Mitigating Microbiologically Influenced Corrosion of Iron Caused by Sulphate-Reducing Bacteria Using ZnO Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
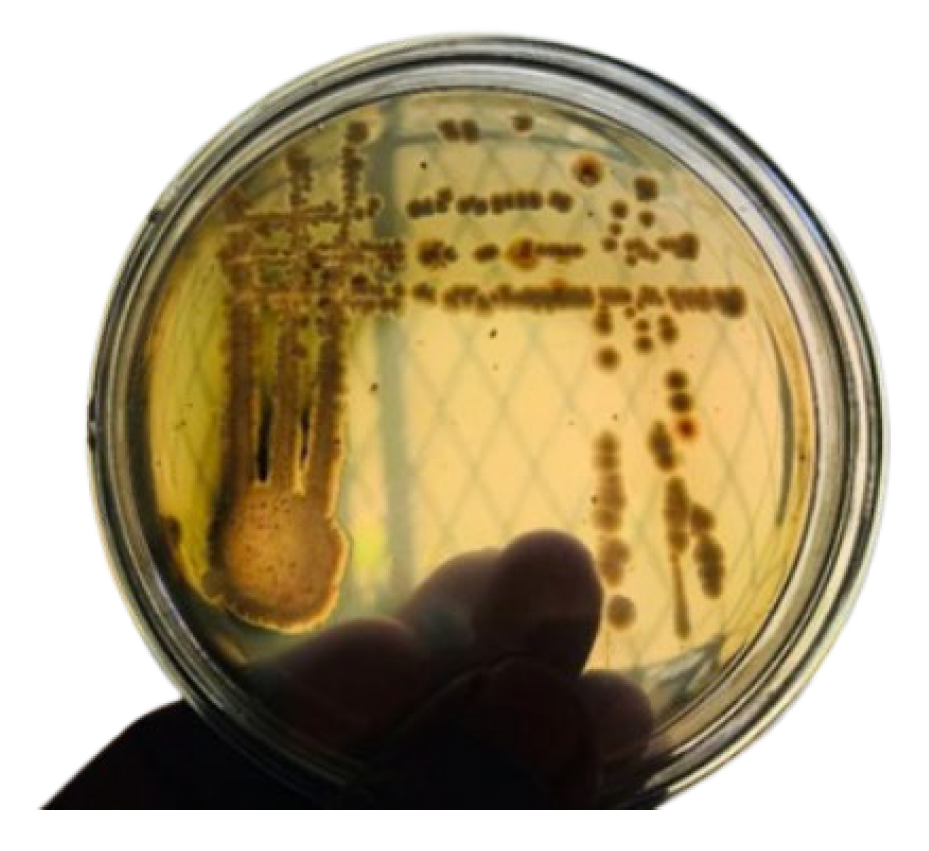
2.1. Sample Collection and Microorganism Isolation
2.2. Molecular Characterization of the Sulfur Reducing Bacterium
2.3. Preparation of Artificial Seawater Medium (ATSM) and Gas-Absorbent Sachets
2.4. Synthesis and Characterization of ZnO Nanoparticles
2.5. Microbial Fuel Cell (MFC) Setup
2.6. Electrochemical Measurements
2.7. Surface Morphology and Elemental Analysis
2.8. Corrosion Rate Determination
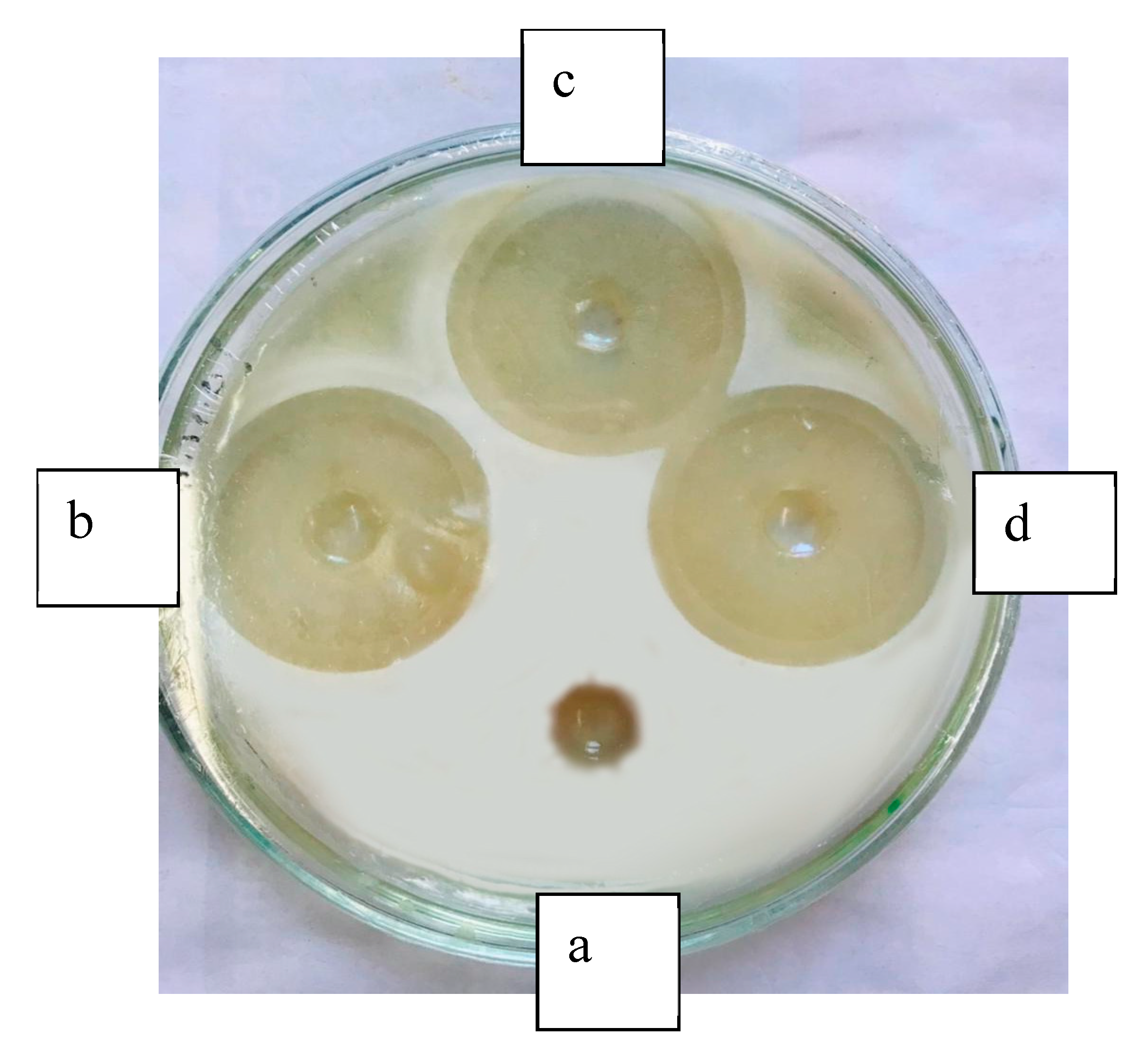
2.9. Antibiotic Susceptibility Testing of Isolated Microorganisms
3. Results
3.1. Initial Microbial Community Stability
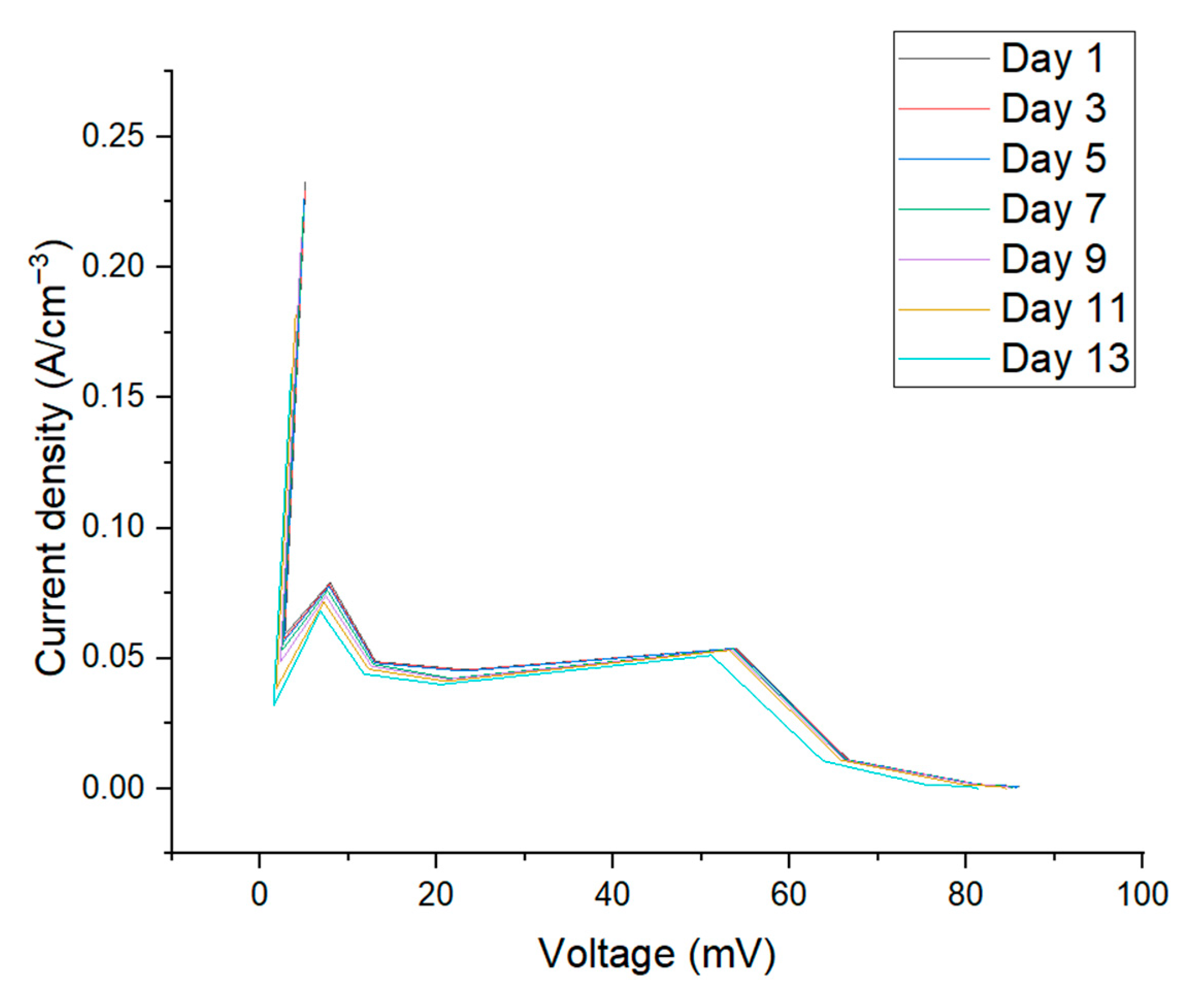
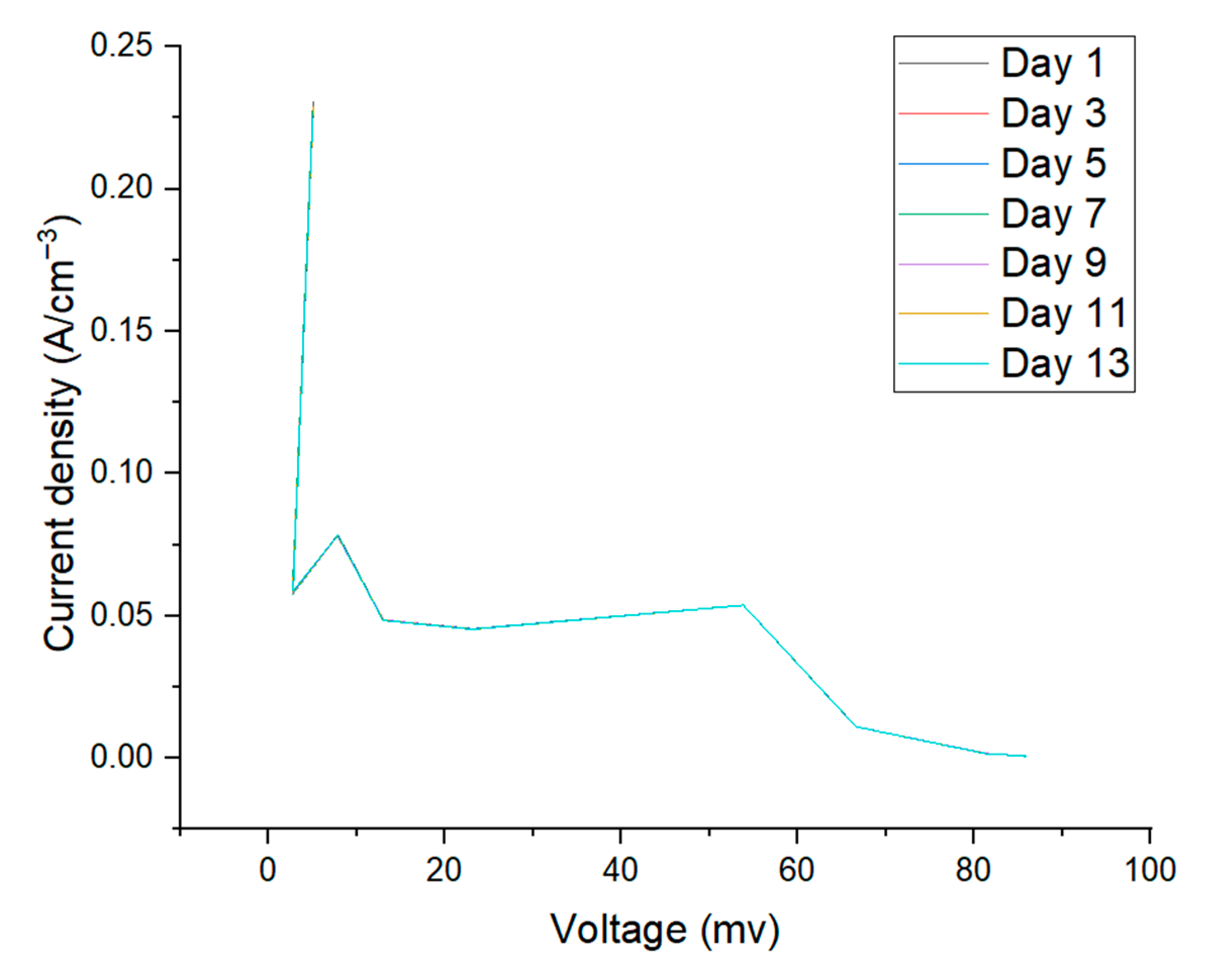
3.2. Electrochemical Characterization: Current vs. Voltage Plot
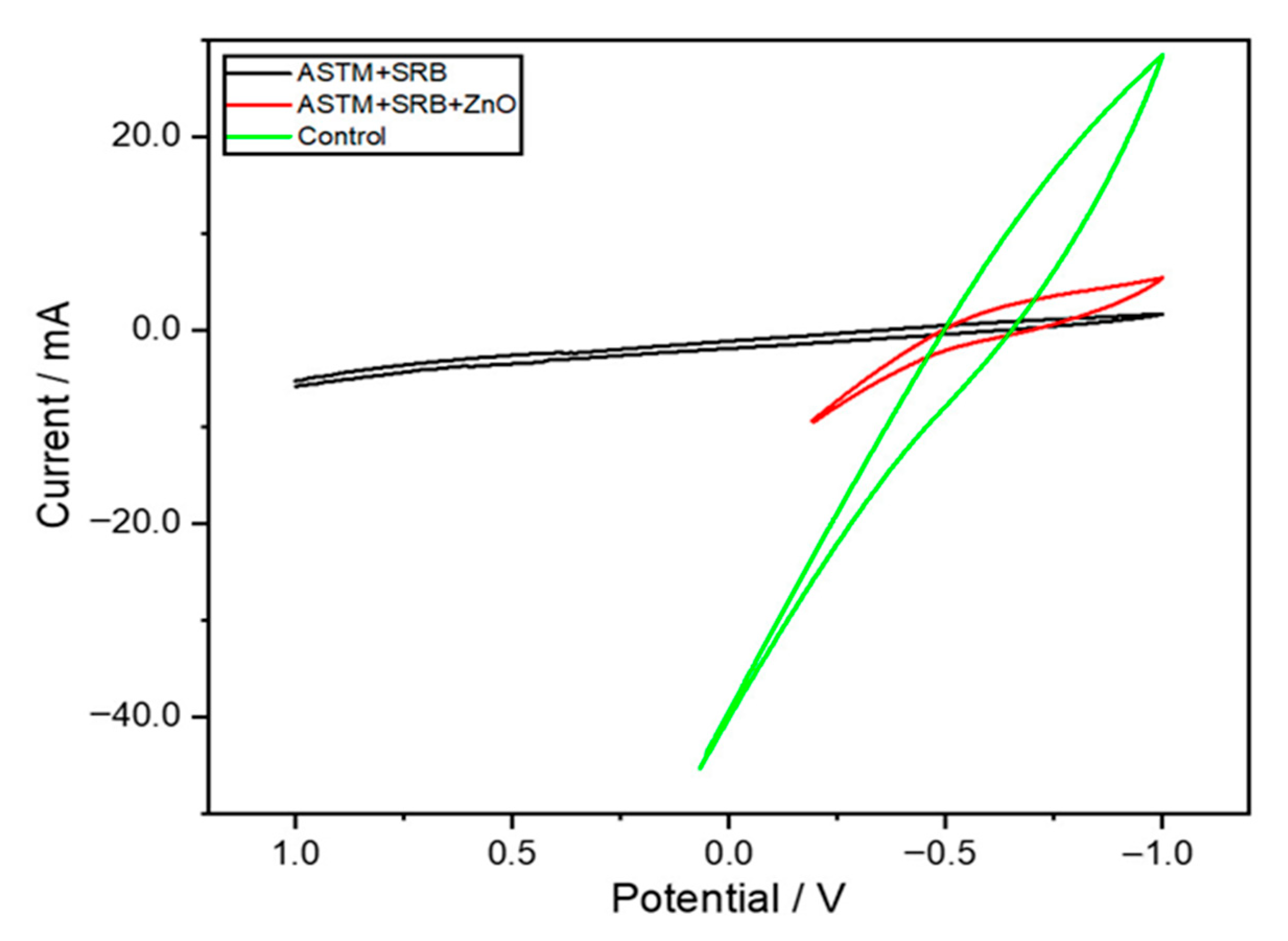
3.3. Electrochemical Characterization of Metal Electrodes Using Cyclic Voltammetry (CV)
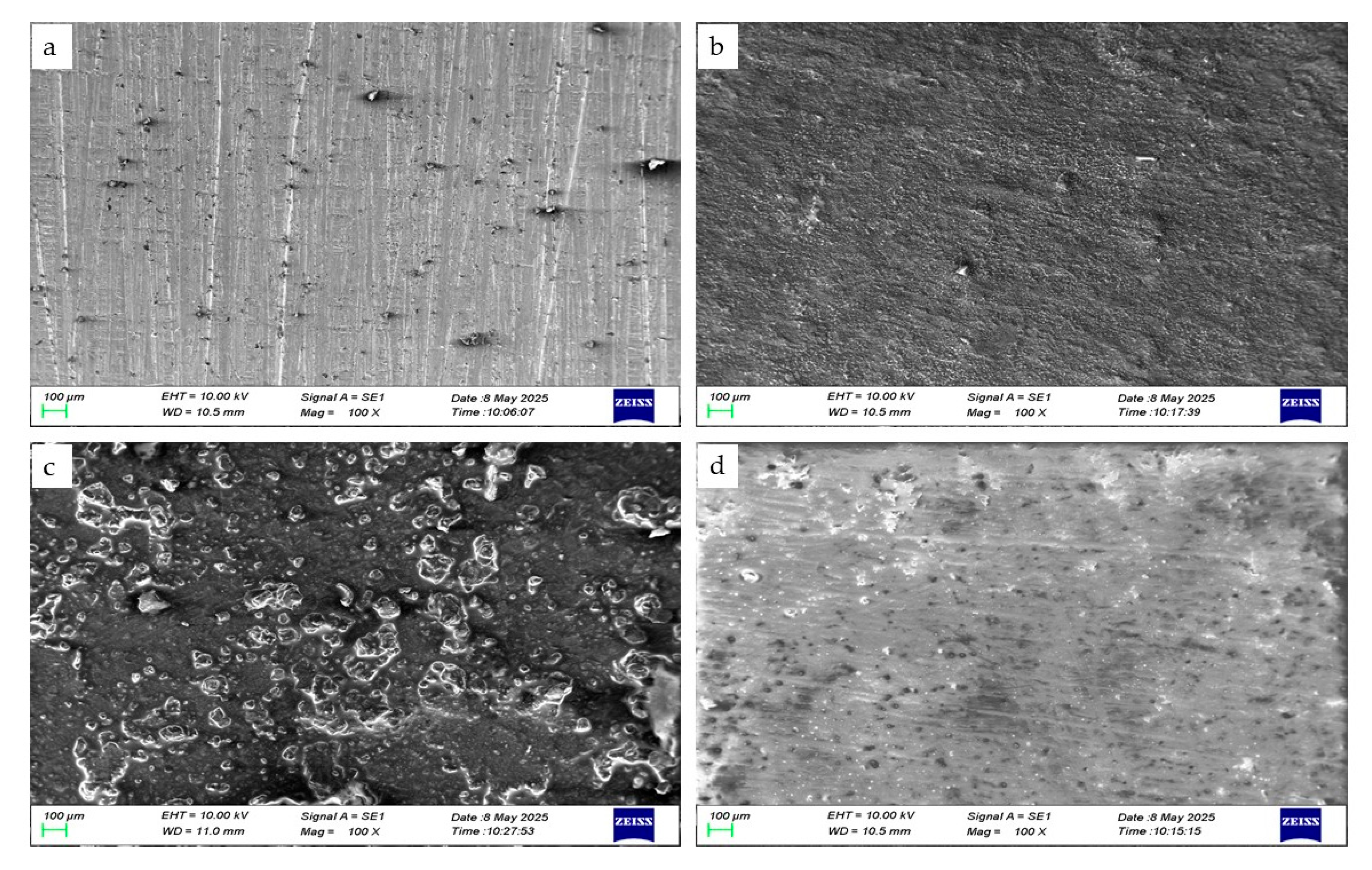
3.4. Characterization of Surface Morphology and Corrosion Analysis by Scanning Electron Microscopy
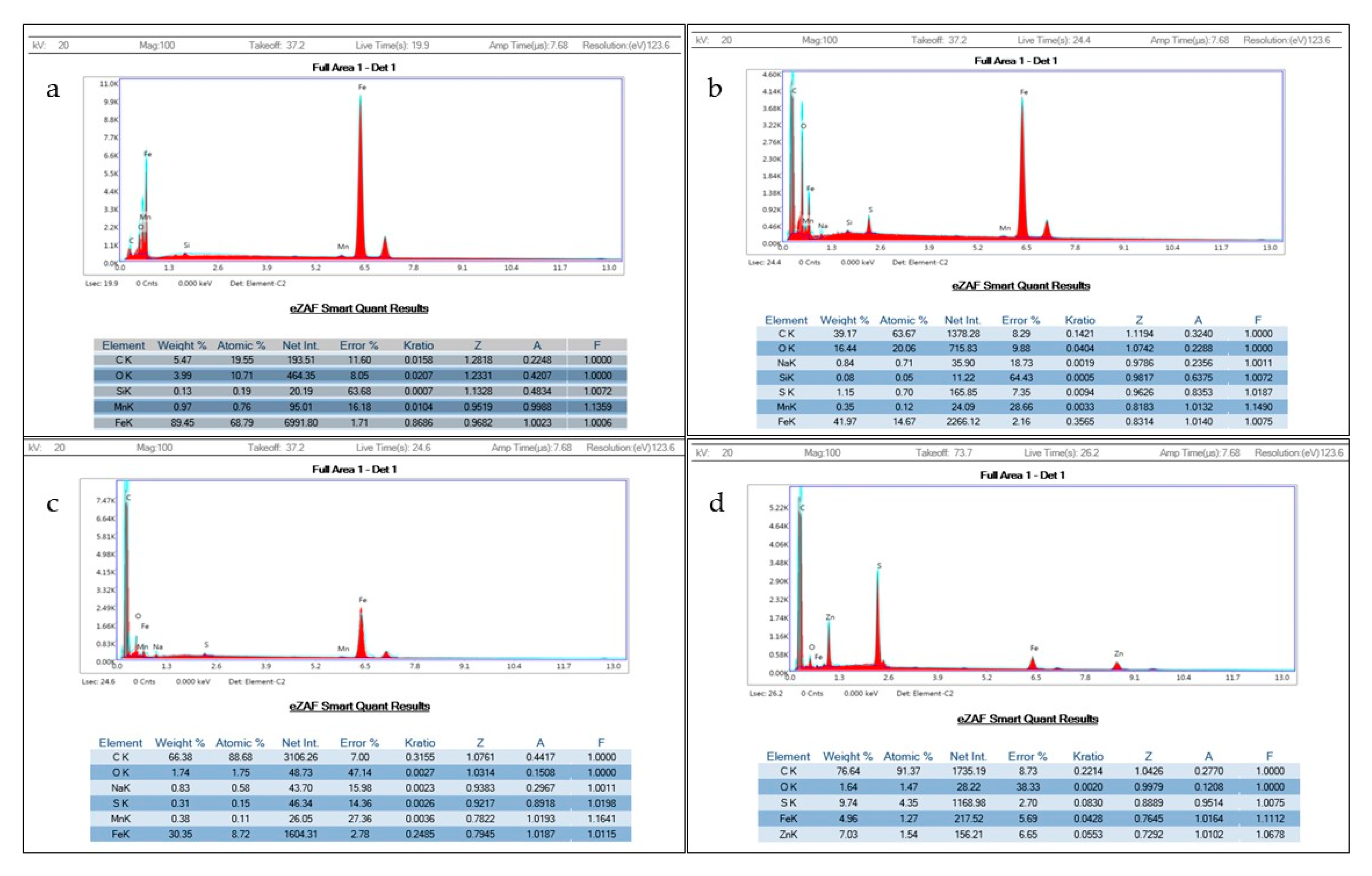
3.5. Identification and Quantification of Elemental Composition of Electrodes Using Energy Dispersive X-Ray Spectroscopy (EDS)
3.6. Mass Loss Observations and Corrosion Rate Calculation
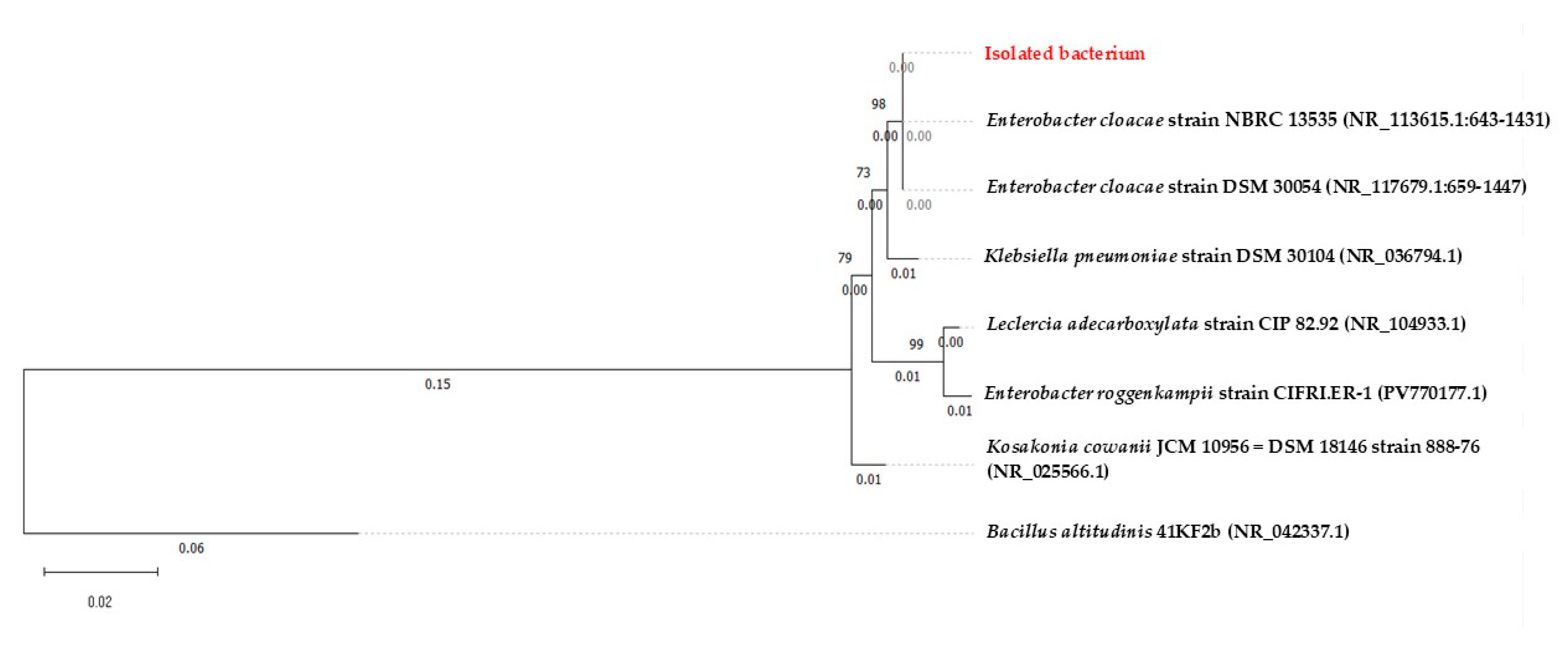
3.7. Molecular Characterization of Isolated Microorganisms
3.8. Antibiotic Susceptibility Test of Isolated Microorganisms Against ZnO Nanoparticles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIC | Microbiologically Influenced Corrosion |
| SRB | Sulphate-Reducing Bacteria |
| ZnO | Zinc Oxide Nanoparticles |
| ATSM | Artificial Seawater Medium |
References
- Wang, D.; Unsal, T.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.; Saleh, M.A.; Gu, T. Sulfate reducing bacterium Desulfovibrio vulgaris caused severe microbiologically influenced corrosion of zinc and galvanized steel. Int. Biodeterior. Biodegrad. 2021, 157, 105160. [Google Scholar] [CrossRef]
