle Size
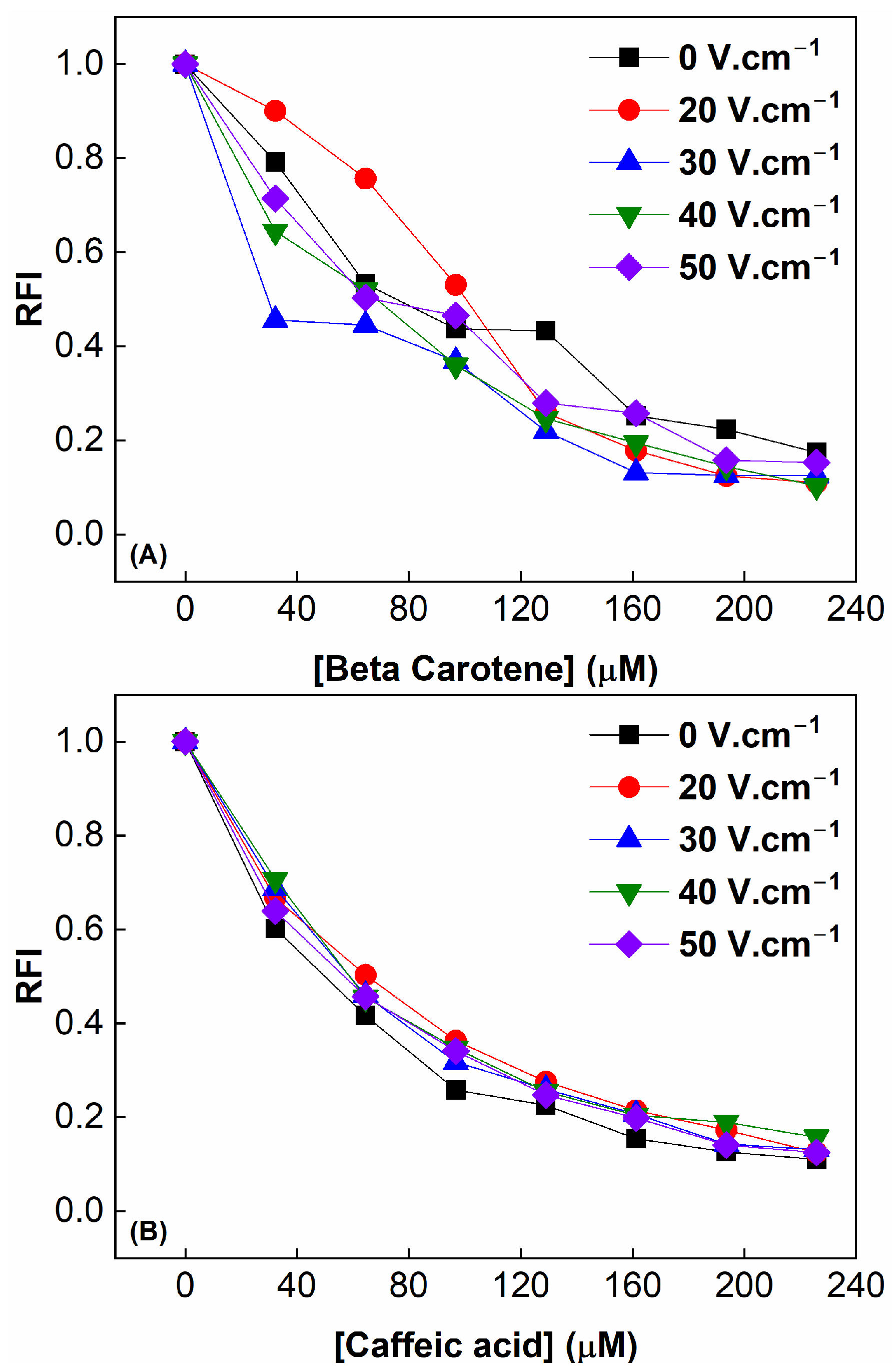
3.8. Relative Fluorescence Intrinsic
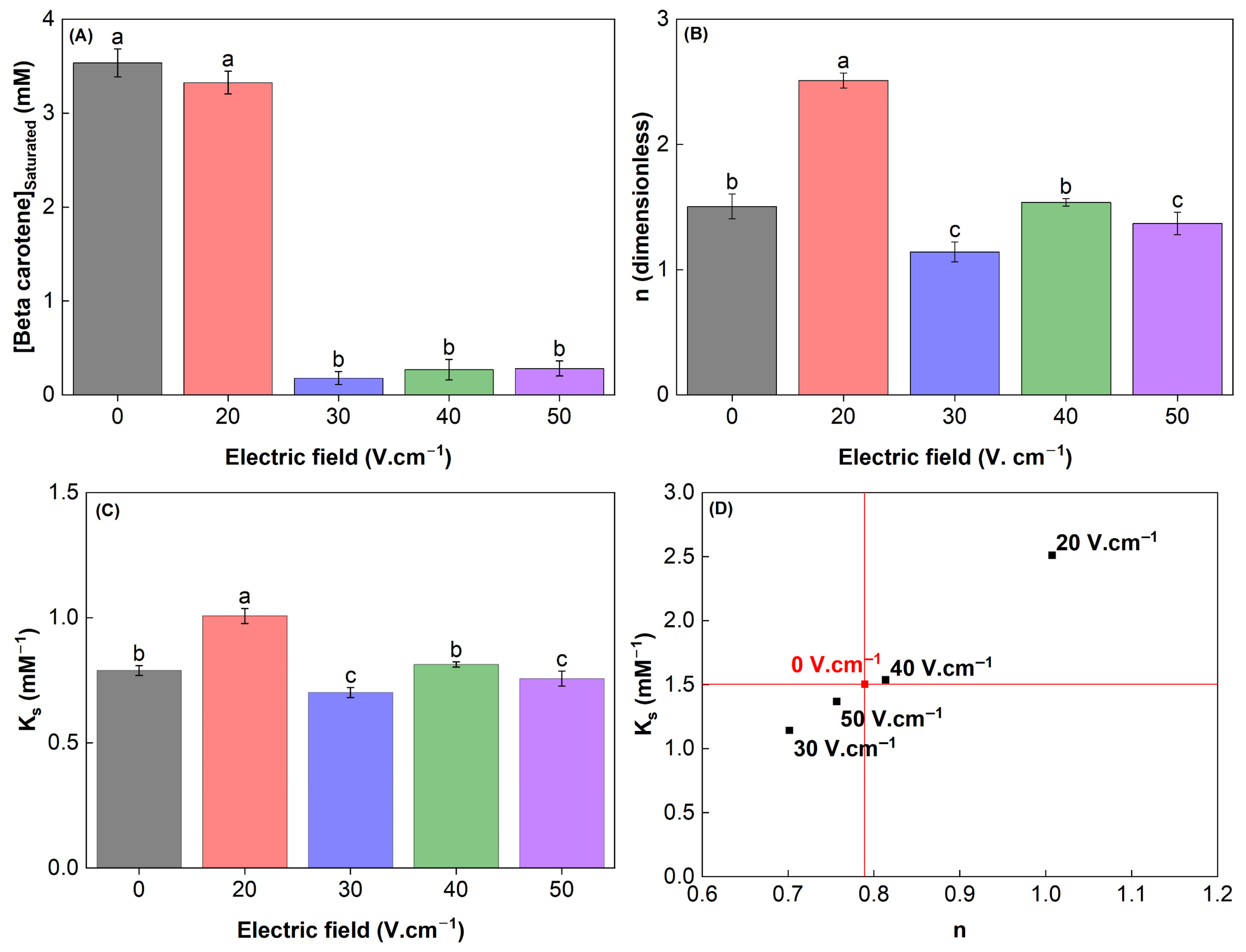
3.9. Interaction Between Modified WPC and β-Carotene
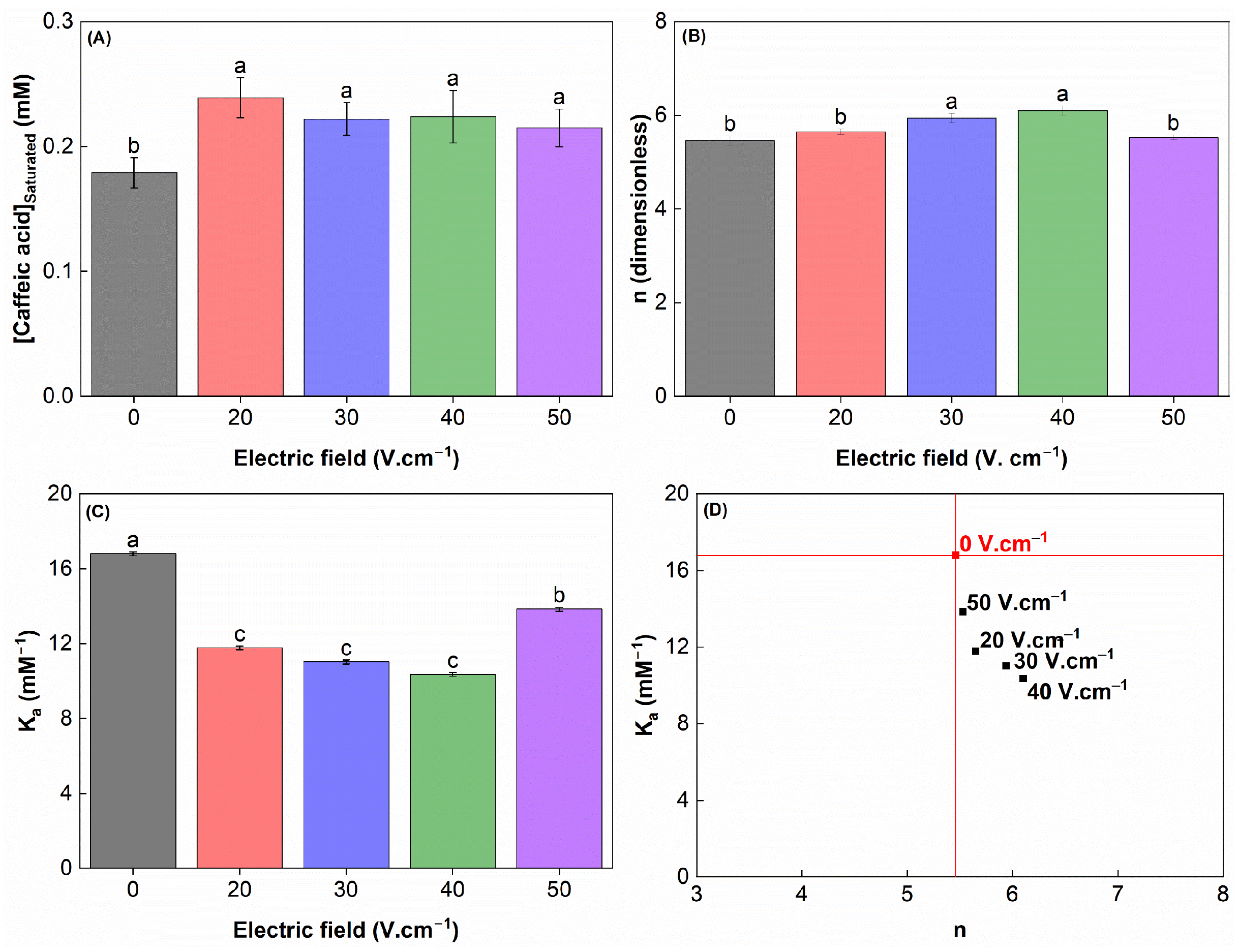
3.10. Interaction Between Modified WPC and Caffeic Acid
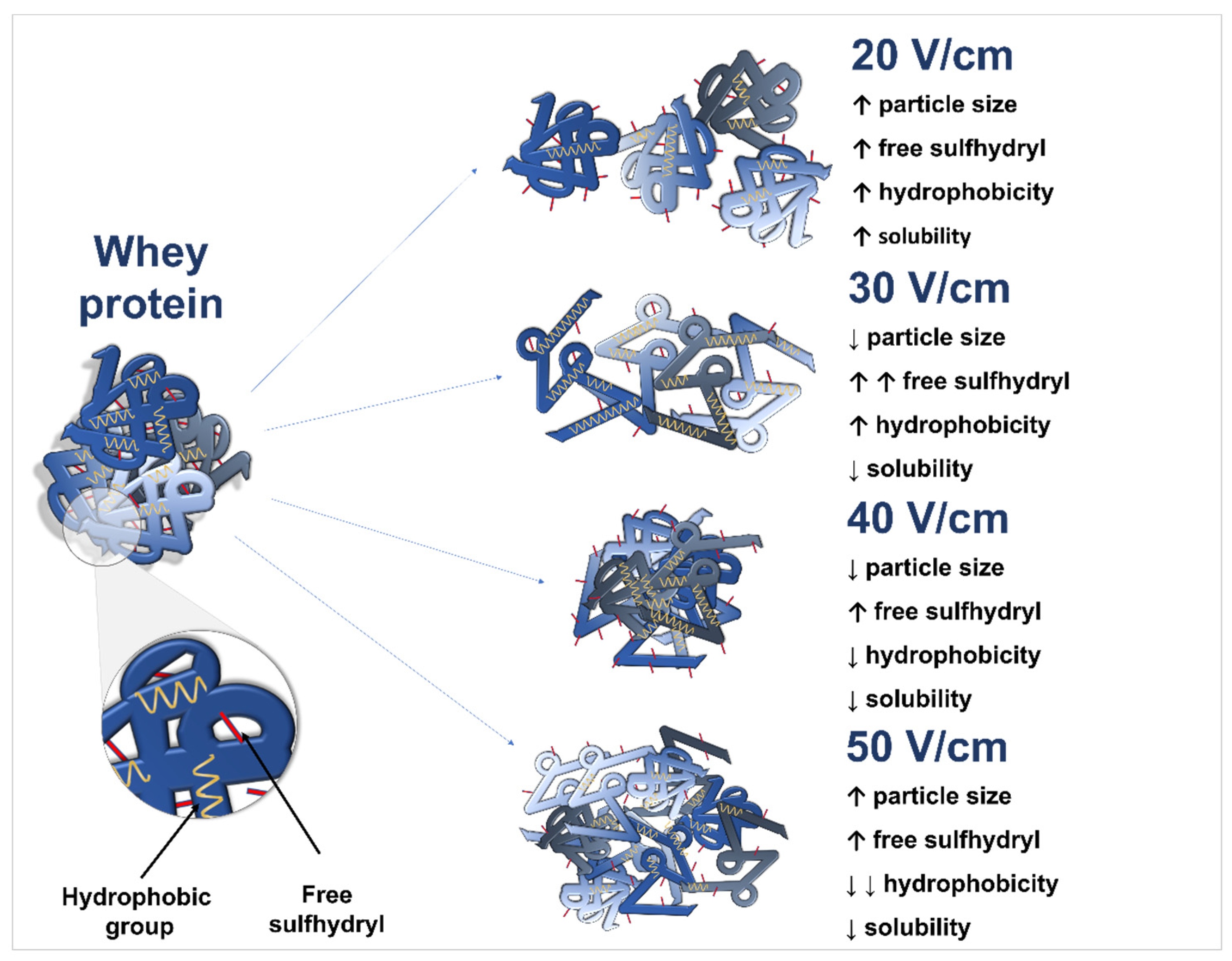
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frydenberg, R.P.; Hammershøj, M.; Andersen, U.; Greve, M.T.; Wiking, L. Protein Denaturation of Whey Protein Isolates (WPIs) Induced by High Intensity Ultrasound during Heat Gelation. Food Chem. 2016, 192, 415–423. [Google Scholar] [CrossRef]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI Aggregates: Formation, Structure and Applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, Y.; Zhang, A.; Dong, Y.; Wang, X.; Xu, N.; Chen, Q. The Effect of Preheated WPI Interaction with AN on Its Complexes Based on Protein Structure and Function. Food Meas. 2023, 17, 3272–3282. [Google Scholar] [CrossRef]
- Dos Santos, I.F.; Pimentel, T.C.; Da Cruz, A.G.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Ohmic Heating in Food Processing: An Overview of Plant-Based Protein Modification. Processes 2024, 12, 1800. [Google Scholar] [CrossRef]
- Rocha, R.S.; Silva, R.; Ramos, G.L.P.; Cabral, L.A.; Pimentel, T.C.; Campelo, P.H.; Blumer Zacarchenco, P.; Freitas, M.Q.; Esmerino, E.A.; Silva, M.C.; et al. Ohmic Heating Treatment in High-Protein Vanilla Flavored Milk: Quality, Processing Factors, and Biological Activity. Food Res. Int. 2022, 161, 111827. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Rocha, R.S.; Ramos, G.L.P.A.; Xavier-Santos, D.; Pimentel, T.C.; Lorenzo, J.M.; Henrique Campelo, P.; Cristina Silva, M.; Esmerino, E.A.; Freitas, M.Q.; et al. What Are the Challenges for Ohmic Heating in the Food Industry? Insights of a Bibliometric Analysis. Food Res. Int. 2022, 157, 111272. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.; Avelar, Z.; Leite, A.C.; Leal, R.; Pereira, R.S.; Vicente, A. Electrical Fields in the Processing of Protein-Based Foods. Foods 2024, 13, 577. [Google Scholar] [CrossRef]
