Abstract
Zinc oxide nanoparticles (ZnO-NPs) have gained significant interest in the agricultural and food industry as a means of killing or reducing the activity of microorganisms. The antibacterial properties of ZnO-NPs may improve food quality, which has a direct impact on human health. ZnO-NPs are one of the most investigated inorganic nanoparticles and have been used in various related sectors, with the potential to rapidly gain attention and increase interest in the agriculture and food industries. In this review, we describe various methods for preparing ZnO-NPs, their characterizations, modifications, applications, antimicrobial activity, testing procedures, and effects, including bactericidal and bacteriostatic mechanisms. It is hoped that this review could provide a better understanding of the preparation and application of ZnO nanoparticles in the field of food and agriculture, and promote their development to advance the field of food and agriculture.
1. Introduction
Although Norio Taniguchi, a Japanese scientist, initially developed “nanotechnology” in 1974, its roots may be found as far back as 1959 [1]. Because of its unique characteristics and the notable significance of nanoparticles, it has become the most recent, revolutionary, inventive, and prominent hotspot of study in modern science. Nanoparticles are employed extensively in the fields of electronics, optics, biomedicine, and materials science. They have gained unexpected popularity in recent years by offering creative solutions in several scientific fields [2]. Due to the high surface area-to-volume ratio and distinctive physicochemical properties such as color, dispersion, and thermodynamics, they demonstrate possessions related to size that are remarkably distinct from bulk materials and have unique properties in comparison to their macroscale counterparts [3,4]. Advanced uses of nanotechnology are being made in the field of food science, and it has emerged as a key factor in production, processing, storage, and quality control of foods [4,5].
Zinc oxide nanoparticles (ZnO-NPs) are one of the metal oxide nanomaterials and a valuable and versatile inorganic compound due to its unique physical and chemical characteristics. They possess high chemical stability, a broadened radiation absorption spectrum, high electrochemical coupling coefficient, and high photostability with the molecular formula ZnO [6]. ZnO-NPs have been widely manufactured and utilized in various commercial and additive products, including ceramics, cement, plastics, glass, ointments, lubricants, adhesives, sealants, pigments, batteries, ferrites, fire retardants, cosmetics, and sunscreens, as well as in foods as a source of zinc nutrient [7,8]. Nanosized ZnO particles demonstrate significant antibacterial capabilities due to their small size, which can stimulate different bactericidal mechanisms once inside the bacterial cell, including the bacterial surface or bacterial core, generate ROS (reactive oxygen species), release Zn2+, and even be endocytosed by cells. (Figure 1) [7,9,10].
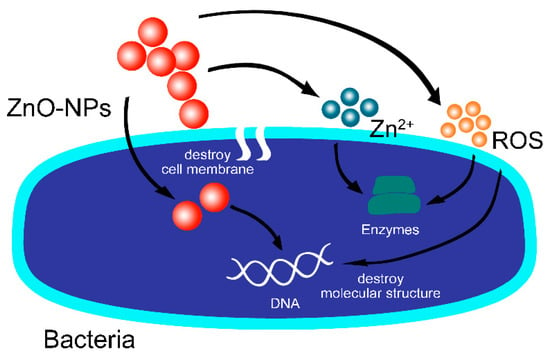
Figure 1.
Antibacterial mechanism of ZnO-NPs. (ROS formation, Zn2+ release, internalized ZnO-NPs, and electrostatic interactions.).
The objective of this review is to provide an overview of the application of ZnO nanotechnology in the food industry and agriculture, and to offer food and agriculture scientists a comprehensive perspective on ZnO nanotechnology. This technology enables rapid, sensitive, and useful detection for defense against microbial contamination, harmful chemicals, and pesticides in food and agriculture.
2. Structure
As zinc and oxygen are members of the second and sixth groups of the periodic table, respectively, ZnO is a recognized II-VI semiconductor in the field of materials science. The ZnO semiconductor has many exceptional and beneficial properties, such as good transparency, antimicrobial agents, high electron mobility, wide bandgap, high thermal and mechanical stability at room temperature, and strong room-temperature luminescence. Its large bandgap, or 3.37 eV, is on the borderline between ionic and covalent semiconductors [6,11].
The crystalline of ZnO has a wurtzite (B4) crystal structure, having a hexagonal unit cell with two lattice parameters a = 0.325 nm and c = 0.521 nm. With its hexagonal wurtzite structure, each anion is surrounded by four cations at the corners of the tetrahedron, which displays the tetrahedral coordination and hence exhibits the sp3 covalent bonding. The tetrahedral shape of ZnO gives rise to a non-centrosymmetric structure (Figure 2) [12,13].
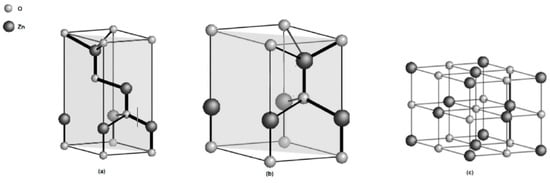
Figure 2.
Crystal structure models of ZnO (a) zinc blende (b) wurtzite and (c) rock salt [14].
3. Preparation Method
In this review, we will briefly discuss a few key techniques for preparing and obtaining ZnO-NPs. By manipulating the synthesis settings, ZnO-NPs might be produced using a variety of techniques. The preferred application largely determines the method to be used, as several techniques produce zinc oxide particles with various morphologies and sizes [15,16,17,18]. There are several methods to prepare ZnO-NPs as follows.
3.1. Conventional Synthesis Methods
Hydrothermal method. This method involves the reaction of precursor chemicals in an aqueous solution under high pressure and temperature conditions, causing the precursor chemicals to react and form nanoparticles. Because of its low process temperature, eco-friendliness, low cost, scalability, use of basic equipment, and simplicity of handling, it is an effective alternative synthetic technique that has attracted a lot of interest. When the temperature, duration, and precursor concentration of the hydrothermal process are altered, the shape and size of the particles can be adjusted. The basic procedure went as follows: Make zinc acetate (dehydrate) solutions (Zn(CH3COO)2(H2O)2), dissolve NaOH in wood alcohol, and, while stirring, add to the (CH3COO)2·2H2O solution. Adjust the pH to between 8 and 11. The collected suspension is then placed in a Teflon-lined stainless steel autoclave and kept there for 6 to 12 h at 120 °C. The resultant compound is cleaned with water and wood alcohol. The suspension is finally freeze-dried to obtain zinc oxide nanoparticle powder [19]. In some reports, ZnO-NPs with more uniform particle size and better dispersion could be obtained by ultrasonic-assisted methods [20].
ZnO-NPs will transform from spherical to rod-shaped if ethanol is used in lieu of water in the reaction system. This is because of the easy cleavage of the C-O bond of the alcohols, which takes place on the zinc metal’s surface. The following was a procedure: 10 mL of ethanol was mixed with 5 mg of Zn metal powder. The reaction mixture was sonically cranked for 20 min before being placed in an inert stainless steel autoclave. The reaction mixture was slowly brought to 200 °C (2 °C/min) and maintained there for 24 to 48 h. To create a rod-shaped nano-zinc oxide powder, the recovered solution was centrifuged, cleaned, and then vacuum-dried [21]. Microwave could be used in ZnO-NPs solvothermal synthesis, and the nanoparticles’ size, ranging from 20 nm to 120 nm, could be collected by controlling the power of microwave radiation [20,22].
Precipitation method. In this method, a precursor solution of zinc salts is mixed with a suitable reagent. This could be a base or an acid, under controlled conditions, resulting in the formation of ZnO nanoparticles. Zinc nitrate and urea might be used as precursors in chemical processes that produce ZnO-NPs under extreme heat and pressure in a sealed aqueous solution. The usefulness of the precipitation approach and its distinct advantages, such as speed, economy, and simplicity, were taken into account. However, the simultaneous occurrence of nucleation and growth in the ZnO growth process made it challenging to investigate the process in detail. The following is the basic procedure: Urea was dissolved in distilled water for 30 min while stirring regularly, and the urea solution serves as a precipitating agent. After vigorous swirling of the zinc nitrate solution for two hours at 70 °C, the mixed solution finally turned into a whitish cloudy solution. Furthermore, this white precursor product was centrifuged at 8000 rpm for 10 min and washed with distilled water to remove any impurities or absorbed unpolymerized ions. The calcination of the obtained product could be performed at 500 °C in air conditions for 3 h using a muffle furnace [23,24].
There was a simpler and costless effective method that did not require a solvent or calcinations after drying. Different morphologies could be obtained from the growth in alkaline solutions using NaOH; in addition, this pathway could be scaled up. However, it required considerable amounts of water. Zinc acetate of aqueous dehydrate dissolved in 50 mL of distilled water under continued stirring. Then a NaOH solution was added dropwise to reach pH 12 at room temperature and kept stirring for 2 h. After completion of the reaction, the harvested white precipitate was washed with a very well and thoroughly with distilled water and ethanol to remove any remaining impurities. The precipitate could then be dried in an air oven for a single day at 60 °C, during which the conversion of Zn(OH)2 to ZnO-NPs could occur [25,26].
Chemical vapor transport method. In this method, the powder is heated to a high temperature in the presence of a transport agent such as graphite or iodine, then transported to a cooler region, where it condenses to form nanoparticles. Bunsen initially introduced this technique in 1852. Heterogeneous reactions that have a common characteristic are referred to as “Chemical Vapor Transfer”. ZnO nanostructures are formed as a result of the process that results from the vapors of oxygen and zinc or oxygen mixture being transported and reacting with one zone after another. That was a straightforward, simple, and easy way to obtain the ZnO decomposition. It also required heating the zinc powder while the oxygen was flowing, which required careful control of the oxygen pressure to zinc vapor pressure ratio in order to produce suitable ZnO nanostructures. Changes in this ratio led to significant variations in the morphology of ZnO, including the size and geometry of nanostructures, which have been observed [27,28,29].
3.2. Biological/Green Synthesis Methods
A more promising and different form of synthesis from chemical and physical synthesis is the biological or “green” production of NPs. ZnO was used primarily as a food additive, dietary supplement, and component of medications. The use of safe reagents, such as water and natural extracts, eliminated the need to employ hazardous ingredients and was an amazing way to create metal nanoparticles [30,31,32]. Due to the exceptional achievements in the field of nanobiotechnology, the production of ZnO-NP using biotechnological methods offers a wide variety of potential applications in the realm of medicine. Proteins, DNA and plants or plant extracts (roots, stems, leaves, flowers, and fruits) [33,34,35] have been investigated in biological ways as safe substitutes for the chemical and physical methods of ZnO-NP, which used the appropriate biochemical and enzymatic pathways in microorganisms [36]. Proteins, amino acids, DNA, enzymes, phages, and marker genes in different microorganisms could also be used for the synthesis of ZnO-NPs, where DNA is used as a guide for the synthesis of ZnO-NPs chains and to control their growth. Moreover, the synthesis of ZnO-NPs by biotechnological tools has great potential in biological applications such as biological labelling, cell cultures, gene delivery, drug delivery, and nanomedicines [37,38,39].