- Alasvand Zarasvand, K.; Rai, V.R. Microorganisms: Induction and inhibition of corrosion in metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74. [Google Scholar] [CrossRef]
- Pal, M.K.; Lavanya, M. Microbial Influenced Corrosion: Understanding Bioadhesion and Biofilm Formation. J. Bio-Tribo-Corros. 2022, 8, 76. [Google Scholar] [CrossRef]
- Little, B.J.; Lee, J.S. Microbiologically influenced corrosion: An update. Int. Mater. Rev. 2014, 59, 384–393. [Google Scholar] [CrossRef]
- Perchikov, R.; Cheliukanov, M.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; Butusov, D.; Arlyapov, V.; Nakamura, H.; Reshetilov, A. Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices. Biosensors 2024, 14, 302. [Google Scholar] [CrossRef]
- Skovhus, T.; Eckert, R.; Rodrigues, E. Management and control of microbiologically influenced corrosion (MIC) in the oil and gas industry—Overview and a North Sea case study. J. Biotechnol. 2017, 256, 31–45. [Google Scholar] [CrossRef]
- Gu, T.; Xu, D.; Zhang, P.; Li, Y.; Lindenberger, A. Microbiologically Influenced Corrosion and Its Impact on Metals and Other Materials. Microbiol. Miner. Met. Mater. Environ. 2015, 25, 383–408. [Google Scholar]
- Mulijani, S.; Rizky, G.; Wulanawati, A.; Handayani, A.W.; Asih, F.N. Synthesis of zinc oxide nanoparticle as corrosion resistance of steel metal. AIP Conf. Proc. 2022, 2638, 020006. [Google Scholar] [CrossRef]
- Sanyal, S.; Park, S.; Chelliah, R.; Yeon, S.-J.; Barathikannan, K.; Vijayalakshmi, S.; Jeong, Y.-J.; Rubab, M.; Oh, D.H. Emerging trends in smart self-healing coatings: A focus on micro/nanocontainer technologies for enhanced corrosion protection. Coatings 2024, 14, 324. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Qiao, Y.; Zheng, Y. Synergistic effects of deposits and sulfate reducing bacteria on the corrosion of carbon steel. Corros. Sci. 2022, 199, 110210. [Google Scholar] [CrossRef]
