Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Model Systems
2.3. Quantification of Proteins
2.4. Simulation of Malaxation Process
2.5. Thermal Treatment
2.6. Ultrasound Treatment
2.7. Enzyme Activity
2.8. Statistical Evaluation
3. Results and Discussion
3.1. Enzyme Activity during Simulated Malaxation Process
3.2. Influence of Thermal Treatment on Enzyme Activity
3.3. Influence of Ultrasound on Enzyme Activity
3.4. Effect of Heating Source on Enzyme Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mehmood, A.; Usman, M.; Patil, P.; Zhao, L.; Wang, C. A Review on Management of Cardiovascular Diseases by Olive Polyphenols. Food Sci. Nutr. 2020, 8, 4639–4655. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.Á.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus Nutrition Intervention Randomized Trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kalogianni, E.P.; Georgiou, D.; Hasanov, J.H. Olive Oil Processing: Current Knowledge, Literature Gaps, and Future Perspectives. J. Am. Oil Chem. Soc. 2019, 96, 481–507. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical Strategies to Increase Nutritional and Sensory Quality of Virgin Olive Oil by Modulating the Endogenous Enzyme Activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Tamborrino, A. Olive Paste Malaxation. The Extra-Virgin Olive Oil Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 127–137. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Malaxation: Influence on Virgin Olive Oil Quality. Past, Present and Future—An Overview. Trends Food Sci. Technol. 2012, 25, 13–23. [Google Scholar] [CrossRef]
- Volk, J.; Sarafeddinov, A.; Unver, T.; Marx, S.; Tretzel, J.; Zotzel, J.; Warzecha, H. Two Novel Methylesterases from Olea Europaea Contribute to the Catabolism of Oleoside-Type Secoiridoid Esters. Planta 2019, 250, 2083–2097. [Google Scholar] [CrossRef]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The Influence of the Malaxation Temperature on the Activity of Polyphenoloxidase and Peroxidase and on the Phenolic Composition of Virgin Olive Oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef]
- Miho, H.; Moral, J.; López-González, M.A.; Díez, C.M.; Priego-Capote, F. The Phenolic Profile of Virgin Olive Oil Is Influenced by Malaxation Conditions and Determines the Oxidative Stability. Food Chem. 2020, 314, 126183. [Google Scholar] [CrossRef]