Plant-mediated synthesis. An interesting alternative to traditional chemical approaches is the production of ZnO-NP using plants and plant extracts [34]. The use of plants and plant extracts is an appealing, innovative, and safe method choice. A typical ZnO-NP production process is given in Figure 3, which avoids the use of harmful and hazardous substances [40,41]. For the synthesis of ZnO-NPs, we have a variety of plants and processes at our disposal. We will talk about the most popular, accessible, and simple synthesis methods in this section. Tomato fruits, chamomile flowers, and olive leaves were rinsed with double-distilled water and then allowed to air-dry. 200 mL of water was extracted for 4 h at 60–70 °C from powdered ground leaves, flowers, and fruits. Afterwards, the extracts were cooled to room temperature and filtered by filter paper. The collected plant extracts and ZnO were then mixed in a separate flask for the synthesis of ZnO-NPs. The mixture was stirred for 4 h at 100 rpm under the heating condition. The reaction solution was centrifuged at 10,000× g for 20 min, the supernatant was discarded, and the precipitation was collected. The precipitate obtained was washed with distilled water and freeze-dried to obtain ZnO NP [42,43].
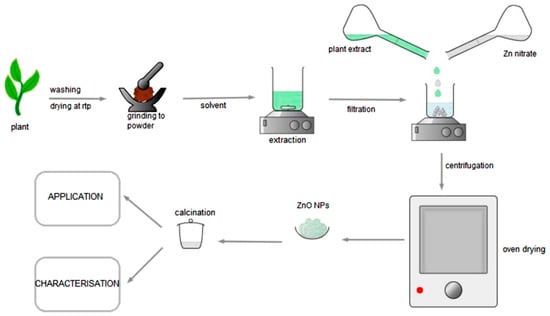
Figure 3.
Schematic of the most common ZnO-NPs synthesis procedure [14].
Microorganisms-mediated synthesis. There has been a lot of interest in employing microbes, and numerous studies have been done using various models of microorganisms. However, maintaining certain processes, such as cell cultures, intracellular synthesis, and multiple purification steps, made microbially assisted nanoparticle synthesis somewhat challenging [44,45], and the mechanism of biological synthesis of ZnO-NP using microbes is not yet fully understood [45,46]. Many studies have been conducted on biologically mediated production of ZnO-NP using microorganisms such as fungus, bacteria, yeast, algae, and phage [47]. The choice of microorganisms, ideal cell growth conditions, and the route of biosynthesis, intracellular or extracellular, are the primary requirements for the production of ZnO-NPs. Most of the time, Sphingobacterium thalpophilum, Staphylococcus aureus, and Bacillus megaterium NCIM2326 were used to produce rod/cubic, multiform, triangular, and acicular ZnO-NP with a particle size of 10–95 nm. The fungi of Aspergillus niger and Candida albicans were used to prepare spherical or quasi-spherical ZnO-NPs with a particle size of 61 nm and 25 nm, respectively [45]. The yeast, Pichia kudriavzevii and Pichia fermentans JA2, was used to prepare hexagonal wurtzite and smooth/elongated ZnO-NPs with a particle size of 10–61 nm. The algae, Chlamydomonas reinhardtii and Sargassum muticum have been used safely. We have summarized the application of fungi, bacteria, yeast, and phages in the synthesis of ZnO-NP, including their size, shape, and activity carried out, as shown in Table 1.

Table 1.
Microorganisms-mediated synthesis of ZnO-NPs.
Algae-mediated synthesis. As it reduces the toxicity of the chemical ingredients used to produce the nanoparticles, algae are one of the safest and most stable natural materials used in the green synthesis of nanoparticles. According to reports, ZnO-NPs can be produced using a variety of marine macro- and microalgae, including chlorella, brown Sargassum muticum, green caulerpa peltata, and red gracilaria gracilis [57,58,59,60].
3.3. Physical Synthesis Methods
These processes were mainly known for their use of chemically pure reagents and simple technology, which makes them ideal for performing industrial processes at high production rates.
Arc plasma. The most popular physical approach for transforming bulk materials into nanomaterials by condensation and evaporation was electrical arc discharge-mediated synthesis. Islam et al. used an arc-discharge-mediated gas evaporation method to synthesized ZnO-NPs, the zinc rod was used as zinc source, dry air was used as oxygen source, and carbon rod was acted as cathode [61]. Thermal evaporation. This was a commonly used method for creating ZnO nanostructures. At a temperature of 1000–1100 °C, graphite was often combined with ZnO powder as a reducing agent to create a pure nanostructure ZnO. The addition of carbon caused the ZnO precursor, which has a melting point of 1975 °C, to be reduced, resulting in the formation of Zn and ZnOx, which evaporate at 500 °C and is then mixed with oxygen to produce ZnO products. Using the thermal evaporation approach to create pure ZnO nano/microstructures, several metallic elements might be employed as a good supply of reducing agents [62].
Physical vapor deposition. This was a fairly straightforward procedure to create ZnO nanowires at a low temperature of 450 °C. The ZnO nanowires followed a catalyst-free growth process, as evidenced by the fact that the diameter of the nanowires grew as the temperature increased [63,64]. These findings were promising for the construction of ZnO-based nanoscale devices onto diverse low-temperature-endurance substrates.
Ultrasonic irradiation. This technique has been utilized often to create nanoparticles in solution phase operations [65]. It differs from surfactant procedures because it is an effective natural way that does not change the character of the particles [66]. The best and most practical approach to produce pure materials with a wide range of regulated characteristics is ultrasonic irradiation [67,68]. In Zhang’s study, the physical mechanism of ultrasound-assisted synthesis of ZnO-NPs was uncovered. Four stages of changes caused by ultrasound were visible through voltage changes in the acoustic signal, cycles of cavitation bubble oscillation, cycles of collapse, maximum voltage amplitude, and acoustic intensity (Figure 4) [69].
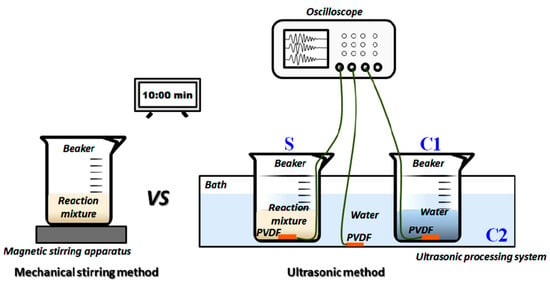
Figure 4.
Schematic diagram of monitoring of ultrasonic fields during synthesis process of nano-ZnO [69].
Laser ablation. This is an easy and reliable approach for creating nanoparticles made of almost any material, including semiconductors. The laser ablation synthesis method has been shown to be an effective way for producing diverse nanoscale materials and allows for the investigation of a solid target in liquids. This method has several benefits, including the ability to control the ablation atmosphere with low-cost equipment. In addition, the size of the synthesized material can be altered by adjusting various parameters, including pulse laser duration, laser wavelength, solution pH, temperature, and the addition of surfactants [70,71].
3.4. A Non-Conventional Method
Microfluidic reactor-based method. This is a process that may be utilized to fabricate materials on a benchtop [47]. Due to its special qualities in the synthesis of nanoparticles as opposed to conventional and macroscale synthesis, the technique demonstrated a number of benefits. Moreover, it was employed to make ZnO-NPs. The procedure uses a minimal amount of chemicals and offers selectivity, environmental protection, quick response times, as well as small environmental impact and increased health and safety [4,72].
4. Modifications
A product can be modified to modify certain characteristics such as size, structure, morphology, colour, hardness, softness, and strength. The modification might improve the compatibility of ZnO-NPs with organic (carboxylic acid, silanes), inorganic (metal oxides), and polymers, etc., (Figure 5), which could improve ZnO-NP performance characteristics, such as consumable time, life cycle, optical properties, and also make it more beneficial to obtain more benefits and significantly reduce defects [73,74,75,76]. Thus, various potential modification techniques stated in the publications of ZnO-NPs are included in this section as follows.
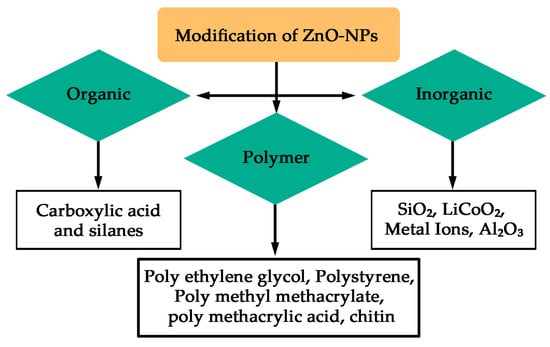
Figure 5.
Schematic diagram of the most popular modifications methods of ZnO.
Increasing numbers of studies have shown that silica and trimethyl siloxane (TMS) may modify ZnO. The precursor Zn carbonate hydroxide (ZCH) was calcinated to produce the purest ZnO particles. Precipitation techniques were used to produce ZCH from a variety of substrates, including ammonium solution (NH4OH), ammonium bicarbonate (NH4HCO3) and zinc sulfate heptahydrate (ZnSO4·7H2O). Hexamethyldisilazane (HMDS) and TEOS were used to modify the ZCH surface in situ while it was being processed in water. To produce ultrafine ZnO particles, the ZCH was calcinated and functionalized. The ZnO particle modification found a workable answer to the agglomeration problem. The photocatalytic activity of an inorganic component (silica) on the ZnO surface was decreased, but an organic molecule increased the compatibility of ZnO with an organic matrix. In order to use these modifying agents to promote important benefits, the modified ZnO surface was explored as an incredibly clear, transparent material that offered outstanding protection against UV radiation. The main mechanisms and contributing factors to the antiyeast and antialgal actions of ZnO-NPs were investigated [77,78,79]. By employing TEOS and CTAB to build a mesoporous silica layer on the surface of ZnO-NPS, we were able to successfully produce and characterize ZnO@mSiO2 nanocomposites and prevent ZnO-NPs from coming into contact with maize directly while exerting their antibacterial activity. Furthermore, it could decrease the ZnO-NP adsorption aggregation and improve their dispersion (Figure 6) [21].
