Electron Microscopy Reveals Variation in Starch Granules in Rice Grains Related to Glycemic Index
Abstract
1. Introduction
2. Materials and Methods
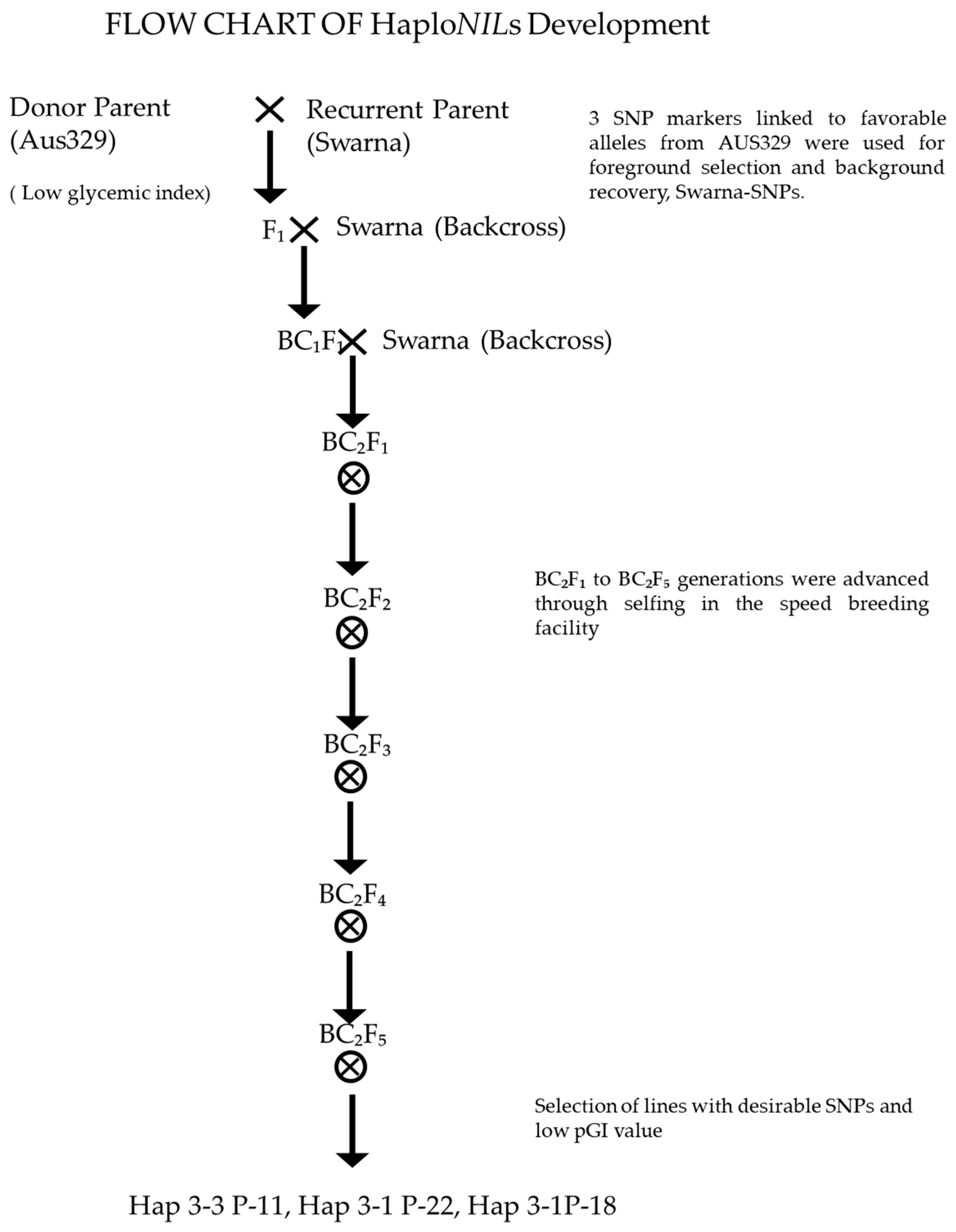
2.1. Plant Materials
2.2. Preparation and Extraction of Starch
2.3. Scanning Electron Microscopy (SEM)
2.4. Estimation of Different Starch Biochemical Properties of Rice
2.4.1. Amylose Content
2.4.2. Total Starch
2.4.3. Glycemic Index
2.4.4. Gel Consistency
2.4.5. Optimum Cooking Time
2.4.6. Water Uptake Ratio
2.5. Grain Appearance and Textural Quality
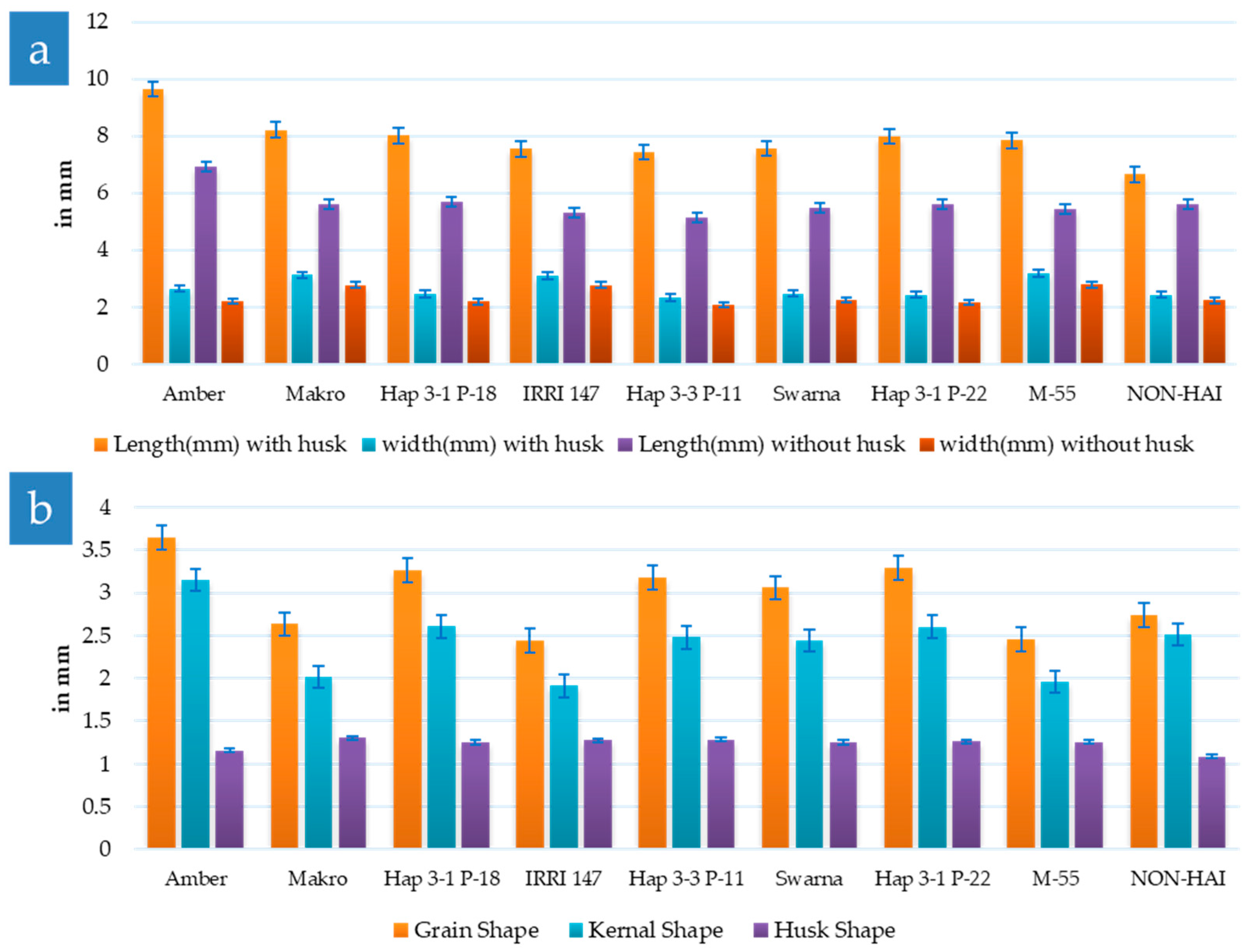
2.5.1. Grain Length and Breadth
2.5.2. Grain Shape
2.5.3. Kernel Shape
2.5.4. Husk Shape
2.5.5. Bulk Density
2.6. Expression Analysis of Genes Related to Amylose and Amylopectin Synthesis in Rice Genotypes
2.6.1. Collection of Leaf Samples
2.6.2. RNA Isolation and cDNA Preparation
2.6.3. Semi-Quantitative RT-PCR
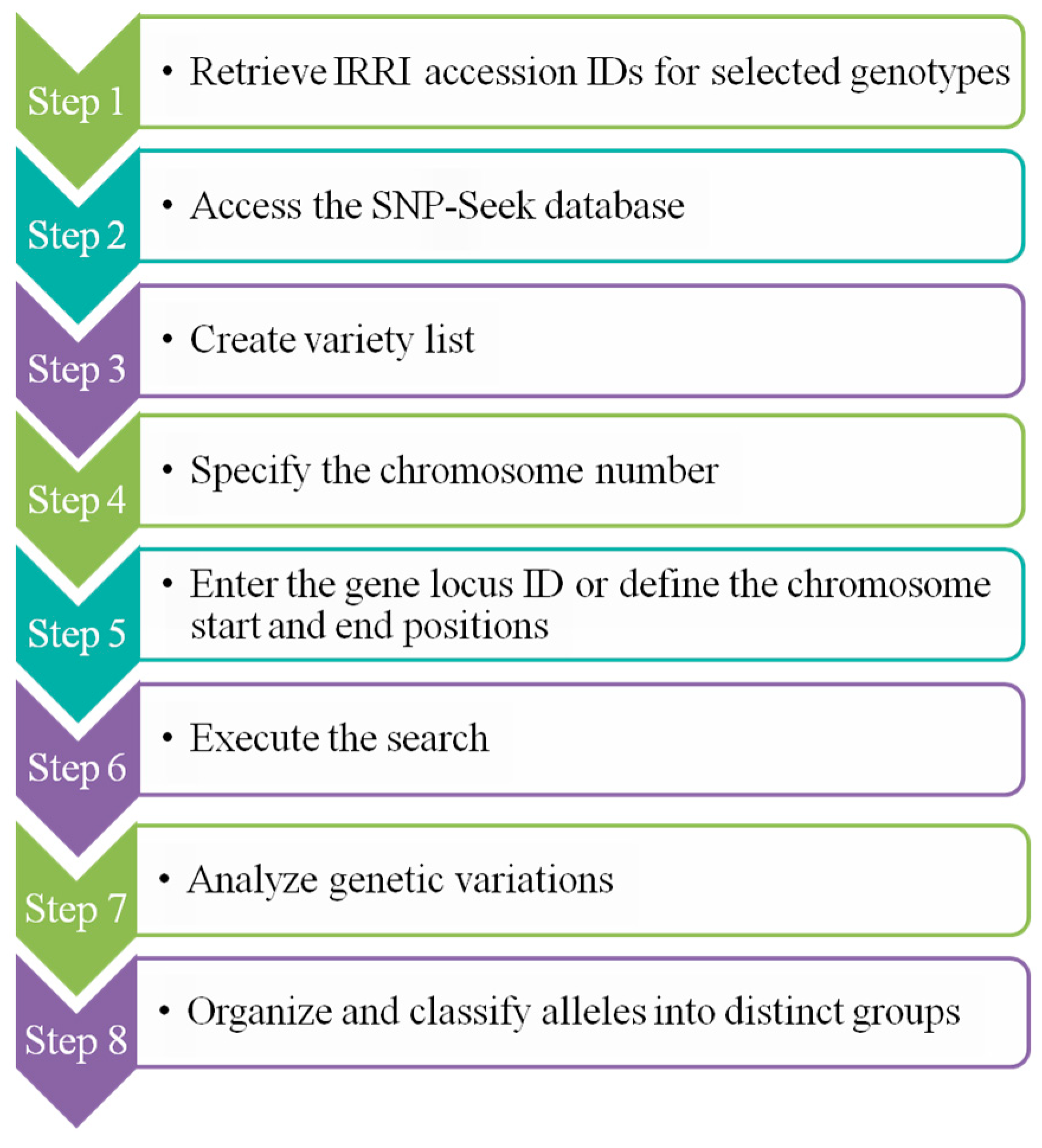
2.7. Analysis of Genetic Variability for Starch Biosynthesis-Related Genes and Identification of Alleles for Low and High Amylose Content
2.8. Statistical Analysis
3. Results
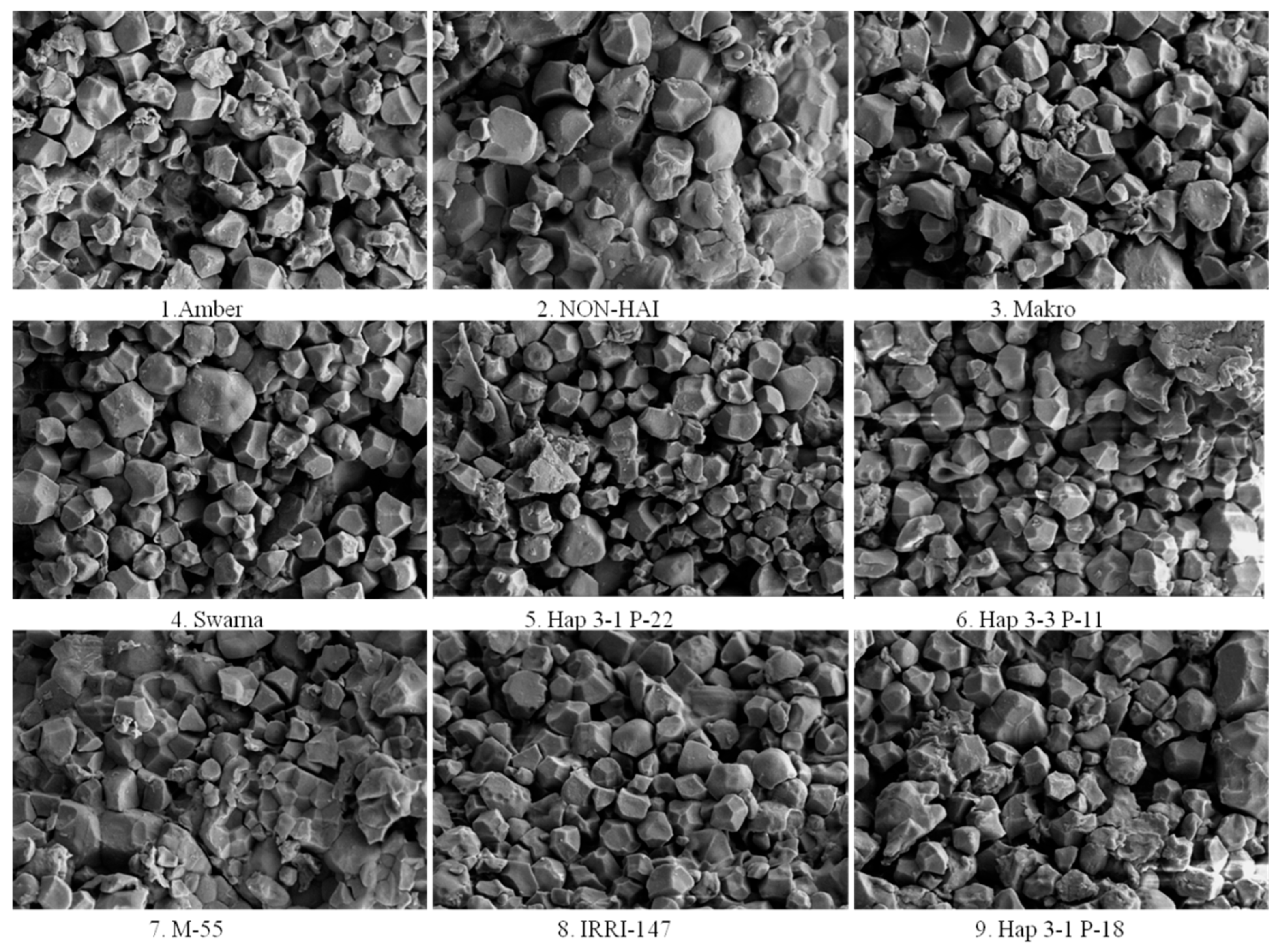
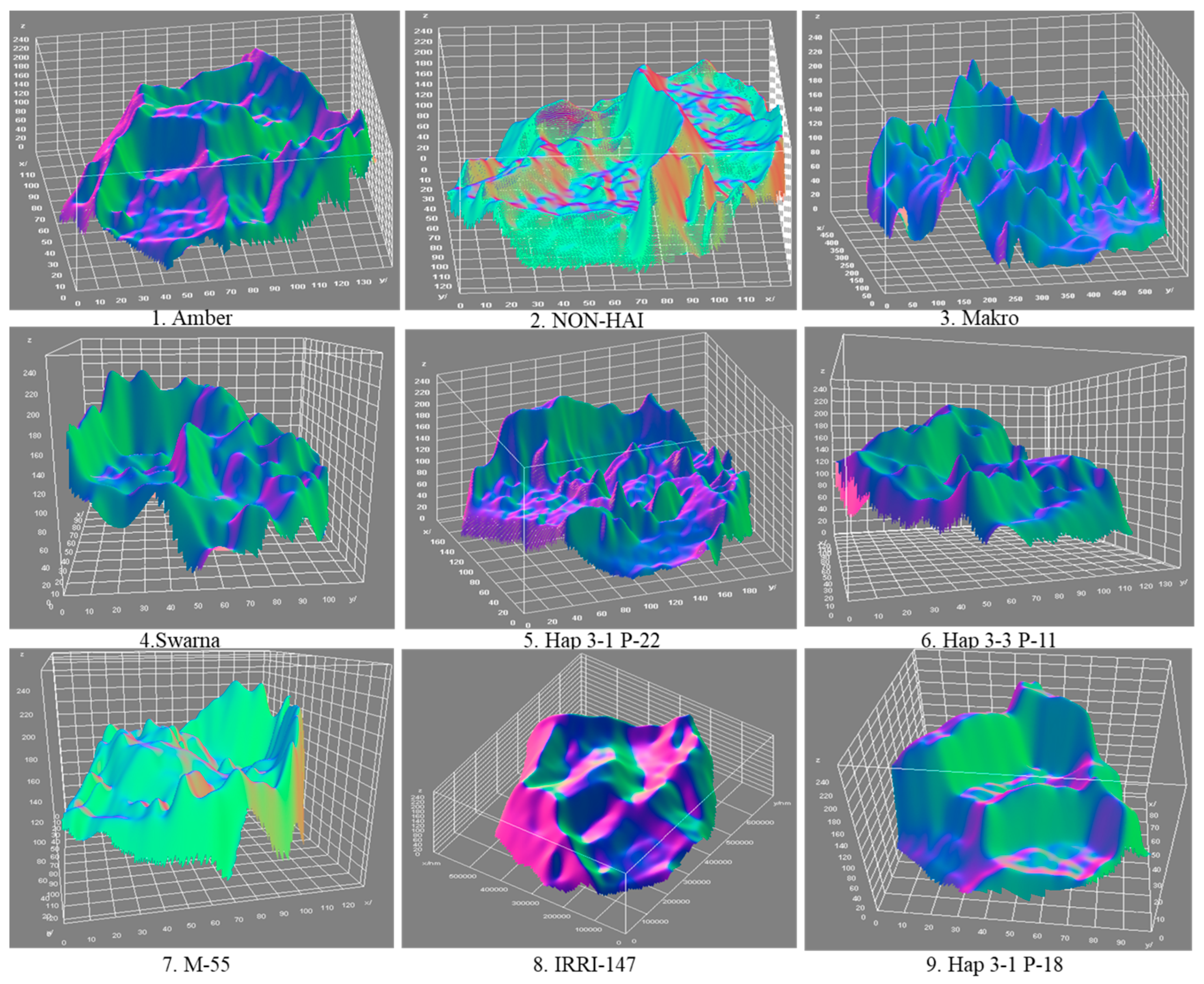
3.1. Starch Granule Size and Morphology
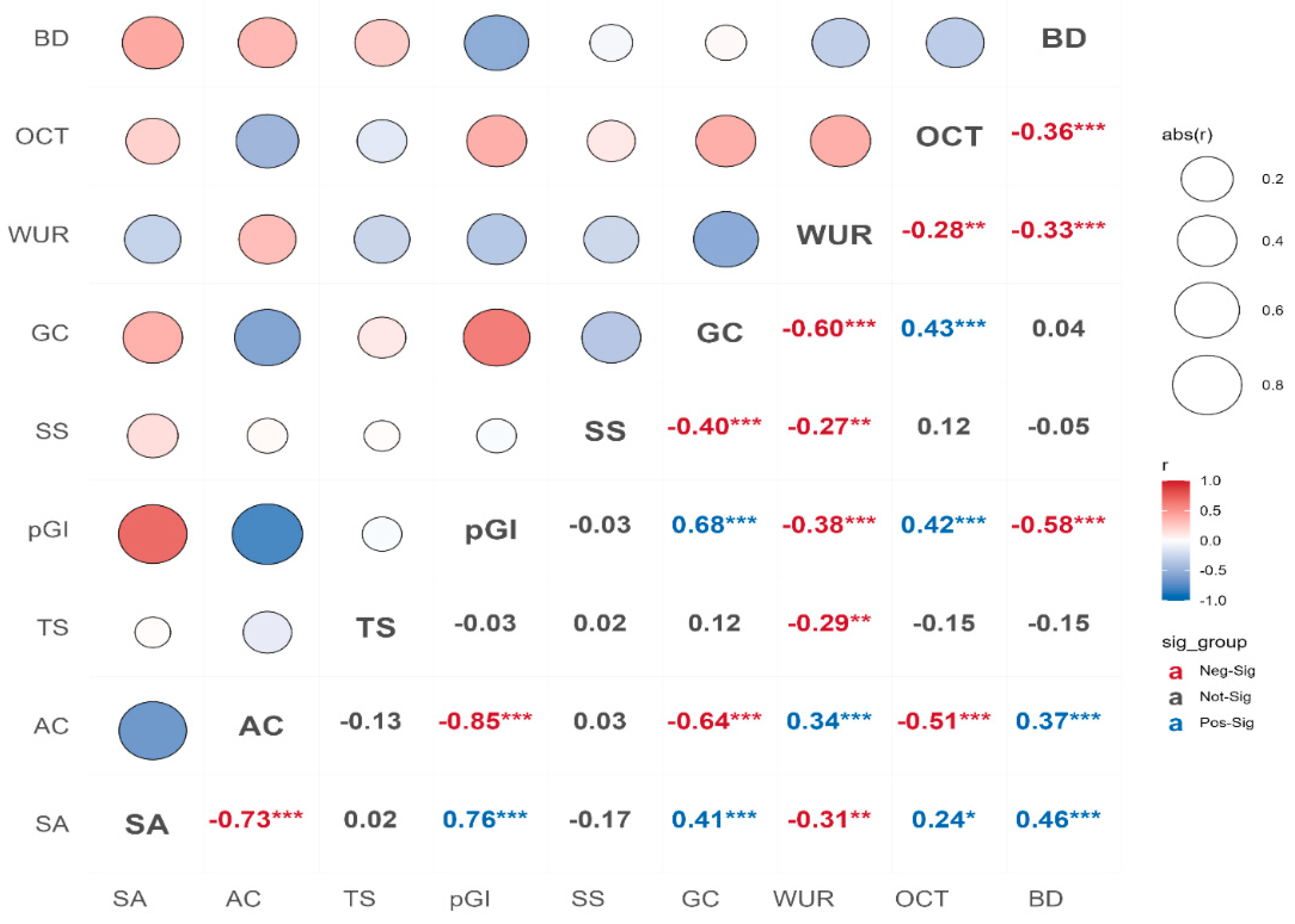
3.2. Amylose Content, Total Starch, and Glycemic Index
3.3. Gel Consistency, Optimum Cooking Time, and Water Uptake Ratio
3.4. Physio-Morphology of Rice Grains
3.5. Expression Analysis of Genes Related to Amylose and Amylopectin Synthesis
3.6. Genetic Variability and Identification of Allele