- Ferreira, S.; Machado, L.; Pereira, R.N.; Vicente, A.A.; Rodrigues, R.M. Unraveling the Nature of Ohmic Heating Effects in Structural Aspects of Whey Proteins—The Impact of Electrical and Electrochemical Effects. Innov. Food Sci. Emerg. Technol. 2021, 74, 102831. [Google Scholar] [CrossRef]
- Sereechantarerk, C.; Hongsprabhas, P.; Chanput, W.; Kamonpatana, P. Effects of Ohmic Heating on Structural and Physicochemical Changes of Whey Proteins. Agric. Nat. Resour. 2021, 55, 464–472. [Google Scholar] [CrossRef]
- Černý, J.; Hobza, P. Non-Covalent Interactions in Biomacromolecules. Phys. Chem. Chem. Phys. 2007, 9, 5291. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.-H.; Sun, D.-W. Effects of Electric Fields and Electromagnetic Wave on Food Protein Structure and Functionality: A Review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Rodrigues, R.; Teixeira, J.A.; Vicente, A.A.; Xavier Malcata, F. Physical Effects upon Whey Protein Aggregation for Nano-Coating Production. Food Res. Int. 2014, 66, 344–355. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Hu, X.; Zhu, X.; Wang, L.; Zhang, N.; Yu, D. Structural and Physical Properties of Soybean Protein Isolate Films with Ohmic Heating Treatment: Impacts of Electric Field. Innov. Food Sci. Emerg. Technol. 2022, 82, 103213. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A.; Cappato, L.P.; da Silva Ferreira, M.V.; da Silva Rocha, R.; da Cruz, A.G. Ohmic Heating for the Dairy Industry: A Potential Technology to Develop Probiotic Dairy Foods in Association with Modifications of Whey Protein Structure. Curr. Opin. Food Sci. 2018, 22, 95–101. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Altinok, E.; Ramos, Ó.L.; Xavier Malcata, F.; Maresca, P.; Ferrari, G.; Teixeira, J.A.; Vicente, A.A. Development of Iron-Rich Whey Protein Hydrogels Following Application of Ohmic Heating—Effects of Moderate Electric Fields. Food Res. Int. 2017, 99, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Altay, I.; Kominami, Y.; Queiroz, L.S.; Huppertz, T.; Sloth, J.J.; Mohammadifar, M.A. Combined Effect of Ultrasonication and Ohmic Heating on Protein Functionality of Cheddar Cheese. Int. Dairy J. 2025, 160, 106116. [Google Scholar] [CrossRef]
- Icier, F. Influence of Ohmic Heating on Rheological and Electrical Properties of Reconstituted Whey Solutions. Food Bioprod. Process. 2009, 87, 308–316. [Google Scholar] [CrossRef]
- Cheng, W.; Li, C.; Xiao, F.; He, J.; Liu, L.; Niu, H.; Ma, J. Elucidating Binding Mechanisms of Caffeic Acid and Resveratrol by Beta-Lactoglobulin: Insights into Hydrophobic Interactions and Complex Formation. Food Hydrocoll. 2024, 146, 109269. [Google Scholar] [CrossRef]
- Qin, J.; Yang, M.; Wang, Y.; Wa, W.; Zheng, J. Interaction between Caffeic Acid/Caffeic Acid Phenethyl Ester and Micellar Casein. Food Chem. 2021, 349, 129154. [Google Scholar] [CrossRef]
- Stănciuc, N.; Râpeanu, G.; Bahrim, G.E.; Aprodu, I. The Interaction of Bovine β-Lactoglobulin with Caffeic Acid: From Binding Mechanisms to Functional Complexes. Biomolecules 2020, 10, 1096. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Guo, H.; Ding, Q.; Liu, R.; Ren, F. Interaction of Milk Whey Protein with Common Phenolic Acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Krichanã, D.; Rigolon, T.C.B.; Santos, T.A.; Renhe, I.R.T.; Felisberto, M.H.F.; De Araújo Bezerra, J.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Effects of Ohmic Heating on Interfacial, Rheology and Structure of Aquafaba Proteins and Products. Innov. Food Sci. Emerg. Technol. 2025, 104, 104081. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical Note: A Simplified Procedure for Myofibril Hydrophobicity Determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Moreira, T.C.P.; Pereira, R.N.; Vicente, A.A.; Da Cunha, R.L. Effect of Ohmic Heating on Functionality of Sodium Caseinate—A Relationship with Protein Gelation. Food Res. Int. 2019, 116, 628–636. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Avelar, Z.; Saraiva, J.A.; Vicente, A.A.; Rodrigues, R.M. Unravelling the Impact of Ohmic Heating on Commercial Pea Protein Structure. Food Hydrocoll. 2024, 150, 109748. [Google Scholar] [CrossRef]
- Visentini, F.F.; Sponton, O.E.; Perez, A.A.; Santiago, L.G. Formation and Colloidal Stability of Ovalbumin-Retinol Nanocomplexes. Food Hydrocoll. 2017, 67, 130–138. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein Nanovehicles Produced from Egg White. Part 1: Effect of pH and Heat Treatment Time on Particle Size and Binding Capacity. Food Hydrocoll. 2017, 73, 67–73. [Google Scholar] [CrossRef]
- Le Maux, S.; Bouhallab, S.; Giblin, L.; Brodkorb, A.; Croguennec, T. Complexes between Linoleate and Native or Aggregated β-Lactoglobulin: Interaction Parameters and in Vitro Cytotoxic Effect. Food Chem. 2013, 141, 2305–2313. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Cabral, L.; Guimarães, J.T.; Noronha, M.F.; Cappato, L.P.; Cruz, A.G.; Sant’Ana, A.S. Conventional and Ohmic Heating Pasteurization of Fresh and Thawed Sheep Milk: Energy Consumption and Assessment of Bacterial Microbiota during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2022, 76, 102947. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mahmoudi, M.R.; Roohi, R.; Torres, I.; Saraiva, J.A. Statistical Modeling of the Inactivation of Spoilage Microorganisms during Ohmic Heating of Sour Orange Juice. LWT 2019, 111, 821–828. [Google Scholar] [CrossRef]