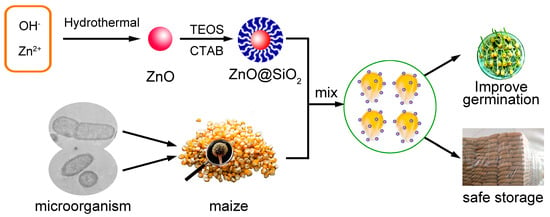
Figure 6.
Schematic illustration of the synthesis and surface modification of ZnO and its application in inhibiting the growth of bacteria and fungi of maize [21].
Mana M. N. Yung et al., performed a modification on the surface of ZnO-NP by commercial sunscreens with the property of easily being released into the water, and raising concerns about their possible impacts on aquatic life. To assess the hazardous potential of three freshwater microalgae and three marine microalgae, the authors evaluated the chemical science characteristics of silane-coated and untreated ZnO-NPs. The ZnO-NP surfaces were modified by covering them with 3-aminopropyl-trimethoxysilane, which produced many surface particles that were deliquescent or dodecyltrichlorosilane, which makes the particles hydrophobic. Compared to uncoated ZnO-NP, coated ZnO-NP produced fewer aggregates and released less Zn2+. Uncoated ZnO-NPs were more effective than coated ZnO-NPs in inhibiting algal cell growth because different algal species had different sensitivities between algal species; uncoated ZnO-NPs were more effective in inhibiting algal cell expansion than coated ZnO-NPs after 96 h exposed to ZnO, uncoated ZnO-NPs, all coated ZnO-NPs, or ZnSO4 at 10 concentrations ranging from 0.1 to 100 mg/L. Due to the exposure of marine diatoms, Thalassiosira pseudonana to nanoparticles showed the distinction within gene expressions, suggesting that ZnO-NPs acted through completely different mechanisms of toxic/harmful action [75].
Wysokowski et al., modified ZnO-containing blended material using β-chitin from Sepia Officinalis cephalopod mollusk. Their study steered a morphological application to outline β-chitin as a layout for biomimetic ZnO deposition that was extremely engaging with the technological side because it eliminated or reduced the challenges related to the production of polysaccharide chitin to chitosan and the process to membranes or scaffolds [74].
Chen et al., and co-researchers developed a unique treatment method to obtain hydrophobic ZnO-NPs to obtain extremely distributed and long-run stable ZnO-NPs in an organic matrix. They grafted aminopropyltriethoxysilane (APS) onto the surface of ZnO-NP, and introduced a protracted carbon chain of stearic acid (SA) through a condensation reaction between APS, and also activated SA with N, N′-carbonyldiimidazole (CDI). FTIR, TGA, SEM, and a sedimentation test are commonly used to analyze ZnO-NPs. SEM observations and the sedimentation test indicate that the new surface treatment would significantly reduce particle aggregates and promote long-term stability in an organic matrix [73].
5. Common Tools and Techniques for Characterization
There are several tools and techniques to identify the characteristics of synthesized ZnO-NPs. These techniques are necessary and specifically used to distinguish the ZnO-NPs accordingly to their appearance, size, distribution, shape, morphology, specific surface area, and all the measurements related to physicochemical property evaluation [80]. Here, we are going to discuss the most common and important tools and techniques used in the characterizations of ZnO-NPs.
5.1. UV-Vis Spectrophotometer (UV-Vis)
The light wavelength in the region of 300–800 nm is often utilized for the evaluation of metal nanoparticles in the size range of 2–100 nm [34,81]. This approach is used for the characterization of nanoparticles for their maximum absorbance and optical characteristics of nanosized particles. Somu et al., used brown alga Chaetomorpha Linum as a reductant to prepare ZnO nanoparticles; its absorption peak maxima was observed at 356.8 nm [82]. Moreover, it provided a significant contribution to the characterization of ZnO-NPs, indicating that the distribution of nanoparticles was monodispersed [83].
5.2. X-ray Diffractometer (XRD)
Determining the form, size, and crystal structures of carbon-based, inorganic, or complicated crystalline materials is a well established and widely used approach. High spatial resolution at the atomic scale is provided by X-ray diffractometers, although they are limited to crystalline materials and have lower intensities than electron diffraction [19,84,85]. The two patterns were exactly matched when we generated a ZnO-NP and compared the XRD pattern with the reference PDF card (01-070-2551) of zinc oxide (Figure 7).
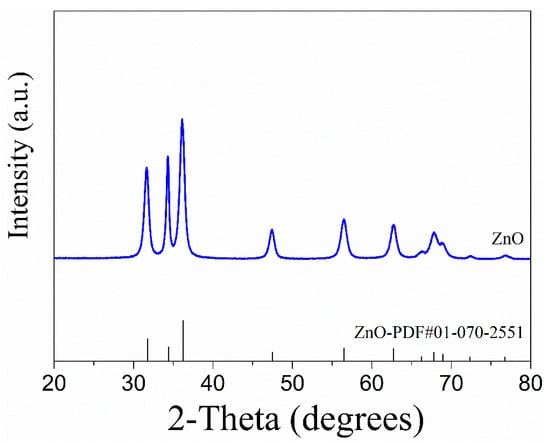
Figure 7.
XRD pattern of ZnO-NPs and ZnO standard PDF card.
5.3. Fourier Transform Infrared Spectroscopy (FT-IR)
With this method, the absorption of IR radiation by a material is measured and plotted versus wavelength. The dried powder was utilized for the FT-IR spectroscopy study of synthesised ZnO-NPs. Interpretation and connection of chemical constituents in the sample and absorption bands (vibrational bands) are required for IR spectra [34,86]. To identify the composition of functional groups in green-produced ZnO-NPs, Alahmdi et al. [87], employed FT-IR. Several peaks were seen in the FT-IR spectra at 3455, 1640, 1400, 1070.1, 960, and 846 cm−1. In the report by Somu et al. [82], the bands for ZnO at 448.6 cm−1 were attributed to ZnO flexing vibrations, and there were bands attributed to -C-O stretching (1026.6 cm−1), hydroxyl grops (1358.2 cm−1) and C-H stretching (2889.1 cm−1).
5.4. Atomic Force Microscopy (AFM)
This is a very new, adaptable, and strong approach that offers a variety of surface measurements to show the resolution of sample fractions at the nanoscale. Moreover, materials seen by AFM do not require specific conditions, such as metal or carbon coatings, that would irreversibly alter or harm the sample [88]. It provides a true three-dimensional surface profile. The Fe2O3/ZnO heterostructure was captured in 3D and 2D RMS (459 nm) AFM micrographs by Sevda et al. [89], using this technique. AFM provided high-resolution images that were easily able to estimate the forces acting between the probe and the sample surface area [90,91].
5.5. Scanning Electron Microscopy (SEM)
This approach is frequently used for high-resolution nanomaterials and is effective in determining ZnO-NP crystallinity, as well as their structural surface morphology, size, size distribution, shape, and dispersion [92]. It may also be used to examine complicated, inorganic, biological, carbon-based, and organic materials, as well as to identify the morphological condition of nanomaterials [93,94].
5.6. Transmission Electron Microscopy (TEM)
This is used for the structural characterization of nanoparticles, as well as for the study of their particle parameters, such as size and shape [34]. Compared to SEM, high-resolution microscopy enables measurement of size and size distribution and validates the morphologies of nanomaterials [95]. At some point during the synthesis process, the size, length distribution, crystalline structures, aggregates, and morphology of ZnO-NPs were widely used and examined [96].
5.7. X-ray Photoelectron Spectroscopy (XPS)
XPS characterization is performed to elucidate the chemical composition component with chemical bonding state and surface chemistry for the confirmation of doping. In the ZnO spectrum, Zn and O elements would be observed, and in the Zn 2p spectrum, two bands were observed at 1045.0 eV and 1021.6 eV would be observed for ZnO in the study by Somu et al. [17,82].
6. Morphological Impacts
Several strategies have been documented by researchers to quantify the morphological effects of ZnO-NPs, which have a significant impact on their antibacterial activity. The circumstances of synthesis determine the shape of ZnO-NPs. ZnO-NPs have uncountable shapes (nano rods, nanowires, nanotubes, nano spheres, nano needles, drums, nano rings, spirals, polyhedrons, flowers, discs, plates, stars, and boxes) (Figure 8), each of which exhibits morphologically dependent physicochemical properties, according to some distinctive synthesis mechanisms that have been previously reported [97]. Therefore, by manipulating key variables including precursor types, solvents, pH and temperature, it is possible to obtain desperately manufactured ZnO-NP structures for the optimal response to antibacterial activity [98,99]. Surface activity controls the morphology beneath regulated growth circumstances, such as the surface. The fraction of active sides in the nanoparticles was used to characterize the shape-dependent activity. Methods of synthesis and growth produce nanoparticles with a variety of active surfaces. However, investigations have shown that morphologies with completely distinct active characteristics have varied antibacterial activity [100,101]. Rods and wires penetrate the walls of bacterial cells more easily than spherical ZnO-NPs, demonstrating how the forms of ZnO-nanostructures will affect how they are internalized. However, ZnO-NPs in flower form exhibited more biocidal activity against E. coli and S. aureus than those in rod or spherical form [102].

Figure 8.
Scanning electron microscopy (SEM) morphology of ZnO nanoparticles (A) spherical ZnO, (B) bicone ZnO, (C) hexagonal sectional ZnO, (D) ZnO plates [97].
ZnO-NPs showed destructing bacterial cell integrity by direct contact with cell walls [103,104]. The liberation of antimicrobial ions in the main Zn2+ ions and ROS formation [104,105,106]. Thus, morphology has been found to play a great and important role in antibacterial activity to make food safe and healthy.
7. Advantages and Possible Risk
7.1. Advantages
In the field of nanotechnology, ZnO-NPs are considered one of the most promising areas of the current industrial revolution [107]. The unique features of ZnO-NPs have motivated scientists, researchers, and technologists to develop various simple, easy, safe and cost-effective techniques to construct ZnO-NPs for industrial applications [98]. ZnO-NPs are considered an important ingredient for several enzymes, sunscreen, and ointments for itches and pain relief [43]. ZnO-NPs are a promising and versatile inorganic material with a wide range of applications. They are low-cost, functional, and adjustable, making them particularly important among other metal nanoparticles. ZnO-NPs are utilized in numerous areas such as agriculture, food, biomedical, drug-delivery systems, biosensors, gas sensors, cosmetics, rubber industry, wastewater management, textile, and medicine [108,109].