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GI | Glycemic index |
| pGI | Predicted glycemic index |
| AC | Amylose content |
| SG | Starch granule |
| GC | Gel consistency |
| OsSSIIb | Rice Starch Synthase IIb |
| OsGBP | Rice Granule-Binding Protein |
| OsFLO6 | Rice Granule-Binding Protein FLO6 |
| OsISA3 | Rice Isoamylase 3 |
| SSIIa | Starch Synthase IIa |
| SBE | Starch Branching Enzyme |
| SBEIIb | Starch Branching Enzyme IIb |
| OsBt1 | Brittle 1 |
| RS | Resistant starch |
| GBSSI (Wx) | Granule-Bound Starch Synthase I (Waxy Gene) |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| Wx | Waxy gene (in relation to starch synthesis) |
| Mins | Minutes |
| NILs | Near isogenic lines |
| IGKV | Indira Gandhi KrishiVishwavidyalaya |
| NaOH | Sodium hydroxide |
| HCl | Hydrochloric acid |
| AMG | Amyloglucosidase |
| GOPOD | Glucose oxidase peroxidase |
| WUR | Water uptake ratio |
| KOH | Potassium hydroxide |
| DEPC | Diethylpyrocarbonate |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SNP | Single nucleotide polymorphism |
| NCBI | National Center for Biotechnology Information |
| L/W | Length-to-width ratio |
References
- Butardo, V.M., Jr.; Sreenivasulu, N. Improving rice grain quality: State-of-the-art approaches and future prospects. In Rice Grain Quality: Methods and Protocols; Springer: Cham, Switzerland, 2016; pp. 139–179. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Smith, A.M. The biosynthesis of starch granules. Biomacromolecules 2001, 2, 335–341. [Google Scholar] [CrossRef]
