Prediction of Molecular Weight of Petroleum Fluids by Empirical Correlations and Artificial Neuron Networks
Abstract
1. Introduction
| Source (Year of Publication) | Correlation | Range of Applicability | Eq. |
|---|---|---|---|
| Hariu, and Sage [21] (1969) | (1) | ||
| Kesler and Lee [13] (1976) | (2) | ||
| Riazi and Daubert [15] (1980) | (3) | ||
| Twu [20] (1984) | (4) | ||
| Rao and Bardon [19] (1985) | (5) | ||
| Soreide [12] (1989) | (6) | ||
| Goosens [9], (1996) | /d | (7) | |
| Riazi and Daubert for MW ≤ 300 g/mol [18] (2005) | (8) | ||
| Riazi and Daubert for MW ≤ 700 g/mol [18], (2005) | (9) | ||
| Liñan et al. [23] (2011) | (10) | ||
| Liñan et al. [23] (2011) (API) | (11) | ||
| Lemus et al. [2] (2016) | (12) | ||
| Hosseinifar, and Shahverdi [10] (2021) | (13) |
2. Materials and Methods
- T50–TBP boiling point at 50% evaporate, °C;
- T70–TBP boiling point at 70% evaporate, °C;
- T30–TBP boiling point at 30% evaporate, °C;
- T10–TBP boiling point at 10% evaporate, °C;
- T90–TBP boiling point at 90% evaporate, °C;
- T5–TBP boiling point at 5% evaporate, °C;
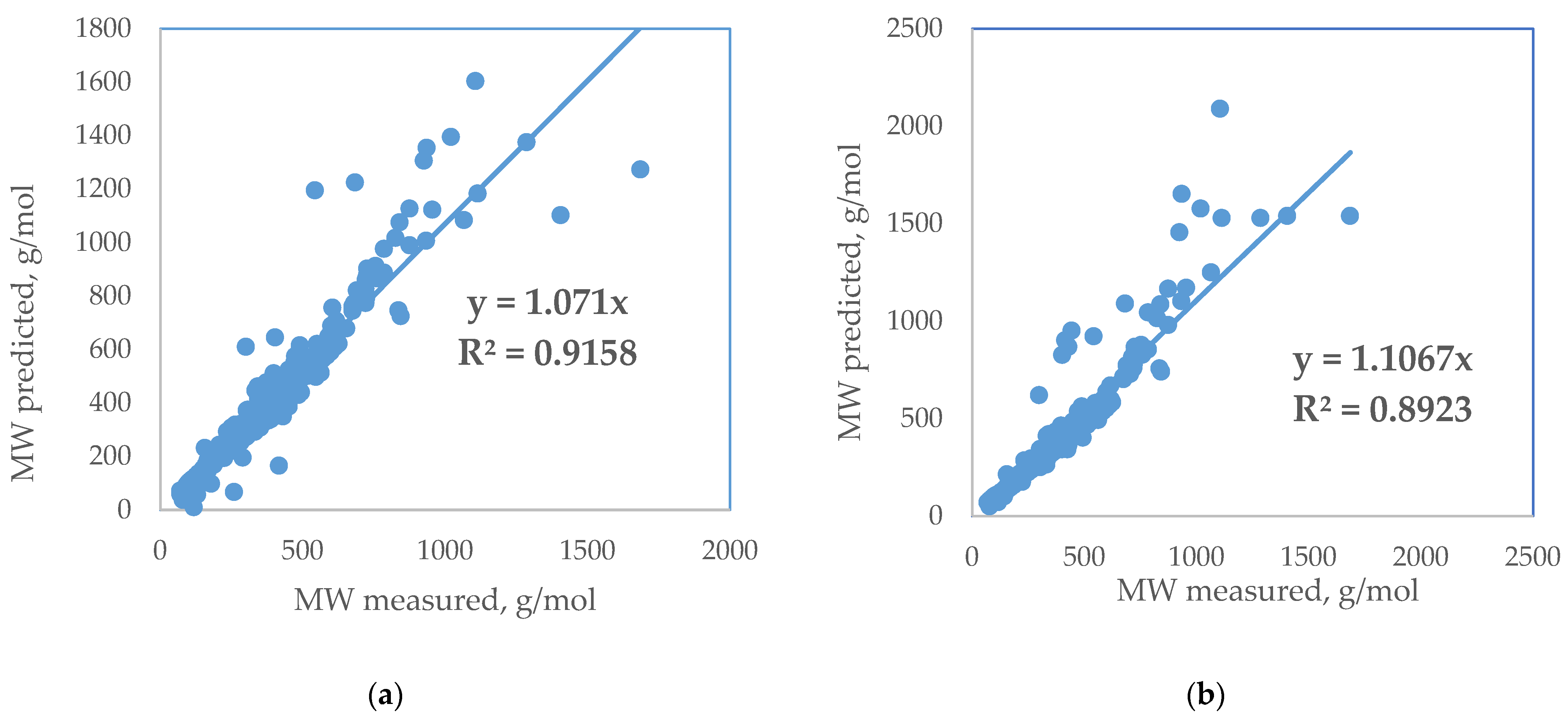
3. Results
- BP = boiling point of the petroleum fluid or individual hydrocarbon, K;
- SG = specific gravity at 15.6 °C.
4. Discussion
- P is an entry network’s vector;
- am is the exit of the m-th layer of the neural network, where;
- wm is a matrix of the coefficients of all inputs;
- bm is neuron’s input bias;
- Fm is the transfer function of the m-th layer exit.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ANN | Artificial neural network |
| %AAD | % Average absolute deviation |
| E | Error |
| Kw | Kw-characterization factor of petroleum fluids |
| MW | Molecular weight |
| RSE | Relative standard error |
| SE | Standard error |
| SRE | Sum of relative errors |
| SSE | Sum of squared errors |
| RSE | Dichloromethane |
| Tb | Boiling point |
References
- Lemus, M.C.S. Extended Distillation and Property Correlations for Heavy Oil. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, December 2015. [Google Scholar]
- Lemus, M.C.S.; Schoeggl, F.; Taylor, S.D.; Yarranton, H.W. Physical properties of heavy oil distillation cuts. Fuel 2016, 180, 457–472. [Google Scholar] [CrossRef]
- Nji, G.N. Characterization of heavy oils and bitumens. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, January 2010. [Google Scholar]
- Al-Mhanna, N.M. Simulation of High Pressure Separator Used in Crude Oil Processing. Processes 2018, 6, 219. [Google Scholar] [CrossRef]
- Aladwani, H.A.; Riazi, M.R. Some guidelines for choosing a characterization method for petroleum fractions in process simulators. Trans IChemE Part A Chem. Eng. Res. Des. 2005, 83, 160–166. [Google Scholar] [CrossRef]
- Schneider, D.F. Select the Right Hydrocracbon Molecular Weight Correlation. Available online: https://www.stratusengr.com/Articles/MoleWt.pdf (accessed on 17 November 2022).