- Agarwal, H.; Gurnani, B.; Pippal, B.; Jain, N. Capturing the micro-communities: Insights into biogenesis and architecture of bacterial biofilms. BBA Adv. 2025, 7, 100133. [Google Scholar] [CrossRef]
- Mechmechani, S.; Yammine, J.; Alhuthali, S.; El Mouzawak, M.; Charvourou, G.; Gharsallaoui, A.; Chihib, N.E.; Doulgeraki, A.; Karam, L. Study of the Resistance of Staphylococcus aureus Biofilm, Biofilm-Detached Cells, and Planktonic Cells to Microencapsulated Carvacrol Used Alone or Combined with Low-pH Treatment. Int. J. Mol. Sci. 2024, 25, 7222. [Google Scholar] [CrossRef]
- Puentes, E.; Tapia-Perdomo, V.; Espinosa-Valbuena, D.; Reyes Reyes, M.; Quintero-Santander, D.; Vasquez-Dallos, S.; Salazar, H.; Santamaría-Galvis, P.; Silva-Rodríguez, R.; Castillo Villamizar, G. Microbiologically influenced corrosion: The gap in the field. Front. Environ. Sci. 2022, 10, 924842. [Google Scholar] [CrossRef]
- Brauer, J.I.; Makama, Z.; Bonifay, V.; Aydin, E.; Kaufman, E.D.; Beech, I.B.; Sunner, J. Mass spectrometric metabolomic imaging of biofilms on corroding steel surfaces using laser ablation and solvent capture by aspiration. Biointerphases 2015, 10, 019003. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, C.; Huang, H. Research Progress of Intelligent Anti-Corrosion Coatings and Their Healing Agents. Adv. Mater. Technol. 2025, 10, 2401669. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of iron by sulfate-reducing bacteria: New views of an old problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef]
- Yan, M.; Wei, B.; Xu, J.; Li, Y.; Hu, Y.; Cai, Z.; Sun, C. Insight into sulfate-reducing bacteria corrosion behavior of X80 pipeline steel welded joint in a soil solution. J. Mater. Res. Technol. 2023, 24, 5839–5863. [Google Scholar] [CrossRef]
- Salgar-Chaparro, S.; Lepkova, K.; Pojtanabuntoeng, T.; Darwin, A.; Machuca, L. Microbiologically influenced corrosion as a function of environmental conditions: A laboratory study using oilfield multispecies biofilms. Corros. Sci. 2020, 169, 108595. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B. Long-term external microbiologically influenced corrosion of buried cast iron pipes in the presence of sulfate-reducing bacteria (SRB). Eng. Fail. Anal. 2020, 115, 104657. [Google Scholar] [CrossRef]