- Asare, E.K.; Jaiswal, S.; Maley, J.; Båga, M.; Sammynaiken, R.; Rossnagel, B.G.; Chibbar, R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agric. Food Chem. 2011, 59, 4743–4754. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Zeng, D.L.; Cui, X.; Zhou, Y.H.; Yan, M.X.; Huang, D.N.; Li, J.Y.; Qian, Q. Map-based cloning of the ALK gene, which controls the gelatinization temperature of rice. Sci. China Ser. C 2003, 46, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.K.; Båga, M.; Rossnagel, B.G.; Chibbar, R.N. Polymorphism in the barley granule bound starch synthase 1 (GBSS1) gene associated with grain starch variant amylose concentration. J. Agric. Food Chem. 2012, 60, 10082–10092. [Google Scholar] [CrossRef]
- Nakamura, Y.; Francisco, P.B.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Botella, J.R.; Zhao, Y. Genome editing—Principles and applications for functional genomics research and crop improvement. Crit. Rev. Plant Sci. 2017, 36, 291–309. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, D.; Zhao, D.; Li, Y.; Li, P.; Wu, B.; Gao, G.; Zhang, Q.; Wang, G.; Xiao, J.; et al. The origin of Wxla provides new insights into the improvement of grain quality in rice. J. Integr. Plant Biol. 2020, 63, 878–888. [Google Scholar] [CrossRef]
- Fitzgerald, M.A.; Rahman, S.; Resurreccion, A.P.; Concepcion, J.; Daygon, V.D.; Dipti, S.S.; Kabir, K.A.; Klingner, B.; Morell, M.K.; Bird, A.R. Identification of a Major Genetic Determinant of Glycaemic Index in Rice. Rice 2011, 4, 66–74. [Google Scholar] [CrossRef]
