Computational Fluid Dynamics, Transport, and Chemical Kinetics-Based Monolith Catalyst Dimensioning Methodology for Cost-Effective Performance
Abstract
1. Introduction
2. Methods
2.1. Initial Calculations
2.2. CFD Modelling
2.3. Model Order Reduction
2.4. Dimensioning Optimization Algorithm
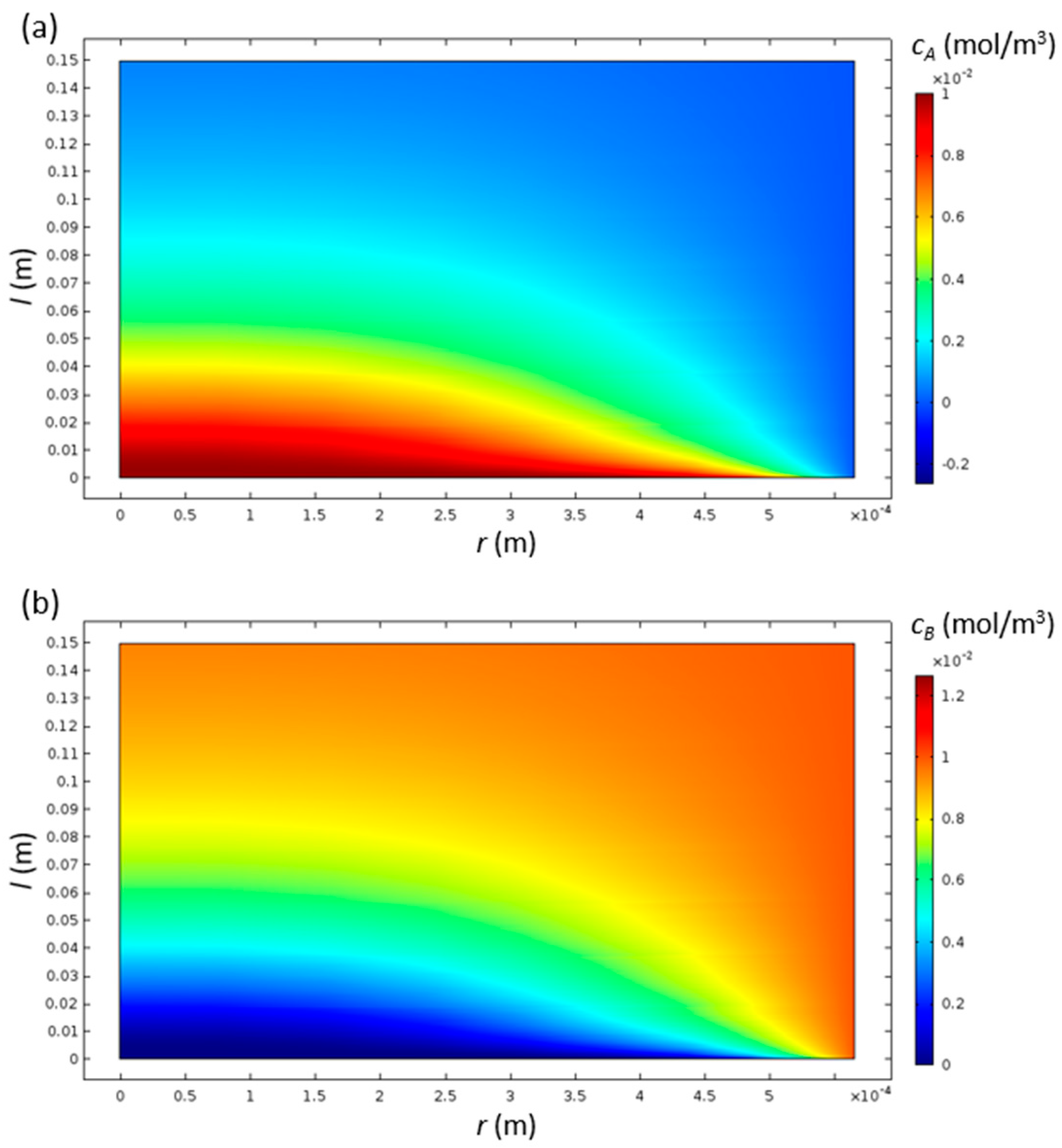
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. CFD Results and MOR Data Summary
| Index (/) | R (mm) | N (/) | Δp (Pa) | ConA (/) | Re (/) | kc (1/m) | R2 (/) | kp (Pa/m) | R2 (/) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.500 | 6000 | 874 | 0.9812 | 143 | 30.45 | 0.995052 | 5829.2 | 1.000000 |
| 2 | 0.502 | 5950 | 867 | 0.9810 | 144 | 30.19 | 0.995086 | 5780.2 | 1.000000 |
| 3 | 0.504 | 5900 | 860 | 0.9801 | 145 | 30.00 | 0.995251 | 5732.7 | 1.000000 |
| 4 | 0.506 | 5850 | 852 | 0.9793 | 145 | 29.56 | 0.994750 | 5683.9 | 1.000000 |
| 5 | 0.509 | 5800 | 845 | 0.9793 | 146 | 29.55 | 0.994571 | 5635.2 | 1.000000 |
| 6 | 0.511 | 5750 | 838 | 0.9786 | 146 | 29.22 | 0.994666 | 5586.6 | 1.000000 |
| 7 | 0.513 | 5700 | 831 | 0.9776 | 147 | 29.06 | 0.994395 | 5539.0 | 1.000000 |
| 8 | 0.515 | 5650 | 824 | 0.9774 | 148 | 28.79 | 0.994704 | 5490.8 | 1.000000 |
| 9 | 0.518 | 5600 | 816 | 0.9772 | 148 | 28.77 | 0.994608 | 5443.5 | 1.000000 |
| 10 | 0.520 | 5550 | 809 | 0.9764 | 149 | 28.50 | 0.994424 | 5394.4 | 1.000000 |
| 11 | 0.522 | 5500 | 802 | 0.9753 | 150 | 28.21 | 0.994275 | 5345.4 | 1.000000 |
| 12 | 0.525 | 5450 | 794 | 0.9743 | 150 | 27.89 | 0.994155 | 5297.1 | 1.000000 |
| 13 | 0.527 | 5400 | 787 | 0.9737 | 151 | 27.64 | 0.994292 | 5248.7 | 1.000000 |
| 14 | 0.530 | 5350 | 780 | 0.9724 | 152 | 27.41 | 0.993711 | 5199.0 | 1.000000 |
| 15 | 0.532 | 5300 | 773 | 0.9716 | 153 | 27.16 | 0.993869 | 5151.5 | 1.000000 |
| 16 | 0.535 | 5250 | 765 | 0.9704 | 153 | 26.84 | 0.993438 | 5102.4 | 1.000000 |
| 17 | 0.537 | 5200 | 758 | 0.9700 | 154 | 26.73 | 0.993488 | 5053.8 | 1.000000 |
| 18 | 0.540 | 5150 | 751 | 0.9690 | 155 | 26.39 | 0.993145 | 5006.0 | 1.000000 |
| 19 | 0.542 | 5100 | 743 | 0.9682 | 156 | 26.28 | 0.993280 | 4957.4 | 1.000000 |
| 20 | 0.545 | 5050 | 736 | 0.9669 | 156 | 25.90 | 0.993056 | 4908.4 | 1.000000 |
| 21 | 0.548 | 5000 | 729 | 0.9660 | 157 | 25.68 | 0.992873 | 4860.0 | 1.000000 |
| 22 | 0.550 | 4950 | 722 | 0.9654 | 158 | 25.56 | 0.992800 | 4812.1 | 1.000000 |
| 23 | 0.553 | 4900 | 715 | 0.9644 | 159 | 25.32 | 0.992759 | 4764.0 | 1.000000 |
| 24 | 0.556 | 4850 | 707 | 0.9631 | 160 | 25.09 | 0.992401 | 4714.6 | 1.000000 |
| 25 | 0.559 | 4800 | 700 | 0.9626 | 160 | 24.93 | 0.992461 | 4666.9 | 1.000000 |
| 26 | 0.562 | 4750 | 693 | 0.9610 | 161 | 24.56 | 0.991998 | 4618.3 | 1.000000 |
| 27 | 0.565 | 4700 | 686 | 0.9622 | 162 | 24.91 | 0.992596 | 4571.0 | 1.000000 |
| 28 | 0.568 | 4650 | 678 | 0.9612 | 163 | 24.68 | 0.992391 | 4523.2 | 1.000000 |
| 29 | 0.571 | 4600 | 671 | 0.9604 | 164 | 24.55 | 0.992352 | 4475.5 | 1.000000 |
| 30 | 0.574 | 4550 | 664 | 0.9596 | 165 | 24.30 | 0.992217 | 4426.9 | 1.000000 |
| 31 | 0.577 | 4500 | 657 | 0.9583 | 166 | 24.08 | 0.992139 | 4378.2 | 1.000000 |