ZnO-NPs are used as an artificial food additive in many foods and are generally considered safe (generally recognized as safe, GRAS) by the US Food and Drug Administration, making them less dangerous and more useful. ZnO-NPs are widely employed in biomedicine, since it has been claimed that they have therapeutic benefits against a variety of ailments (including microbial and cancer-causing disorders). For normal cells to operate properly, endogenous ROS levels must be controlled [110]. Certain regions have an effect that is both large enough to stop the aggregation of nanoparticles and small enough not to cause quick clearance into all nearby tissues and cause significant cytotoxicity through ROS and dissolution [111]. P. Kaur et al., found that ZnO-NPs play a crucial and important role in inhibiting and killing common pathogens. They investigated the antibacterial activity of ZnO-NPs against some human pathogens (E. coli, E. faecal, P. aeruginosa, and S. aureus), and reported that ZnO-NPs have well-built antimicrobial activity toward the above-mentioned human pathogens [112].
7.2. Possible Risk
One of the key areas of concern with ZnO-NPs is safety. The use of ZnO-NPs in the agricultural and food industries has sparked a growing interest among researchers. The first ways that individuals are exposed to accidental nanoparticles are by skin contact, inhalation, or ingestion. Nanoparticles spread throughout the body after entering the circulatory system. ZnO-NPs could show significantly different biological and physicochemical characteristics at specific locations compared to their typical type, and these unknown characteristics could create unexpected risks to human health [4,113]. Although the nanoencapsulation process of bioactive combinations has been extensively researched in the food industry [114], direct oral consumption of nanoparticles by people still has to be addressed. A sophisticated approach should be used to remove dangerous solvents to protect the finished product from unanticipated residual solvents that might have negative effects on safety if their concentration is unknown. Organizations, including the World Health Organization (WHO), the European Food Safety Authority (EFSA), and the Food and Drug Administration (FDA), have recorded acceptable usage limits for organic, inorganic solvents, and emulsifiers. The evaluation of risk requires more research on the safety of ZnO-NP in relation to hazardous risks [72].
However, ZnO-NPs have demonstrated great economic use and are found in a variety of commercial goods. Nevertheless, it is evident that there is growing public concern about the toxicological consequences of ZnO-NPs.
Although further research is still being done, it has been revealed that the significant consumption of organic solvents and emulsifiers during the manufacturing of nano carriers poses dangers due to their possible toxicological effects on public health [115]. However, there are several concerns associated with using nanoparticles that should be considered, including their potential toxicity and buildup in feed and food, which might indicate that they have entered the food chain. In particular, their ability to produce free radicals or ROS, can subject living things to oxidative stress [116].
Based on its examination of the end-points of subtoxic processes, such as inflammation, aerophilic stress response, or gene expression profiling, spanning greatly different timelines, the research demonstrates that the hazardous potentials of nano- and micro-particles of ZnO are the same during acute and chronic toxicity. Inhaling ZnO fume and dust beyond the recommended exposure limit of 5 mg m−3 appears to be the most risky hazardous exposure, as Zn fume fever can be lethal [117].
In particular, ZnO-NPs are useful in sun-screens, having intrinsic capability to filter UVA and UVB radiation. This exceptional property provides broader protection than other sun-screening agents. However, they also have the possibility to penetrate the skin and achieve viable cells, potentially leading to toxicity [51,107].
7.3. Regulations
In the world today, smart food regulations are necessary to ensure that food products meet high safety standards. Numerous global organizations are working towards this goal, which poses many regulatory challenges related to consumer health, some of which may be applied to nanotechnology employed in food, food control, food safety, and food trade. Notwithstanding the present lack of particular rules, regulations, and risk management for nanotechnology, the use and legislation of novel nanotechnology is notable in the food sector. The protection of nanomaterials in the food industry could be ensured by maintaining strict food regulations, implementing new specific regulations for nanotechnology, ensuring transparency of information, and being willing to share data with the public [118,119].
8. Applications
Due to their distinctive characteristics and broad range of uses, ZnO-NPs have been the subject of a great deal of scientific investigation in recent years, particularly in the pharmaceutical, medicinal, cosmetic, food, agricultural and biomedical sectors [34]. In this part, we focus on summarize the main applications in the agriculture and food industry. In Table 2, we list some recent patents related to the application of ZnO nanoparticles in food and agriculture.

Table 2.
The patents on the application of ZnO nanoparticles in food and agriculture.
8.1. Role in Agriculture
We all know that agriculture is a basic necessity and the foundation of every nation’s economy, but sadly, this industry is currently facing a number of global challenges, including urbanization, climate change, sustainable resource use, environmental issues, runoff, and the buildup of pesticides and fertilizers. Additionally, the world’s population is expected to increase by a number that could be calculated on the basis of current trends [120]. According to research, applying ZnO-NPs to various plants at various stages of their development cycles accelerates plant production by supplementing them with micronutrients.
Significantly, research has shown that Zn is a crucial nutrient for living things. Nandhini et al., emphasized the beneficial effects of ZnO-NPs on pearl millet germination and development as well as the enhanced activity of the plant defense enzymes, such as lipoxygenase, phenylalanine, polyphenol oxidase, ammonia-lyase, and peroxidase [121]. Moreover, the production of chlorophyll pigment from maize and wheat plants was positively impacted by ZnO-NPs. The beneficial effects of ZnO-NP on quantitative, dietary, and physiological parameters of wheat (Triticum aestivum L.) and maize (Zea mays L.) were examined by Singh et al. In addition, these comprised the total protein, carbohydrate, and oil content [122].
In order to increase crop growth and production in global agriculture, and especially in food crops, the fertilizer application rate is very accumulated [123]. According to several studies, ZnO-NPs have the ability to increase food crop output, diversity, and promote healthy growth. Many studies have effectively demonstrated and suggested the use of ZnO-NPs as a foliar fertilizer in addition to a Zn fertilizer in the soil [122]. ZnO-NPs have been recommended as a crucial micronutrient and are intended as a cofactor for the mobilization and activation of nutrient enzymes due to their special characteristics [124].
Raliya et al., studied the influence of ZnO-NP (25 ± 3.5 nm) on the herb (tomato plant). They covered some parameters, such as the whole life cycle of the plant, flower, chlorophyl content, fruit yield, total biomass, and nutritional values. They studied plant development by an optimal concentration of ZnO-NPs, and reported no stimulation on the far side of the optimal concentration. The foliar application was more effective and simpler than the soil treatments. They ended with a maximum lycopene content and fruit yield of 100 mg kg−1, and a concentration of 250 mg kg−1 was observed for the best stimulation of plant height, root length, and biomass and improved chlorophyl content at a concentration of 750 mg kg−1 [124].
In another study, ZnO-NPs were termed novel fertilizer nutrients for crops. In a greenhouse, the organic compound (urea) was coated with ZnO-NP (1%) or bulk ZnO (2%) and evaluated in wheat (Triticum aestivum L.) under drought (40% field moisture capacity; FMC) and non-drought conditions (80% FMC), compared to urea not coated with ZnO (control) and urea with separate ZnO-NP (1%) or bulk ZnO (2%) distinction. The crops were exposed to <2.2 mg/kg of ZnO NP and <4.4 mg/kg of bulk ZnO, indicating exposure to a better Zn rate of bulk ZnO (2%). ZnO-NP and bulk-ZnO showed similar vital efficiencies of urea coating of 74–75%. However, drought considerably (p < 0.05) exaggerated the time to panicle initiation, reduced grain yield, and inhibited the uptake of Zn, phosphorus (P) and nitrogen (N). Within the drought conditions, scientists noticed that ZnO-NPs remarkably reduced the average typical time to panicle initiation by five days, which was regardless of the coating, and relative to the management control. In distinction, bulk ZnO did not have an effect on time to panicle initiation. The grain yield was significantly exaggerated to 51 or 39%, with a ZnO-NP-coated or uncoated organic compound (urea), respectively, compared to the control. As balanced with both control and ZnO-NP treatments, the yield of bulk-ZnO-coated or uncoated urea increased insignificantly [125,126].
8.2. As Antimicrobial Agent against Food-Borne Pathogens
ZnO-NPs had a significant influence on the control of foodborne diseases. Foodborne infections are putting the entire planet in a dangerous situation. 20,000 people are hospitalized for foodborne illnesses each year in the United Kingdom, and 56 people die as a result, according to the UK Food Standards Agency (FSA). Foodborne illnesses cause a variety of challenges, including health concerns, poverty, and even economic problems [7,127,128]. ZnO-NPs are becoming more effective at combating hazardous microbes and preventing food contamination through adsorption-induced membrane damage and ROS-mediated cellular toxicity. The ZnO-NPs have been shown to be an effective antibacterial agent against harmful microorganisms found in food, including Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Pseudomonas vulgaris, Bacillus megaterium, Candida albicans, Klebsiella pneumonia, Aspergillus Niger and so on [129,130,131,132]. Metal compound (oxide) particles have been studied to generate active oxygen species that could be the main mechanism of their antibacterial activity [133,134]. Firouzabadi et al. [135], studied the practical application of a ZnO suspension containing 0.3% citric acid at different concentrations (0, 1, 3, 5, 8 mM) in Listeria monocytogenes, Escherichia coli, Staphylococcus aureus and Bacillus cereus. It significantly inhibited the growth of all strains during 12 h of culture, and the suspension of ZnO with 5 mM and 8 mM citric acid was the most effective for all strains, and 5 mM and 8 mM ZnO were selected for further study in mango juice. The ZnO-NPs could reduce the initial growth count of all of the strains described above in the mango solution.
ZnO-NPs have direct interaction with cell surface poignant cell membranes, which subsequently ZnO-NPs enter and induce oxidative stress in microorganism cells, which results in inhibition of cell growth to cell death; this study recommends ZnO-NPs as a active antimicrobial agent within the food preservation field. ZnO-NPs are often used as a disinfecting and sterilizing agent for the types of equipment and containers utilized in the food industry against the strike and contamination with foodborne pathogenic microbes [136].