- Sakr, M.; Liu, S. A Comprehensive Review on Applications of Ohmic Heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Halder, U.C.; Chakraborty, J.; Das, N.; Bose, S. Tryptophan Dynamics in the Exploration of Micro-Conformational Changes of Refolded β-Lactoglobulin after Thermal Exposure: A Steady State and Time-Resolved Fluorescence Approach. J. Photochem. Photobiol. B Biol. 2012, 109, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.M.; Vicente, A.A.; Petersen, S.B.; Pereira, R.N. Electric Field Effects on β-Lactoglobulin Thermal Unfolding as a Function of pH—Impact on Protein Functionality. Innov. Food Sci. Emerg. Technol. 2019, 52, 1–7. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Martins, A.J.; Ramos, O.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A.; Pereira, R.N. Influence of Moderate Electric Fields on Gelation of Whey Protein Isolate. Food Hydrocoll. 2015, 43, 329–339. [Google Scholar] [CrossRef]
- Miranda, C.G.; Rodrigues, R.M.; Pereira, R.N.; Speranza, P.; Kurozawa, L.E.; Vicente, A.A.; Sato, A.C.K. Influence of Ohmic Heating on Lentil Protein Structure and Protein-Pectin Interactions. Innov. Food Sci. Emerg. Technol. 2023, 87, 103413. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, Emulsifying, and Interfacial Properties of Different Whey Protein Aggregates Obtained by Thermal Treatment. LWT 2021, 149, 111904. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, H.; Wang, H.; Wang, L.; Zhang, N.; Yu, D. Effects of Electric Field Intensity Regulation on Protein Aggregation Behaviour and Foaming Property of Soybean 7S Globulin. Int. J. Biol. Macromol. 2023, 248, 125784. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.-H.; Wen, Q.-H.; He, F.; Xu, F.-Y.; Chen, B.-R.; Zeng, X.-A. Combination of Pulsed Electric Field and pH Shifting Improves the Solubility, Emulsifying, Foaming of Commercial Soy Protein Isolate. Food Hydrocoll. 2023, 134, 108049. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Y.; Li, Z.; Li, T.; Shi, Y.; Xie, H.; Li, Y.; Su, H.; Li, Z. Application of Whey Protein Isolate Fibrils in Encapsulation and Protection of β-Carotene. Food Chem. 2021, 346, 128963. [Google Scholar] [CrossRef]