| 32 | 0.581 | 4450 | 649 | 0.9563 | 167 | 23.72 | 0.991320 | 4329.4 | 1.000000 |
| 33 | 0.584 | 4400 | 642 | 0.9550 | 167 | 23.49 | 0.991487 | 4280.6 | 1.000000 |
| 34 | 0.587 | 4350 | 635 | 0.9524 | 168 | 23.15 | 0.991095 | 4232.2 | 1.000000 |
| 35 | 0.591 | 4300 | 627 | 0.9513 | 169 | 22.86 | 0.990751 | 4183.1 | 1.000000 |
| 36 | 0.594 | 4250 | 620 | 0.9495 | 170 | 22.72 | 0.990327 | 4133.9 | 1.000000 |
| 37 | 0.598 | 4200 | 613 | 0.9472 | 171 | 22.30 | 0.989910 | 4084.7 | 1.000000 |
| 38 | 0.601 | 4150 | 605 | 0.9454 | 172 | 22.04 | 0.989998 | 4036.0 | 1.000000 |
| 39 | 0.605 | 4100 | 598 | 0.9438 | 173 | 21.87 | 0.989740 | 3987.0 | 1.000000 |
| 40 | 0.609 | 4050 | 591 | 0.9411 | 175 | 21.49 | 0.989604 | 3938.0 | 1.000000 |
| 41 | 0.612 | 4000 | 583 | 0.9374 | 176 | 20.96 | 0.989082 | 3888.7 | 1.000000 |
| 42 | 0.616 | 3950 | 576 | 0.9341 | 177 | 20.62 | 0.988426 | 3839.5 | 1.000000 |
| 43 | 0.620 | 3900 | 568 | 0.9303 | 178 | 20.24 | 0.987491 | 3790.2 | 1.000000 |
| 44 | 0.624 | 3850 | 561 | 0.9279 | 179 | 19.93 | 0.987649 | 3741.2 | 1.000000 |
| 45 | 0.628 | 3800 | 554 | 0.9245 | 180 | 19.59 | 0.987004 | 3691.9 | 1.000000 |
| 46 | 0.632 | 3750 | 546 | 0.9223 | 181 | 19.37 | 0.986508 | 3643.1 | 1.000000 |
| 47 | 0.637 | 3700 | 539 | 0.9205 | 183 | 19.15 | 0.986923 | 3595.3 | 1.000000 |
| 48 | 0.641 | 3650 | 532 | 0.9178 | 184 | 18.94 | 0.986445 | 3546.3 | 1.000000 |
| 49 | 0.645 | 3600 | 525 | 0.9155 | 185 | 18.71 | 0.986071 | 3497.8 | 0.999999 |
| 50 | 0.650 | 3550 | 517 | 0.9126 | 186 | 18.44 | 0.985406 | 3449.2 | 0.999999 |
| 51 | 0.655 | 3500 | 510 | 0.9100 | 188 | 18.21 | 0.985677 | 3400.8 | 0.999999 |
| 52 | 0.659 | 3450 | 503 | 0.9072 | 189 | 17.96 | 0.984682 | 3352.3 | 0.999999 |
| 53 | 0.664 | 3400 | 496 | 0.9048 | 190 | 17.79 | 0.984604 | 3304.4 | 1.000000 |
| 54 | 0.669 | 3350 | 488 | 0.9025 | 192 | 17.58 | 0.984443 | 3255.7 | 0.999999 |
| 55 | 0.674 | 3300 | 481 | 0.9002 | 193 | 17.40 | 0.984127 | 3207.8 | 0.999999 |
| 56 | 0.679 | 3250 | 474 | 0.8975 | 195 | 17.19 | 0.983736 | 3159.5 | 0.999999 |
| 57 | 0.685 | 3200 | 467 | 0.8938 | 196 | 16.93 | 0.983234 | 3110.9 | 0.999999 |
| 58 | 0.690 | 3150 | 459 | 0.8924 | 198 | 16.86 | 0.983105 | 3062.5 | 0.999999 |
| 59 | 0.696 | 3100 | 452 | 0.8877 | 200 | 16.51 | 0.982191 | 3013.9 | 0.999999 |
| 60 | 0.701 | 3050 | 445 | 0.8867 | 201 | 16.43 | 0.982221 | 2966.3 | 0.999999 |
| 61 | 0.707 | 3000 | 438 | 0.8839 | 203 | 16.25 | 0.982359 | 2917.7 | 0.999999 |
| 62 | 0.713 | 2950 | 430 | 0.8817 | 205 | 16.10 | 0.982212 | 2869.0 | 0.999999 |
| 63 | 0.719 | 2900 | 423 | 0.8779 | 206 | 15.85 | 0.981770 | 2820.5 | 0.999999 |
| 64 | 0.725 | 2850 | 416 | 0.8735 | 208 | 15.61 | 0.980241 | 2772.4 | 1.000000 |
| 65 | 0.732 | 2800 | 409 | 0.8699 | 210 | 15.36 | 0.980929 | 2724.0 | 0.999999 |
| 66 | 0.739 | 2750 | 401 | 0.8657 | 212 | 15.12 | 0.980276 | 2675.0 | 0.999999 |
| 67 | 0.745 | 2700 | 394 | 0.8571 | 214 | 14.69 | 0.978628 | 2625.7 | 0.999999 |
| 68 | 0.752 | 2650 | 387 | 0.8510 | 216 | 14.33 | 0.977858 | 2577.2 | 1.000000 |
| 69 | 0.760 | 2600 | 379 | 0.8451 | 218 | 14.08 | 0.977024 | 2528.3 | 0.999999 |