- Powers, D.P.; Sadeghi, H.; Yarranton, H.W.; van den Berg, F.G.A. Regular solution based approach to modeling asphaltene precipitation from native and reacted oils: Part 1, molecular weight, density, and solubility parameter distributions of asphaltenes. Fuel 2016, 178, 218–233. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Powers, D.P.; Okafor, J.C.; van den Berg, F.G.A. Regular solution based approach to modeling asphaltene precipitation from native and reacted oils: Part 2, molecular weight, density, and solubility parameter of saturates, aromatics, and resins. Fuel 2018, 215, 766–777. [Google Scholar] [CrossRef]
- Goosens, A.G. Prediction of molecular weight of petroleum fractions. Ind. Eng.Chem. Res. 1996, 35, 985–988. [Google Scholar] [CrossRef]
- Hosseinifar, P.; Shahverdi, H. A predictive method for constructing the distillation curve of petroleum fluids using their physical bulk properties. J. Petrol. Sci. Eng. 2021, 200, 108403. [Google Scholar] [CrossRef]
- Altgelt, K.H.; Boduszynski, M.M. Composition and Analysis of Heavy Petroleum Fractions; Marcel Dekker: New York, NY, USA, 1994; pp. 1–495. [Google Scholar]
- Soreide, I. Improved Phase Behavior Predictions of Petroleum Reservoir Fluids from a Cubic Equation of State. Ph.D. Thesis, Norwegian Institute of Technology, Department of Petroleum Technology and Applied Geophysics, Trondheim, Norway, 1989. [Google Scholar]
- Lee, B.I.; Kesler, M.G. A Generalized thermodynamic correlation based on the three-parameter corresponding states. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Kesler, M.G.; Lee, B.I. Improve prediction of enthalpy of fractions. Hydrocarb. Process 1976, 55, 153–158. [Google Scholar]
- Riazi, M.R.; Daubert, T.E. Simplify property predictions. Hydrocarb. Process 1980, 59, 115–116. [Google Scholar]
