Correlations of HTSD to TBP and Bulk Properties to Saturate Content of a Wide Variety of Crude Oils
Abstract
1. Introduction
2. Materials and Methods
3. Results
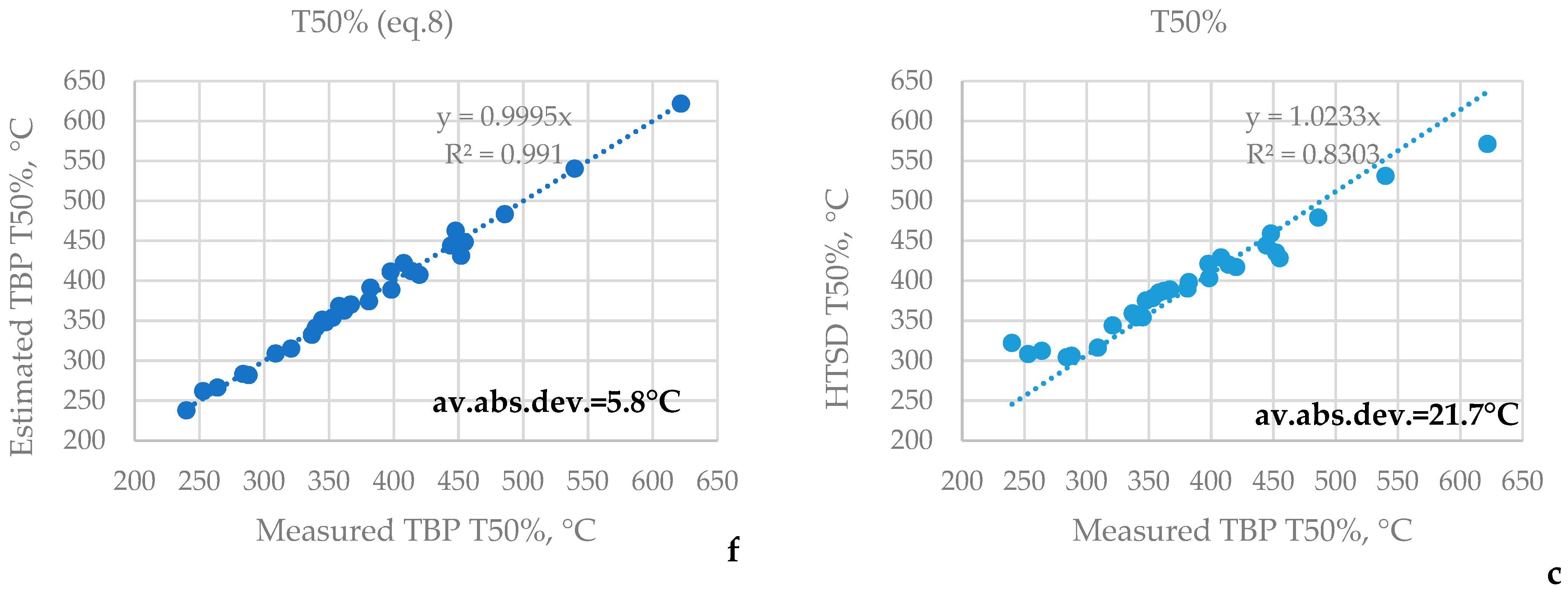
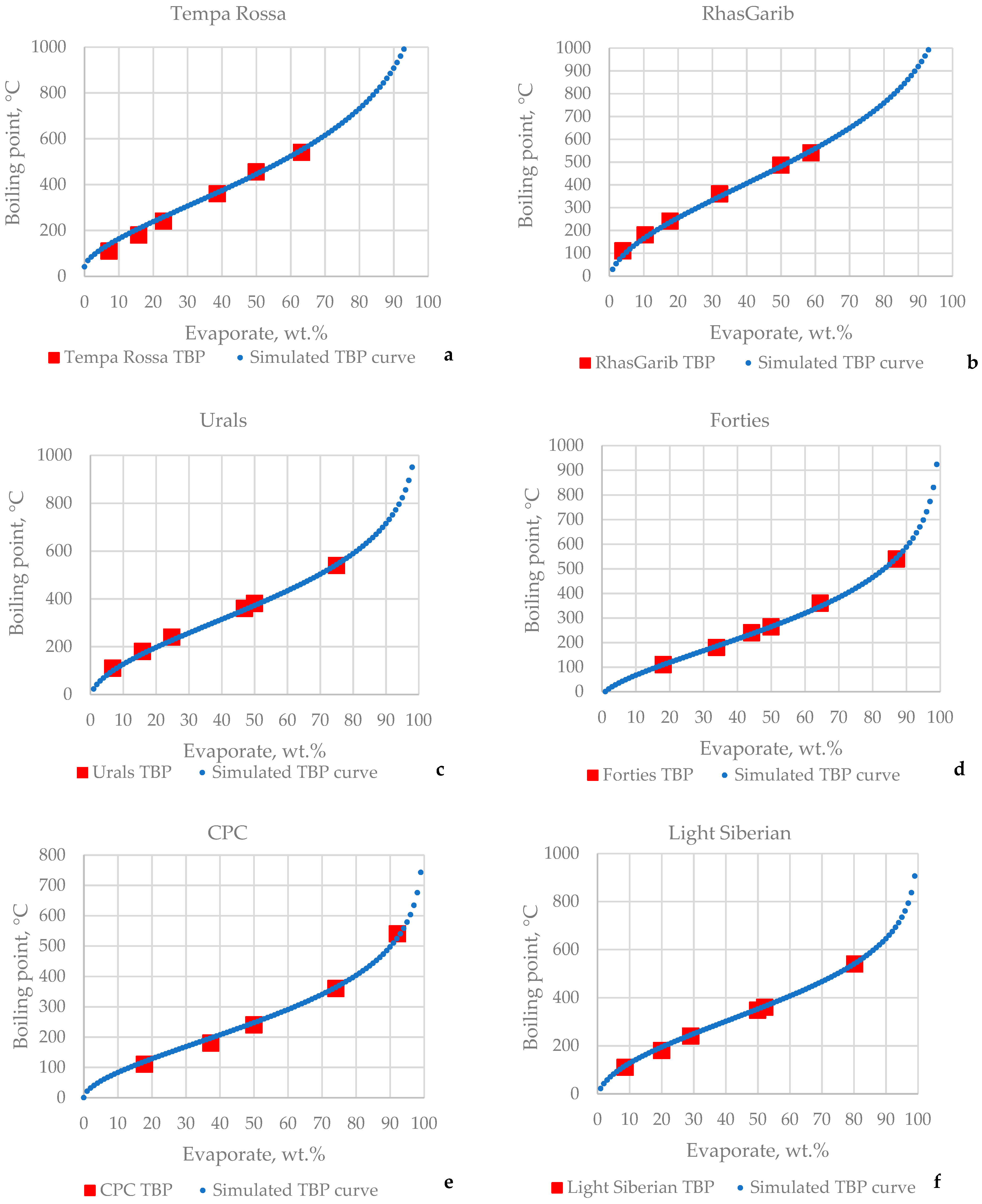
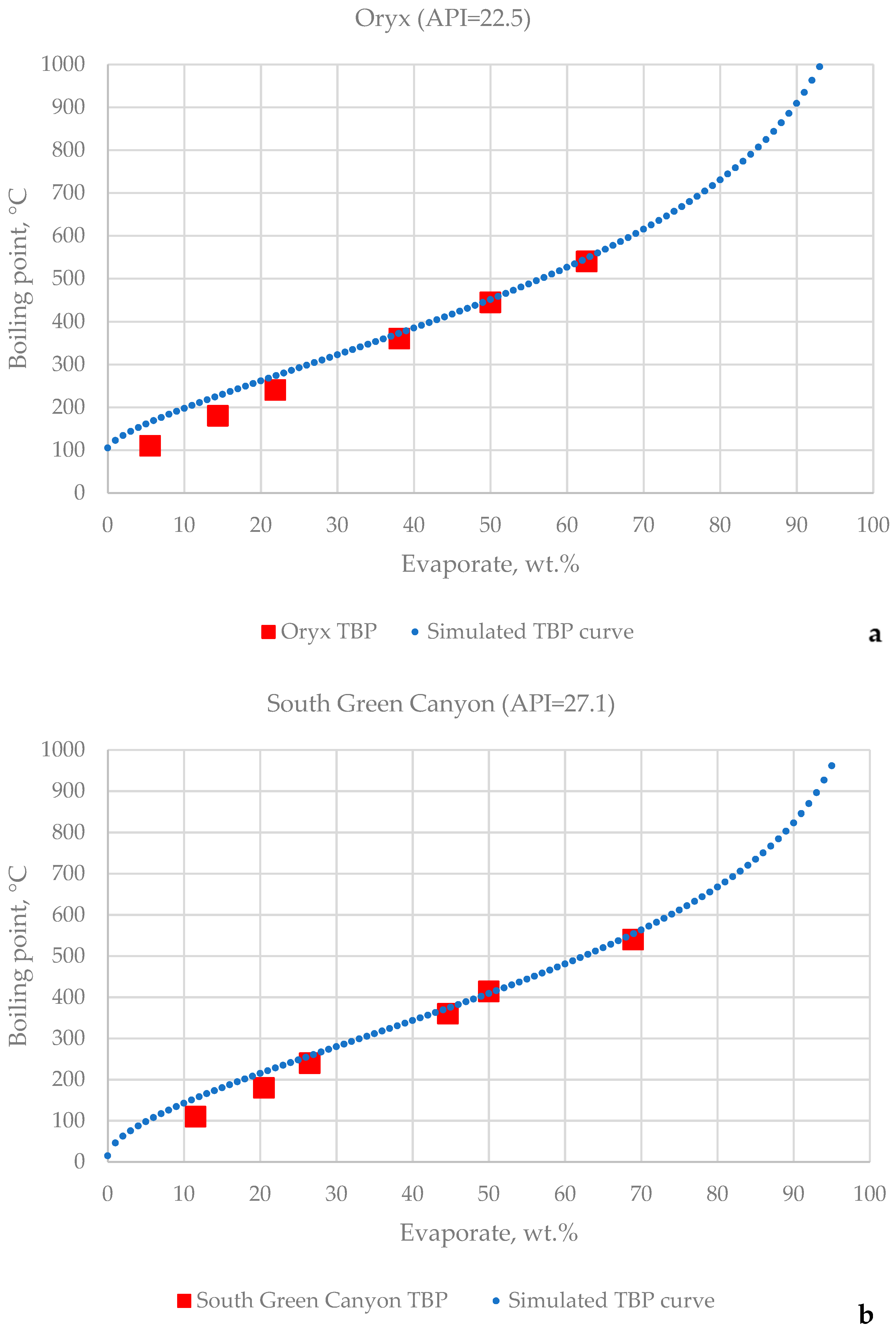
3.1. HTSD and TBP of Crude Oils
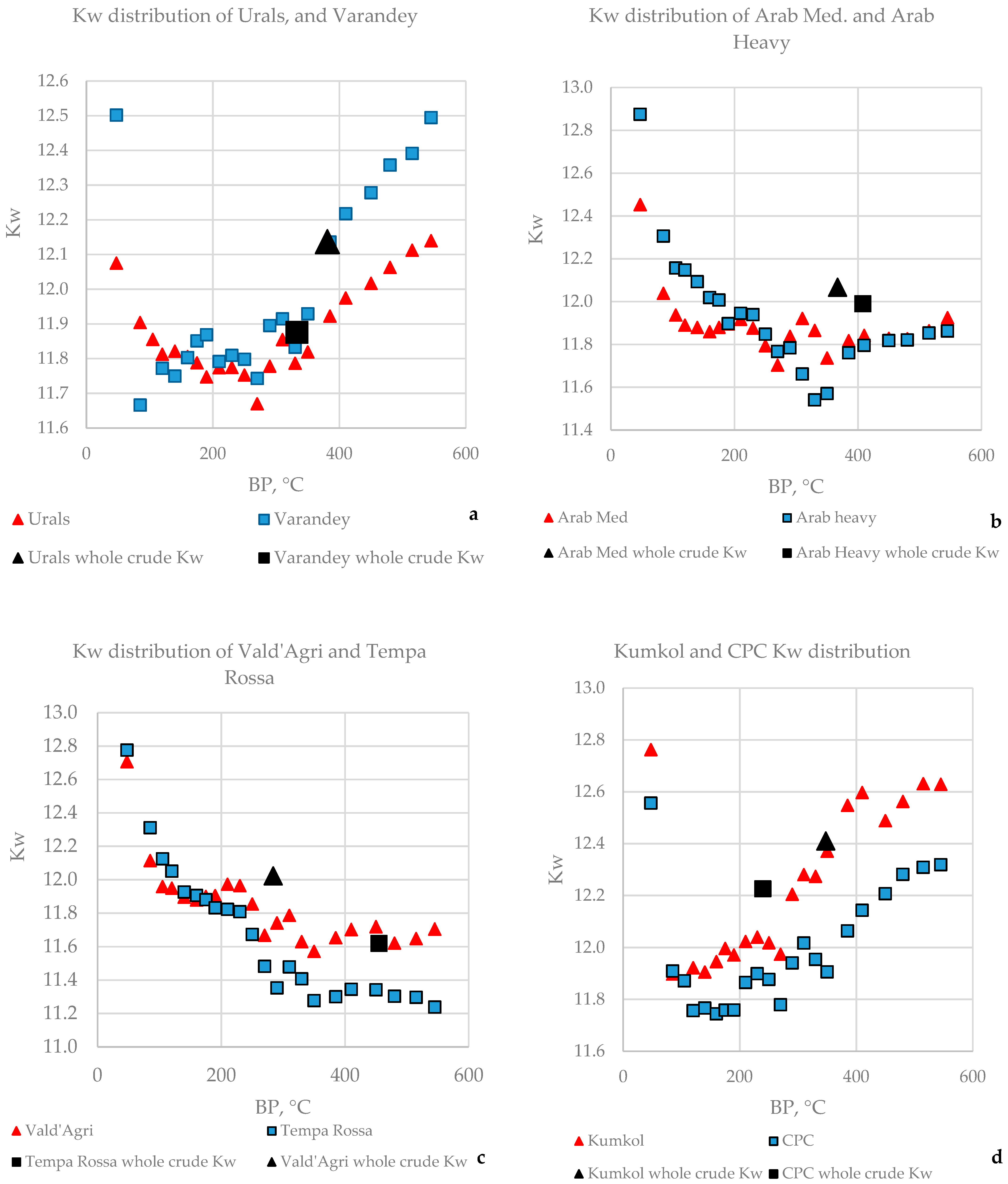
3.2. Kw Characterization Factor, and Sulphur Distributions of Narrow Fractions in the Crude Oils
3.3. SARA Composition and Bulk Properties of Studied Crude Oils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| API | American Petroleum Institute gravity |
| Aro | Aromatics |
| As | Asphaltenes |
| ASTM | American Society for Testing and Materials |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| HP | Hewlett Packard |
| HPLC | High-performance liquid chromatography |
| HTSD | High-temperature simulated distillation |