| 70 | 0.767 | 2550 | 372 | 0.8385 | 220 | 13.75 | 0.975798 | 2479.5 | 0.999999 |
| 71 | 0.775 | 2500 | 365 | 0.8335 | 222 | 13.53 | 0.974831 | 2430.5 | 0.999999 |
| 72 | 0.782 | 2450 | 357 | 0.8269 | 224 | 13.25 | 0.973394 | 2381.7 | 0.999999 |
| 73 | 0.791 | 2400 | 350 | 0.8208 | 227 | 13.05 | 0.971437 | 2333.0 | 0.999999 |
| 74 | 0.799 | 2350 | 343 | 0.8157 | 229 | 12.81 | 0.971131 | 2284.5 | 0.999999 |
| 75 | 0.808 | 2300 | 335 | 0.8087 | 232 | 12.51 | 0.970381 | 2235.8 | 0.999999 |
| 76 | 0.816 | 2250 | 328 | 0.8049 | 234 | 12.40 | 0.969208 | 2187.7 | 0.999999 |
| 77 | 0.826 | 2200 | 321 | 0.8007 | 237 | 12.21 | 0.968941 | 2139.1 | 0.999999 |
| 78 | 0.835 | 2150 | 314 | 0.7953 | 240 | 12.00 | 0.968407 | 2090.7 | 0.999999 |
| 79 | 0.845 | 2100 | 306 | 0.7879 | 242 | 11.76 | 0.966999 | 2042.4 | 0.999999 |
| 80 | 0.855 | 2050 | 299 | 0.7808 | 245 | 11.51 | 0.965259 | 1993.6 | 0.999999 |
| 81 | 0.866 | 2000 | 292 | 0.7752 | 248 | 11.31 | 0.964688 | 1945.2 | 0.999999 |
| 82 | 0.877 | 1950 | 285 | 0.7711 | 252 | 11.17 | 0.964817 | 1897.3 | 0.999999 |
| 83 | 0.889 | 1900 | 277 | 0.7634 | 255 | 10.93 | 0.962795 | 1848.8 | 0.999999 |
| 84 | 0.900 | 1850 | 270 | 0.7580 | 258 | 10.76 | 0.962640 | 1800.3 | 0.999999 |
| 85 | 0.913 | 1800 | 263 | 0.7501 | 262 | 10.51 | 0.961581 | 1751.9 | 0.999999 |
| 86 | 0.926 | 1750 | 255 | 0.7418 | 266 | 10.27 | 0.959729 | 1703.3 | 0.999999 |
| 87 | 0.939 | 1700 | 248 | 0.7319 | 269 | 9.99 | 0.957383 | 1653.9 | 0.999999 |
| 88 | 0.953 | 1650 | 241 | 0.7226 | 273 | 9.76 | 0.955118 | 1605.0 | 0.999999 |
| 89 | 0.968 | 1600 | 233 | 0.7067 | 278 | 9.33 | 0.951942 | 1555.5 | 0.999999 |
| 90 | 0.984 | 1550 | 226 | 0.6929 | 282 | 8.97 | 0.948455 | 1506.5 | 0.999999 |
| 91 | 1.000 | 1500 | 219 | 0.6860 | 287 | 8.83 | 0.947314 | 1458.2 | 0.999999 |
| 92 | 1.017 | 1450 | 211 | 0.6813 | 292 | 8.74 | 0.945683 | 1410.2 | 0.999999 |
| 93 | 1.035 | 1400 | 204 | 0.6692 | 297 | 8.45 | 0.944485 | 1361.6 | 0.999998 |
| 94 | 1.054 | 1350 | 197 | 0.6626 | 302 | 8.28 | 0.943402 | 1313.4 | 0.999998 |
| 95 | 1.074 | 1300 | 190 | 0.6525 | 308 | 8.07 | 0.941091 | 1264.9 | 0.999998 |
| 96 | 1.095 | 1250 | 182 | 0.6434 | 314 | 7.89 | 0.939183 | 1216.4 | 0.999998 |
| 97 | 1.118 | 1200 | 175 | 0.6324 | 321 | 7.67 | 0.937808 | 1167.3 | 0.999998 |
| 98 | 1.142 | 1150 | 168 | 0.6167 | 328 | 7.35 | 0.932660 | 1118.6 | 0.999998 |
| 99 | 1.168 | 1100 | 160 | 0.5970 | 335 | 6.99 | 0.925107 | 1069.3 | 0.999998 |
| 100 | 1.195 | 1050 | 153 | 0.5843 | 343 | 6.75 | 0.922493 | 1020.7 | 0.999998 |
| 101 | 1.225 | 1000 | 146 | 0.5735 | 351 | 6.56 | 0.919182 | 972.5 | 0.999998 |
| 102 | 1.257 | 950 | 139 | 0.5609 | 360 | 6.35 | 0.914481 | 923.9 | 0.999998 |
| 103 | 1.291 | 900 | 131 | 0.5490 | 370 | 6.15 | 0.912582 | 875.5 | 0.999998 |
| 104 | 1.328 | 850 | 124 | 0.5355 | 381 | 5.92 | 0.909780 | 826.9 | 0.999998 |
| 105 | 1.369 | 800 | 117 | 0.5161 | 393 | 5.62 | 0.901627 | 777.7 | 0.999997 |
| 106 | 1.414 | 750 | 109 | 0.4981 | 406 | 5.35 | 0.893601 | 728.8 | 0.999997 |
| 107 | 1.464 | 700 | 102 | 0.4856 | 420 | 5.16 | 0.890363 | 680.4 | 0.999997 |