- Yin, K.; Liu, H.; Cheng, Y.F. Microbiologically influenced corrosion of X52 pipeline steel in thin layers of solution containing sulfate-reducing bacteria trapped under disbonded coating. Corros. Sci. 2018, 145, 271–282. [Google Scholar] [CrossRef]
- Javed, M.A.; Neil, W.C.; McAdam, G.; Wade, S. Effect of sulphate-reducing bacteria on the microbiologically influenced corrosion of ten different metals using constant test conditions. Int. Biodeterior. Biodegrad. 2017, 125, 75–83. [Google Scholar] [CrossRef]
- Javaherdashti, R. Impact of sulphate-reducing bacteria on the performance of engineering materials. Appl. Microbiol. Biotechnol. 2011, 91, 1507–1517. [Google Scholar] [CrossRef]
- Abdus Samad, U.; Alam, M.A.; Anis, A.; Sherif, E.M.; Al-Mayman, S.I.; Al-Zahrani, S.M. Effect of Incorporated ZnO Nanoparticles on the Corrosion Performance of SiO(2) Nanoparticle-Based Mechanically Robust Epoxy Coatings. Materials 2020, 13, 3767. [Google Scholar] [CrossRef]
- Şomoghi, R.; Semenescu, A.; Pasăre, V.; Chivu, O.R.; Nițoi, D.F.; Marcu, D.F.; Florea, B. The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers 2024, 16, 2054. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Fan, Y.; Xu, D. Microbially Influenced Corrosion of Steel in Marine Environments: A Review from Mechanisms to Prevention. Microorganisms 2023, 11, 2299. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Rasool, K.; Pandey, R.P.; Sliem, M.H.; Helal, M.; Samara, A.; Abdullah, A.M.; Mahmoud, K.A. Controlling the biocorrosion of sulfate-reducing bacteria (SRB) on carbon steel using ZnO/chitosan nanocomposite as an eco-friendly biocide. Corros. Sci. 2019, 148, 397–406. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Houskova, V.; Stengl, V.; Bakardjieva, S.; Murafa, N.; Kalendova, A.; Oplustil, F. Zinc oxide prepared by homogeneous hydrolysis with thioacetamide, its destruction of warfare agents, and photocatalytic activity. J. Phys. Chem. A 2007, 111, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.A.M.; Almeida, J.S.; Lemos, P.C.; Carrondo, M.J.T. Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol. Bioeng. 1992, 40, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Yahaya, N.; Bakar, A.A.; Noor, N.N.N. Cultivation of sulphate reducing bacteria in different media. Malays. J. Civ. Eng. 2014, 26, 456–465. [Google Scholar] [CrossRef]
- Ghazy, E.; Mahmoud, M.; Asker, M.; Mahmoud, M.; Abo Elsoud, M.; Abdel Sami, M. Cultivation and detection of sulfate reducing bacteria (SRB) in sea water. J. Am. Sci. 2011, 7, 604–608. [Google Scholar]
- Narkiewicz, U.; Sibera, D.; Kuryliszyn-Kudelska, I.; Kilański, L.; Dobrowolski, W.; Romčević, N. Synthesis by Wet Chemical Method and Characterization of Nanocrystalline ZnO Doped with Fe_2O_3. Acta Phys. Pol. A 2008, 113, 1695–1700. [Google Scholar] [CrossRef]
- Somasiri, M.; Amandani, T.; Basnayaka, C.; Ahsan, A.; Dilangani, G.P.; Herath, A.C.; Bandara, S.; Kyazze, G.; Fernando, E.Y. Direct synthesis of nanomaterials on carbon microfibre electrode material for superior electrocatalysis in lake sediment microbial fuel cells. Energy Nexus 2024, 13, 100280. [Google Scholar] [CrossRef]
- Basnayaka, C.; Somasiri, M.; Ahsan, A.; Nazeer, Z.; Thilini, N.; Bandara, S.; Fernando, E.Y. Marine Photosynthetic Microbial Fuel Cell for Circular Renewable Power Production. BioEnergy Res. 2024, 17, 2299–2310. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford university press: Oxford, UK, 2000. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Gascuel, O. BIONJ: An improved version of the NJ algorithm based on a simple model of sequence data. Mol. Biol. Evol. 1997, 14, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Vakilipour Takaloo, A. Corrosion Behavior of Heat Treated Nickel-Aluminum Bronze Alloy in Artificial Seawater. Mater. Sci. Appl. 2011, 02, 1542–1555. [Google Scholar] [CrossRef]