- Xi, C.; Kang, N.; Zhao, C.; Song, H.; Liu, Y.; Zhang, T. Effect of Reaction Temperature on the Protein Structure and the Ability to Encapsulate Β-carotene of WPI-dextran Conjugates. J. Food Sci. 2020, 85, 1707–1716. [Google Scholar] [CrossRef]
- Wang, R.; Guo, P.-F.; Yang, J.-P.; Huang, Y.-Y.; Wang, L.-H.; Li, J.; Lin, S.-Y.; Sheng, Q.-L.; Zeng, X.-A.; Teng, Y.-X. Exploration of the Regulatory Mechanism of Pulsed Electric Field on the Aggregation Behavior of Soybean Protein Isolates. Food Hydrocoll. 2025, 160, 110761. [Google Scholar] [CrossRef]
- Campelo, P.H.; Junqueira, L.A.; Resende, J.V.D.; Zacarias, R.D.; Fernandes, R.V.D.B.; Botrel, D.A.; Borges, S.V. Stability of Lime Essential Oil Emulsion Prepared Using Biopolymers and Ultrasound Treatment. Int. J. Food Prop. 2017, 20, S564–S579. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Chen, X.; Wu, Z.; Zhong, W.; Yu, D.; Zhang, H. Effects of Moderate Electric Field on the Structural Properties and Aggregation Characteristics of Soybean Protein Isolate. Food Hydrocoll. 2022, 133, 107911. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Machado, L.; Ferreira, S.; Costa, J.; Villa, C.; Barreiros, M.P.; Mafra, I.; Teixeira, J.A.; Vicente, A.A. Influence of Ohmic Heating on the Structural and Immunoreactive Properties of Soybean Proteins. LWT 2021, 148, 111710. [Google Scholar] [CrossRef]
- Allahdad, Z.; Varidi, M.; Zadmard, R.; Saboury, A.A.; Haertlé, T. Binding of β-Carotene to Whey Proteins: Multi-Spectroscopic Techniques and Docking Studies. Food Chem. 2019, 277, 96–106. [Google Scholar] [CrossRef]
- Protte, K.; Bollow, C.; Sonne, A.; Menéndez-Aguirre, O.; Weiss, J.; Hinrichs, J. Impacts on Micro- and Macro-Structure of Thermally Stabilised Whey Protein-Pectin Complexes: A Fluorescence Approach. Food Biophys. 2016, 11, 226–234. [Google Scholar] [CrossRef]
- Relkin, P.; Shukat, R. Food Protein Aggregates as Vitamin-Matrix Carriers: Impact of Processing Conditions. Food Chem. 2012, 134, 2141–2148. [Google Scholar] [CrossRef]