| 108 | 1.519 | 650 | 95 | 0.4707 | 436 | 4.94 | 0.888446 | 632.1 | 0.999997 |
| 109 | 1.581 | 600 | 88 | 0.4540 | 453 | 4.70 | 0.880629 | 583.6 | 0.999997 |
References
- Cybulski, A.; Moulijn, J.A. Monoliths in Heterogeneous Catalysis. Catal. Rev.—Sci. Eng. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Roy, S.; Bauer, T.; Al-Dahhan, M.; Lehner, P.; Turek, T. Monoliths as Multiphase Reactors: A Review. AIChE J. 2004, 50, 2918–2938. [Google Scholar] [CrossRef]
- Boger, T.; Heibel, A.K.; Sorensen, C.M. Monolithic Catalysts for the Chemical Industry. Ind. Eng. Chem. Res. 2004, 43, 4602–4611. [Google Scholar] [CrossRef]
- Gokalp, B. Using the Three-Way Catalyst Monolith Reactor for Reducing Exhaust Emissions. J. Renew. Sustain. Energy 2012, 4, 43114. [Google Scholar] [CrossRef]
- Nandi, S.; Arango, P.; Chaillou, C.; Dujardin, C.; Granger, P.; Laigle, E.; Nicolle, A.; Norsic, C.; Richard, M. Relationship between Design Strategies of Commercial Three-Way Monolithic Catalysts and Their Performances in Realistic Conditions. Catal. Today 2022, 384, 122–132. [Google Scholar] [CrossRef]
- Rico-Pérez, V.; García-Cortés, J.M.; De Lecea, C.S.-M.; Bueno-López, A. NOx Reduction to N2 with Commercial Fuel in a Real Diesel Engine Exhaust Using a Dual Bed of Pt/Beta Zeolite and RhOx/Ceria Monolith Catalysts. Chem. Eng. Sci. 2013, 104, 557–564. [Google Scholar] [CrossRef]
- Dupont, V.; Moallemi, F.; Williams, A.; Zhang, S. Combustion of Methane in Catalytic Honeycomb Monolith Burners. Int. J. Energy Res. 2000, 24, 1181–1201. [Google Scholar] [CrossRef]
- Tacchino, S.; Vella, L.D.; Specchia, S. Catalytic Combustion of CH4 and H2 into Micro-Monoliths. Catal. Today 2010, 157, 440–445. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Chen, G.-B.; Hsu, H.-W.; Hsu, J.-R. Catalytic Combustion of Gasified Biomass in a Platinum Monolith Honeycomb Reactor. Appl. Catal. A Gen. 2004, 261, 99–107. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.; Ji, S.; Lang, B.; Habimana, F.; Li, C. Steam Reforming of Methane to Hydrogen over Ni-Based Metal Monolith Catalysts. Catal. Today 2009, 146, 82–86. [Google Scholar] [CrossRef]
- Roh, H.-S.; Lee, D.K.; Koo, K.Y.; Jung, U.H.; Yoon, W.L. Natural Gas Steam Reforming for Hydrogen Production over Metal Monolith Catalyst with Efficient Heat-Transfer. Int. J. Hydrogen Energy 2010, 35, 1613–1619. [Google Scholar] [CrossRef]
- Moraes, T.S.; Borges, L.E.P.; Farrauto, R.; Noronha, F.B. Steam Reforming of Ethanol on Rh/SiCeO2 Washcoated Monolith Catalyst: Stable Catalyst Performance. Int. J. Hydrogen Energy 2018, 43, 115–126. [Google Scholar] [CrossRef]
- Bustinza, A.; Frías, M.; Liu, Y.; García-Bordejé, E. Mono-and Bimetallic Metal Catalysts Based on Ni and Ru Supported on Alumina-Coated Monoliths for CO2 Methanation. Catal. Sci. Technol. 2020, 10, 4061–4071. [Google Scholar] [CrossRef]
- García-Moncada, N.; Navarro, J.C.; Odriozola, J.A.; Lefferts, L.; Faria, J.A. Enhanced Catalytic Activity and Stability of Nanoshaped Ni/CeO2 for CO2 Methanation in Micro-Monoliths. Catal. Today 2022, 383, 205–215. [Google Scholar] [CrossRef]