8.3. Role in Food Processing and Storage
ZnO-NPs have brought revolutionary advances to the food industry. In recent years, scientific researchers have shown great interest in the study of ZnO-NPs, along with other nanoparticles, in food processing and storage. These particles not only exhibit antimicrobial activity, as discussed previously, but also remain stable under adverse conditions, such as high temperature, extreme pH values, and more. Furthermore, they are considered “generally recognized as safe (GRAS)” by the US FDA for both human and animal consumption in their bulk form [137,138,139]. Biosensors of ZnO-NPs are being utilized successfully and greatly helped to detect the different pathogens and contaminants of food and water throughout storage [139]. Researchers are interested in enhancing the food processing and storage industry to extend the shelf life of processed food, control food-borne pathogens, and ensure food safety. Achieving these objectives entails improving the nutritional value, activity, and overall quality of food. These goals are critical for the food industry to meet [7,140]. Researchers are particularly concerned about the negative impact of pathogens on stored grain, which can also pose a risk to human health. ZnO-NPs have demonstrated antimicrobial activity and could play a significant role in controlling other important properties of food materials, such as moisture content, absorption, monolayer, solubility, and physical properties [43]. Therefore, reducing the chances of contamination by infectious agents and ensuring safe storage of products with an extended shelf life are critical objectives. To mitigate the risk of contamination or spoilage of food by microbes before or after processing, it is essential to understand the impact of storage time and temperature on microbial populations of minimally processed foods. ZnO-NPs have played a crucial role in protecting and preserving the freshness and nutritional value of food. Moreover, they could be used as a source of zinc supplements in functional foods for consumers [141,142,143]. Hakimian et al. [144], conducted a study on the use of ZnO-NPs (0, 0.1, 0.5, 1 g/kg) as a preservative to control microbial and physicochemical deterioration of mayonnaise during cold storage over a period of 6 months. The addition of ZnO-NPs to mayonnaise resulted in delayed growth of microorganisms, preservation of physical and chemical properties, and increased shelf life compared to the control (0 g/kg). In another study, Lili He and colleagues investigated the antifungal activity of ZnO-NPs against two postharvest fruit fungal diseases, B. cinerea and P. expansum. The mechanism of action of ZnO-NPs on the development of fungal hyphae was also examined. The results showed that a concentration of ZnO-NPs greater than 3 mmol L−1 significantly inhibited the growth of both B. cinerea and P. expansum. However, the nanoparticles were found to be more effective against B. cinerea than P. expansum [145].
8.4. Role in Food Packaging
A very important and in-demand area in the food sector may be related to the use of antibacterial agents in food packaging and with respect to foodborne pathogens [7]. Therefore, nanofood packaging technology is resulting in a brand new mode of safe, active, healthy, and intelligent food packaging. ZnO-NPs offer a safer and more cost-effective alternative compared to other metal oxides for pathogen-free food packaging and processed ingredients [139]. ZnO-NPs are utilized in processed food as a fount of metallic elements (Zn) that help play a crucial and important role in the growth, progress, and welfare of humans and animals. ZnO-NPs are an important antimicrobial supplement in food packaging to counter foodborne pathogens to increase shelf life, inhibit and inactivate or kill pathogens and reduce spoilage, contamination, post-harvest losses to maintain good quality and safety insurance of food products [98,146]. Safe, active, and smart food packaging aligns with customer’s desires by enhancing safety and extending shelf life, while also providing a healthier solution [138]. ZnO-nanostructures could be used in the food packaging industry for the composition of filters that are efficient in maintaining microorganisms before packaging [147]. Food poisoning represents microbial contamination that causes significant health, social, and economic problems. Smart food packaging and similar preservative measures can prevent or inhibit the growth of microbials in food, as well as prevent their attachment, colonization, and spreading [141,143]. Microscopic and spectroscopic techniques, such as chemical analysis, can help create optimal food packaging materials to ensure the formation of a composite that enhances food safety and preservation [43].A group of researchers in China investigated the potential migration of ZnO-NPs from industrial products, which is often a mutual concern when it comes to their application in food packaging. They evaluated the migration of ZnO-NPs from polypropylene food containers to food-simulating solutions, taking into account various experimental factors, including the type of food simulant, storage time, and temperature. The experimental results showed significant migration of nano-sized zinc oxide into aqueous, oily, and acidic simulants. The quantity of migrated ZnO increased with storage time and temperature. However, it was observed that ZnO showed a sporadic tendency to migrate into food simulants [148]. Zafar et al. [149], have shown that the ZnO-2.5% composite membrane has strong antibacterial activity and high antioxidant activity against foodborne pathogenic bacteria, Escherichia coli, and Lactobacillus mononucleus. According to the results, compared to the CMC/GEL blend film, ZnO nanocomposite film could be used to prevent photooxidation, ensure food safety, and improve the shelf life of packaging products in active food packaging.
Using the solution casting method, Insoo Kim et al., created PLA/Zn-ONPs containing volatile oil in collaboration with Menthe piperita and Zataria multiflora Boiss. In comparison with five common foodborne bacteria including Salmonella enterica, S. aureus, Pseudomonas aeruginosa, E. coli, and Bacillus cereus, the nanocomposite film showed high antimicrobial activity. The PLA/ZnO-NP containing essential oil extended the shelf life of Otolithes ruber fish during storage. They jointly reported that Zn2+ migration was below the NIH limit for food contact material quality requirements [150].
Silvestre et al., introduced the utilization of compound nanotechnology (polymer) in food packaging that mainly meets the requirements of protection against bacteria to attain a completely unique method of packaging. Active packaging, smart packaging, and intelligent packaging are new materials with improved antimicrobial properties that jointly allow the trailing of food throughout storage and transfer [127].
8.5. Role in Food Flavor
This section aims to explain, discuss and analyze research works that have utilized ZnO-NPs for the improvement, enhancement, and protection of food flavor, an important ingredient in altering or enhancing the taste of any food. ZnO-NPs’ mechanism of action and applications on food flavor are also discussed, particularly in relation to food packaging applications. As previously mentioned, ZnO-NPs are a highly intriguing inorganic compound that is considered safe, non-toxic, and has numerous functions. It is widely used in various industries, including cosmetics, pharmaceuticals, textiles, rubber, and medical fields. Therefore, it holds particular importance in the food and agricultural industry due to its numerous advantages [151]. Sensory quality via ZnO-NPs playing a significant role to make it toxin-free helps in influencing and increasing the interest in quality, stability, acceptability, consumption of different types of food-linked flavors [152,153]. As a result of its difficulty in controlling and stabilizing during the storage and production processes, ZnO-NPs help limit the deterioration or loss during processing and storage. It is advantageous to encapsulate flavors before using ZnO-NPs in food because it increases chemical stability and allows for regulated release. Protective carrier encapsulation prevents oxidation, reactions induced by light, and interactions between tastes. Biopolymers such as polysaccharides (starch, dextrose and maltodextrins), proteins (gelatine and whey proteins), gums (gum arabic, alginates and carrageenan), and chitosan are therefore preferred transporters [154,155]. Some other protein-based materials, e.g., soy protein, polypeptone, or gelatine derivatives, are considered to form stable emulsions with volatiles compounds [156,157].
Brian D. et al., conducted some experiments on diacetyl substitutes 2,3-heptanedione and 2,3-hexanedione. On the contrary, aldehydes seem to be omnipresent in food production, and almost in every sample acetaldehyde was detected and classified as a possible human carcinogen [152].
9. Summary and Future Perspectives
In this review, we have summarized the properties of ZnO-NPs, including their chemical and green synthesis, characterizations, antimicrobial activity, benefits, and risks, as well as their applications in the food industry and agricultural values. ZnO-NPs exhibit good antimicrobial properties against foodborne pathogens and are a promising substitute for antibiotics. The aim of this review is to provide a succinct reference for researchers interested in the antibacterial activities and functional applications of ZnO-NPs, and considering the nanotechnological principles that are associated with the noble and hazardous properties of ZnO-NPs.
Furthermore, the incorporation of ZnO-NPs into biodegradable polymeric matrices could enhance material performance, including mechanical, thermal, and barrier properties. However, further research is necessary to determine the potential hazardous effects of ZnO-NPs and assess their impact on consumers. Additionally, there are challenges associated with exploring the broader applications of ZnO-NPs in the food industry with alternative emerging technologies.
Author Contributions
Writing (original draft preparation): Z.H., M.-Y.L. and S.H.; Conceptualization, editing and fund acquisition, D.-D.Z.; Writing (review and editing): X.-Q.Z., Q.W., Y.-F.C. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32001745, Young Elite Scientists Sponsorship Program by CAST, grant number 2019QNRC001, and China Agriculture Research System of MOF and MARA, grant number CARS-03.