- Nardella, M.; Moscetti, R.; Bedini, G.; Bandiera, A.; Chakravartula, S.S.N.; Massantini, R. Impact of Traditional and Innovative Malaxation Techniques and Technologies on Nutritional and Sensory Quality of Virgin Olive Oil—A Review. Food Chem. Adv. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Angerosa, F. Virgin Olive Oil Odour Notes: Their Relationships with Volatile Compounds from the Lipoxygenase Pathway and Secoiridoid Compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex Interactive Effects of Ripening Degree, Malaxation Duration and Temperature on Oblica Cv. Virgin Olive Oil Phenols, Volatiles and Sensory Quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Cevik, S.; Ozkan, G.; Kıralan, M. Optimization of Malaxation Process of Virgin Olive Oil Using Desired and Undesired Volatile Contents. LWT 2016, 73, 514–523. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Sakellaropoulos, N. Olive Fruit Harvest and Processing and Their Effects on Oil Functional Compounds. In Olives and Olive Oil as Functional Foods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 127–146. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Clodoveo, M.L. Industrial Ultrasound Applications in The Extra-Virgin Olive Oil Extraction Process: History, Approaches, and Key Questions. Foods 2019, 8, 121. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S.; di Maio, I.; Sordini, B.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Oil Mechanical Extraction Process: Cultivar Impact on the Phenolic and Volatile Composition of Virgin Olive Oil. J. Agric. Food Chem. 2015, 63, 6066–6074. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Tamborrino, A.; Esposto, S.; Berardi, A.; Servili, M. Investigation on the Effects of a Pulsed Electric Field (PEF) Continuous System Implemented in an Industrial Olive Oil Plant. Foods 2022, 11, 2758. [Google Scholar] [CrossRef]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A. A New Designer Malaxer to Improve Thermal Exchange Enhancing Virgin Olive Oil Quality. Acta Hortic. 2012, 949, 455–462. [Google Scholar] [CrossRef]
- Leone, A.; Esposto, S.; Tamborrino, A.; Romaniello, R.; Taticchi, A.; Urbani, S.; Servili, M. Using a Tubular Heat Exchanger to Improve the Conditioning Process of the Olive Paste: Evaluation of Yield and Olive Oil Quality. Eur. J. Lipid Sci. Technol. 2016, 118, 308–317. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; di Maio, I.; Sordini, B.; Servili, M. Cooling Treatment of Olive Paste during the Oil Processing: Impact on the Yield and Extra Virgin Olive Oil Quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef]
- Aydar, A.Y.; Bağdatlıoğlu, N.; Köseoğlu, O. Effect of Ultrasound on Olive Oil Extraction and Optimization of Ultrasound-Assisted Extraction of Extra Virgin Olive Oil by Response Surface Methodology (RSM). Grasas Aceites 2017, 68, 189. [Google Scholar] [CrossRef]