- Riazi, M.R.; Daubert, T.E. Analytical correlations interconvert distillation-curve types. Oil Gas J. 1986, 84, 50–57. [Google Scholar]
- Riazi, M.R.; Daubert, T.E. Characterization parameters for petroleum fractions. Ind. Eng. Chem. Res. 1987, 26, 755–759. [Google Scholar] [CrossRef]
- Riazi, M.R. Characterization and Properties of Petroleum Fractions, 1st ed; ASTM International: West Conshohocken, PA, USA, 2005; pp. 1–407. [Google Scholar]
- Rao, V.K.; Bardon, M.F. Estimating the molecular weight of petroleum fractions. Ind. Eng. Chem. Proc. Des. Dev. 1985, 24, 498. [Google Scholar] [CrossRef]
- Twu, C.H. An internally consistent correlation for predicting the critical properties and molecular weight of petroleum and coal-tar liquids. Fluid Phase Equilibria 1984, 16, 137–150. [Google Scholar] [CrossRef]
- Hariu, O.H.; Sage, R.C. Crude split figured by computer. Hydrocarb. Process 1969, 4, 143–148. [Google Scholar]
- Katz, D.L.; Firoozabadi, B. Predicting phase behavior of condensate/crude oil systems using methane interaction coefficients. J. Pet. Tech. 1978, 228, 1649–1655. [Google Scholar] [CrossRef]
- Liñan, L.Z.; Lima, N.M.N.; Maciel, M.R.W.; Filho, R.M.; Medina, L.C.; Embiruçu, M. Correlation for predicting the molecular weight of Brazilian petroleum residues and cuts: An application for the simulation of a molecular distillation process. J. Pet. Sci. Eng. 2011, 78, 78–85. [Google Scholar] [CrossRef]
- Liu, Y.A.; Chang, A.-F.; Kiran, P. Chapter 1: Characterization, physical and thermodynamic properties of oil fractions. In Petroleum Refinery Process Modeling: Integrated Optimization Tools and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 1–58. [Google Scholar]