- Kaur, B.; Ranawana, V.; Henry, J. The Glycemic Index of Rice and Rice Products: A Review, and Table of GI Values. Crit. Rev. Food Sci. Nutr. 2016, 56, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Syahariza, Z.A.; Sar, S.; Hasjim, J.; Tizzotti, M.J.; Gilbert, R.G. The importance of amylose and amylopectin fine structures for starch digestibility in cooked rice grains. Food Chem. 2013, 136, 742–749. [Google Scholar] [CrossRef]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. Instrumental measurement of cooked rice texture by dynamic rheological testing and its relation to the fine structure of rice starch. Carbohydr. Polym. 2016, 146, 253–263. [Google Scholar] [CrossRef]
- Anacleto, R.; Cuevas, R.P.; Jimenez, R.; Llorente, C.; Nissila, E.; Henry, R.; Sreenivasulu, N. Prospects of breeding high-quality rice using post-genomic tools. Theor. Appl. Genet. 2015, 128, 1449–1466. [Google Scholar] [CrossRef]
- Juliano, B.O. Rice in human nutrition. Food and Agriculture Organization of the United Nations (FAO). 1993. Available online: https://www.fao.org/4/t0567e/t0567e00.htm (accessed on 2 July 2025).
- Bao, J. (Ed.) Rice: Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Huang, L.; Tan, H.; Zhang, C.; Li, Q.; Liu, Q. Starch biosynthesis in cereal endosperms: An updated review over the last decade. Plant Commun. 2021, 2, 100237. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Q.; Wang, J.; Song, Y.; Ye, H.; Zhang, X.; He, Y.; Sun, M.; Zhang, R.; Shi, G. Large-field objective lens for multi-wavelength microscopy at mesoscale and submicron resolution. Opto-Electron. Adv. 2024, 7, 230212. [Google Scholar] [CrossRef]