- Sahai, A.; Goswami, N. Structural and vibrational properties of ZnO nanoparticles synthesized by the chemical precipitation method. Phys. E Low-Dimens. Syst. Nanostructures 2014, 58, 130–137. [Google Scholar] [CrossRef]
- Kımuya, A. The modified OHM’S law and its implications for electrical circuit analysis. Eurasian J. Sci. Eng. Technol. 2023, 4, 59–70. [Google Scholar] [CrossRef]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- Zaafarany, I. Cyclic Voltammetric Behavior of Iron Electrode in Sodium Hydroxide Solutions. Mater. Sci. Res. India 2009, 6, 241–250. [Google Scholar] [CrossRef]
- Hassan Basri, H.; Talib, R.A.; Sukor, R.; Othman, S.H.; Ariffin, H. Effect of Synthesis Temperature on the Size of ZnO Nanoparticles Derived from Pineapple Peel Extract and Antibacterial Activity of ZnO-Starch Nanocomposite Films. Nanomaterials 2020, 10, 1061. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Li, Z.; Huang, J.; Wu, J.; Zhang, Y.; Ge, H.; Zhao, Y. Antibacterial Effect of Low-Concentration ZnO Nanoparticles on Sulfate-Reducing Bacteria under Visible Light. Nanomaterials 2023, 13, 2033. [Google Scholar] [CrossRef]
- Yu, S.; Lou, Y.; Zhang, D.; Zhou, E.; Li, Z.; Du, C.; Qian, H.; Xu, D.; Gu, T. Microbiologically influenced corrosion of 304 stainless steel by nitrate reducing Bacillus cereus in simulated Beijing soil solution. Bioelectrochemistry 2020, 133, 107477. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Guan, F.; Yang, Z.; Zhai, X.; Zhang, Y.; Tang, X.; Duan, J.; Xiao, H. The Isolation of Anaerobic and Facultative Anaerobic Sulfate-Reducing Bacteria (SRB) and a Comparison of Related Enzymes in Their Sulfate Reduction Pathways. Microorganisms 2023, 11, 2019. [Google Scholar] [CrossRef]

| Date | Day1 | Day3 | Day5 | Day7 | Day9 | Day11 | Day13 | |
|---|---|---|---|---|---|---|---|---|
| R (Om) | Voltage (mV) | |||||||
| 22 | 5.12 | 5.04 | 4.98 | 4.86 | 4.65 | 4 | 3.5 | |
| 41 | 2.78 | 2.7 | 2.65 | 2.5 | 2.29 | 1.8 | 1.5 | |