- Hosseinifar, P.; Shahverdi, H. Prediction of the ASTM and TBP distillation curves and specific gravity distribution curve for fuels and petroleum fluids. Can. J. Chem. Eng. 2022, 100, 3288–3310. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Dinkov, R.; Nenov, S.; Sotirov, S.; Sotirova, E.; Kolev, I.; Ivanov, V.; Ribagin, S.; Atanassov, K.; et al. Prediction of petroleum viscosity from molecular weight and density. Fuel 2023, 331, 125679. [Google Scholar] [CrossRef]
- Riazi, M.R.; Daubert, T.E. Prediction of molecular-type analysis of petroleum fractions and coal liquids. Ind. Eng. Chem. Res. 1986, 25, 1009–1015. [Google Scholar] [CrossRef]
- Riazi, M.R.; Daubert, T.E. Improved characterization of wide boiling range undefined petroleum fractions. Ind. Eng. Chem. Res. 1987, 26, 629–632. [Google Scholar] [CrossRef]
- White, C.M.; Perry, M.B.; Schmidt, C.E.; Douglas, L.J. Relationship between refractive indices and other properties of coal hydrogenation distillates. Energy Fuels 1987, 1, 99–105. [Google Scholar] [CrossRef]
- Bollas, G.M.; Vasalos, I.A.; Lappas, A.A.; Iatridis, D.K.; Tsioni, G.K. Bulk molecular characterization approach for the simulation of FCC feedstocks. Ind. Eng. Chem. Res. 2004, 43, 3270–3281. [Google Scholar] [CrossRef]
- Vargas, F.M.; Chapman, W.G. Application of the one-third rule in hydrocarbon and crude oil systems. Fluid Phase Equilib. 2010, 290, 103–108. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Okafor, J.C.; Ortiz, D.P.; van den Berg, F.G.A. Density and refractive index of petroleum, cuts, and mixtures. Energy Fuels 2015, 29, 5723–5736. [Google Scholar] [CrossRef]
- Carbognani, L.; Díaz-Gómez, L.; Oldenburg, T.B.P.; Pereira-Almao, P. Determination of molecular masses for petroleum distillates by simulated distillation. Cienc. Tecnol. Futuro 2012, 4, 43–55. [Google Scholar]