| IBP | Initial boiling point |
| ICrA | Intercriteria analysis |
| Kw | Watson characterization factor |
| PIANO | Paraffins, iso-paraffins, aromatics, naphthenes, and olefins |
| PONA | Paraffins, olefins, naphthenes, and aromatics |
| PP | Pour point |
| SARA | Saturates, aromatics, resins, asphaltenes |
| Sat. | saturates |
| SG | Specific gravity |
| Slope | Slope in Walther equation [65] for double logarithm dependence on logarithm of temperature |
| T0 | Boiling point at zero yield of distillate |
| TBP | True boiling point |
| Ti | Boiling point of i-weight fraction of distillation curve |
| TLC | Thin-layer chromatography |
| VIS | Kinematic viscosity |
| VR | Vacuum residue |
| xi | Weight fraction of i-component. |
References
- Liu, Y.; Chang, A.; Pashikanti, K. Petroleum Refinery Process Modeling: Integrated Optimization Tools and Applications; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Gao, C. Petroleum Production Technology; Science Press: Beijing, China, 2017. [Google Scholar]
- Hsu, C.S.; Robinson, P.R. Petroleum Science and Technology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kaiser, M.J.; De Klerk, A.; Gary, J.H.; Handwerk, G.E. Petroleum Refining. Technology, Economics, and Markets, 6th ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Lopes, M.S.; Savioli Lopes, M.; Maciel Filho, R.; Wolf Maciel, M.R.; Medina, L.C. Extension of the TBP curve of petroleum using the correlation DESTMOL. Procedia Eng. 2012, 42, 726–732. [Google Scholar] [CrossRef]
- Chapter 5. Heptanes Plus Characterization. Available online: http://www.ipt.ntnu.no/~curtis/courses/PVT-Flow/Plus-Characterization/Molar-Distribution/SPE-Phase-Behavior-Monograph-Ch-5.pdf (accessed on 23 December 2022).