- Jiménez, A.; Beltrán, G.; Uceda, M. High-Power Ultrasound in Olive Paste Pretreatment. Effect on Process Yield and Virgin Olive Oil Characteristics. Ultrason. Sonochem. 2007, 14, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Durante, V.; la Notte, D.; Punzi, R.; Gambacorta, G. Ultrasound-Assisted Extraction of Virgin Olive Oil to Improve the Process Efficiency. Eur. J. Lipid Sci. Technol. 2013, 115, 1062–1069. [Google Scholar] [CrossRef]
- Almeida, B.; Valli, E.; Bendini, A.; Gallina Toschi, T. Semi-industrial Ultrasound-assisted Virgin Olive Oil Extraction: Impact on Quality. Eur. J. Lipid Sci. Technol. 2017, 119, 1600230. [Google Scholar] [CrossRef]
- Wang, D.; Yan, L.; Ma, X.; Wang, W.; Zou, M.; Zhong, J.; Ding, T.; Ye, X.; Liu, D. Ultrasound Promotes Enzymatic Reactions by Acting on Different Targets: Enzymes, Substrates and Enzymatic Reaction Systems. Int. J. Biol. Macromol. 2018, 119, 453–461. [Google Scholar] [CrossRef]
- Axelrod, B.; Cheesbrough, T.M.; Laakso, S. [53] Lipoxygenase from Soybeans. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1981; Volume 71, pp. 441–451. [Google Scholar]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Romero-Segura, C.; Sanz, C.; Perez, A.G. Purification and Characterization of an Olive Fruit β-Glucosidase Involved in the Biosynthesis of Virgin Olive Oil Phenolics. J. Agric. Food Chem. 2009, 57, 7983–7988. [Google Scholar] [CrossRef] [PubMed]
- Luaces, P.; Sanz, C.; Pérez, A.G. Thermal Stability of Lipoxygenase and Hydroperoxide Lyase from Olive Fruit and Repercussion on Olive Oil Aroma Biosynthesis. J. Agric. Food Chem. 2007, 55, 6309–6313. [Google Scholar] [CrossRef]
- XLSTAT. Statistical and Data Analysis Solution; Lumivero: Denver, CO, USA, 2022. [Google Scholar]
- Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G. Volatile Compounds and Phenolic Composition of Virgin Olive Oil: Optimization of Temperature and Time of Exposure of Olive Pastes to Air Contact during the Mechanical Extraction Process. J. Agric. Food Chem. 2003, 51, 7980–7988. [Google Scholar] [CrossRef]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of Fruit Ripening on ‘Cornicabra’ Virgin Olive Oil Quality A Study of Four Successive Crop Seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of Malaxation Temperature and Time on the Quality of Virgin Olive Oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Fauconnier, M.L.; Marlier, M. An Efficient Procedure for the Production of Fatty Acid Hydroperoxides from Hydrolyzed Flax Seed Oil and Soybean Lipoxygenase. Biotechnol. Technol. 1996, 10, 839–844. [Google Scholar] [CrossRef]