Data Availability Statement
The data presented in this study are available on request from author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bokhari, H. Exploitation of Microbial Forensics and Nanotechnology for the Monitoring of Emerging Pathogens. Crit. Rev. Microbiol. 2018, 44, 504–521. [Google Scholar] [CrossRef]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A Critical Review of Synthesis Parameters Affecting the Properties of Zinc Oxide Nanoparticle and Its Application in Wastewater Treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A State of the Art Review on the Synthesis, Antibacterial, Antioxidant, Antidiabetic and Tissue Regeneration Activities of Zinc Oxide Nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An Overview of Nanotechnology in Food Science: Preparative Methods, Practical Applications, and Safety. J. Chem. 2018, 2018, 5427978. [Google Scholar] [CrossRef]
- Prabha, R.-K.; Jajoo, A. Priming with Zinc Oxide Nanoparticles Improve Germination and Photosynthetic Performance in Wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar]
- Agnieszka, K.-R.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Eixenberger, J.E.; Anders, C.B.; Hermann, R.J.; Brown, R.J.; Reddy, K.M.; Punnoose, A.; Wingett, D.G. Rapid Dissolution of ZnO Nanoparticles Induced by Biological Buffers Significantly Impacts Cytotoxicity. Chem. Res. Toxicol. 2017, 30, 1641–1651. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles—An Antimicrobial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial Applications of Nanotechnology: Methods and Literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia Coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Tseng, T.Y.; Li, S.Y.; Lin, P. Effect of Phosphorus Dopant on Photoluminescence and Field-Emission Characteristics of Mg0.1Zn0.9O Nanowires. J. Appl. Phys. 2006, 99, 024303. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012, 2012, 624520. [Google Scholar] [CrossRef]
- Mutukwa, D.; Taziwa, R.; Khotseng, L.E. A Review of the Green Synthesis of ZnO Nanoparticles Utilising Southern African Indigenous Medicinal Plants. Nanomaterials 2022, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Noman, M.T.; Amor, N.; Petru, M. Synthesis and Applications of ZnO Nanostructures (Zonss): A Review. Crit. Rev. Solid State 2022, 47, 99–141. [Google Scholar] [CrossRef]
- Thakral, F.; Bhatia, G.K.; Tuli, H.S.; Sharma, A.K.; Sood, S. Zinc Oxide Nanoparticles: From Biosynthesis, Characterization, and Optimization to Synergistic Antibacterial Potential. Curr. Pharmacol. Rep. 2021, 7, 15–25. [Google Scholar] [CrossRef]
- Rohani, R.; Dzulkharnien, N.S.F.; Harun, N.H.; Ilias, I.A. Green Approaches, Potentials, and Applications of Zinc Oxide Nanoparticles in Surface Coatings and Films. Bioinorg. Chem. Appl. 2022, 2022, 3077747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Hu, S.; Wu, Q.; Zhao, J.-F.; Su, K.-R.; Tan, L.-Q.; Zhou, X.-Q. Construction of ZnO@mSiO2 Antibacterial Nanocomposite for Inhibition of Microorganisms During Zea Mays Storage and Improving the Germination. LWT-Food Sci. Technol. 2022, 168, 113907. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Gierlotka, S.; Lojkowski, W. Effect of Microwave Radiation Power on the Size of Aggregates of ZnO Nps Prepared Using Microwave Solvothermal Synthesis. Nanomaterials 2018, 8, 343. [Google Scholar] [CrossRef]
- Yaseri, S.; Verki, V.M.; Mahdikhani, M. Utilization of High Volume Cement Kiln Dust and Rice Husk Ash in the Production of Sustainable Geopolymer. J. Clean Prod. 2019, 230, 592–602. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size Control Mechanism of ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef]
- Adam, R.E.; Pozina, G.; Willander, M.; Nur, O. Synthesis of ZnO Nanoparticles by Co-Precipitation Method for Solar Driven Photodegradation of Congo Red Dye at Different Ph. Photonics Nanostruct.-Fundam. Appl. 2018, 32, 11–18. [Google Scholar] [CrossRef]
- Cruz-Hernandez, C.; Goeuriot, S.; Giuffrida, F.; Thakkar, S.K.; Destaillats, F. Direct Quantification of Fatty Acids in Human Milk by Gas Chromatography. J. Chromatogr. A. 2013, 1284, 174–179. [Google Scholar] [CrossRef]
- Koutu, V.; Shastri, L.; Malik, M.M. Effect of Naoh Concentration on Optical Properties of Zinc Oxide Nanoparticles. Mater. Sci. 2016, 34, 819–827. [Google Scholar] [CrossRef]
- Xue, X.; Zhou, Z.; Peng, B.; Zhu, M.M.; Zhang, Y.J.; Ren, W.; Ye, Z.G.; Chen, X.; Liu, M. Review on Nanomaterials Synthesized by Vapor Transport Method: Growth and Their Related Applications. RSC Adv. 2015, 5, 79249–79263. [Google Scholar] [CrossRef]
- Colibaba, G.V. Sintering Highly Conductive ZnO:Hcl Ceramics by Means of Chemical Vapor Transport Reactions. Ceram Int. 2019, 45, 15843–15848. [Google Scholar] [CrossRef]
- Güell, F.; Cabot, A.; Claramunt, S.; Moghaddam, A.O.; Martínez-Alanis, P.R. Influence of Colloidal Au on the Growth of ZnO Nanostructures. Nanomaterials 2021, 11, 870. [Google Scholar] [CrossRef]
- Barani, M.; Masoudi, M.; Mashreghi, M.; Makhdoumi, A.; Eshghi, H. Cell-Free Extract Assisted Synthesis of ZnO Nanoparticles Using Aquatic Bacterial Strains: Biological Activities and Toxicological Evaluation. Int. J. Pharm. 2021, 606, 120878. [Google Scholar] [CrossRef]
- Sana, S.S.; Li, H.; Zhang, Z.; Sharma, M.; Usmani, Z.; Hou, T.; Netala, V.R.; Wang, X.; Gupta, V.K. Recent Advances in Essential Oils-Based Metal Nanoparticles: A Review on Recent Developments and Biopharmaceutical Applications. J. Mol. Liq. 2021, 333, 115951. [Google Scholar] [CrossRef]
- Maťátková, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial Properties and Applications of Metal Nanoparticles Biosynthesized by Green Methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of Zinc Oxide Nanoparticles Usingmangifera Indica Leaves and Evaluation of Their Antioxidant and Cytotoxic Properties in Lung Cancer (A549) Cells. Enzyme Microb. Tech. 2018, 117, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green Synthesis of Zinc Oxide Nanoparticles Using Different Plant Extracts and Their Antibacterial Activity against Xanthomonas Oryzae Pv. Oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, L.; Han, F.; Wang, A.; Cai, W.; Yu, J.; Yang, J.; Peng, F. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Corymbia Citriodora Leaf Extract and Their Photocatalytic Activity. Green Chem. Lett. Rev. 2015, 8, 59–63. [Google Scholar] [CrossRef]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. ZnO Nanoparticles Enhanced Antibacterial Activity of Ciprofloxacin against Staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 557–561. [Google Scholar] [CrossRef]
- Żelechowska, K.; Karczewska-Golec, J.; Karczewski, J.; Łoś, M.; Kłonkowski, A.M.; Węgrzyn, G.; Golec, P. Phage-Directed Synthesis of Photoluminescent Zinc Oxide Nanoparticles under Benign Conditions. Bioconjug. Chem. 2016, 27, 1999–2006. [Google Scholar] [CrossRef]
- Iravani, S.; Zolfaghari, B. Plant Viruses and Bacteriophages for Eco-Friendly Synthesis of Nanoparticles: Recent Trends and Important Challenges. Comment Inorg. Chem. 2022, 42, 226–248. [Google Scholar] [CrossRef]
- Bao, Z.Q.; Lan, C.Q. Advances in Biosynthesis of Noble Metal Nanoparticles Mediated by Photosynthetic Organisms—A Review. Colloid Surface B. 2019, 184, 110519. [Google Scholar] [CrossRef]
- Araya-Sibaja, A.M.; Wilhelm-Romero, K.; Quirós-Fallas, M.I.; Huertas, L.F.V.; Vega-Baudrit, J.R.; Navarro-Hoyos, M. Bovine Serum Albumin-Based Nanoparticles: Preparation, Characterization, and Antioxidant Activity Enhancement of Three Main Curcuminoids from Curcuma Longa. Molecules 2022, 27, 2758. [Google Scholar] [CrossRef]
- Dixon, S.C.; Scanlon, D.O.; Carmalt, C.J.; Parkin, I.P. N-Type Doped Transparent Conducting Binary Oxides: An Overview. J. Mater. Chem. C 2016, 4, 6946–6961. [Google Scholar]
- Paul, S.K.; Dutta, H.; Sarkar, S.; Sethi, L.N.; Ghosh, S.K. Nanosized Zinc Oxide: Super-Functionalities, Present Scenario of Application, Safety Issues, and Future Prospects in Food Processing and Allied Industries. Food Rev. Int. 2019, 35, 505–535. [Google Scholar] [CrossRef]
- Chen, C.C.; Yu, B.H.; Liu, P.; Liu, J.F.; Wang, L. Investigation of Nano-Sized ZnO Particles Fabricated by Various Synthesis Routes. J. Ceram. Process. Res. 2011, 12, 420–425. [Google Scholar]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, Morphological and Biological Analysis of Zinc Oxide Nanoparticles (ZnO Nps) Using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Khan, M.R. Synthesis of Schiff’s Base Magnetic Crosslinked Chitosan-Glyoxal/ZnO/Fe3O4 Nanoparticles for Enhanced Adsorption of Organic Dye: Modeling and Mechanism Study. Sustain. Chem. Pharm. 2021, 20, 100379. [Google Scholar] [CrossRef]
- Jin, S.-E.; Jin, H.-E. Synthesis, Characterization, and Three-Dimensional Structure Generation of Zinc Oxide-Based Nanomedicine for Biomedical Applications. Pharmaceutics 2019, 11, 575. [Google Scholar] [CrossRef]
- Rajan, A.; Cherian, E.; Baskar, G. Biosynthesis of Zinc Oxide Nanoparticles Using Aspergillus Fumigatus Jcf and Its Antibacterial Activity. Int. J. Mod. Sci. Technol. 2016, 1, 52–57. [Google Scholar]
- Hefny, M.E.; El-Zamek, F.I.; El-Fattah, H.I.A.; Mahgoub, S.A. Biosynthesis of Zinc Nanoparticles Using Culture Filtrates of Aspergillus, Fusarium and Penicillium Fungal Species and Their Antibacterial Properties against Gram-Positive and Gram-Negative Bacteria. Zagazig J. Agric. Res. 2019, 46, 2009–2021. [Google Scholar] [CrossRef]
- Shamsuzzaman; Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological Synthesis of ZnO Nanoparticles Using C. Albicans and Studying Their Catalytic Performance in the Synthesis of Steroidal Pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar] [CrossRef]
- Rauf, M.A.; Owais, M.; Rajpoot, R.; Ahmad, F.; Khan, N.; Zubair, S. Biomimetically Synthesized ZnO Nanoparticles Attain Potent Antibacterial Activity against Less Susceptible S. Aureus Skin Infection in Experimental Animals. RSC Adv. 2017, 7, 36361–36373. [Google Scholar] [CrossRef]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO Nanoparticles by Halomonas Elongata Ibrc-M 10214 in Different Conditions of Medium. Bioimpacts 2018, 8, 81–89. [Google Scholar] [CrossRef]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Shafiei, M.; Zahiri, H.S.; Noghabi, K.A. A Bio-Inspired Strategy for the Synthesis of Zinc Oxide Nanoparticles (ZnO Nps) Using the Cell Extract of Cyanobacterium Nostoc Sp. Ea03: From Biological Function to Toxicity Evaluation. RSC Adv. 2019, 9, 23508–23525. [Google Scholar] [CrossRef]