| 100 | 7.91 | 7.83 | 7.77 | 7.61 | 7.4 | 7.17 | 6.8 | |
| 268 | 13.08 | 13 | 12.94 | 12.8 | 12.59 | 12.3 | 11.78 | |
| 510 | 23.25 | 23.17 | 23 | 21.6 | 21.39 | 21.01 | 20.42 | |
| 1000 | 53.88 | 53.8 | 53.74 | 53.58 | 53.37 | 53.08 | 51.12 | |
| 6000 | 66.67 | 66.59 | 66.51 | 66.35 | 66.14 | 65.8 | 63.76 | |
| 47,000 | 81.32 | 81.24 | 81.18 | 81.02 | 80.77 | 79.5 | 75.32 | |
| 100,000 | 85.91 | 85.84 | 85.78 | 84.92 | 84.66 | 83.78 | 80.52 | |
| 1,000,000 | 85.56 | 85.48 | 85.42 | 85.26 | 85 | 84.6 | 81.33 | |
| Date | Day1 | Day3 | Day5 | Day7 | Day9 | Day11 | Day13 | |
|---|---|---|---|---|---|---|---|---|
| R (Om) | Voltage (mV) | |||||||
| 22 | 5.1 | 5.07 | 5.04 | 4.99 | 5 | 4.9 | 4.75 | |
| 41 | 2.76 | 2.73 | 2.7 | 2.65 | 2.65 | 2.55 | 2.3 | |
| 100 | 7.89 | 7.86 | 7.83 | 7.78 | 7.73 | 7.64 | 7.54 | |
| 268 | 13.06 | 13.03 | 12.99 | 12.86 | 11.9 | 11.79 | 11.68 | |
| 510 | 23.23 | 23.2 | 23.17 | 23.09 | 22.92 | 22.83 | 22.73 | |
| 1000 | 53.86 | 53.82 | 53.79 | 53.74 | 53.5 | 53.41 | 53.2 | |
| 6000 | 66.65 | 66.61 | 66.57 | 66.52 | 66.4 | 66.33 | 66.12 | |
| 47,000 | 81.3 | 81.26 | 81.22 | 81.17 | 81 | 79.98 | 79.75 | |
| 100,000 | 85.89 | 85.84 | 85.81 | 85.76 | 85.5 | 84 | 83.79 | |
| 1,000,000 | 85.54 | 85.5 | 85.47 | 85.42 | 85.32 | 85.11 | 84.9 | |
| Date | Day1 | Day3 | Day5 | Day7 | Day9 | Day11 | Day13 | |
|---|---|---|---|---|---|---|---|---|
| R (Om) | Voltage (mV) | |||||||
| 22 | 5.07 | 5.02 | 5.03 | 5.01 | 5.02 | 5.04 | 5 | |
| 41 | 2.73 | 2.71 | 2.72 | 2.7 | 2.73 | 2.75 | 2.76 | |
| 100 | 7.86 | 7.83 | 7.84 | 7.83 | 7.8 | 7.83 | 7.84 | |
| 268 | 13.03 | 13.01 | 13 | 13.02 | 13.05 | 13 | 12.98 | |
| 510 | 23.2 | 23.17 | 23.19 | 23.17 | 23.13 | 23.12 | 23.11 | |
| 1000 | 53.83 | 53.82 | 53.83 | 53.84 | 53.82 | 53.83 | 53.82 | |
| 6000 | 66.62 | 66.6 | 66.6 | 66.6 | 66.58 | 66.57 | 66.58 | |
| 47,000 | 81.27 | 81.25 | 81.27 | 81.28 | 81.3 | 81.27 | 81.25 | |
| 100,000 | 85.86 | 85.84 | 85.83 | 85.81 | 85.83 | 85.85 | 85.86 | |
| 1,000,000 | 85.51 | 85.49 | 85.51 | 85.52 | 85.53 | 85.52 | 85.53 | |