- Gardner, H.W. Decomposition of Linoleic Acid Hydroperoxides. Enzymic Reactions Compared with Nonenzymic. J. Agric. Food Chem. 1975, 23, 129–136. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of Malaxation Conditions on Phenol and Volatile Profiles in Olive Paste and the Corresponding Virgin Olive Oils (Olea europaea L. Cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Nucciarelli, D.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Tomasone, R.; Pagano, M.; Servili, M. Application of Low Temperature during the Malaxation Phase of Virgin Olive Oil Mechanical Extraction Processes of Three Different Italian Cultivars. Foods 2021, 10, 1578. [Google Scholar] [CrossRef]
- Ludikhuyze, L.; Indrawati; van den Broeck, I.; Weemaes, C.; Hendrickx, M. Effect of Combined Pressure and Temperature on Soybean Lipoxygenase. 1. Influence of Extrinsic and Intrinsic Factors on Isobaric−Isothermal Inactivation Kinetics. J. Agric. Food Chem. 1998, 46, 4074–4080. [Google Scholar] [CrossRef]
- Fan, X.-H.; Zhang, X.-Y.; Zhang, Q.-A.; Zhao, W.-Q.; Shi, F.-F. Optimization of Ultrasound Parameters and Its Effect on the Properties of the Activity of Beta-Glucosidase in Apricot Kernels. Ultrason. Sonochem. 2019, 52, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, J.; Yang, A.; Chen, H. The Ultrasound-Treated Soybean Seeds Improve Edibility and Nutritional Quality of Soybean Sprouts. Food Res. Int. 2015, 77, 704–710. [Google Scholar] [CrossRef]


| Source of Variation | β-GLU Activity (µmol p-NP/mg Protein) | LOX Activity (µmol HPOT/mg Protein) | ||
|---|---|---|---|---|
| TT Treatment | TT Treatment and Incubation | TT Treatment | TT Treatment and Incubation | |
| Temperature (°C) | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.002 |
| 15 | 0.00 ± 0.00 d | 66.11 ± 1.34 d | 0.10 ± 0.01 d | 1.13 ± 0.02 a |
| 20 | 0.00 ± 0.00 d | 81.02 ± 2.12 bc | 0.08 ± 0.02 d | 1.08 ± 0.02 a |
| 25 | 0.43 ± 0.60 cd | 74.61 ± 0.52 c | 0.14 ± 0.01 d | 1.06 ± 0.01 ab |
| 30 | 2.54 ± 0.56 bc | 89.09 ± 2.17 ab | 0.26 ± 0.00 c | 1.11 ± 0.00 a |
| 35 | 13.00 ± 2.60 ab | 86.12 ± 3.51 abc | 0.33 ± 0.01 b | 1.12 ± 0.04 a |
| 40 | 23.22 ± 1.35 a | 91.58 ± 0.56 a | 0.52 ± 0.02 a | 0.98 ± 0.00 b |
| Source of Variation | β-GLU Activity (µmol p-NP/mg Protein) | LOX Activity (µmol HPOT/mg Protein) | ||
|---|---|---|---|---|
| US Treatment | US Treatment and Incubation | US Treatment | US Treatment and Incubation | |
| Time (min) * | p < 0.001 | p = 0.319 | p < 0.001 | p = 0.006 |
| 1 | 19.36 ± 0.62 c | 99.09 ± 1.31 a | 0.24 ± 0.01 c | 1.40 ± 0.02 ab |
| 5 | 39.77 ± 0.49 b | 101.85 ± 2.34 a | 0.80 ± 0.08 b | 1.28 ± 0.03 b |
| 12 | 73.15 ± 2.48 a | 99.80 ± 1.52 a | 1.78 ± 0.13 a | 1.61 ± 0.12 a |
| Power (W) ** | p < 0.001 | p = 0.016 | p = 0.014 | p < 0.001 |
| 128 | 24.85 ± 0.67 c | 97.16 ± 1.77 b | 1.11 ± 0.03 a | 1.76 ± 0.03 a |
| 320 | 42.00 ± 0.41 b | 99.88 ± 2.24 ab | 0.94 ± 0.13 ab | 1.29 ± 0.11 b |
| 640 | 65.43 ± 2.49 a | 103.70 ± 1.14 a | 0.77 ± 0.08 b | 1.24 ± 0.05 b |
| Time (min) × Power (W) | p < 0.001 | p = 0.033 | p = 0.086 | p = 0.005 |