- Du, H.; Lin, Y.; Stanton, C.; Daniloski, D.; Zannini, E.; Ross, R.P.; Miao, S. Characterization and Functional Properties of pH- and Heated Time-Induced Aggregates from Red Lentil Protein. Food Struct. 2023, 37, 100342. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Balaso Watharkar, R.; Ding, T.; Ye, X.; Liu, D. Effect of pH-Shifting Treatment on Structural and Functional Properties of Whey Protein Isolate and Its Interaction with (−)-Epigallocatechin-3-Gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Ramos, Ó.L.; Xavier Malcata, F.; Teixeira, J.A.; Vicente, A.A. Production of Whey Protein-Based Aggregates Under Ohmic Heating. Food Bioprocess Technol. 2016, 9, 576–587. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Liu, B.; Han, C.; Wang, T.; Pan, M.; Yu, D. Effect of Ohmic Heating-Assisted Glycation Reaction on the Properties of Soybean Protein Isolate-Chitosan Complexes. Int. J. Biol. Macromol. 2024, 283, 137859. [Google Scholar] [CrossRef] [PubMed]
- Sponton, O.E.; Perez, A.A.; Carrara, C.R.; Santiago, L.G. Linoleic Acid Binding Properties of Ovalbumin Nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ghumro, J.A.; Su, J.; Li, C.; He, Z.; Yuan, J. Sustainable Treatment of Amoxicillin-Contaminated Wastewater Using Fe2+/H2O2/AC: Performance, Stability, and Environmental Impact. Processes 2025, 13, 1054. [Google Scholar] [CrossRef]
- Aprodu, I.; Ursache, F.-M.; Turturică, M.; Râpeanu, G.; Stănciuc, N. Thermal Stability of the Complex Formed between Carotenoids from Sea Buckthorn (Hippophae Rhamnoides L.) and Bovine β-Lactoglobulin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 562–571. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Rasera, M.L.; Vidotto, D.C.; Mercadante, A.Z.; Tavares, G.M. Binding of Carotenoids to Milk Proteins: Why and How. Trends Food Sci. Technol. 2021, 110, 280–290. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Muhoza, B.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X.; Ho, C.-T. Whey Protein Isolate-Dextran Conjugates: Decisive Role of Glycation Time Dependent Conjugation Degree in Size Control and Stability Improvement of Colloidal Nanoparticles. LWT 2021, 148, 111766. [Google Scholar] [CrossRef]