- Riazi, M.R. Characterization and Properties of Petroleum Fractions; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Speight, J. Rules of Thumb for Petroleum Engineers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Stratiev, D.S.; Marinov, I.; Nedelchev, A.; Velkov, I.; Stratiev, D.D.; Veli, A.; Mitkova, M.; Stanulov, K. Evaluation of approaches for conversion of ASTM into TBP distillation data of oil fractions. OGEM 2014, 40, 216–221. [Google Scholar]
- Villalanti, D.C.; Raia, J.C.; Maynard, J.B. High-temperature simulated distillation applications in petroleum characterization. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- ASTM D2892-20; Standard Test Method for Distillation of Crude Petroleum (15-Theoretical Plate Column). ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D5236-18a; Standard Test Method for Distillation of Heavy Hydrocarbon Mixtures (Vacuum Potstill Method). ASTM International: West Conshohocken, PA, USA, 2018.
- Stratiev, D.; Shishkova, I.; Ivanov, M.; Dinkov, R.; Argirov, G.; Vasilev, S.; Yordanov, D. Validation of diesel fraction content in heavy oils measured by high temperature simulated distillation and physical vacuum distillation by performance of commercial distillation test and process simulation. Appl. Sci. 2022, 12, 11824. [Google Scholar] [CrossRef]
- Durand, J.-P.; Bré, A.; Béboulène, J.-J.; Ducrozet, A.; Carbonneaux, S. Improvement of simulated distillation methods by gas chromatography in routine analysis. Oil Gas Sci. Technol. 1999, 54, 431–438. [Google Scholar] [CrossRef]
- Characterization of Crude Oil by Simulated Distillation. Patent WO2016111965A1, 14 July 2016.
- Diaz, O.C.; Yarranton, H.W. Applicability of simulated distillation for heavy Oils. Energy Fuels 2019, 33, 6083–6087. [Google Scholar] [CrossRef]
- Villalanti, D.C.; Raia, J.C.; Subramanian, M.; Williams, B. Application of high-temperature simulated distillation to the residuum oil supercritical extraction process in petroleum refining. J. Chromatogr. Sci. 2000, 38, 1–5. [Google Scholar]
- Jennerwein, M.K.; Eschner, M.S.; Wilharm, T.; Zimmermann, R.; Gröger, T.M. Proof of concept of high-temperature comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry for two-dimensional simulated distillation of crude oils. Energy Fuels 2017, 31, 11651–11659. [Google Scholar] [CrossRef]
- Espinosa-Pena, M.; Figueroa-Gomez, Y.; Jimenez-Cruz, F. Simulated distillation yield curves in heavy crude oils: A comparison of precision between ASTM D-5307 and ASTM D-2892 physical distillation. Energy Fuels 2004, 18, 1832–1840. [Google Scholar] [CrossRef]
- Rodrigues, E.V.A.; Silva, S.R.C.; Romão, W.; Castro, E.V.R.; Filgueiras, P.R. Determination of crude oil physicochemical properties by high-temperature gas chromatography associated with multivariate calibration. Fuel 2018, 220, 389–395. [Google Scholar] [CrossRef]
- Coutinho, D.M.; França, D.; Vanini, G.; Gomes, A.O.; Azevedo, D.A. Understanding the molecular composition of petroleum and its distillation cuts. Fuel 2022, 311, 122594. [Google Scholar] [CrossRef]
- Azinfar, B.; Zirrahi, M.; Hassanzadeh, H.; Abedi, J. Characterization of heavy crude oils and residues using combined Gel Permeation Chromatography and simulated distillation. Fuel 2018, 233, 885–893. [Google Scholar] [CrossRef]
- Shishkova, I.; Stratiev, D.; Kolev, I.V.; Nenov, S.; Nedanovski, D.; Atanassov, K.; Ivanov, V.; Ribagin, S. Challenges in petroleum characterization—A review. Energies 2022, 15, 7765. [Google Scholar] [CrossRef]
- Abutaqiya, M.I.L.; Sisco, C.J.; Khemka, Y.; Safa, M.A.; Ghloum, E.F.; Rashed, A.M.; Gharbi, R.; Santhanagopalan, S.; Al-Qahtani, M.; Al-Kandari, E.; et al. Accurate Modeling of Asphaltene Onset Pressure in Crude Oils Under Gas Injection Using Peng−Robinson Equation of State. Energy Fuels 2020, 34, 4055–4070. [Google Scholar] [CrossRef]
- David Ting, P.; Hirasaki, G.J.; Chapman, W.G. Modeling of Asphaltene Phase Behavior with the SAFT Equation of State. Pet. Sci. Technol. 2003, 21, 647–661. [Google Scholar] [CrossRef]
- Panuganti, S.R.; Vargas, F.M.; Gonzalez, D.L.; Kurup, A.S.; Chapman, W.G. PC-SAFT characterization of crude oils and modeling of asphaltene phase behavior. Fuel 2012, 93, 658–669. [Google Scholar] [CrossRef]
- Punnapala, S.; Vargas, F.M. Revisiting the PC-SAFT characterization procedure for an improved asphaltene precipitation prediction. Fuel 2013, 108, 417–429. [Google Scholar] [CrossRef]
- Abutaqiya, M.I.L.; Sisco, C.J.; Wang, J.; Vargas, F.M. Systematic Investigation of Asphaltene Deposition in the Wellbore and Near-Wellbore Region of a Deepwater Oil Reservoir Under Gas Injection. Part 1: Thermodynamic Modeling of the Phase Behavior of Polydisperse Asphaltenes. Energy Fuels 2019, 33, 3632–3644. [Google Scholar] [CrossRef]