- El Sawi, M.; Frusteri, F.; Parmaliana, A.; Formisano, B.; Giordano, N. A Kinetic Study of Cyclohexane Dehydrogenation on Pt Monolithic Catalyst. J. Chem. Technol. Biotechnol. 1986, 36, 122–128. [Google Scholar] [CrossRef]
- Parmaliana, A.; Crisafulli, C.; Maggiore, R.; Bart, J.C.J.; Giordano, N. Catalytic Activity of Honeycomb Catalysts, I. The Benzene-Cyclohexane (de) Hydrogenation Reaction. React. Kinet. Catal. Lett. 1981, 18, 295–299. [Google Scholar] [CrossRef]
- Tronconi, E.; Cavanna, A.; Orsenigo, C.; Forzatti, P. Transient Kinetics of SO2 Oxidation over SCR-DeNO X Monolith Catalysts. Ind. Eng. Chem. Res. 1999, 38, 2593–2598. [Google Scholar] [CrossRef]
- Subbanna, P.; Greene, H.; Desai, F. Catalytic Oxidation of Polychlorinated Biphenyls in a Monolithic Reactor System. Environ. Sci. Technol. 1988, 22, 557–561. [Google Scholar] [CrossRef]
- Michael, B.C.; Nare, D.N.; Schmidt, L.D. Catalytic Partial Oxidation of Ethane to Ethylene and Syngas over Rh and Pt Coated Monoliths: Spatial Profiles of Temperature and Composition. Chem. Eng. Sci. 2010, 65, 3893–3902. [Google Scholar] [CrossRef]
- Portela, R.; Wolf, P.; Marinkovic, J.M.; Serrano-Lotina, A.; Riisager, A.; Haumann, M. Tailored Monolith Supports for Improved Ultra-Low Temperature Water-Gas Shift Reaction. React. Chem. Eng. 2021, 6, 2114–2124. [Google Scholar] [CrossRef]
- Quiney, A.S.; Germani, G.; Schuurman, Y. Optimization of a Water–Gas Shift Reactor over a Pt/Ceria/Alumina Monolith. J. Power Sources 2006, 160, 1163–1169. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Li, C.; Ji, S. Conceptual Design and CFD Simulation of a Novel Metal-Based Monolith Reactor with Enhanced Mass Transfer. Catal. Today 2005, 105, 401–406. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Di Sarli, V. CFD Modeling and Simulation of a Catalytic Micro-Monolith. Int. J. Chem. React. Eng. 2011, 9, e2526. [Google Scholar] [CrossRef]
- Shin, S.B.; Skau, K.I.; Menon, M.; Maroor, S.; Spatenka, S. A Modelling Approach to Kinetics Study and Novel Monolith Channel Design for Selective Catalytic Reduction (SCR) Applications. Chem. Eng. Res. Des. 2019, 142, 412–428. [Google Scholar] [CrossRef]
- Shaikh, S.K.; Pathan, K.A.; Chaudhary, Z.I.; Khan, S.A. CFD Analysis of an Automobile Catalytic Converter to Obtain Flow Uniformity and to Minimize Pressure Drop across the Monolith. CFD Lett. 2020, 12, 116–128. [Google Scholar] [CrossRef]
- Sandu, V.-C.; Cormos, A.-M.; Dumbrava, I.-D.; Imre-Lucaci, A.; Cormos, C.-C.; de Boer, R.; Boon, J.; Sluijter, S. Assessment of CO2 Capture Efficiency in Packed Bed versus 3D-Printed Monolith Reactors for SEWGS Using CFD Modeling. Int. J. Greenh. Gas Control 2021, 111, 103447. [Google Scholar] [CrossRef]
- Sadeghi, F.; Tirandazi, B.; Khalili-Garakani, A.; Nasseri, S.; Nodehi, R.N.; Mostoufi, N. Investigating the Effect of Channel Geometry on Selective Catalytic Reduction of NOx in Monolith Reactors. Chem. Eng. Res. Des. 2017, 118, 21–30. [Google Scholar] [CrossRef]
