Natural Antioxidant Enrichment of Goat Meat Pates with Portulaca oleracea and Honey Improves Oxidative Stability and Color Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Fatty Acid Composition
2.3. Determination of Color Characteristics
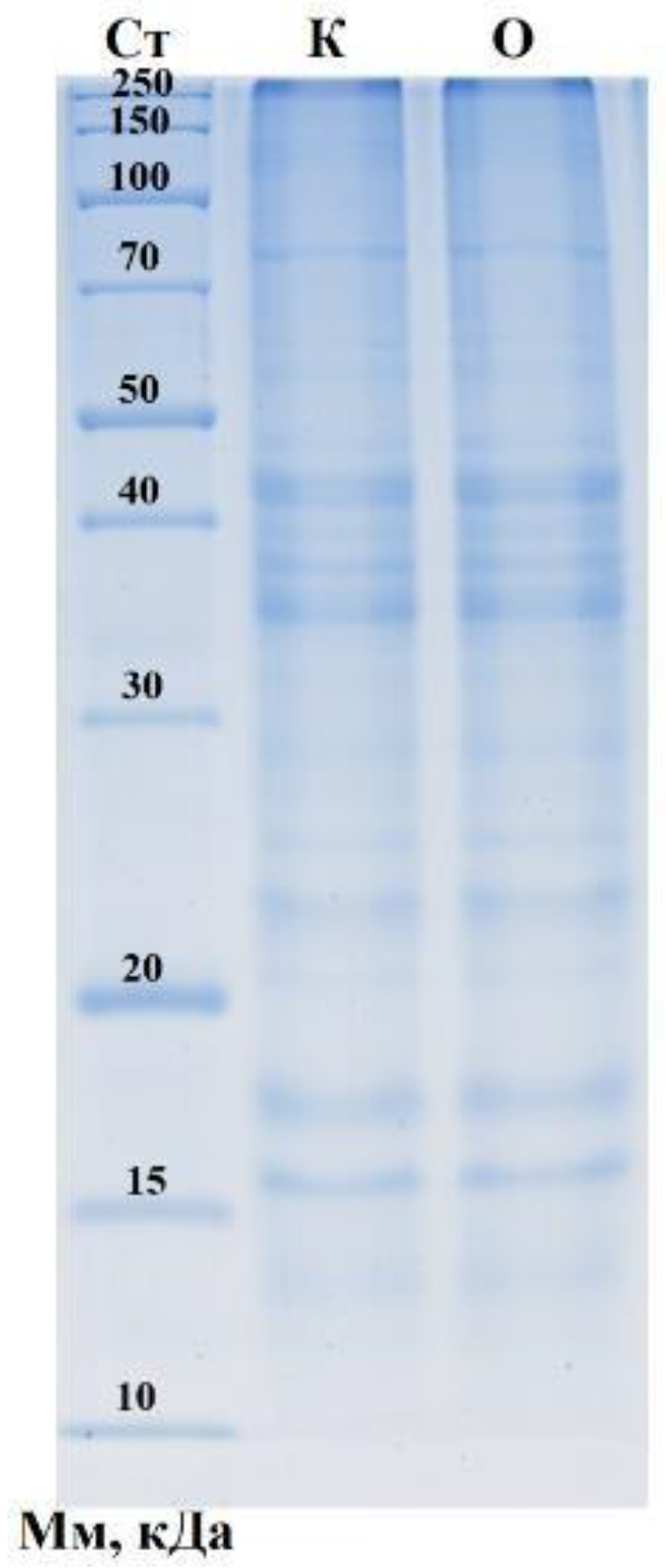
2.4. Analysis of Molecular Weight Distribution of Protein Fractions in Samples Carried Out Using One-Dimensional Electrophoresis
2.5. Determination of Ferric-Reducing Antioxidant Power (FRAP) and Antioxidant Activity Using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.6. Statistical Analyses
3. Results and Discussion
3.1. Analysis of Fatty Acid Composition Results
3.2. Analysis of Color Characteristic Results
3.3. Analysis of Molecular Weight Distribution of Protein Fractions in Samples Using One-Dimensional Electrophoresis
3.4. Evaluation of Antioxidant Capacity: Ferric-Reducing Antioxidant Power (FRAP) and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay Results
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M.A. Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants 2020, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-Derived Natural Antioxidants in Meat and Meat Products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef] [PubMed]
- Selani, M.M.; Herrero, A.M.; Ruiz-Capillas, C. Plant Antioxidants in Dry Fermented Meat Products with a Healthier Lipid Profile. Foods 2022, 11, 3558. [Google Scholar] [CrossRef]
- Velázquez, L.; Quiñones, J.; Díaz, R.; Pateiro, M.; Lorenzo, J.M.; Sepúlveda, N. Natural Antioxidants from Endemic Leaves in the Elaboration of Processed Meat Products: Current Status. Antioxidants 2021, 10, 1396. [Google Scholar] [CrossRef]
- Bumsted, J.; Ford, E.; Blair, A.; Underwood, K.; Zuelly, S.M.S. Instrumental Color Measurements Have Relationships to Fat Smearing in Fresh Sausage. Foods 2023, 12, 2813. [Google Scholar] [CrossRef]
- Echegaray, N.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M.; Chabani, Z.; Farag, M.A.; Domínguez, R. Measurement of Antioxidant Capacity of Meat and Meat Products: Methods and Applications. Molecules 2021, 26, 3880. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Sun, W.; Ma, F.; Wang, X.; Yang, Z. New Techniques of Meat Quality Assessment for Detecting Meat Texture. Processes 2025, 13, 640. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Ngadi, M. Recent Developments in Hyperspectral Imaging for Assessment of Food Quality and Safety. Sensors 2014, 14, 7248–7276. [Google Scholar] [CrossRef]