- Choe, S.-W.; Kim, B.; Kim, M. Progress of microfluidic continuous separation techniques for micro-/nanoscale bioparticles. Biosensors 2021, 11, 464. [Google Scholar] [CrossRef]
- Qin, F.; Man, J.; Xu, B.; Hu, M.; Gu, M.; Liu, Q.; Wei, C. Structural properties of hydrolyzed high-amylose rice starch by α-amylase from Bacillus licheniformis. J. Agric. Food Chem. 2011, 59, 12667–12673. [Google Scholar] [CrossRef]
- Juliano, B.O.; Bautista, G.M.; Lugay, J.C.; Reyes, A.C. Rice quality, Studies on physicochemical properties of rice. J. Agric. Food Chem. 1964, 12, 131–138. [Google Scholar] [CrossRef]
- Megazyme International Ireland Ltd. Total Starch Assay Kit (Amyloglucosidase/Alpha-Amylase Method) Assay Procedure. 2017. Available online: https://www.megazyme.com/total-starch-assay-kit (accessed on 2 July 2025).
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Cagampang, G.B.; Perez, C.M.; Juliano, B.O. A gel consistency test for eating quality of rice. J. Sci. Food Agric. 1973, 24, 1589–1594. [Google Scholar] [CrossRef]
- Wu, K.; Gunaratne, A.; Gan, R.; Bao, J.; Corke, H.; Jiang, F. Relationships between cooking properties and physicochemical properties in brown and white rice. Starch-Starke 2018, 70, 1700167. [Google Scholar] [CrossRef]
- Bhattacharya, K.R.; Sowbhagya, C.M. Water uptake by rice during cooking. Cereal Sci. Today 1971, 16, 420–424. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19721607559 (accessed on 2 July 2025).