- Wang, S.; Dong, X.; Sun, R. Predicting saturates of sour vacuum gas oil using artificial neural networks and genetic algorithms. Expert Syst. Appl. 2010, 37, 4768–4771. [Google Scholar]
- Wang, G.; Liu, Y.; Wang, X.; Xu, C.; Gao, J. Studies on the catalytic cracking performance of coker gas oil. Energy Fuels 2009, 23, 1942–1949. [Google Scholar] [CrossRef]
- Pitault, I.; Nevicato, D.; Forissier, M.; Bernard, J.-R. Kinetic model based on a molecular description for catalytic cracking of vacuum gas oil. Chem.Eng.Sci. 1994, 49, 4249–4262. [Google Scholar] [CrossRef]
- Sheng, Q.; Wang, G.; Duan, M.; Ren, A.; Yao, L.; Hu, M.; Gao, J. Determination of the hydrogen-donating ability of industrial distillate narrow fractions. Energy Fuels 2016, 30, 10314–10321. [Google Scholar] [CrossRef]
- Altgelt, K.H.; Boduszynski, M.M. Composition of heavy petroleums. 3. An improved boiling point-molecular weight relation. Energy Fuels 1992, 6, 68–72. [Google Scholar] [CrossRef]
- Dominguez, M. FCC feed fractionation. Pet. Coal 2003, 45, 113–118. [Google Scholar]
- Van Camp, C.E.; Van Damme, P.S.; Froment, G.F. Thermal cracking of kerosene. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 155–162. [Google Scholar] [CrossRef]
- Nace, D.M.; Voltz, S.E.; Weekman, V.W., Jr. Application of a kinetic model for catalytic cracking. Effects of charge stocks. Ind. Eng. Chem. Process Des. Develop. 1971, 10, 530–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Schuler, B.; Fatayer, S.; Gross, L.; Harper, M.R.; Kushnerick, J.D. Understanding the effects of sample preparation on the chemical structures of petroleum imaged with non-contact atomic force microscopy. Ind. Eng. Chem. Res. 2018, 57, 15935–15941. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Nedanovski, D.; Shishkova, I.; Dinkov, R.; Stratiev, D.D.; Stratiev, D.D.; Sotirov, S.; Sotirova, E.; Atanassova, V.; et al. Different Nonlinear Regression Techniques and Sensitivity Analysis as Tools to Optimize Oil Viscosity Modeling. Resources 2021, 10, 99. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Sotirov, S.; Shishkova, I.; Palichev, G.; Sotirova, E.; Ivanov, V.; Atanassov, K.; Ribagin, S.; Angelova, N. Petroleum viscosity modeling using least squares and ANN methods. J. Pet. Sci. Eng. 2022, 212, 110306. [Google Scholar] [CrossRef]
- Sinha, U.; Dindoruk, B.; Soliman, M. Machine learning augmented dead oil viscosity model for all oil types. J. Pet. Sci. Eng. 2020, 195, 107603. [Google Scholar] [CrossRef]
- Sinha, U.; Dindoruk, B.; Soliman, M.Y. Physics augmented correlations and machine learning methods to accurately calculate dead oil viscosity based on the available inputs. SPE J. 2022, 27, 3240–3253. [Google Scholar] [CrossRef]
- Stratiev, D.; Marinov, I.; Dinkov, R.; Shishkova, I.; Velkov, I.; Sharafutdinov, I.; Nenov, S.; Tsvetkov, T.; Sotirov, S.; Mitkova, M.; et al. Opportunity to improve diesel fuel cetane number prediction from easy available physical properties and application of the least squares method and the artificial neural networks. Energy Fuels 2015, 29, 1520–1533. [Google Scholar] [CrossRef]
- Shishkova, I.; Stratiev, D.; Kolev, I.V.; Nenov, S.; Nedanovski, D.; Atanassov, K.; Ivanov, V.; Ribagin, S. Challenges in Petroleum Characterization—A Review. Energies 2022, 15, 7765. [Google Scholar] [CrossRef]
- D’Addona, D.M. Neural Network. In CIRP Encyclopedia of Production Engineering; Laperrière, L., Reinhart, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Yang, Z.R.; Yang, Z. 6.01—Artificial Neural Networks. Compr. Biomed. Phys. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Hadavimoghaddam, F.; Ostadhassan, M.; Heidaryan, E.; Sadri, M.A.; Chapanova, I.; Popov, E.; Cheremisin, A.; Rafieepour, S. Prediction of dead oil viscosity: Machine learning vs. classical correlations. Energies 2021, 14, 930. [Google Scholar] [CrossRef]