- Sisco, C.J.; Abutaqiya, M.I.L.; Wang, F.; Zhang, J.; Tavakkoli, M.; Vargas, F.M. Asphaltene Precipitation Modeling. In Asphaltene Deposition: Fundamentals, Prediction, Prevention, and Remediation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 111–159. [Google Scholar]
- Klein, G.C.; Angström, A.; Rodgers, R.P.; Marshall, A.G. Use of saturates/aromatics/resins/asphaltenes (SARA) fractionation to determine matrix effects in crude oil analysis by electrospray ionization fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2006, 20, 668–672. [Google Scholar] [CrossRef]
- Efimov, I.; Povarov, V.G.; Rudko, V.A. Comparison of UNIFAC and LSER models for calculating partition coefficients in the hexane–acetonitrile system using middle distillate petroleum products as an example. Ind. Eng. Chem. Res. 2022, 61, 9575–9585. [Google Scholar] [CrossRef]
- Efimov, I.; Povarov, V.G.; Rudko, V.A. Use of partition coefficients in a hexane–acetonitrile system in the GC–MS analysis of polyaromatic hydrocarbons in the example of delayed coking gas oils. ACS Omega 2021, 6, 9910–9919. [Google Scholar] [CrossRef]
- Benassi, M.; Berisha, A.; Romão, W.; Babayev, E.; Römpp, A.; Spengler, B. Petroleum crude oil analysis using low-temperature plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 825–834. [Google Scholar] [CrossRef]
- Rakhmatullin, I.; Efimov, S.; Tyurin, V.; Gafurov, M.; Al-Muntaser, A.; Varfolomeev, M.; Klochkov, V. Qualitative and quantitative analysis of heavy crude oil samples and their sara fractions with 13C nuclear magnetic resonance. Processes 2020, 8, 995. [Google Scholar] [CrossRef]
- Afanasjeva, N.; González-Córdoba, A.; Palencia, M. Mechanistic approach to thermal production of new materials from asphaltenes of Castilla crude oil. Processes 2020, 8, 1644. [Google Scholar] [CrossRef]
- Zheng, F.; Shi, Q.; Vallverdu, G.S.; Giusti, P.; Bouyssiere, B. Fractionation and characterization of petroleum asphaltene: Focus on metalopetroleomics. Processes 2020, 8, 1504. [Google Scholar] [CrossRef]
- ASTM D4052-18a; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D4294-21; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometr. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D97-12; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D445-21e2; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- Stratiev, D.; Shishkova, I.; Nikolova, R.; Tsaneva, T.; Mitkova, M.; Yordanov, D. Investigation on precision of determination of SARA analysis of vacuum residual oils from different origin. Pet Coal. 2016, 58, 109–119. [Google Scholar]
- Stratiev, D.; Shishkova, I.; Tsaneva, T.; Mitkova, M.; Yordanov, D. Investigation of relations between properties of vacuum residual oils from different origin, and of their deasphalted and asphaltene fractions. Fuel 2016, 170, 115–129. [Google Scholar] [CrossRef]
- Atanassov, K.; Mavrov, D.; Atanassova, V. Intercriteria decision making: A new approach for multicriteria decision making, based on index matrices and intuitionistic fuzzy sets. Issues Intuit. Fuzzy Sets Gen. Nets 2014, 11, 1–8. [Google Scholar]
- Atanassov, K.; Atanassova, V.; Gluhchev, G. Intercriteria analysis: Ideas and problems. Notes Intuit. Fuzzy Sets 2015, 21, 81–88. [Google Scholar]
- Atanassov, K. Index Matrices: Towards an Augmented Matrix Calculus; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Stratiev, D.S.; Sotirov, S.; Shishkova, I.; Nedelchev, A.; Sharafutdinov, I.; Anife, V.; Mitkova, M.; Yordanov, D.; Sotirova, E.; Atanassova, K.; et al. Investigation of relationships between bulk properties and fraction properties of crude oils by application of the Intercriteria analysis. Petrol. Sci. Technol. 2016, 34, 1113–1120. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Nedelchev, A.; Kirilov, K.; Nikolaychuk, E.; Ivanov, A.; Sharafutdinov, I.; Veli, A.; Mitkova, M.; Tsaneva, T.; et al. Investigation of relationships between petroleum properties and their impact on crude oil compatibility. Energy Fuels 2015, 29, 7836–7854. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Shishkova, I.; Georgiev, B.; Argirov, G.; Dinkov, R.; Yordanov, D.; Atanassova, V.; Vassilev, P.; Atanassov, K. Commercial investigation of the ebullated-bed vacuum residue hydrocracking in the conversion range of 55–93%. ACS Omega 2020, 51, 33290. [Google Scholar] [CrossRef] [PubMed]
- Stratiev, D.; Shishkova, I.; Dinkov, R.; Kolev, I.; Argirov, G.; Ivanov, V.; Ribagin, S.; Atanassova, V.; Atanassov, K.; Stratiev, D.D.; et al. Intercriteria analysis to diagnose the reasons for increased fouling in a commercial ebullated bed vacuum residue hydrocracker. ACS Omega 2022, 7, 30462–30476. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7169-20; Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2020.