| 1 × 128 | 8.79 ± 1.22 g | 98.05 ± 2.60 ab | 0.27 ± 0.01 d | 1.84 ± 0.06 a |
| 1 × 320 | 15.08 ± 1.22 g | 101.92 ± 2.78 ab | 0.17 ± 0.03 d | 1.04 ± 0.03 b |
| 1 × 640 | 34.20 ± 0.69 f | 97.32 ± 0.95 b | 0.27 ± 0.01 d | 1.33 ± 0.01 ab |
| 5 × 128 | 14.81 ± 1.44 g | 99.64 ± 4.51 ab | 1.11 ± 0.06 bc | 1.77 ± 0.01 a |
| 5 × 320 | 42.67 ± 0.00 e | 97.62 ± 5.29 ab | 0.71 ± 0.04 cd | 1.06 ± 0.02 b |
| 5 × 640 | 61.85 ± 0.30 c | 108.30 ± 0.87 a | 0.58 ± 0.23 cd | 1.02 ± 0.10 b |
| 12 × 128 | 50.95 ± 0.69 d | 93.79 ± 1.08 b | 1.95 ± 0.07 a | 1.68 ± 0.07 a |
| 12 × 320 | 68.26 ± 0.22 b | 100.12 ± 3.09 ab | 1.93 ± 0.38 a | 1.77 ± 0.34 a |
| 12 × 640 | 100.26 ± 7.42 a | 105.5 ± 3.17 ab | 1.46 ± 0.10 ab | 1.37 ± 0.12 ab |
| β-GLU Activity (µmol p-NP/mg Protein) | ||||
|---|---|---|---|---|
| TT Treatment | US Treatment | TT Treatment and Incubation | US Treatment and Incubation | |
| Figure 2a | Figure 2b | Figure 2c | Figure 2d | |
| Model * | β-GLU activity = a × e(k×T) | |||
| a | 0.0277 | 0.8650 | 60.1699 | 90.6092 |
| k | 0.1690 | 0.1258 | 0.0108 | 0.0031 |
| R2 | 0.963 | 0.929 | 0.727 | 0.113 |
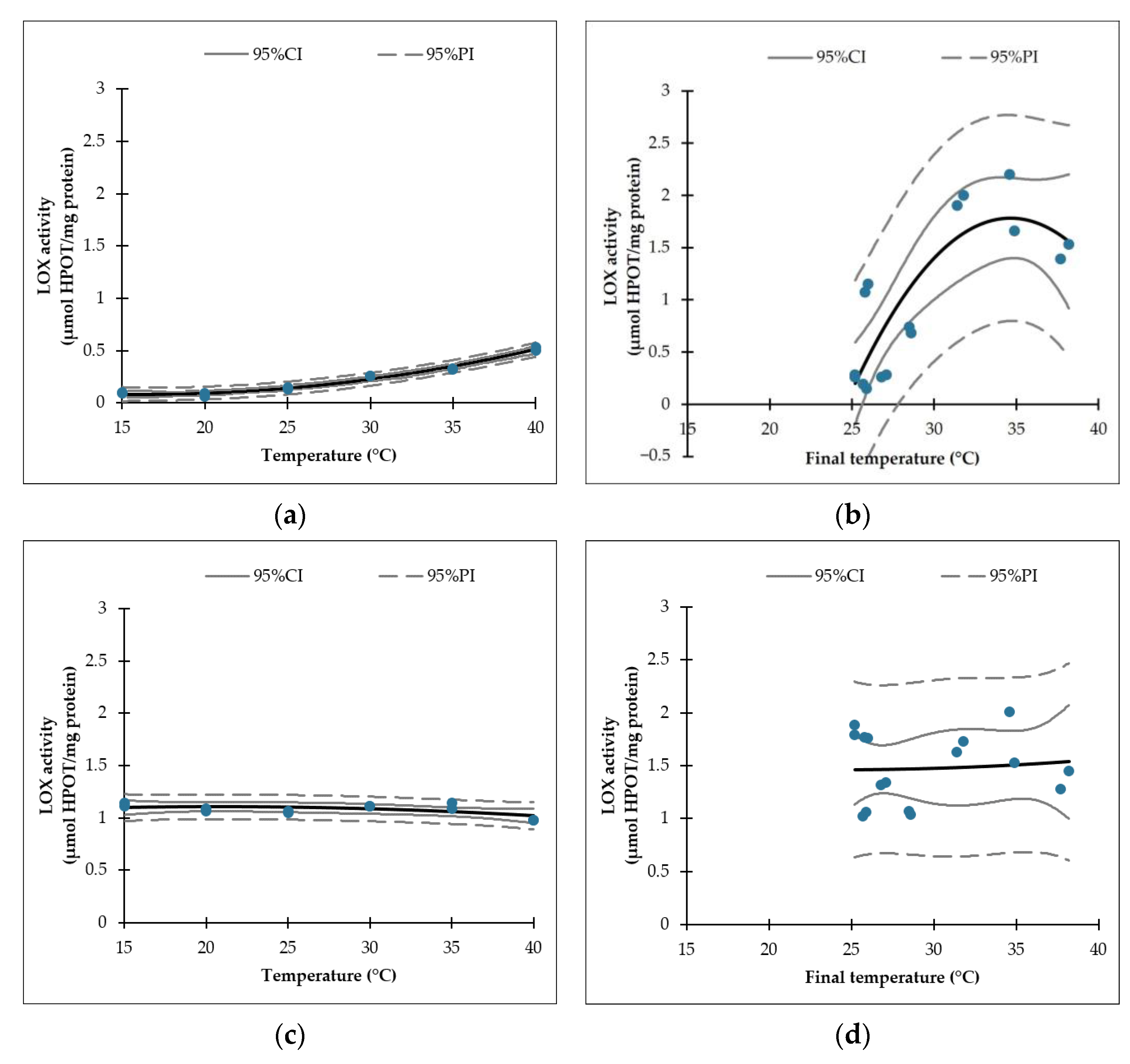
| LOX Activity (µmol HPOT/mg Protein) | ||||
|---|---|---|---|---|
| TT Treatment | US Treatment | TT Treatment and Incubation | US Treatment and Incubation | |
| Figure 3a | Figure 3b | Figure 3c | Figure 3d | |
| Model * | LOX activity = a + b × T + c × T2 | |||
| a | 0.2808 | −19.4915 | 1.0045 | 1.6760 |
| b | −0.0243 | 1.2277 | 0.0096 | −0.0178 |
| c | 0.0008 | −0.0177 | −0.0002 | 0.0004 |
| R2 | 0.980 | 0.707 | 0.352 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraljić, K.; Balbino, S.; Filipan, K.; Herceg, Z.; Ivanov, M.; Vukušić Pavičić, T.; Stuparević, I.; Pavlić, K.; Škevin, D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques. Processes 2023, 11, 1194. https://doi.org/10.3390/pr11041194
Kraljić K, Balbino S, Filipan K, Herceg Z, Ivanov M, Vukušić Pavičić T, Stuparević I, Pavlić K, Škevin D. Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques. Processes. 2023; 11(4):1194. https://doi.org/10.3390/pr11041194
Chicago/Turabian StyleKraljić, Klara, Sandra Balbino, Katarina Filipan, Zoran Herceg, Mia Ivanov, Tomislava Vukušić Pavičić, Igor Stuparević, Kristian Pavlić, and Dubravka Škevin. 2023. "Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques" Processes 11, no. 4: 1194. https://doi.org/10.3390/pr11041194
APA StyleKraljić, K., Balbino, S., Filipan, K., Herceg, Z., Ivanov, M., Vukušić Pavičić, T., Stuparević, I., Pavlić, K., & Škevin, D. (2023). Innovative Approaches to Enhance Activity of Endogenous Olive Enzymes—A Model System Experiment: Part I—Thermal Techniques. Processes, 11(4), 1194. https://doi.org/10.3390/pr11041194