- Chauhan, R.; Reddy, A.; Abraham, J. Biosynthesis of Silver and Zinc Oxide Nanoparticles Using Pichia Fermentans Ja2 and Their Antimicrobial Property. Appl. Nanosci. 2015, 5, 63–71. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Namvar, F.; Navaderi, M.; Mohamad, R. Biosynthesis of ZnO Nanoparticles by a New Pichia Kudriavzevii Yeast Strain and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Golec, P.; Karczewska-Golec, J.; Łoś, M.; Węgrzyn, G. Novel ZnO-Binding Peptides Obtained by the Screening of a Phage Display Peptide Library. J. Nanopart. Res. 2012, 14, 1218. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and Antiapoptotic Effects of Green-Synthesized Zinc Oxide Nanoparticles Using Sargassum Muticum Algae Extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Synthesis and Modification of Bio-Derived Antibacterial Ag and ZnO Nanoparticles by Plants, Fungi, and Bacteria. Drug Discov. Today 2021, 26, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Yoshida, T.; Fujita, Y. Effects of Ambience on Thermal-Diffusion Type Ga-Doping Process for Zno Nanoparticles. Coatings 2022, 12, 57. [Google Scholar] [CrossRef]
- Lv, H.; Sang, D.D.; Li, H.D.; Du, X.B.; Li, D.M.; Zou, G.T. Thermal Evaporation Synthesis and Properties of ZnO Nano/Microstructures Using Carbon Group Elements as the Reducing Agents. Nanoscale Res. Lett. 2010, 5, 620. [Google Scholar] [CrossRef]
- Lyu, S.C.; Zhang, Y.; Lee, C.J.; Ruh, H.; Lee, H.J. Low-Temperature Growth of ZnO Nanowire Array by a Simple Physical Vapor-Deposition Method. Chem. Mater. 2003, 15, 3294–3299. [Google Scholar] [CrossRef]
- Eremina, A.; Kargina, Y.V.; Kharin, A.Y.; Petukhov, D.; Timoshenko, V.Y. Mesoporous Silicon Nanoparticles Covered with Peg Molecules by Mechanical Grinding in Aqueous Suspensions. Microporous Mesoporous Mater. 2022, 331, 111641. [Google Scholar] [CrossRef]
- Hajnorouzi, A.; Afzalzadeh, R.; Ghanati, F. Ultrasonic Irradiation Effects on Electrochemical Synthesis of ZnO Nanostructures. Ultrason. Sonochem. 2014, 21, 1435–1440. [Google Scholar] [CrossRef]
- Xu, J.; Ji, W.; Lin, J.; Tang, S.; Du, Y. Preparation of Zns Nanoparticles by Ultrasonic Radiation Method. Appl. Phys. A 1998, 66, 639–641. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Elmanama, A.A.; El Ashgar, N.M.; Amara, N.; Selmane, M.; Chehimi, M.M. Stabilization of Nano-Structured ZnO Particles onto the Surface of Cotton Fibers Using Different Surfactants and Their Antimicrobial Activity. Ultrason. Sonochem. 2017, 38, 478–487. [Google Scholar] [CrossRef]
- Barabaszová, K.; Holešová, S.; Šulcová, K.; Hundáková, M.; Thomasová, B. Effects of Ultrasound on Zinc Oxide/Vermiculite/Chlorhexidine Nanocomposite Preparation and Their Antibacterial Activity. Nanomaterials 2019, 9, 1309. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, X.; Zhang, A.; Gao, X.; Yagoub, A.E.-G.A.; Ma, H.; Zhou, C. Ultrasound-Assisted Synthesis of Potentially Food-Grade Nano-Zinc Oxide in Ionic Liquids: A Safe, Green, Efficient Approach and Its Acoustics Mechanism. Foods 2022, 11, 1656. [Google Scholar] [CrossRef]
- Farahani, S.V.; Mahmoodi, A.; Goranneviss, M. The Effect of Laser Environment on the Characteristics of ZnO Nanoparticles by Laser Ablation. Int. Nano Lett. 2016, 6, 45–49. [Google Scholar] [CrossRef]
- Al-Nassar, S.I.; Hussein, F.I.; Adel, K.M. The Effect of Laser Pulse Energy on ZnO Nanoparticles Formation by Liquid Phase Pulsed Laser Ablation. J. Mater. Res. Technol. 2019, 8, 4026–4031. [Google Scholar] [CrossRef]
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. 2011, 51, 285–330. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Z.; Jia, L. Preparation and Surface Modification of Highly Dispersed Nano-ZnO with Stearic Acid Activated by N,N′-Carbonyldiimidazole. Mater. Lett. 2012, 82, 167–170. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme Biomimetic Approach for Developing Novel Chitin-GeO2 Nanocomposites with Photoluminescent Properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Yung, M.M.N.; Fougères, P.-A.; Leung, Y.H.; Liu, F.; Djurišić, A.B.; Giesy, J.P.; Leung, K.M.Y. Physicochemical Characteristics and Toxicity of Surface-Modified Zinc Oxide Nanoparticles to Freshwater and Marine Microalgae. Sci. Rep. 2017, 7, 15909. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Surface-Modified Zinc Oxide Nanoparticles for Antialgal and Antiyeast Applications. Acs Appl. Nano Mater. 2020, 3, 440–451. [Google Scholar] [CrossRef]
- Gu, T.; Yao, C.; Zhang, K.; Li, C.; Ding, L.; Huang, Y.; Wu, M.; Wang, Y. Toxic Effects of Zinc Oxide Nanoparticles Combined with Vitamin C and Casein Phosphopeptides on Gastric Epithelium Cells and the Intestinal Absorption of Mice. RSC Adv. 2018, 8, 26078–26088. [Google Scholar] [CrossRef] [PubMed]
- Rtesani, A.; Dozzi, M.V.; Toniolo, L.; Valentini, G.; Comelli, D. Experimental Study on the Link between Optical Emission, Crystal Defects and Photocatalytic Activity of Artist Pigments Based on Zinc Oxide. Minerals 2020, 10, 1129. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Pérez-Díaz, M.A.; Mendoza-Mendoza, E.; Peralta-Rodríguez, R.D.; Ojeda-Galván, H.J.; Perez, D.P.P.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Martinez-Gutierrez, F. Green, Novel, and One-Step Synthesis of Silver Oxide Nanoparticles: Antimicrobial Activity, Synergism with Antibiotics, and Cytotoxic Studies. New J. Chem. 2022, 46, 17841–17853. [Google Scholar] [CrossRef]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of Metal Nanoparticles and Their Pharmacological Applications: Present Status and Application Prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Somu, P.; Khanal, H.D.; Gomez, L.A.; Shim, J.J.; Lee, Y.R. Multifunctional Biogenic Al-Doped Zinc Oxide Nanostructures Synthesized Using Bioreductant Chaetomorpha Linum Extricate Exhibit Excellent Photocatalytic and Bactericidal Ability in Industrial Effluent Treatment. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Liang, Y.; Wicker, S.; Wang, X.; Erichsen, E.S.; Fu, F. Organozinc Precursor-Derived Crystalline ZnO Nanoparticles: Synthesis, Characterization and Their Spectroscopic Properties. Nanomaterials 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, V.N.; Rajeswari, V.D. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO Nps. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Poovizhi, J.; Krishnaveni, B. Synthesis, Characterization and Antimicrobial Activity of Zinc Oxide Nanoparticles Synthesized from Calotropis Procera. Int. J. Pharm. Sci. Res. 2015, 7, 425–431. [Google Scholar]
- Alahmdi, M.I.; Khasim, S.; Vanaraj, S.; Panneerselvam, C.; Mahmoud, M.A.A.; Mukhtar, S.; Alsharif, M.A.; Zidan, N.S.; Abo-Dya, N.E.; Aldosari, O.F. Green Nanoarchitectonics of ZnO Nanoparticles from Clitoria ternatea Flower Extract for In Vitro Anticancer and Antibacterial Activity: Inhibits Mcf-7 Cell Proliferation Via Intrinsic Apoptotic Pathway. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2146–2159. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Çakıcı, T.; Muğlu, G.M.; Yıldırım, M. Investigation of Optical, Structural, and Electrical Properties of Heterostructure Fe2O3 Deposited by Rf Magnetron Sputtering on ZnO Layer by Spray Pyrolysis. J. Mater. Sci. Mater. Electron. 2022, 33, 11246–11256. [Google Scholar] [CrossRef]
- Amakali, T.; Daniel, L.S.; Uahengo, V.; Dzade, N.Y.; de Leeuw, N.H. Structural and Optical Properties of ZnO Thin Films Prepared by Molecular Precursor and Sol–Gel Methods. Crystals 2020, 10, 132. [Google Scholar] [CrossRef]
- Beinik, I.; Kratzer, M.; Wachauer, A.; Wang, L.; Lechner, R.T.; Teichert, C.; Motz, C.; Anwand, W.; Brauer, G.; Chen, X.Y.; et al. Electrical Properties of ZnO Nanorods Studied by Conductive Atomic Force Microscopy. J. Appl. Phys. 2011, 110, 052005. [Google Scholar] [CrossRef]
- Liou, T.-H.; Wang, S.-Y.; Lin, Y.-T.; Yang, S. Sustainable Utilization of Rice Husk Waste for Preparation of Ordered Nanostructured Mesoporous Silica and Mesoporous Carbon: Characterization and Adsorption Performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128150. [Google Scholar] [CrossRef]
- Zhang, D.-D.; Liu, J.-M.; Song, N.; Liu, Y.-Y.; Dang, M.; Fang, G.-Z.; Wang, S. Fabrication of Mesoporous La3Ga5Geo14: Cr3+, Zn2+ Persistent Luminescence Nanocarriers with Super-Long Afterglow for Bioimaging-Guided in Vivo Drug Delivery to the Gut. J. Mater. Chem. B 2018, 6, 1479–1488. [Google Scholar] [CrossRef]
- Mao, X.; Xiao, W.; Wan, Y.; Li, Z.; Luo, D.; Yang, H. Dispersive Solid-Phase Extraction Using Microporous Metal-Organic Framework Uio-66: Improving the Matrix Compounds Removal for Assaying Pesticide Residues in Organic and Conventional Vegetables. Food Chem. 2021, 345, 128807. [Google Scholar] [CrossRef]
- Li, X.; Cheng, S.; Deng, S.; Wei, X.; Zhu, J.; Chen, Q. Direct Observation of the Layer-by-Layer Growth of ZnO Nanopillar by in Situ High Resolution Transmission Electron Microscopy. Sci. Rep. 2017, 7, 40911. [Google Scholar] [CrossRef]