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the Physicochemical Stability of β-Carotene Solid Lipid Nanoparticle (SLNP) Using Whey Protein Isolate. Food Res. Int. 2018, 105, 962–969. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L. Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37291-4. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, I.F.; Bieler, P.D.; Horta, G.O.; Rigolon, T.C.B.; da Cruz, A.G.; Stringheta, P.C.; Martins, E.; Campelo, P.H. Tuning Whey Protein Properties: Ohmic Heating Effects on Interfacial Properties and Hydrophobic and Hydrophilic Interactions. Processes 2025, 13, 3305. https://doi.org/10.3390/pr13103305
dos Santos IF, Bieler PD, Horta GO, Rigolon TCB, da Cruz AG, Stringheta PC, Martins E, Campelo PH. Tuning Whey Protein Properties: Ohmic Heating Effects on Interfacial Properties and Hydrophobic and Hydrophilic Interactions. Processes. 2025; 13(10):3305. https://doi.org/10.3390/pr13103305
Chicago/Turabian Styledos Santos, Israel Felipe, Philippe Defáveri Bieler, Gabriel Oliveira Horta, Thais Caroline Buttow Rigolon, Adriano Gomes da Cruz, Paulo Cesar Stringheta, Evandro Martins, and Pedro Henrique Campelo. 2025. "Tuning Whey Protein Properties: Ohmic Heating Effects on Interfacial Properties and Hydrophobic and Hydrophilic Interactions" Processes 13, no. 10: 3305. https://doi.org/10.3390/pr13103305
APA Styledos Santos, I. F., Bieler, P. D., Horta, G. O., Rigolon, T. C. B., da Cruz, A. G., Stringheta, P. C., Martins, E., & Campelo, P. H. (2025). Tuning Whey Protein Properties: Ohmic Heating Effects on Interfacial Properties and Hydrophobic and Hydrophilic Interactions. Processes, 13(10), 3305. https://doi.org/10.3390/pr13103305