- Anagnostou, G.; Ferragina, A.; Crofton, E.C.; Frias Celayeta, J.M.; Hamill, R.M. The Development of Optical Sensing Techniques as Digital Tools to Predict the Sensory Quality of Red Meat: A Review. Appl. Sci. 2025, 15, 1719. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Du, L.; Shang, X.; Shi, J. Hyperspectral Imaging for Foreign Matter Detection in Foods: Advances, Challenges, and Future Directions. Foods 2025, 14, 3026. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as Potential Source of Natural Additives for Meat Industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Pollonio, M.A.R.; Sepúlveda, N.; Andres, S.C.; Reyes, J.; Santos, E.M.; Lorenzo, J.M. Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review. Foods 2021, 10, 2094. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.; Pascual, J.A.; Ros, M.; Petropoulos, S.A.; Alguacil, M.d.M. Agronomical Practices and Management for Commercial Cultivation of Portulaca oleracea as a Crop: A Review. Plants 2023, 12, 1246. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidants 2019, 8, 293. [Google Scholar] [CrossRef]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and Comparison of the Antioxidant Component of Portulaca oleracea Leaves Obtained by Different Solid-Liquid Extraction Techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Zengin, G.; Tzortzakis, N. Purslane (Portulaca oleracea L.) Growth, Nutritional, and Antioxidant Status under Different Nitrogen Levels in Hydroponics. Horticulturae 2024, 10, 1007. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.d.P.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Phytochemical Composition and Antioxidant Activity of Portulaca oleracea: Influence of the Steaming Cooking Process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Nkhumeleni, Z.; Phoswa, W.; Mokgalaboni, K. Purslane Ameliorates Inflammation and Oxidative Stress in Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12276. [Google Scholar] [CrossRef]
- Makangali, K.; Tultabayeva, T.; Zamaratskaia, G.; Ospankulova, G.; Tokysheva, G.; Abzhanova, S.; Zhakupova, G.; Ergalikyzy, A. Enhancing the Antioxidant Capacity and Oxidative Stability of Cooked Sausages Through Portulaca oleracea (Purslane) Supplementation: A Natural Alternative to Synthetic Additives. Appl. Sci. 2024, 14, 9986. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Dar, S.A.; Ahmed, M.M.M.; Aly, M.M.; Vlainić, J. Antioxidant Capacity and Therapeutic Applications of Honey: Health Benefits, Antimicrobial Activity and Food Processing Roles. Antioxidants 2025, 14, 959. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, M.C.; Izah, S.C. Honey as a Natural Antimicrobial. Antibiotics 2025, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, R.; Onopiuk, A.; Chmiel, M.; Piotrowska, A.; Kostyra, E.; Lipińska, E.; Bryś, J.; Samborska, K.; Pietrzak, D. The Effect of Honey Powder Addition on Chosen Quality Properties of Model Chicken Products. Foods 2024, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- Petcu, C.D.; Tăpăloagă, D.; Mihai, O.D.; Gheorghe-Irimia, R.