- Štěpánek, J.; Kočí, P.; Marek, M.; Kubíček, M. Catalyst Simulations Based on Coupling of 3D CFD Tool with Effective 1D Channel Models. Catal. Today 2012, 188, 87–93. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Wang, N.; Ring, Z.; Dabros, T. Mathematical Modeling of Monolith Catalysts and Reactors for Gas Phase Reactions. Appl. Catal. A Gen. 2008, 345, 1–11. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Bhattacharya, M.; Balakotaiah, V. Reduced Order Models for Describing Dispersion and Reaction in Monoliths. Chem. Eng. Sci. 2012, 83, 77–92. [Google Scholar] [CrossRef]
- Nien, T.; Mmbaga, J.P.; Hayes, R.E.; Votsmeier, M. Hierarchical Multi-Scale Model Reduction in the Simulation of Catalytic Converters. Chem. Eng. Sci. 2013, 93, 362–375. [Google Scholar] [CrossRef]
- Tu, M.; Ratnakar, R.; Balakotaiah, V. Reduced Order Models with Local Property Dependent Transfer Coefficients for Real Time Simulations of Monolith Reactors. Chem. Eng. J. 2020, 383, 123074. [Google Scholar] [CrossRef]
- Pérez-Cadenas, A.F.; Kapteijn, F.; Moulijn, J.A.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Moreno-Castilla, C. Pd and Pt Catalysts Supported on Carbon-Coated Monoliths for Low-Temperature Combustion of Xylenes. Carbon 2006, 44, 2463–2468. [Google Scholar] [CrossRef]
- Consentino, L.; Pantaleo, G.; Parola, V.L.; Migliore, C.; Greca, E.L.; Liotta, L.F. NH3-NO SCR Catalysts for Engine Exhaust Gases Abatement: Replacement of Toxic V2O5 with MnOx to Improve the Environmental Sustainability. Top. Catal. 2023, 66, 850–859. [Google Scholar] [CrossRef]
- Ferella, F. A Review on Management and Recycling of Spent Selective Catalytic Reduction Catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and Kinetic Modeling Study of NH3-SCR of NOx on Fe-ZSM-5, Cu-Chabazite and Combined Fe- and Cu-Zeolite Monolithic Catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E. Detailed Kinetic Modeling of the NH3–NO/NO2 SCR Reactions over a Commercial Cu-Zeolite Catalyst for Diesel Exhausts after Treatment. Catal. Today 2012, 197, 243–255. [Google Scholar] [CrossRef]
- Benjamin, S.F.; Liu, Z.; Roberts, C.A. Automotive Catalyst Design for Uniform Conversion Efficiency. Appl. Math. Model. 2004, 28, 559–572. [Google Scholar] [CrossRef]
- Baharudin, L.; Watson, M.J. Monolithic Substrate Support Catalyst Design Considerations for Steam Methane Reforming Operation. Rev. Chem. Eng. 2018, 34, 481–501. [Google Scholar] [CrossRef]
- Chang, H.-C.; Wang, L.-C. A Simple Proof of Thue’s Theorem on Circle Packing. arXiv 2010, arXiv:1009.4322. [Google Scholar]
- Tomašić, V. Application of the Monoliths in DeNOx Catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Comsol. CFD Module User’s Guide; Version 5.4; Comsol AB: Stockholm, Sweden, 2018; Available online: https://doc.comsol.com/5.4/doc/com.comsol.help.cfd/CFDModuleUsersGuide.pdf (accessed on 28 October 2023).
- Comsol. Chemical Reaction Engineering Module User’s Guide; Version 5.4; Comsol AB: Stockholm, Sweden, 2018; Available online: https://doc.comsol.com/5.4/doc/com.comsol.help.chem/ChemicalReactionEngineeringModuleUsersGuide.pdf (accessed on 28 October 2023).