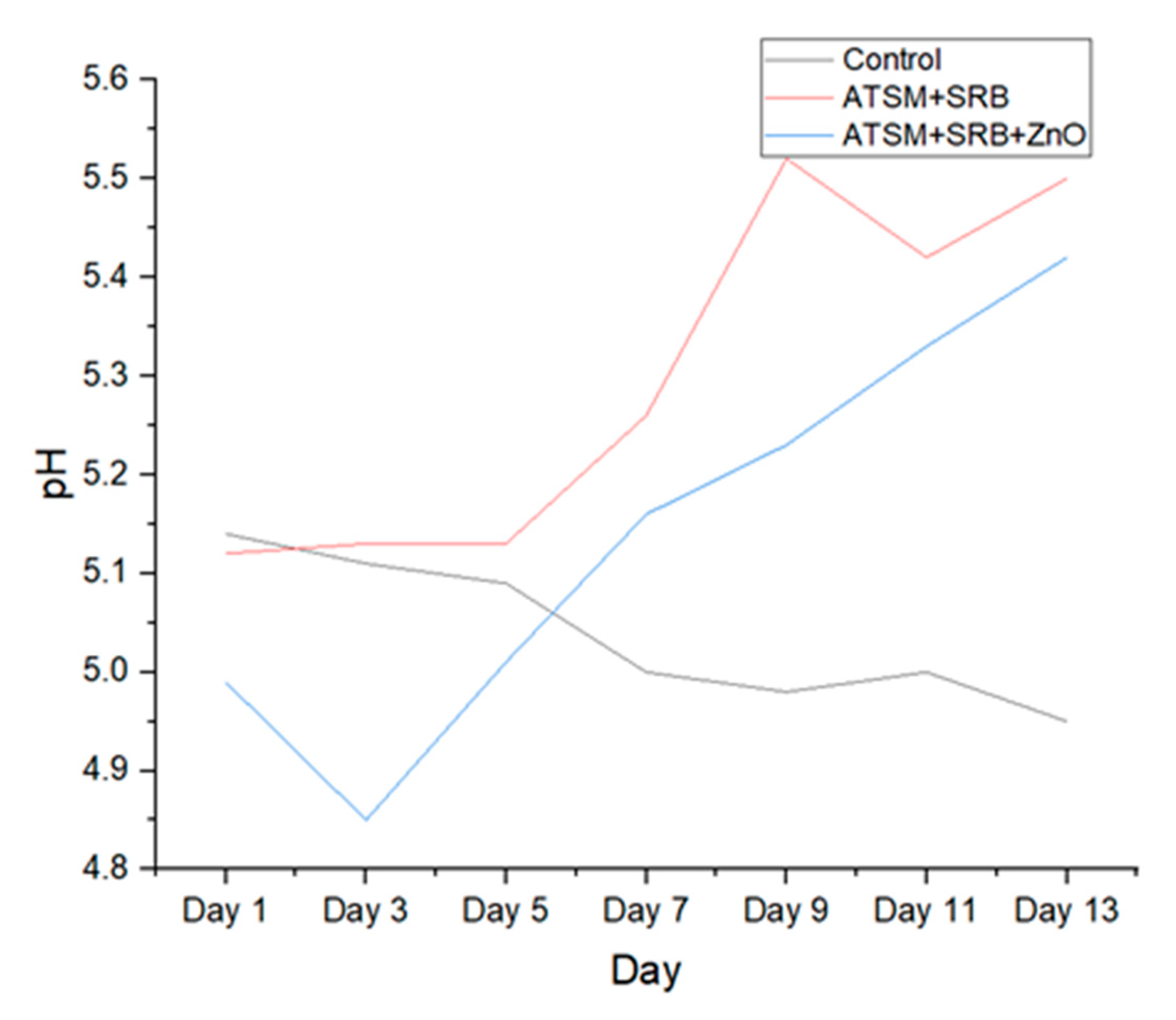
| Date | Control | ATSM + Isolated Bacterium | ATSM + Isolated Bacterium + ZnO |
|---|---|---|---|
| 01/10 | 5.14 | 5.12 | 4.99 |
| 01/12 | 5.11 | 5.13 | 4.85 |
| 01/14 | 5.09 | 5.13 | 5.01 |
| 01/16 | 5 | 5.26 | 5.16 |
| 01/18 | 4.98 | 5.52 | 5.23 |
| 01/20 | 5 | 5.42 | 5.33 |
| 01/22 | 4.95 | 5.5 | 5.42 |
| Electrode | Initial Weight (g) | Final Weight (g) | Difference (g) |
|---|---|---|---|
| Control | 3.6984 | 3.6998 | 0.0014 |
| Isolated bacterium | 3.7029 | 3.7046 | 0.0017 |
| ZnO + isolated bacterium | 3.7123 | 3.7134 | 0.0011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambepitiya, H.; Rathnayaka, S.; Perera, Y.; Jayathilake, C.; Ferdinandez, H.; Herath, A.; Sanjula, U.; Rathnayake, A.; Basnayaka, C.; Fernando, E. Mitigating Microbiologically Influenced Corrosion of Iron Caused by Sulphate-Reducing Bacteria Using ZnO Nanoparticles. Processes 2025, 13, 3239. https://doi.org/10.3390/pr13103239
Ambepitiya H, Rathnayaka S, Perera Y, Jayathilake C, Ferdinandez H, Herath A, Sanjula U, Rathnayake A, Basnayaka C, Fernando E. Mitigating Microbiologically Influenced Corrosion of Iron Caused by Sulphate-Reducing Bacteria Using ZnO Nanoparticles. Processes. 2025; 13(10):3239. https://doi.org/10.3390/pr13103239
Chicago/Turabian StyleAmbepitiya, Harith, Supun Rathnayaka, Yashodha Perera, Chamindu Jayathilake, Himashi Ferdinandez, Ajith Herath, Udul Sanjula, Aishwarya Rathnayake, Charitha Basnayaka, and Eustace Fernando. 2025. "Mitigating Microbiologically Influenced Corrosion of Iron Caused by Sulphate-Reducing Bacteria Using ZnO Nanoparticles" Processes 13, no. 10: 3239. https://doi.org/10.3390/pr13103239
APA StyleAmbepitiya, H., Rathnayaka, S., Perera, Y., Jayathilake, C., Ferdinandez, H., Herath, A., Sanjula, U., Rathnayake, A., Basnayaka, C., & Fernando, E. (2025). Mitigating Microbiologically Influenced Corrosion of Iron Caused by Sulphate-Reducing Bacteria Using ZnO Nanoparticles. Processes, 13(10), 3239. https://doi.org/10.3390/pr13103239