-A.; Negoiță, C.; Georgescu, I.M.; Tăpăloagă, P.R.; Borda, C.; Ghimpețeanu, O.M. Harnessing Natural Antioxidants for Enhancing Food Shelf Life: Exploring Sources and Applications in the Food Industry. Foods 2023, 12, 3176. [Google Scholar] [CrossRef]
- Tkacz, K.; Modzelewska-Kapituła, M.; Więk, A.; Nogalski, Z. The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times. Appl. Sci. 2020, 10, 8215. [Google Scholar] [CrossRef]
- Soulage, C.O.; Pelletier, C.C.; Florens, N.; Lemoine, S.; Dubourg, L.; Juillard, L.; Guebre-Egziabher, F. Two Toxic Lipid Aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), Accumulate in Patients with Chronic Kidney Disease. Toxins 2020, 12, 567. [Google Scholar] [CrossRef]
- Salzano, A.; Damiano, S.; D’Angelo, L.; Ballistreri, G.; Claps, S.; Rufrano, D.; Maggiolino, A.; Neglia, G.; De Palo, P.; Ciarcia, R. Productive Performance and Meat Characteristics of Kids Fed a Red Orange and Lemon Extract. Animals 2021, 11, 809. [Google Scholar] [CrossRef]
- Dragoev, S.G. Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health. Foods 2024, 13, 797. [Google Scholar] [CrossRef]
- Schaur, R.; Siems, W.; Bresgen, N.; Eckl, P. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Arteaga, A.; Krell, J.; Gibis, M.; Heinz, V.; Terjung, N.; Tomasevic, I. Intrinsic and Extrinsic Factors Affecting the Color of Fresh Beef Meat-Comprehensive Review. Appl. Sci. 2023, 13, 4382. [Google Scholar] [CrossRef]
- Silva, S.R.; Teixeira, A.; Guedes, C. Sheep and Goat Meat Processing and Quality. Foods 2023, 12, 2033. [Google Scholar] [CrossRef] [PubMed]
- Reitznerová, A.; Šuleková, M.; Nagy, J.; Marcinčák, S.; Semjon, B.; Čertík, M.; Klempová, T. Lipid Peroxidation Process in Meat and Meat Products: A Comparison Study of Malondialdehyde Determination between Modified 2-Thiobarbituric Acid Spectrophotometric Method and Reverse-Phase High-Performance Liquid Chromatography. Molecules 2017, 22, 1988. [Google Scholar] [CrossRef]
- Wei, X.; Lam, S.; Bohrer, B.M.; Uttaro, B.; López-Campos, O.; Prieto, N.; Larsen, I.L.; Juárez, M. A Comparison of Fresh Pork Colour Measurements by Using Four Commercial Handheld Devices. Foods 2021, 10, 2515. [Google Scholar] [CrossRef]
- Iftikhar, A.; Dupas-Farrugia, C.; De Leonardis, A.; Macciola, V.; Moiz, A.; Martin, D. Antioxidant potential of olive leaf (Olea europaea L.) sustainable extracts evaluated in vitro and minced beef meat. Ital. J. Food Sci. 2024, 36, 305–316. [Google Scholar] [CrossRef]