- Krocher, O.; Elsener, M. Combination of V2O5/WO3− TiO2, Fe− ZSM5, and Cu− ZSM5 Catalysts for the Selective Catalytic Reduction of Nitric Oxide with Ammonia. Ind. Eng. Chem. Res. 2008, 47, 8588–8593. [Google Scholar] [CrossRef]
- Wilke, C.R. A Viscosity Equation for Gas Mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]
- Voglar, J.; Teržan, J.; Kroflič, A.; Huš, M.; Likozar, B. Mechanistic Modelling of Catalytic NOX Reduction Reactions after Hydrogen or Ammonia Combustion on Multiple Scales. Renew. Sustain. Energy Rev. 2023, 186, 113666. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009; ISBN 0521871212. [Google Scholar]
- Liu, Z.; Yi, Y.; Zhang, S.; Zhu, T.; Zhu, J.; Wang, J. Selective Catalytic Reduction of NOx with NH3 over Mn-Ce Mixed Oxide Catalyst at Low Temperatures. Catal. Today 2013, 216, 76–81. [Google Scholar] [CrossRef]
- Wang, Z.; Lan, J.; Haneda, M.; Liu, Z. Selective Catalytic Reduction of NOx with NH3 over a Novel Co-Ce-Ti Catalyst. Catal. Today 2021, 376, 222–228. [Google Scholar] [CrossRef]
- Gonzo, E.E. Hydrogen from Methanol-Steam Reforming. Isothermal and Adiabatic Monolith Reactors’ Simulation. Int. J. Hydrogen Energy 2008, 33, 3511–3516. [Google Scholar] [CrossRef]
- Heiredal, M.L.; Jensen, A.D.; Thøgersen, J.R.; Frandsen, F.J.; Friemann, J. Pilot-scale Investigation and CFD Modeling of Particle Deposition in Low-dust Monolithic SCR DeNOx Catalysts. AIChE J. 2013, 59, 1919–1933. [Google Scholar] [CrossRef]
- Cutler, A.H.; Antal Jr, M.J.; Jones Jr, M. A Critical Evaluation of the Plug-Flow Idealization of Tubular-Flow Reactor Data. Ind. Eng. Chem. Res. 1988, 27, 691–697. [Google Scholar] [CrossRef]
- Moody, L.F. Friction Factors for Pipe Flow. Trans. Am. Soc. Mech. Eng. 1944, 66, 671–678. [Google Scholar] [CrossRef]
- Gekas, I.; Vressner, A.; Johansen, K. NOx Reduction Potential of V-SCR Catalyst in SCR/DOC/DPF Configuration Targeting Euro VI Limits from High Engine NOx Levels; SAE Technical Paper 2009-01-0626; SAE International: Warrendale, PA, USA, 2009. [Google Scholar]
- Cornejo, I.; Nikrityuk, P.; Hayes, R.E. The Influence of Channel Geometry on the Pressure Drop in Automotive Catalytic Converters: Model Development and Validation. Chem. Eng. Sci. 2020, 212, 115317. [Google Scholar] [CrossRef]
- Lin, K.-S.; Mdlovu, N.V.; Juang, R.-S.; Tang, M.-T. Fine Structural Characterization of Noble Metals in Washcoat of Motorcycle Three-Way Converter Catalysts. J. Environ. Chem. Eng. 2023, 11, 109530. [Google Scholar] [CrossRef]
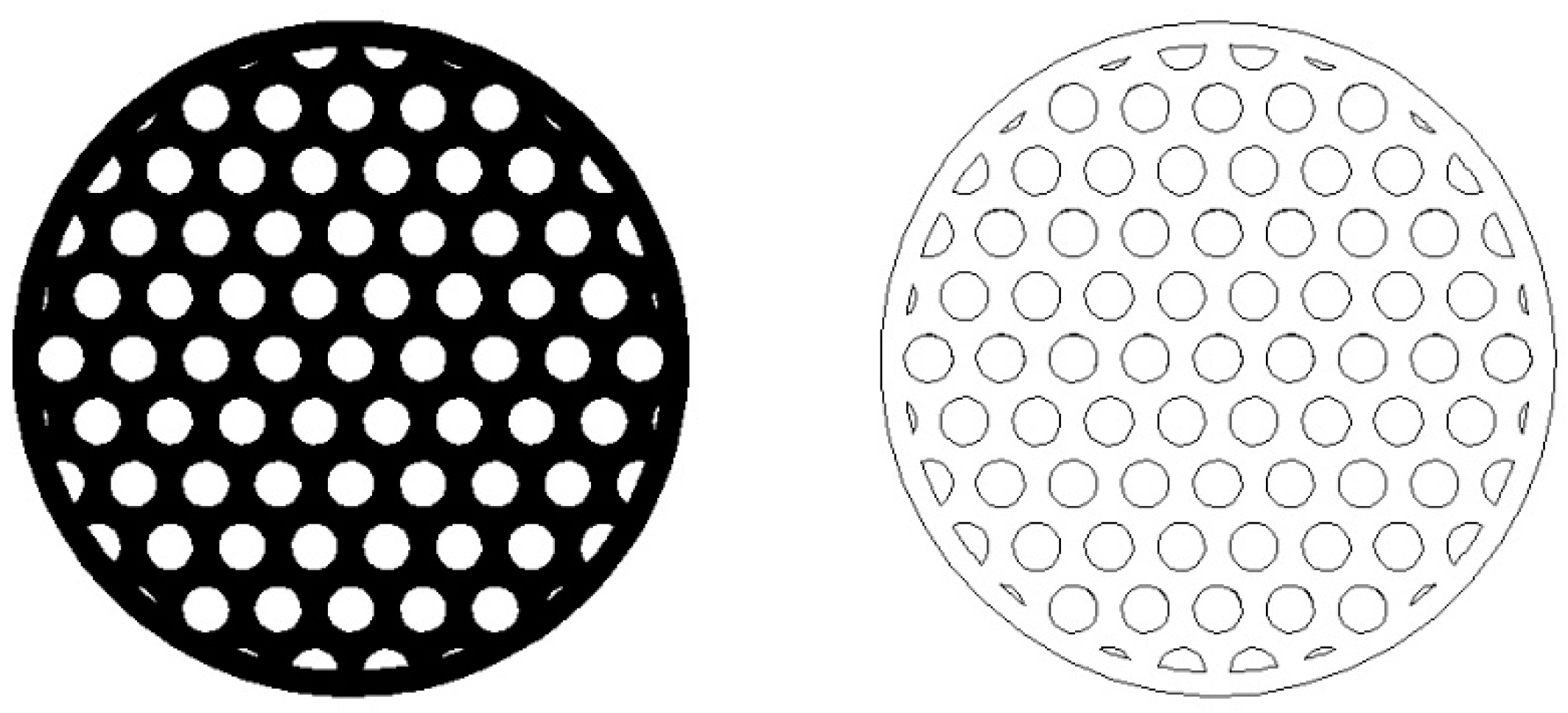


| Index (/) | R (mm) | N (/) | Δp (Pa) | L (mm) | CR (%) |
|---|---|---|---|---|---|
| 1 | 0.500 | 6000 | 573 | 98.4 | 34.4 |
| 2 | 0.502 | 5950 | 574 | 99.2 | 33.8 |
| 3 | 0.504 | 5900 | 572 | 99.8 | 33.4 |
| 4 | 0.506 | 5850 | 576 | 101.3 | 32.4 |
| 5 | 0.509 | 5800 | 571 | 101.4 | 32.4 |
| 6 | 0.511 | 5750 | 573 | 102.5 | 31.6 |