- Fraser, B.M.; Verma, S.S.; Muir, W.E. Some physical properties of fababeans. J. Agric. Eng. Res. 1978, 23, 53–57. [Google Scholar] [CrossRef]
- Hirochika, H.; Guiderdoni, E.; An, G.; Hsing, Y.-I.; Eun, M.Y.; Han, C.-D.; Upadhyaya, N.M.; Ramachandran, S.; Zhang, Q.; Pereira, A.; et al. Rice mutant resources for gene discovery. Plant Mol. Biol. 2004, 54, 325–334. [Google Scholar] [CrossRef]
- Zhong, Y.; Blennow, A.; Kofoed-Enevoldsen, O.; Jiang, D.; Hebelstrup, K.H. Protein Targeting to Starch 1 is essential for starchy endosperm development in barley. J. Exp. Bot. 2018, 70, 485–496. [Google Scholar] [CrossRef]
- Yamanouchi, H.; Nakamura, Y. Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol. 1992, 33, 985–991. [Google Scholar] [CrossRef]
- Kubo, A.; Fujita, N.; Harada, K.; Matsuda, T.; Satoh, H.; Nakamura, Y. The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol. 1999, 121, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, Y.; Utsumi, C.; Sawada, T.; Fujita, N.; Nakamura, Y. Functional diversity of isoamylase oligomers: The ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm. Plant Physiol. 2011, 156, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-S.; Zeeman, S.C.; Thorneycroft, D.; Fulton, D.C.; Dunstan, H.; Lue, W.-L.; Hegemann, B.; Tung, S.-Y.; Umemoto, T.; Chapple, A.; et al. Alpha-amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J. Biol. Chem. 2005, 280, 9773–9779. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, F.; Ren, Y.; Zhou, K.; Lv, J.; Zheng, M.; Zhao, S.; Zhang, L.; Wang, C.; et al. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 2014, 77, 917–930. [Google Scholar] [CrossRef]
- Borule, T.; Pandey, V.K.; Singh, L.; Sathe, A.P.; Akanksha; Raut, P.M.; T.G., A.; Rana, S.S.; Shori, S.; Verulkar, S.B.; et al. Analysis of yield stability in diverse rice genotypes. J. Adv. Biol. Biotechnol. 2024, 27, 79–89. [Google Scholar] [CrossRef]
- Ramadoss, B.R.; Gangola, M.P.; Agasimani, S.; Jaiswal, S.; Venkatesan, T.; Sundaram, G.R.; Chibbar, R.N. Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J. Food Sci. Technol. 2019, 56, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Yang, Y.; Chen, X.; Wang, Q.; Zhang, X.; Ran, L.; Xiong, F. Morphology and physicochemical properties of starch in wheat superior and inferior grains. Starch-Starke 2018, 70, 1700177. [Google Scholar] [CrossRef]
- Zhao, F.; Jing, L.; Wang, D.; Bao, F.; Lu, W.; Wang, G. Grain and starch granule morphology in superior and inferior kernels of maize in response to nitrogen. Sci. Rep. 2018, 8, 6343. [Google Scholar] [CrossRef]
- Dhital, S.; Warren, F.J.; Butterworth, P.J.; Ellis, P.R.; Gidley, M.J. Mechanisms of Starch Digestion by α-Amylase—Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2016, 57, 875–892. [Google Scholar] [CrossRef]
- Chaichoompu, E.; Ruengphayak, S.; Wattanavanitchakorn, S.; Wansuksri, R.; Yonkoksung, U.; Suklaew, P.O.; Chotineeranat, S.; Raungrusmee, S.; Vanavichit, A.; Toojinda, T.; et al. Development of Whole-Grain Rice Lines Exhibiting Low and Intermediate Glycemic Index with Decreased Amylose Content. Foods 2024, 13, 3627. [Google Scholar] [CrossRef]
- Xu, H.; Xu, S.; Xu, Y.; Jiang, Y.; Li, T.; Zhang, X.; Yang, J.; Wang, L. Relationship between the physicochemical properties and amylose content of rice starch in rice varieties with the same genetic background. J. Cereal Sci. 2024, 118, 103932. [Google Scholar] [CrossRef]
- Foster-Powell, K.; Holt, S.C.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not Just a Grain of Rice: The Quest for Quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, R.P.; Fitzgerald, M.A. Genetic Diversity of Rice Grain Quality. In Genetic Diversity in Plants; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Zhang, X.; He, F.; Chen, Y.; Li, R.; Yao, J.; Zhang, M.; Zheng, W.; Yu, G. Genetic improvements in rice grain quality: A review of elite genes and their applications in molecular breeding. Agronomy 2023, 13, 1375. [Google Scholar] [CrossRef]
- Zhou, Y.; Miao, J.; Gu, H.; Peng, X.; Leburu, M.; Yuan, F.; Gu, H.; Gao, Y.; Tao, Y.; Zhu, J.; et al. Natural variations in SLG7 regulate grain shape in rice. Genetics 2015, 201, 1591–1599. [Google Scholar] [CrossRef]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
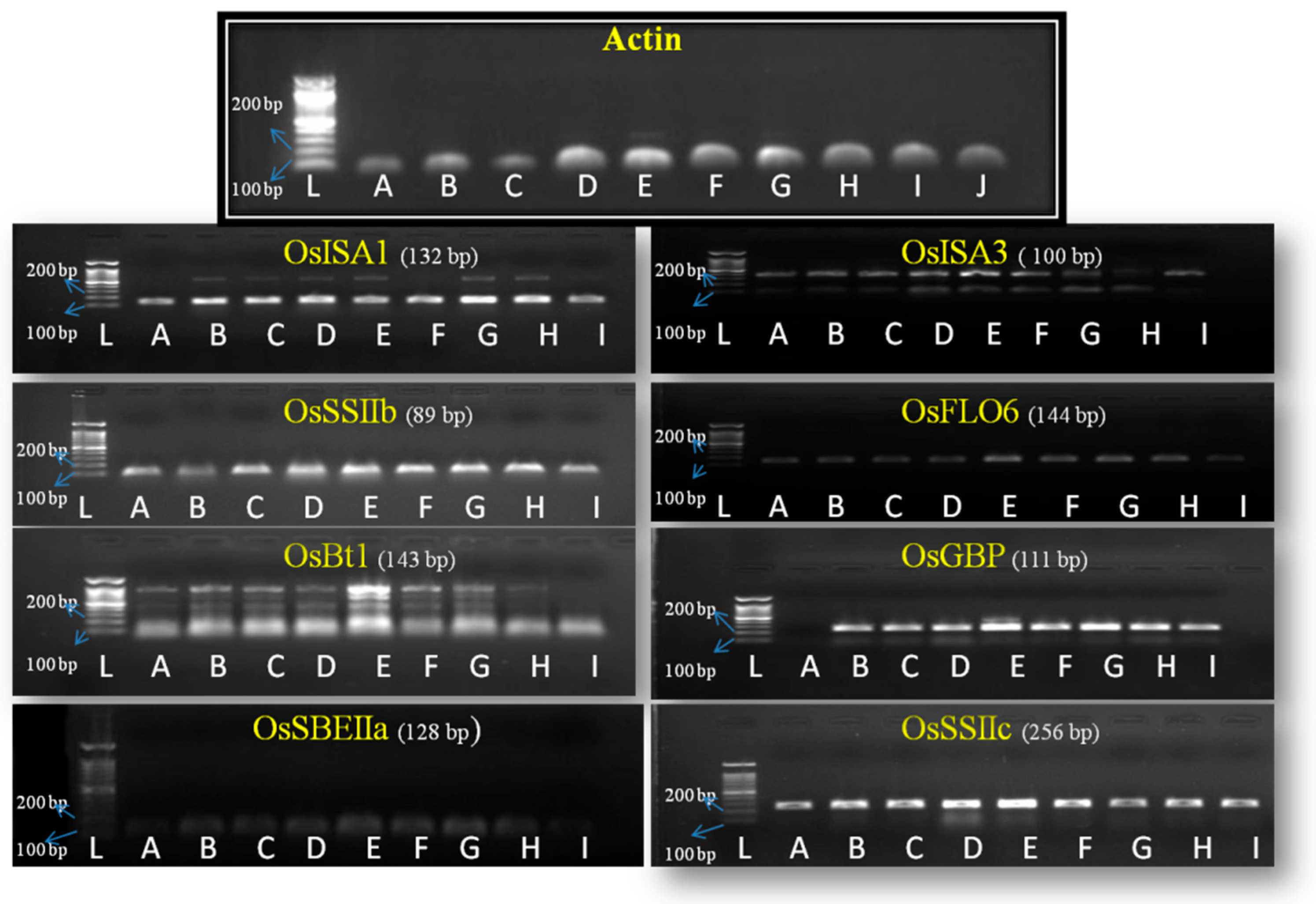
| Gene Name | Locus ID | Chromosome No | Tissue/Stage | Gene Function | Reference |
|---|---|---|---|---|---|
| OsSSIIb/SSII-2 | Os02g0744700 | 2 | Leaf | Elongation of α-glucan chains during amylopectin synthesis. | [9] |