- Comandella, D.; Gottardo, S.; Rio-Echevarria, I.M.; Rauscher, H. Quality of Physicochemical Data on Nanomaterials: An Assessment of Data Completeness and Variability. Nanoscale 2020, 12, 4695–4708. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Zhang, Y.; Wang, J.; Fu, M.; Wu, J.; Ye, D. The Applications of Morphology Controlled ZnO in Catalysis. Catalysts 2016, 6, 188. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; De Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Tech. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Song, X.; Qi, Y.; Zhang, M.; Zhang, G.; Zhan, W. Application and Optimization of Drag Reduction Characteristics on the Flow around a Partial Grooved Cylinder by Using the Response Surface Method. Eng. Appl. Comput. Fluid Mech. 2019, 13, 158–176. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Ramani, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Marsili, E. Amino Acid-Mediated Synthesis of Zinc Oxide Nanostructures and Evaluation of Their Facet-Dependent Antimicrobial Activity. Colloid Surf. B 2014, 117, 233–239. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative Study of Cytotoxicity, Oxidative Stress and Genotoxicity Induced by Four Typical Nanomaterials: The Role of Particle Size, Shape and Composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative Eco-Toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Zhang, L.L.; Jiang, Y.H.; Ding, Y.L.; Daskalakis, N.; Jeuken, L.; Povey, M.; O’Neill, A.J.; York, D.W. Mechanistic Investigation into Antibacterial Behaviour of Suspensions of ZnO Nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Inkielewicz-Stepniak, I. Modified Nanoparticles as Potential Agents in Bone Diseases: Cancer and Implant-Related Complications. Nanomaterials 2020, 10, 658. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef]
- Medina, J.; Bolaños, H.; Mosquera-Sanchez, L.P.; Rodriguez-Paez, J.E. Controlled Synthesis of ZnO Nanoparticles and Evaluation of Their Toxicity in Mus Musculus Mice. Int. Nano Lett. 2018, 8, 165–179. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, M.; Garg, R. An Overview of Beneficiary Aspects of Zinc Oxide Nanoparticles on Performance of Cement Composites. Mater. Today Proc. 2021, 43, 892–898. [Google Scholar] [CrossRef]
- Amin, S.; Yang, P.; Li, Z. Pyruvate Kinase M2: A Multifarious Enzyme in Non-Canonical Localization to Promote Cancer Progression. Acta (BBA)-Rev. Cancer 2019, 1871, 331–341. [Google Scholar] [CrossRef]
- Garner, K.L.; Keller, A.A. Emerging Patterns for Engineered Nanomaterials in the Environment: A Review of Fate and Toxicity Studies. J. Nanopart. Res. 2014, 16, 2503. [Google Scholar] [CrossRef]
- Bedi, P.; Kaur, A. An Overview on Uses of Zinc Oxide Nanoparticles. J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent Advances in the Application of Metabolomics for Food Safety Control and Food Quality Analyses. Crit. Rev. Food Sci. 2021, 61, 1448–1469. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.-M. Nanotechnology in Food Science: Functionality, Applicability, and Safety Assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef]
- Voss, L.; Saloga, P.E.; Stock, V.; Böhmert, L.; Braeuning, A.; Thünemann, A.F.; Lampen, A.; Sieg, H. Environmental Impact of ZnO Nanoparticles Evaluated by in Vitro Simulated Digestion. ACS Appl. Nano Mater. 2020, 3, 724–733. [Google Scholar] [CrossRef]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative Effect of ZnO Nps, ZnO Bulk and ZnSO4 in the Antioxidant Defences of Two Plant Species Growing in Two Agricultural Soils under Greenhouse Conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Sushkova, S.; Behal, A.; Maksimov, A.; Blicharska, E.; Ghazaryan, K.; Movsesyan, H.; Barsova, N. ZnO and Cuo Nanoparticles: A Threat to Soil Organisms, Plants, and Human Health. Environ. Geochem. Hlth. 2020, 42, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, S.; Kumar, A. Potential Risks and Benefits of Zinc Oxide Nanoparticles: A Systematic Review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Barbasz, A. A Review: Zinc Oxide Nanoparticles—Friends or Enemies? Int. J. Environ. Health Res. 2022, 32, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spiegel, M.; Luning, P.; De Boer, W.; Ziggers, G.; Jongen, W. Measuring Effectiveness of Food Quality Management in the Bakery Sector. Total. Qual. Manag. Bus. Excel. 2006, 17, 691–708. [Google Scholar] [CrossRef]
- Nandhini, M.; Rajini, S.; Udayashankar, A.; Niranjana, S.; Lund, O.S.; Shetty, H.; Prakash, H. Biofabricated Zinc Oxide Nanoparticles as an Eco-Friendly Alternative for Growth Promotion and Management of Downy Mildew of Pearl Millet. Crop. Prot. 2019, 121, 103–112. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Afzal, S.; Singh, T.; Hussain, I. Zinc Oxide Nanoparticles: A Review of Their Biological Synthesis, Antimicrobial Activity, Uptake, Translocation and Biotransformation in Plants. J. Mater. Sci. 2018, 53, 185–201. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, S.; Yang, D.; Zou, Z.; Li, J. Physiological Effects of Mgo and ZnO Nanoparticles on the Citrus Maxima. J. Wuhan Univ. Technol. 2019, 34, 243–253. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic Evaluation of Translocation and Physiological Impact of Titanium Dioxide and Zinc Oxide Nanoparticles on the Tomato (Solanum lycopersicum L.) Plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile Coating of Urea with Low-Dose ZnO Nanoparticles Promotes Wheat Performance and Enhances Zn Uptake under Drought Stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Dapkekar, A.; Deshpande, P.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc Use Efficiency Is Enhanced in Wheat through Nanofertilization. Sci. Rep. 2018, 8, 6832. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food Packaging Based on Polymer Nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A.A. Microbial Synthesis of Zinc Oxide Nanoparticles and Their Potential Application as an Antimicrobial Agent and a Feed Supplement in Animal Industry: A Review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Mirhosseini, M.; Firouzabadi, F.B. Antibacterial Activity of Zinc Oxide Nanoparticle Suspensions on Food-Borne Pathogens. Int. J. Dairy Technol. 2013, 66, 291–295. [Google Scholar] [CrossRef]
- Jin, S.-E.; Hwang, W.; Lee, H.J.; Jin, H.-E. Dual Uv Irradiation-Based Metal Oxide Nanoparticles for Enhanced Antimicrobial Activity in Escherichia coli and M13 Bacteriophage. Int. J. Nanomed. 2017, 12, 8057–8070. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Ali, S.G.; Khan, H.M.; Rehman, S. Anticandidal Activity of Bioinspired ZnO Nps: Effect on Growth, Cell Morphology and Key Virulence Attributes of Candida Species. Artif. Cells Nanomed. Biotechnol. 2018, 46, S912–S925. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Gun’Ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Konduru, N.V.; Murdaugh, K.M.; Swami, A.; Jimenez, R.J.; Donaghey, T.C.; Demokritou, P.; Brain, J.D.; Molina, R.M. Surface Modification of Zinc Oxide Nanoparticles with Amorphous Silica Alters Their Fate in the Circulation. Nanotoxicology 2016, 10, 720–727. [Google Scholar] [CrossRef]
- Ye, H.; Yang, Z.; Khan, I.M.; Niazi, S.; Guo, Y.; Wang, Z.; Yang, H. Split Aptamer Acquisition Mechanisms and Current Application in Antibiotics Detection: A Short Review. Crit. Rev. Food Sci. 2022, 137, 108973. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, F.B.; Noori, M.; Edalatpanah, Y.; Mirhosseini, M. ZnO Nanoparticle Suspensions Containing Citric Acid as Antimicrobial to Control Listeria Monocytogenes, Escherichia Coli, Staphylococcus Aureus and Bacillus Cereus in Mango Juice. Food Control 2014, 42, 310–314. [Google Scholar] [CrossRef]
- Joshi, H.; Dave, R.; Venugopalan, V.P. Pumping Iron to Keep Fit: Modulation of Siderophore Secretion Helps Efficient Aromatic Utilization in Pseudomonas Putida Kt2440. Microbiology 2014, 160, 1393–1400. [Google Scholar] [CrossRef]
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles—A Review. Text Res. J. 2010, 80, 1721–1737. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of Nanotechnology in Food Packaging and Food Safety: Barrier Materials, Antimicrobials and Sensors. J. Colloid Interf. Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sahani, S.; Sharma, Y.C. Advancements in Applications of Nanotechnology in Global Food Industry. Food Chem. 2021, 342, 128318. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-Grade Nanoparticles. NPJ Sci. Food. 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Bari, L.; Grumezescu, A.; Ukuku, D.O.; Dey, G.; Miyaji, T. New Food Processing Technologies and Food Safety. J. Food Quality 2017, 2017, 3535917. [Google Scholar] [CrossRef]
- Martirosyan, A.; Schneider, Y.-J. Engineered Nanomaterials in Food: Implications for Food Safety and Consumer Health. Int. J. Environ. Res. Public Health 2014, 11, 5720–5750. [Google Scholar] [CrossRef]
- Hakimian, F.; Emamifar, A.; Karami, M. Evaluation of Microbial and Physicochemical Properties of Mayonnaise Containing Zinc Oxide Nanoparticles. LWT-Food Sci. Technol. 2022, 163, 113517. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal Activity of Zinc Oxide Nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Sorour, N.M.; El-Baz, A.F.; El-Tras, W.F. 14—Nanometals Appraisal in Food Preservation and Food-Related Activities. In Food Preservation; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 487–526. [Google Scholar]
- Omerović, N.; Djisalov, M.; Živojević, K.; Mladenović, M.; Vunduk, J.; Milenković, I.; Knežević, N.; Gadjanski, I.; Vidić, J. Antimicrobial Nanoparticles and Biodegradable Polymer Composites for Active Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2428–2454. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. Applications of Nano-Materials in Food Packaging: A Review. J. Food Process. Eng. 2021, 44, e13708. [Google Scholar] [CrossRef]
- Zafar, A.; Khosa, M.K.; Noor, A.; Qayyum, S.; Saif, M.J. Carboxymethyl Cellulose/Gelatin Hydrogel Films Loaded with Zinc Oxide Nanoparticles for Sustainable Food Packaging Applications. Polymers 2022, 14, 5201. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, M.; Xu, B.; Guo, Z. Fresh-Cut Orange Preservation Based on Nano-Zinc Oxide Combined with Pressurized Argon Treatment. LWT-Food Sci. Technol. 2021, 135, 110036. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour Encapsulation and Controlled Release—A Review. Int. J. Food Sci. Tech. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Curwin, B.D.; Deddens, J.A.; McKernan, L.T. Flavoring Exposure in Food Manufacturing. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 324–333. [Google Scholar] [CrossRef]
- Saffarionpour, S.; Ottens, M. Recent Advances in Techniques for Flavor Recovery in Liquid Food Processing. Food Eng. Rev. 2018, 10, 81–94. [Google Scholar] [CrossRef]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current Applications and Future Scope in Food. Food Front. 2021, 2, 3–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).