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and Goat Meat Processed Products Quality: A Review. Foods 2020, 9, 960. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Tilami, S.K.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Cavallo, M.; Menchetti, L.; Angelucci, E.; Cartoni Mancinelli, A.; Vaudo, G.; Marconi, S.; Camilli, E.; Galli, F.; Castellini, C.; et al. The Healthy Fatty Index Allows for Deeper Insights into the Lipid Composition of Foods of Animal Origin When Compared with the Atherogenic and Thrombogenicity Indexes. Foods 2024, 13, 1568. [Google Scholar] [CrossRef] [PubMed]
- Al Juhaimi, F.; Atasoy, Z.B.; Uslu, N.; Özcan, M.M.; Mohamed Ahmed, I.A.; Walayat, N. The Effect of Roasting on Oil Content, Fatty Acids, Bioactive Compounds and Mineral Contents of Purslane (Portulaca oleracea L.) Seeds. Foods 2025, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of n−6:n−3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Lupuliasa, D.; Neacșu, S.M.; Olteanu, G.; Busnatu, Ș.S.; Mihai, A.; Popovici, V.; Măru, N.; Boroghină, S.C.; Mihai, S.; et al. Polyunsaturated Fatty Acids and Human Health: A Key to Modern Nutritional Balance in Association with Polyphenolic Compounds from Food Sources. Foods 2024, 14, 46. [Google Scholar] [CrossRef]
- Minelli, G.; D’Ambra, K.; Macchioni, P.; Lo Fiego, D.P. Effects of Pig Dietary n−6/n−3 Polyunsaturated Fatty Acids Ratio and Gender on Carcass Traits, Fatty Acid Profiles, Nutritional Indices of Lipid Depots and Oxidative Stability of Meat in Medium-Heavy Pigs. Foods 2023, 12, 4106. [Google Scholar] [CrossRef]
- Terevinto, A.; Cabrera, M.C.; Zaccari, F.; Saadoun, A. The Oxidative and Color Stability of Beef from Steers Fed Pasture or Concentrate during Retail Display. Animals 2023, 13, 2972. [Google Scholar] [CrossRef]
- Sanjulian, L.; Lamas, A.; Barreiro, R.; Martínez, I.; García-Alonso, L.; Cepeda, A.; Fente, C.; Regal, P. Investigating the Dietary Impact on Trans-Vaccenic Acid (Trans-C18:1 n-7) and Other Beneficial Fatty Acids in Breast Milk and Infant Formulas. Foods 2024, 13, 2164. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Agnew, M.P.; Craigie, C.R.; Weralupitiya, G.; Reis, M.M.; Johnson, P.L.; Reis, M.G. Comprehensive Evaluation of Parameters Affecting One-Step Method for Quantitative Analysis of Fatty Acids in Meat. Metabolites 2019, 9, 189. [Google Scholar] [CrossRef]