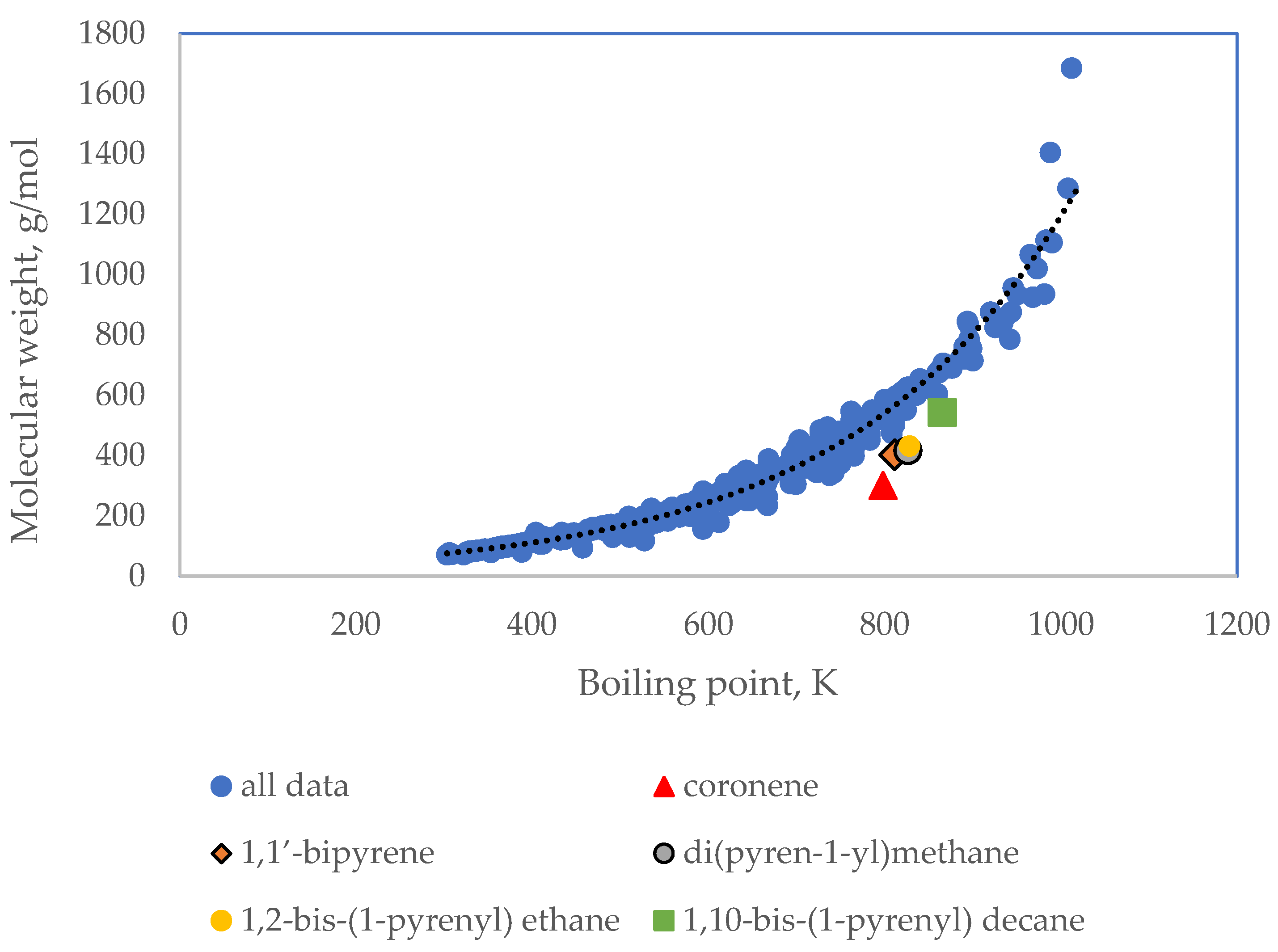

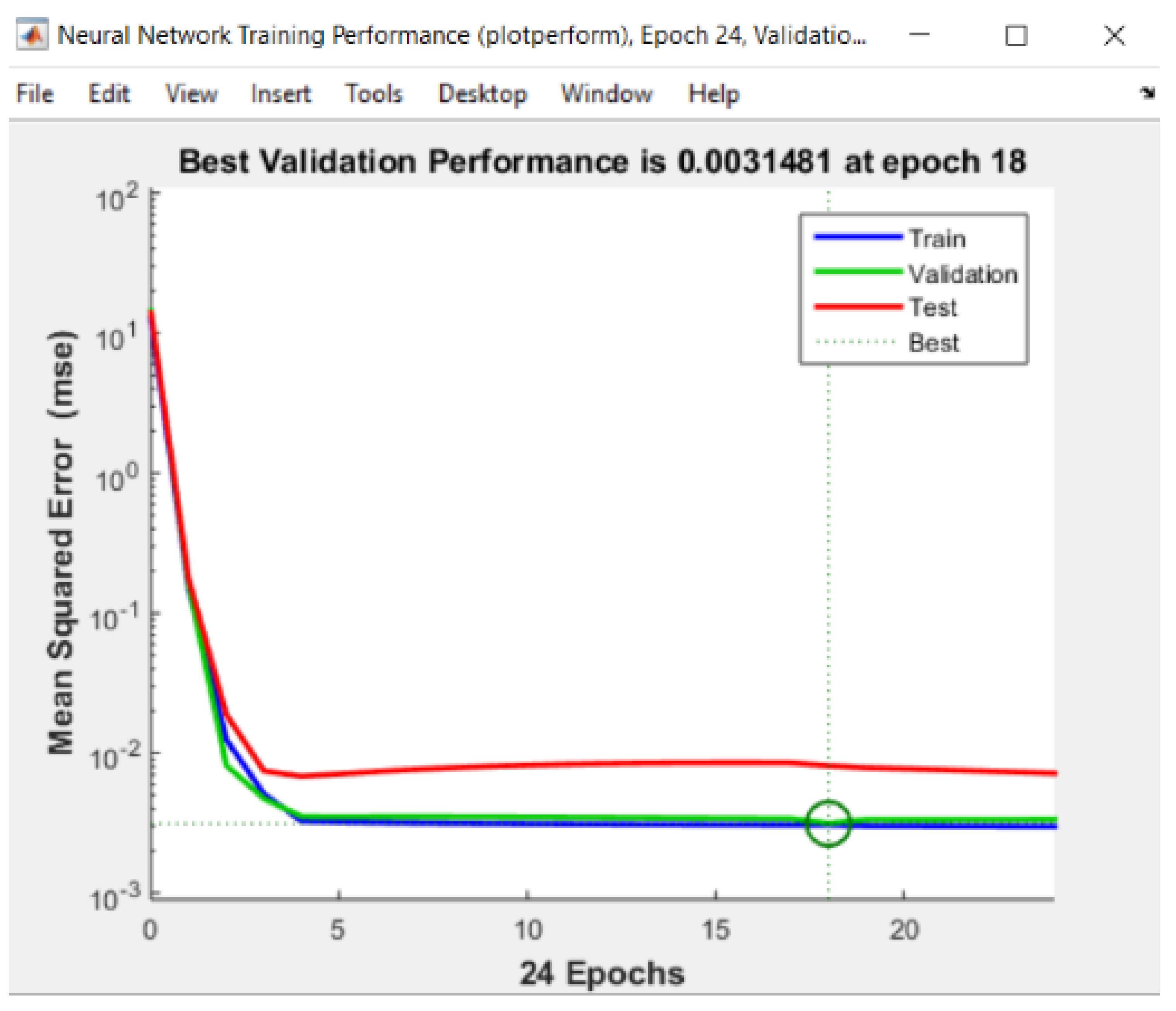
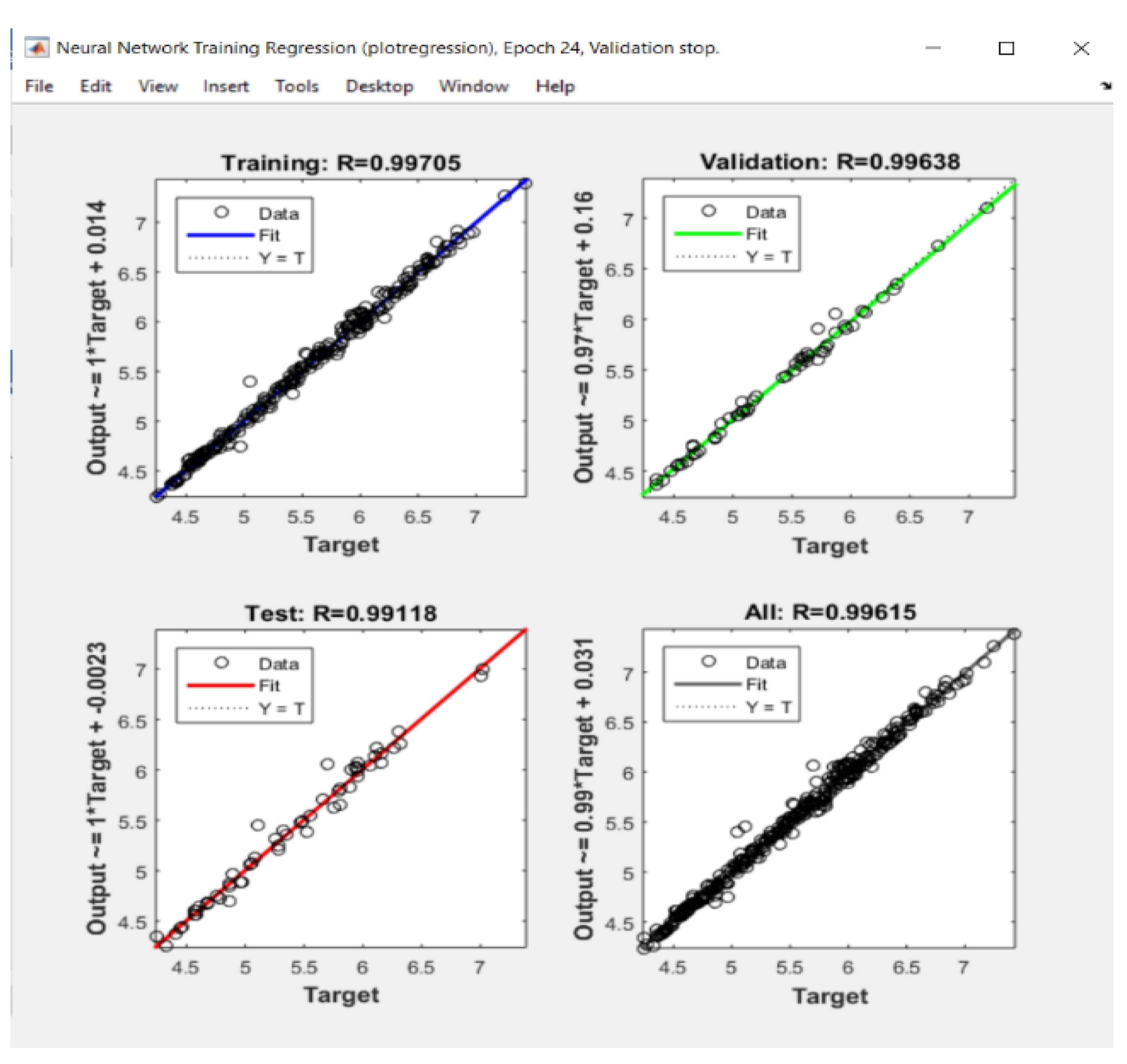
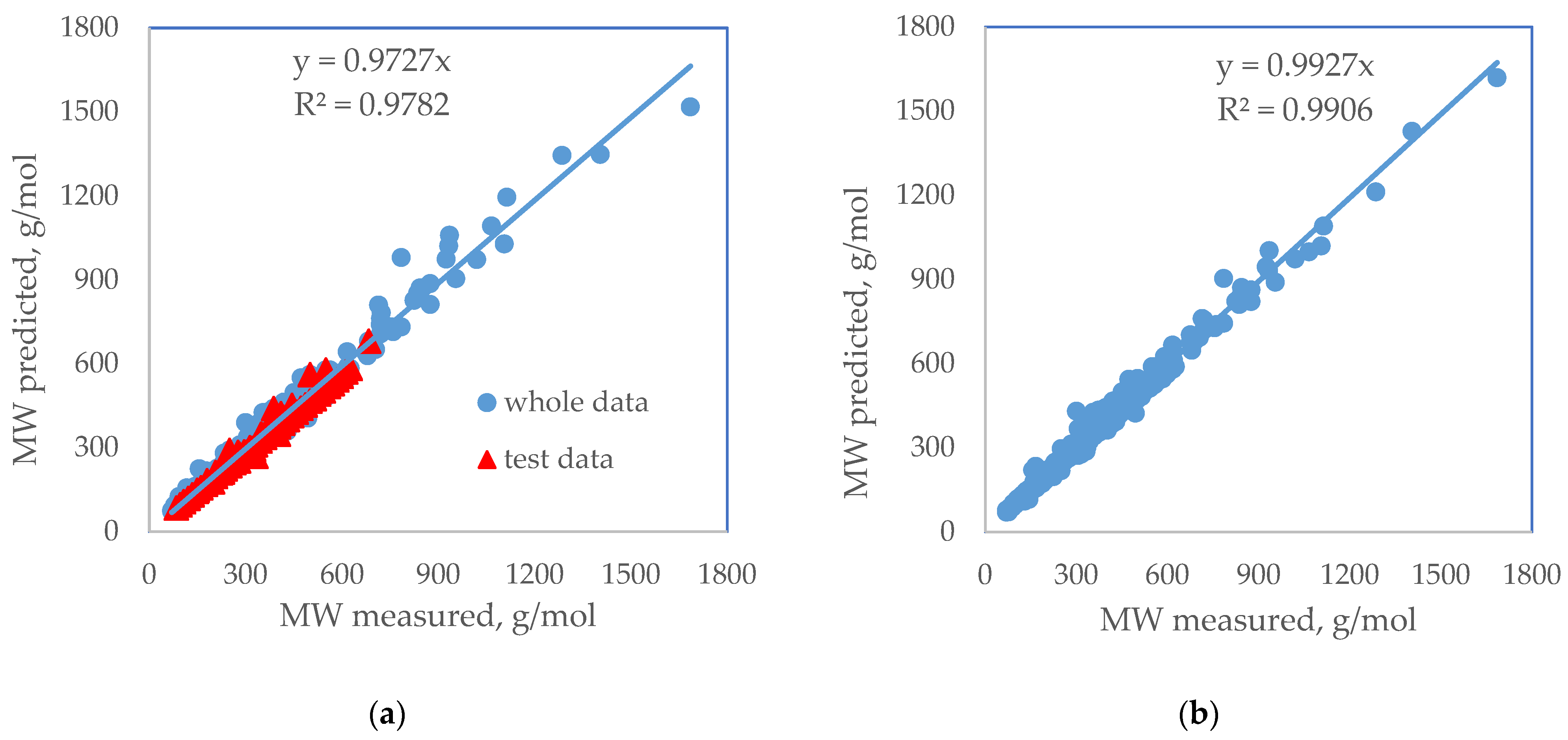
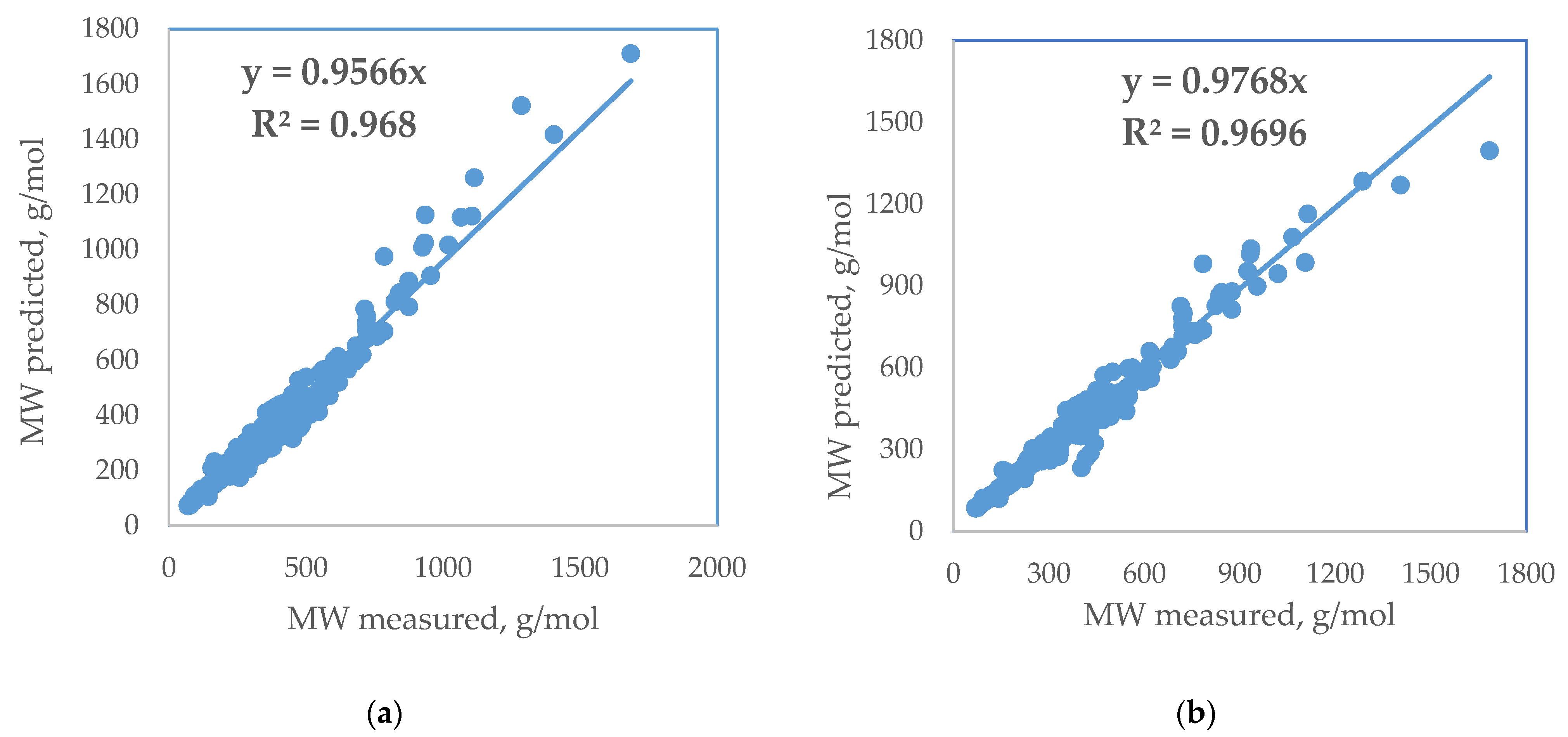
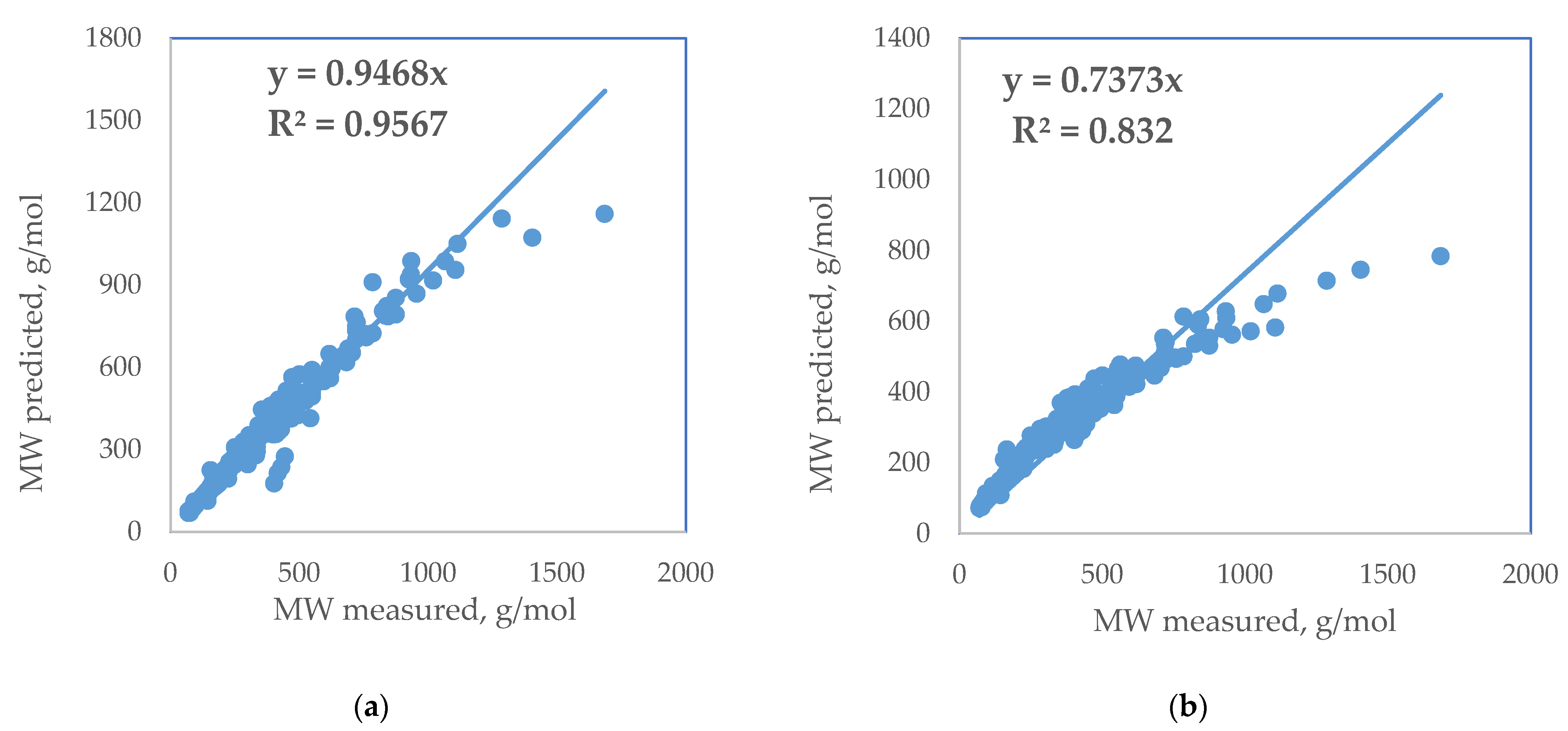
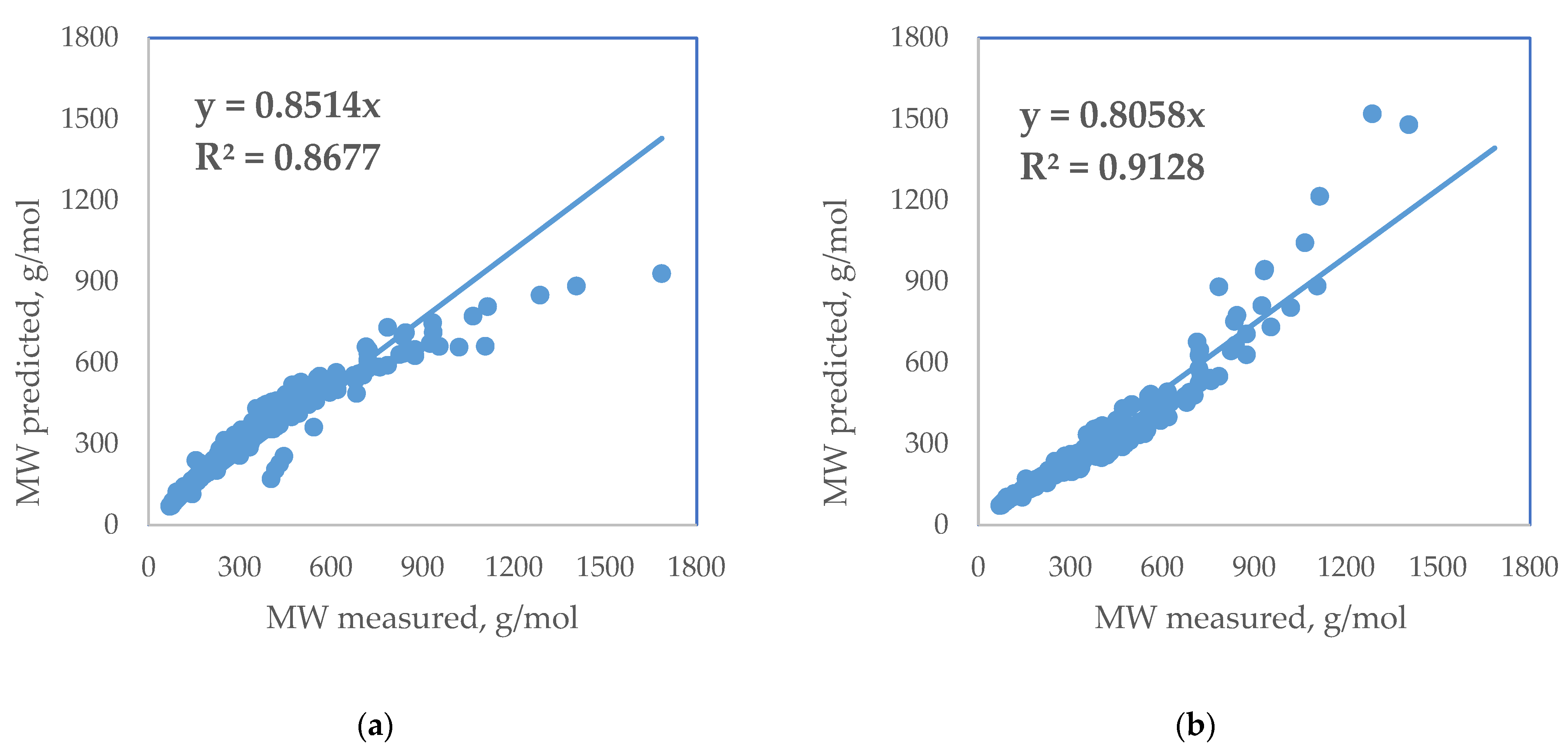
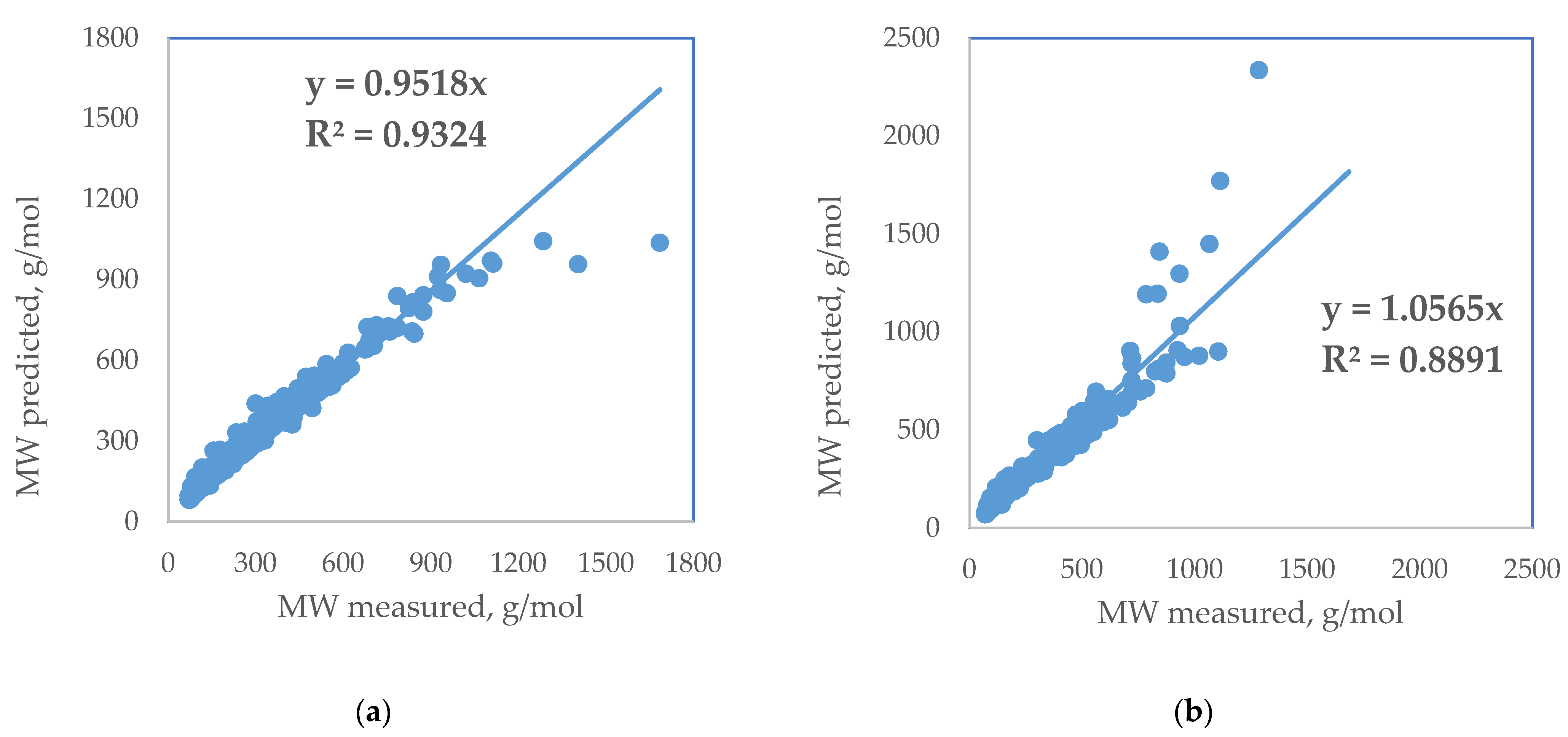

| Standard Error | Rel. St. Error | Sum of Squarred Errors | %AAD | SRE | |
|---|---|---|---|---|---|
| ANN | 23.0 | 7.2 | 1.6 | 4.3 | −126.8 |
| New empirical correlation (this work) | 34.8 | 10.9 | 3.4 | 6.4 | 695.5 |
| Hosseinifar (2021) [10] | 84.4 | 26.6 | 8.6 | 9.4 | −1851.7 |
| Goosens (1996) [9] | 44.1 | 13.9 | 4.5 | 7.6 | 1970.2 |
| Riazi and Daubert MW ≤ 700 g/mol (2005) [18] | 48.3 | 15.2 | 3.9 | 6.3 | 358.2 |
| API (2011) [23] | 39.3 | 12.4 | 5.1 | 8.6 | −999.1 |
| Liñan (2011) [23] | 54.4 | 17.1 | 10.2 | 11.3 | −2973.1 |
| Twu (1984) [20] | 96.4 | 30.3 | 20.6 | 18.7 | 7840.0 |
| Lee–Kesler (1976) [14] | 84.2 | 26.5 | 7.1 | 9.7 | 345.9 |
| Riazi and Daubert ≤ 300 g/mol (2005) [18] | 117.3 | 36.9 | 12.4 | 12.5 | 4155.7 |
| Lemus et al. (2016) [2] | 103.0 | 32.4 | 14.0 | 10.8 | 864.9 |
| Soreide (1989) [12] | 84.1 | 26.5 | 12.2 | 9.4 | −598 |
| Standard Error | Rel. St. Error | %AAD | |
|---|---|---|---|
| ANN | 13.4 | 7.9 | 3.6 |
| New empirical correlation (this work) | 16.5 | 9.7 | 5.1 |
| Hosseinifar (2021) [10] | 18.1 | 10.7 | 8.1 |
| Goosens (1996) [9] | 17.8 | 10.5 | 5.4 |
| Riazi and Daubert MW ≤ 700 g/mol (2005) [18] | 16.0 | 9.4 | 4.0 |
| API (2011) [23] | 17.0 | 10.0 | 5.4 |
| Twu (1984) [20] | 38.8 | 22.9 | 13.0 |
| Lee–Kesler (1976) [14] | 18.3 | 10.8 | 7.3 |