| OsSSIIc/SSII-3 | Os10g0437600 | 10 | Endosperm | Elongation of α-glucan chains during amylopectin synthesis. | [30] |
| OsBt1 | Os02g0202400 | 2 | Endosperm | Transport of ADPG as OsBT1-ADPG complex from cytoplasm to amyloplast in endosperm | [31] |
| OsSBEIIa/SBE4 | Os04g0409200 | 4 | Leaf | An enzyme that acts on glycan induces branches connected by α-1, 6-glycosidic bonds. | [32] |
| OsISA1 | Os08g0520900 | 8 | Endosperm | ISA1, ISA3 hydrolyze α-1, 6-glucosidic bonds and correct errors in starch synthesis to ensure regular amylopectin synthesis. | [33] |
| OsISA3 | Os09g0469400 | 9 | Leaf | [34] | |
| OsGBP | Os02g0135900 | 2 | Leaf | OsGBP and OsFLO6, homologues to PTST1, interact with GBSSI for its localization on surface of starch granules to drive amylose synthesis after phosphorylation. | [35] |
| OsFLO6 | Os03g0686900 | 3 | Grain/leaf | [36] |
| S.N. | Genotypes | SG Surface Area (µm2) | SG Perimeter (µm) | Amylose Content (%) | Total Starch (%) | pGI | Starch Shape (Circularity | Gel Consistency (mm) | Water Uptake Ratio | Optimum Cooking Time (min) | Bulk Density (kg/m3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IRRI 147 | 23.22 | 18.15 | 30.8 | 76.36 | 56.2 | 0.88 | 56 | 3.09 | 47 | 566.63 |
| 2 | NON-HAI | 47.67 | 26.61 | 6.39 | 82.95 | 82.46 | 0.85 | 95 | 3.11 | 50 | 504.77 |
| 3 | Amber | 34.09 | 22.46 | 31.5 | 78.78 | 63.97 | 0.84 | 34 | 3.53 | 46 | 435.15 |
| 4 | Swarna | 33.09 | 21.97 | 29.6 | 65.84 | 58.06 | 0.86 | 30 | 3.49 | 46 | 516.97 |
| 5 | Makro | 35.64 | 23.58 | 30.6 | 71.88 | 59.06 | 0.80 | 91 | 3.22 | 47 | 561.70 |
| 6 | Hap 3-3 P-11 | 20.05 | 17.46 | 32.8 | 81.7 | 41.07 | 0.82 | 42 | 3.62 | 44 | 587.50 |
| 7 | Hap 3-1 P-22 | 34.29 | 22.40 | 36.9 | 84.11 | 42.34 | 0.85 | 19 | 3.22 | 42 | 601.63 |
| 8 | Hap 3-1 P-18 | 27.46 | 20.06 | 33.1 | 75.34 | 41.79 | 0.85 | 24 | 3.42 | 51 | 531.27 |
| 9 | M-55 | 26.27 | 19.70 | 32.7 | 83.68 | 54.42 | 0.85 | 71 | 3.11 | 48 | 583.44 |
| Mean ± SD | 31.31 ± 8.20 | 21.38 ± 2.85 | 29.38 ± 8.87 | 77.85 ± 6.15 | 55.49 ± 13.17 | 0.85 ± 0.02 | 51.33 ± 28.55 | 3.32 ± 0.20 | 46.78 ± 2.77 | 543.23 ± 52.28 |
| Genotypes | Shape Description | Texture Description | Implications |
|---|---|---|---|
| Amber | The starch granules exhibit predominantly spherical shapes with a high degree of uniformity. The granules are well-rounded, indicating consistent starch biosynthesis. | The surface is smooth with minimal irregularities. There are few to no indentations or protrusions, suggesting a stable and uninterrupted formation process. | The uniform shape and smooth texture may contribute to predictable gelatinization properties, making Amber suitable for products requiring consistent texture and viscosity. |
| NON -HAI | Granules are primarily oval to slightly elongated. Some granules display minor angularity, indicating variability in granule development. | The surface texture is moderately smooth with subtle ridges. These slight variations may reflect differences in amylopectin branching during starch synthesis. | The moderate smoothness and shape variability might affect the granules’ packing, impacting the texture of the final product. |
| Makro | The granules are irregularly polygonal, with a mix of spherical and angular forms. This diversity suggests heterogeneous biosynthetic activity. | Surfaces exhibit mild roughness with noticeable facets and edges. The textural variation may affect the granules’ packing and interaction. | The diverse shapes and textures may influence water absorption rates and gelatinization behavior during cooking. |
| Swarna | Starch granules are predominantly spherical with occasional oval forms. There is also noticeable elongation in some granules, deviating from typical spherical forms. | The surface is smooth with minimal textural deviations. A consistent surface suggests efficient starch synthesis enzymes. | The overall uniformity indicates coordinated starch deposition. |
| Hap 3-1 P-22 | Granules display irregular and elongated shapes, with some appearing rod-like or kidney-shaped. This irregularity hints at genetic variations affecting granule formation. | The surface is rough with pronounced grooves and depressions. These features indicate disruptions or alterations in the starch layering process. | The rough texture and irregular shape may alter gelatinization properties and enzymatic digestibility, affecting cooking quality and nutritional aspects. |