- Frunză, G.; Ciobanu, M.-M.; Murariu, O.C.; Radu-Rusu, R.-M.; Boișteanu, P.-C. The Fatty Acid Content, Health Lipid Indices, and Instrumental, Histological, and Sensory Quality of Hare Meat (Lepus europaeus Pallas). Foods 2025, 14, 310. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Zaldivar-Ortega, A.K.; Cenobio-Galindo Ade, J.; Morfin, N.; Aguirre-Álvarez, G.; Campos-Montiel, R.G.; Esturau-Escofet, N.; Garduño-García, A.; Angeles-Hernandez, J.C. The Physicochemical Parameters, Phenolic Content, and Antioxidant Activity of Honey from Stingless Bees and Apis mellifera: A Systematic Review and Meta-Analysis. Antioxidants 2024, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Orădan, A.C.; Tocai, A.C.M.; Rosan, C.A.; Vicas, S.I. Fruit Extracts Incorporated into Meat Products as Natural Antioxidants, Preservatives, and Colorants. Processes 2024, 12, 2756. [Google Scholar] [CrossRef]
- Martins, A.A.; Andrade, S.; Correia, D.; Matos, E.; Caetano, N.S.; Mata, T.M. Valorization of Agro-Industrial Residues: Bioprocessing of Animal Fats to Reduce Their Acidity. Sustainability 2021, 13, 10837. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; Picard, B. Protein Array-Based Approach to Evaluate Biomarkers of Beef Tenderness and Marbling in Cows: Understanding of the Underlying Mechanisms and Prediction. Foods 2020, 9, 1180. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef]
- Cierach, M.; Niedźwiedź, J. Effects of three lighting intensities during display on discolouration of beef semitendinosus muscle. Eur. Food Res. Technol. 2014, 239, 377–383. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Sujintonniti, N.; Chaum, P.; Ketnawa, S.; Rawdkuen, S. Developments of Plant-Based Emulsion-Type Sausage by Using Grey Oyster Mushrooms and Chickpeas. Foods 2023, 12, 1564. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in Natural Antioxidants for Food Improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef]

| Ingredient | Control Pate | Treatment Pate (Purslane + Honey) |
|---|---|---|
| Goat meat (leg/shoulder) | 76.0 | 71.2 |
| Onion | 10.0 | 10.0 |
| Carrot | 10.0 | 10.0 |
| Mustard | 2.0 | 2.0 |
| Starch | 2.0 | 2.0 |
| Curing mix | 1.5 | 0.8 |
| Purslane extract powder | – | 1.0 |
| Honey | – | 4.0 |
| Total | 100.0 | 100.0 |
| Indicator Name | Control Pate (Without Purslane and Honey) (% of Total FA) | Treatment Pate (with Purslane and Honey) (% of Total FA) |
|---|---|---|
| Butyric, C4:0 | <0.1 | <0.1 |
| Caproic, C6:0 | <0.1 | <0.1 |
| Caprylic, C8:0 | <0.1 | <0.1 |
| Capric, C10:0 | <0.1 | <0.1 |
| Undecanoic, C11:0 | <0.1 | <0.1 |
| Lauric, C12:0 | <0.1 | <0.1 |
| Tridecanoic, C13:0 | <0.1 | <0.1 |
| Myristic, C14:0 | 4.5 ± 0.4 | 4.3 ± 0.4 |
| Pentadecanoic, C15:0 | 0.8 ± 0.4 | 0.8 ± 0.4 |
| Palmitic, C16:0 | 27.1 ± 2.1 | 26.5 ± 2.1 |
| Margaric (Heptadecanoic), C17:0 | 1.6 ± 0.4 | 1.5 ± 0.4 |
| Stearic, C18:0 | 21.2 ± 2.1 | 19.8 ± 2.1 |
| Arachidic, C20:0 | 0.4 ± 0.4 | 0.6 ± 0.4 |