- Combining Simulated Distillation (ASTM D7169) and Detailed Hydrocarbon Analysis (ASTM D7900) for the Full Boiling Point Distribution of Crude Oils. Available online: https://www.petro-online.com/article/analytical-instrumentation/11/scion-instruments/combining-simulated-distillation-astm-d7169-and-detailed-hydrocarbon-analysis-astm-d7900-for-the-full-boiling-point-distribution-of-crude-oils/2943 (accessed on 4 January 2023).
- Golden, S.; Barletta, T.; White, S. Vacuum unit performance. Sour Heavy 2012, 11–15. Available online: www.digitalrefining.com/article/1000565 (accessed on 5 January 2023).
- Golden, S.; Barletta, T. Designing vacuum units. PTQ 2006, Q2, 105–110. [Google Scholar]
- Golden, S.; Villalanti, D.C.; Martin, G.R. Feed characterization and deep cut vacuum columns: Simulation and design. In Proceedings of the AIChE 1994, Spring National Meeting, Atlanta, GA, USA, 17–21 April 1994. [Google Scholar]
- He, P.; Ghoniem, A.F. A Group contribution pseudocomponent method for phase equilibrium modeling of mixtures of petroleum fluids and a solvent. Ind. Eng. Chem. Res. 2015, 54, 8809–8820. [Google Scholar] [CrossRef]