| Hap 3-3 P-11 | The starch granules are highly irregular, with fragmented and angular shapes dominating the sample. Such variability suggests significant genetic divergence. | Surfaces are very rough with sharp edges and pronounced irregularities. This roughness may be due to aberrant starch synthesis or environmental stress during development. | Such granules may behave differently during processing, impacting texture and consistency in food applications. |
| M-55 | Starch granules are predominantly spherical with some oval forms. They are uniform in size, indicating coordinated biosynthesis. | The surface is smooth with slight undulations. Minor surface variations hint at standard starch synthesis with minimal disruptions. | The consistent shape and texture are favorable for applications requiring predictable starch behavior, such as in baking or thickening agents. |
| IRRI-147 | Exhibits a mix of spherical and polygonal granule shapes. Some granules have well-defined edges, while others are more rounded. | The surface is moderately rough, featuring small ridges and facets. Variability may reflect differences in enzyme activities during starch formation. | This genotype may offer unique textural properties suitable for specialized culinary uses or industrial applications. |
| Hap 3-1 P-18 | Primarily spherical granules with some irregularities in shape. There is a mix of sizes, suggesting a heterogeneous granule population. | The surface is rougher compared with other genotypes, with noticeable grooves and indentations. Indicates potential genetic factors affecting surface morphology. | The rough texture may enhance enzymatic access, possibly improving the digestibility of starch and influencing the glycemic index. |
| S.N. | Genotypes | Accession ID | Amylose % | Amylose % | Mean |
|---|---|---|---|---|---|
| 2020 | 2021 | ||||
| 1 | NOROI | IRGC 121069 | 24.34 | 27.91 | 26.12 |
| 2 | SOLOMON RED RICE | IRGC 126312 | 27.12 | 30.09 | 28.60 |
| 3 | GOKAUNG | IRGC 128045 | 21.86 | 30.06 | 25.96 |
| 4 | IR 3839-1 | IRGC 127437 | 23.87 | 29.62 | 26.74 |
| 5 | JIN JUN DAO | IRGC 125613 | 27.02 | 28.68 | 27.85 |
| 6 | NCS 237 | IRGC 125853 | 23.43 | 28.51 | 25.97 |
| 7 | IR 4432-28-5 | IRGC 127438 | 30.20 | 27.81 | 29.01 |
| 8 | CHAKOL | IRGC 125696 | 25.61 | 28.28 | 26.95 |
| 9 | IR 77390-1-6-4-19-1-B | IRGC 127075 | 17.50 | 28.04 | 22.77 |
| 10 | IRRIBINI | IRGC 126215 | 30.21 | 28.01 | 29.11 |
| 11 | BENGALY MORIMO | IRGC 125677 | 25.31 | 21.61 | 23.46 |
| 12 | IRI 339 | IRGC 125778 | 20.42 | 21.51 | 20.96 |
| 13 | IRGA 659-1-2-2-2 | IRGC 120993 | 15.09 | 17.35 | 16.22 |
| 14 | ARC 12576 | IRGC 125645 | 12.27 | 17.15 | 14.71 |
| 15 | RD 15 | IRGC 126003 | 13.45 | 16.55 | 15.00 |
| 16 | ARC 11901 | IRGC 127033 | 18.54 | 15.95 | 17.24 |
| 17 | KHAO DAWK MALI 105 | IRGC 121235 | 7.48 | 10.55 | 9.02 |
| 18 | ARC 13778 | IRGC 125647 | 5.54 | 9.31 | 7.42 |
| 19 | KAM PAI | IRGC 126152 | 4.80 | 9.14 | 6.97 |
| 20 | NEP BA BONG TO | IRGC 128116 | 6.01 | 9.01 | 7.51 |
| K-GROUP | Variety | Amylose Content | chr02-1909569 | chr02-1909678 | chr02-1909917 | chr02-1910147 | chr02-1910189 | chr02-1910611 | chr02-1910815 | chr02-1910818 | chr02-1911075 | chr02-1911438 | chr02-1911890 | chr02-1912215 | chr02-1912277 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | NEP BA BONG TO::IRGC 17005-2 | 9.62 | C | A | A | A | G | C | T | G | T | C | C | T | C |
| RD 15::IRGC 47705-1 | |||||||||||||||
| KHAO DAWK MALI 105::IRGC 27748-2 | |||||||||||||||
| KAM PAI::IRGC 78245-1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Ali, A.; Qutub, M.; Rana, S.S.; Raut, P.; Pandey, V.K.; N, M.; Borule, T.; Dharavath, N.; Adhimoolam, K. Electron Microscopy Reveals Variation in Starch Granules in Rice Grains Related to Glycemic Index. Processes 2025, 13, 3241. https://doi.org/10.3390/pr13103241
Banerjee S, Ali A, Qutub M, Rana SS, Raut P, Pandey VK, N M, Borule T, Dharavath N, Adhimoolam K. Electron Microscopy Reveals Variation in Starch Granules in Rice Grains Related to Glycemic Index. Processes. 2025; 13(10):3241. https://doi.org/10.3390/pr13103241
Chicago/Turabian StyleBanerjee, Shubha, Amiruddin Ali, Maqbool Qutub, Shivani Singh Rana, Pradnya Raut, Vipin Kumar Pandey, Mustafa N, Taruna Borule, Nagaraju Dharavath, and Karthikeyan Adhimoolam. 2025. "Electron Microscopy Reveals Variation in Starch Granules in Rice Grains Related to Glycemic Index" Processes 13, no. 10: 3241. https://doi.org/10.3390/pr13103241
APA StyleBanerjee, S., Ali, A., Qutub, M., Rana, S. S., Raut, P., Pandey, V. K., N, M., Borule, T., Dharavath, N., & Adhimoolam, K. (2025). Electron Microscopy Reveals Variation in Starch Granules in Rice Grains Related to Glycemic Index. Processes, 13(10), 3241. https://doi.org/10.3390/pr13103241