| Heneicosanoic, C21:0 | 0.8 ± 0.4 | 1.0 ± 0.4 |
| Behenic, C22:0 | <0.1 | 0.5 ± 0.4 |
| Tricosanoic, C23:0 | <0.1 | 0.2 ± 0.4 |
| Lignoceric, C24:0 | <0.1 | <0.1 |
| Myristoleic, C14:1 | <0.1 | <0.1 |
| cis-10-Pentadecenoic, C15:1 | 0.4 ± 0.4 | 0.5 ± 0.4 |
| Palmitoleic, C16:1 | 2.5 ± 0.4 | 2.6 ± 0.4 |
| Heptadecenoic, C17:1 | 0.8 ± 0.4 | 0.9 ± 0.4 |
| Oleic, C18:1 | 30.0 ± 2.1 | 29.5 ± 2.1 |
| Elaidic (trans-C18:1) | 3.8 ± 0.4 | 3.8 ± 0.4 |
| Gondoic, C20:1 | 0.6 ± 0.4 | 1.0 ± 0.4 |
| Erucic, C22:1 | <0.1 | 0.2 ± 0.4 |
| Nervonic, C24:1 | <0.1 | 0.3 ± 0.4 |
| α-Linolenic (ALA), C18:3 n−3 | 0.8 ± 0.4 | 1.2 ± 0.4 |
| Timnodonic (EPA), C20:5 n−3 | <0.1 | <0.1 |
| Eicosatrienoic, C20:3 n−3 | <0.1 | <0.1 |
| Docosahexaenoic (DHA), C22:6 n−3 | <0.1 | <0.1 |
| Linoleic, C18:2 n−6 | 4.0 ± 0.4 | 3.3 ± 0.4 |
| Linolelaidic (trans-C18:2 n−6) | 0.5 ± 0.4 | 0.6 ± 0.4 |
| Dihomo-γ-linolenic, C20:3 n−6 | <0.1 | <0.1 |
| Arachidonic, C20:4 n−6 | <0.1 | 0.5 ± 0.4 |
| Eicosadienoic, C20:2 n−6 | <0.1 | 0.3 ± 0.4 |
| Docosadienoic, C22:2 n−6 | <0.1 | <0.1 |
| Parameter Measured | Unit | Control Pate (Without Purslane and Honey) | Treatment Pate (with Purslane and Honey) | p-Value, Treatment Within Storage Time |
|---|---|---|---|---|
| Peroxide value, day 0 | meq O2/kg fat | 3.8 ± 0.4 | 3.5 ± 0.4 | 0.410 |
| Peroxide value, day 2 | meq O2/kg fat | 4.1 ± 0.4 | 4.3 ± 0.4 | 0.573 |
| Peroxide value, day 4 | meq O2/kg fat | 5.9 ± 0.3 | 5.5 ± 0.3 | 0.178 |
| Peroxide value, day 6 | meq O2/kg fat | 7.1 ± 0.4 | 7.0 ± 0.4 | 0.775 |
| Peroxide value, day 8 | meq O2/kg fat | 10.1 ± 0.5 | 9.7 ± 0.5 | 0.383 |
| Peroxide value, day 10 | meq O2/kg fat | 14.0 ± 0.9 | 13.0 ± 0.9 | 0.245 |
| Protein carbonyls (baseline) | nmol/mg protein | 95.73 | 99.19 | 0.686 |
| Parameter Measured | Unit | Control Pate (Without Purslane and Honey) | Treatment Pate (with Purslane and Honey) | p-Value, Treatment Within Storage Time |
|---|---|---|---|---|
| TBARS, day 0 | mg MDA/kg | 0.028 ± 0.003 | 0.028 ± 0.003 | 1.000 |
| TBARS, day 2 | mg MDA/kg | 0.028 ± 0.003 | 0.028 ± 0.003 | 1.000 |
| TBARS, day 4 | mg MDA/kg | 0.028 ± 0.003 | 0.140 ± 0.014 | 0.004 |
| TBARS, day 6 | mg MDA/kg | 0.117 ± 0.012 | 0.546 ± 0.038 | 0.001 |
| TBARS, day 8 | mg MDA/kg | 1.719 ± 0.120 | 0.766 ± 0.054 | 0.002 |
| TBARS, day 10 | mg MDA/kg | 3.289 ± 0.230 | 1.910 ± 0.134 | 0.002 |
| Parameter Measured | Unit | Control Pate (Without Purslane and Honey) | Treatment Pate (with Purslane and Honey) | p-Value, Treatment Within Storage Time |
|---|---|---|---|---|
| Acid value, day 0 | mg KOH/g | 2.4 ± 0.2 | 2.0 ± 0.2 | 0.070 |
| Acid value, day 2 | mg KOH/g | 3.0 ± 0.2 | 2.6 ± 0.2 | 0.070 |