| 7 | 0.513 | 5700 | 571 | 103.1 | 31.3 |
| 8 | 0.515 | 5650 | 571 | 104.1 | 30.6 |
| 9 | 0.518 | 5600 | 567 | 104.1 | 30.6 |
| 10 | 0.520 | 5550 | 567 | 105.1 | 29.9 |
| 11 | 0.522 | 5500 | 568 | 106.2 | 29.2 |
| 12 | 0.525 | 5450 | 569 | 107.4 | 28.4 |
| 13 | 0.527 | 5400 | 569 | 108.4 | 27.8 |
| 14 | 0.530 | 5350 | 568 | 109.3 | 27.1 |
| 15 | 0.532 | 5300 | 568 | 110.3 | 26.5 |
| 16 | 0.535 | 5250 | 570 | 111.6 | 25.6 |
| 17 | 0.537 | 5200 | 566 | 112.1 | 25.3 |
| 18 | 0.540 | 5150 | 568 | 113.5 | 24.3 |
| 19 | 0.542 | 5100 | 565 | 114.0 | 24.0 |
| 20 | 0.545 | 5050 | 568 | 115.7 | 22.9 |
| 21 | 0.548 | 5000 | 567 | 116.7 | 22.2 |
| 22 | 0.550 | 4950 | 564 | 117.2 | 21.9 |
| 23 | 0.553 | 4900 | 564 | 118.3 | 21.1 |
| 24 | 0.556 | 4850 | 563 | 119.4 | 20.4 |
| 25 | 0.559 | 4800 | 561 | 120.2 | 19.9 |
| 26 | 0.562 | 4750 | 563 | 122.0 | 18.7 |
| 27 | 0.565 | 4700 | 550 | 120.3 | 19.8 |
| 28 | 0.568 | 4650 | 549 | 121.4 | 19.1 |
| 29 | 0.571 | 4600 | 546 | 122.0 | 18.7 |
| 30 | 0.574 | 4550 | 546 | 123.3 | 17.8 |
| 31 | 0.577 | 4500 | 545 | 124.4 | 17.1 |
| 32 | 0.581 | 4450 | 547 | 126.3 | 15.8 |
| 33 | 0.584 | 4400 | 546 | 127.5 | 15.0 |
| 34 | 0.587 | 4350 | 548 | 129.4 | 13.7 |
| 35 | 0.591 | 4300 | 548 | 131.1 | 12.6 |
| Index (/) | R (mm) | N (/) | Δp (Pa) | L (mm) | Cost (/) | CR (%) |
|---|---|---|---|---|---|---|
| 27 | 0.565 | 4700 | 550 | 120.3 | 0.3194 | 4.1 |
| 28 | 0.568 | 4650 | 549 | 121.4 | 0.3206 | 3.7 |
| 29 | 0.571 | 4600 | 546 | 122.0 | 0.3205 | 3.7 |
| 30 | 0.574 | 4550 | 546 | 123.3 | 0.3221 | 3.2 |
| 31 | 0.577 | 4500 | 545 | 124.4 | 0.3232 | 2.9 |
| 32 | 0.581 | 4450 | 547 | 126.3 | 0.3263 | 2.0 |
| 33 | 0.584 | 4400 | 546 | 127.5 | 0.3276 | 1.6 |
| 34 | 0.587 | 4350 | 548 | 129.4 | 0.3306 | 0.7 |
| 35 | 0.591 | 4300 | 548 | 131.1 | 0.3329 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voglar, J.; Pavlišič, A.; Likozar, B. Computational Fluid Dynamics, Transport, and Chemical Kinetics-Based Monolith Catalyst Dimensioning Methodology for Cost-Effective Performance. Processes 2024, 12, 1704. https://doi.org/10.3390/pr12081704
Voglar J, Pavlišič A, Likozar B. Computational Fluid Dynamics, Transport, and Chemical Kinetics-Based Monolith Catalyst Dimensioning Methodology for Cost-Effective Performance. Processes. 2024; 12(8):1704. https://doi.org/10.3390/pr12081704
Chicago/Turabian StyleVoglar, Jure, Andraž Pavlišič, and Blaž Likozar. 2024. "Computational Fluid Dynamics, Transport, and Chemical Kinetics-Based Monolith Catalyst Dimensioning Methodology for Cost-Effective Performance" Processes 12, no. 8: 1704. https://doi.org/10.3390/pr12081704
APA StyleVoglar, J., Pavlišič, A., & Likozar, B. (2024). Computational Fluid Dynamics, Transport, and Chemical Kinetics-Based Monolith Catalyst Dimensioning Methodology for Cost-Effective Performance. Processes, 12(8), 1704. https://doi.org/10.3390/pr12081704