| Riazi and Daubert MW ≤ 300 g/mol (2005) [18] | 11.5 | 6.8 | 4.6 |
| Lemus et al. (2016) [2] | 29.4 | 17.3 | 8.0 |
| Soreide (1989) [12] | 32.0 | 18.8 | 7.6 |
| Standard Error | Rel. St. Error | %AAD | |
|---|---|---|---|
| New empirical correlation (this work, test data) | 42.1 | 11.6 | 8.8 |
| Goosens (1996) [9] | 56.7 | 15.6 | 12.1 |
| Riazi and Daubert MW ≤ 700 g/mol (2005) [18] | 52.7 | 14.5 | 8.7 |
| API (2011) [23] | 47.1 | 13.0 | 9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratiev, D.; Sotirov, S.; Sotirova, E.; Nenov, S.; Dinkov, R.; Shishkova, I.; Kolev, I.V.; Yordanov, D.; Vasilev, S.; Atanassov, K.; et al. Prediction of Molecular Weight of Petroleum Fluids by Empirical Correlations and Artificial Neuron Networks. Processes 2023, 11, 426. https://doi.org/10.3390/pr11020426
Stratiev D, Sotirov S, Sotirova E, Nenov S, Dinkov R, Shishkova I, Kolev IV, Yordanov D, Vasilev S, Atanassov K, et al. Prediction of Molecular Weight of Petroleum Fluids by Empirical Correlations and Artificial Neuron Networks. Processes. 2023; 11(2):426. https://doi.org/10.3390/pr11020426
Chicago/Turabian StyleStratiev, Dicho, Sotir Sotirov, Evdokia Sotirova, Svetoslav Nenov, Rosen Dinkov, Ivelina Shishkova, Iliyan Venkov Kolev, Dobromir Yordanov, Svetlin Vasilev, Krassimir Atanassov, and et al. 2023. "Prediction of Molecular Weight of Petroleum Fluids by Empirical Correlations and Artificial Neuron Networks" Processes 11, no. 2: 426. https://doi.org/10.3390/pr11020426
APA StyleStratiev, D., Sotirov, S., Sotirova, E., Nenov, S., Dinkov, R., Shishkova, I., Kolev, I. V., Yordanov, D., Vasilev, S., Atanassov, K., Simeonov, S., & Palichev, G. N. (2023). Prediction of Molecular Weight of Petroleum Fluids by Empirical Correlations and Artificial Neuron Networks. Processes, 11(2), 426. https://doi.org/10.3390/pr11020426