| Acid value, day 4 | mg KOH/g | 3.6 ± 0.3 | 3.1 ± 0.2 | 0.084 |
| Acid value, day 6 | mg KOH/g | 4.2 ± 0.3 | 3.9 ± 0.3 | 0.288 |
| Acid value, day 8 | mg KOH/g | 6.5 ± 0.5 | 5.2 ± 0.3 | 0.026 |
| Acid value, day 10 | mg KOH/g | 8.5 ± 0.6 | 7.0 ± 0.5 | 0.031 |
| Item | Light Exposure | Color Characteristics | p-Value, Color Within Light Exposure | |
|---|---|---|---|---|
| Control Pate (Without Purslane and Honey) | Treatment Pate (with Purslane and Honey) | |||
| L-lightness | Before | 58.37 ± 0.44 | 51.20 ± 0.51 | 0.988 |
| After | 56.95 ± 0.39 | 51.74 ± 0.48 | 0.316 | |
| a-redness | Before | 7.38 ± 0.13 | 7.48 ± 0.58 | <0.0001 |
| After | 6.53 ± 0.36 | 6.07 ± 0.81 | <0.0001 | |
| b-yellowness | Before | 25.31 ± 0.35 | 24.35 ± 0.83 | 0.772 |
| After | 26.27 ± 0.26 | 26.53 ± 0.78 | 0.996 | |
| Color stability, % | 93.17 ± 1.90 | 88.84 ± 1.18 | 0.183 | |
| Indicator | Results | p-Value | |
|---|---|---|---|
| Control Pate (Without Purslane and Honey) | Treatment Pate (with Purslane and Honey) | ||
| Ferric-reducing antioxidant power (FRAP), mg GAE/g | Not detected | 10.5 ± 0.04 | <0.0001 |
| DPPH radical-scavenging activity, % | 22.33 ± 0.007 | 33.02 ± 0.009 | <0.0001 |
| IC50 of DPPH radical-scavenging activity, µg/mL | 138.25 ± 11.15 | 106.10 ± 10.01 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tultabayeva, T.; Tokysheva, G.; Muldasheva, A.; Shoman, A.; Kassenov, A.; Tumenov, S.; Dairova, K.; Battalova, N.; Makangali, K. Natural Antioxidant Enrichment of Goat Meat Pates with Portulaca oleracea and Honey Improves Oxidative Stability and Color Properties. Processes 2025, 13, 3213. https://doi.org/10.3390/pr13103213
Tultabayeva T, Tokysheva G, Muldasheva A, Shoman A, Kassenov A, Tumenov S, Dairova K, Battalova N, Makangali K. Natural Antioxidant Enrichment of Goat Meat Pates with Portulaca oleracea and Honey Improves Oxidative Stability and Color Properties. Processes. 2025; 13(10):3213. https://doi.org/10.3390/pr13103213
Chicago/Turabian StyleTultabayeva, Tamara, Gulzhan Tokysheva, Aknur Muldasheva, Aruzhan Shoman, Amirzhan Kassenov, Serik Tumenov, Kalamkas Dairova, Nuray Battalova, and Kadyrzhan Makangali. 2025. "Natural Antioxidant Enrichment of Goat Meat Pates with Portulaca oleracea and Honey Improves Oxidative Stability and Color Properties" Processes 13, no. 10: 3213. https://doi.org/10.3390/pr13103213
APA StyleTultabayeva, T., Tokysheva, G., Muldasheva, A., Shoman, A., Kassenov, A., Tumenov, S., Dairova, K., Battalova, N., & Makangali, K. (2025). Natural Antioxidant Enrichment of Goat Meat Pates with Portulaca oleracea and Honey Improves Oxidative Stability and Color Properties. Processes, 13(10), 3213. https://doi.org/10.3390/pr13103213