- Mlquel, J.; Hernandez, J.; Castells, F. A New method for petroleum fractions and crude oil characterization. SPE Reserv. Eng. 1992, 7, 265–270. [Google Scholar]
- Wauquier, J.-P. Crude Oil Petroleum Products. Process Flowsheets; Editions Technip: Paris, France, 1995. [Google Scholar]
- Abdel-AalMohammed, H.K.; Alsahlawi, A. Petroleum Economics and Engineering, 3rd ed.; Taylor & Francis Group: Abingdon, UK, 2014. [Google Scholar]
- Gary, J.H.; Handwerk, G.E.; Kaiser, M.J. Petroleum Refining Technology and Economics, 5th ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2007. [Google Scholar]
- Swafford, P.; McCarthy, R. Improving crude oil selection. PTQ 2008, Q3, 125–129. [Google Scholar]
- Stratiev, D.; Shishkova, I.; Palichev, G.N.; Atanassov, K.; Ribagin, S.; Nenov, S.; Nedanovski, D.; Ivanov, V. Study of bulk properties relations to SARA Composition data of various vacuum residues employing intercriteria analysis. Energies 2022, 15, 9042. [Google Scholar] [CrossRef]
- Yarranton, H. Prediction of crude oil saturate content from a simdist assay. Energy Fuels 2022, 36, 8809–8817. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Nedanovski, D.; Shishkova, I.; Dinkov, R.; Stratiev, D.D.; Stratiev, D.D.; Sotirov, S.; Sotirova, E.; Atanassova, V.; et al. Empirical modeling of viscosities and softening points of straight-run vacuum residues from different origins and of hydrocracked unconverted vacuum residues obtained in different conversions. Energies 2022, 15, 1755. [Google Scholar] [CrossRef]
- Stratiev, D.; Nenov, S.; Sotirov, S.; Shishkova, I.; Palichev, G.; Sotirova, E.; Ivanov, V.; Atanassov, K.; Ribagin, S.; Angelova, N. Petroleum viscosity modeling using least squares and ANN methods. J. Pet. Sci. Eng. 2022, 212, 110306. [Google Scholar] [CrossRef]
- Stratiev, D.; Shishkova, I.; Dinkov, R.; Nenov, S.; Sotirov, S.; Sotirova, E.; Kolev, I.; Ivanov, V.; Ribagin, S.; Atanassov, K.; et al. Prediction of petroleum viscosity from molecular weight and density. Fuel 2023, 331, 125679. [Google Scholar] [CrossRef]
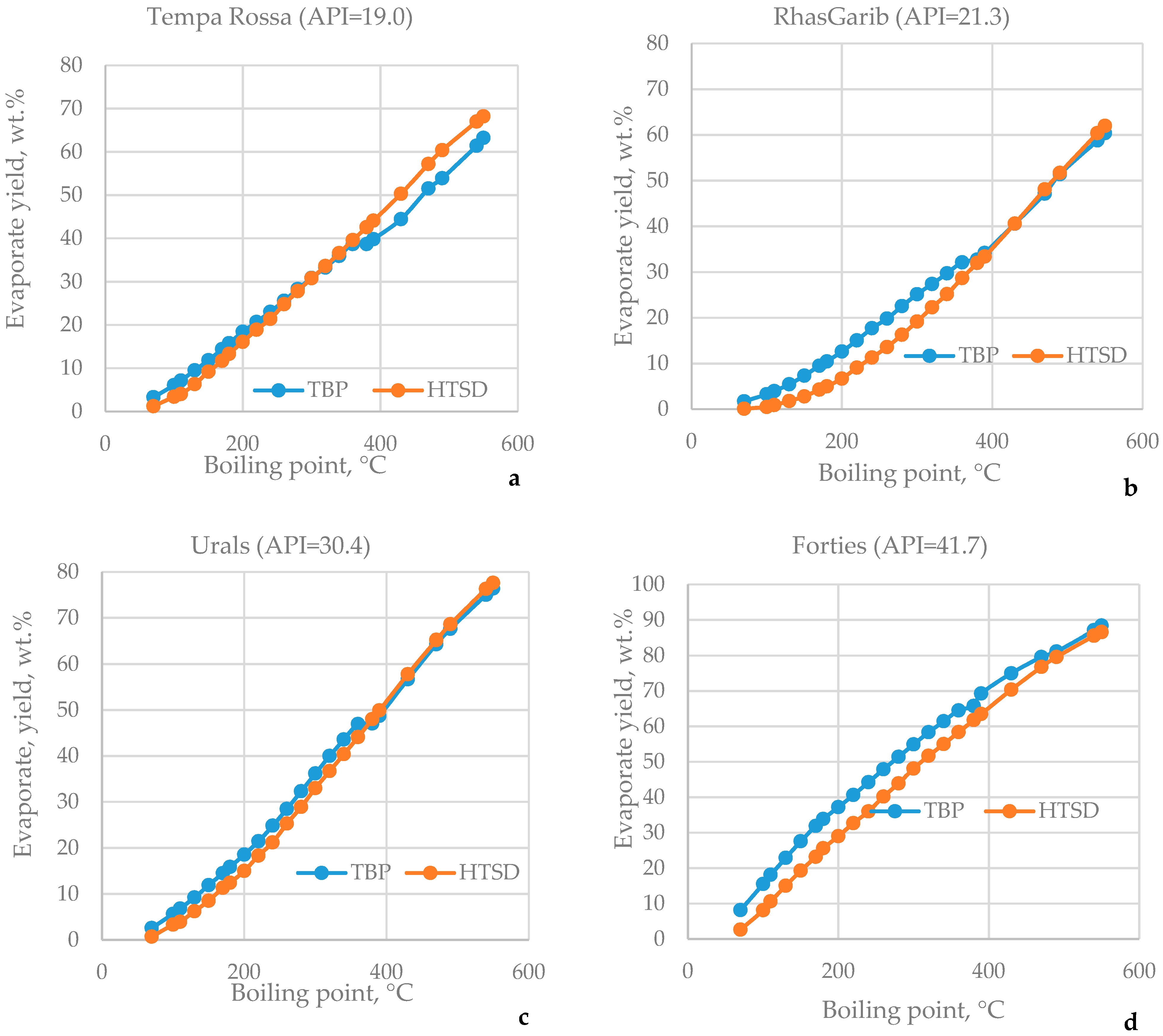
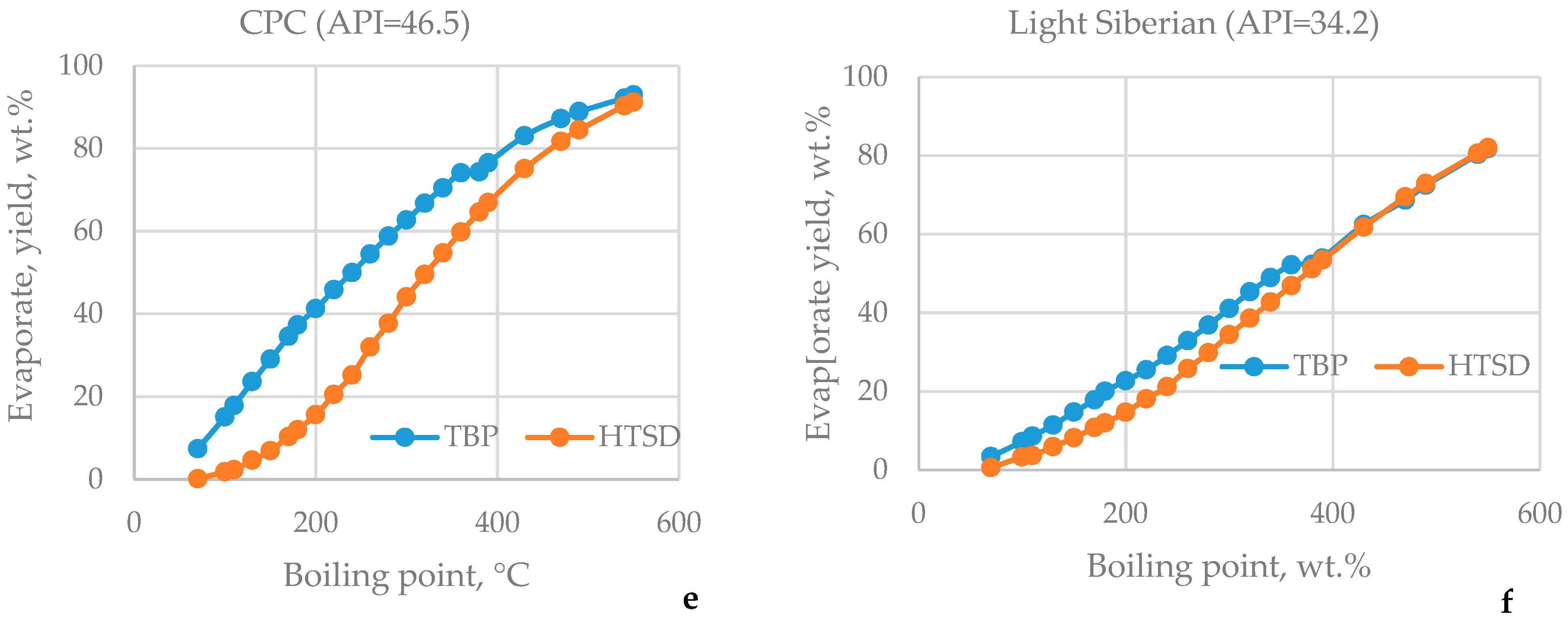
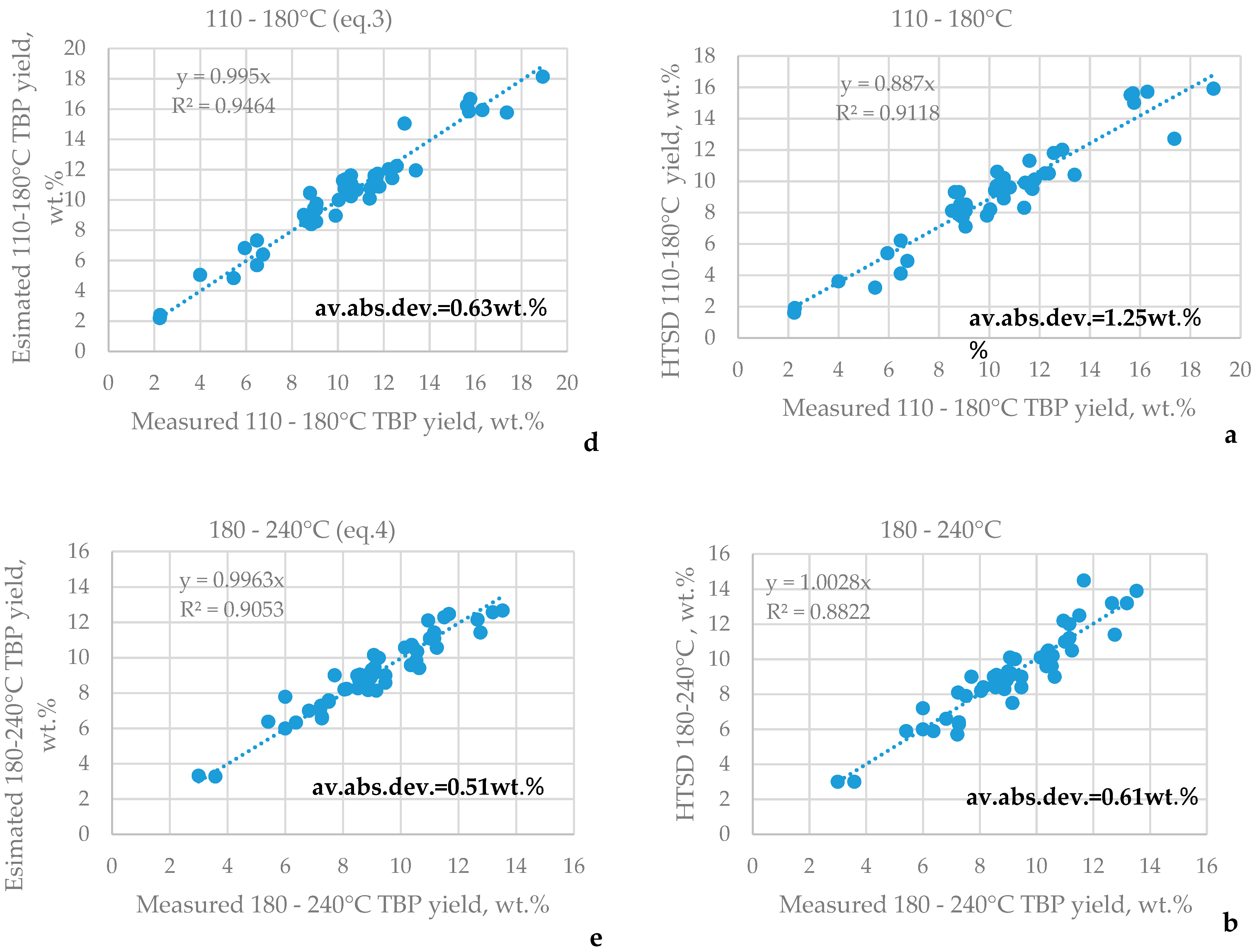
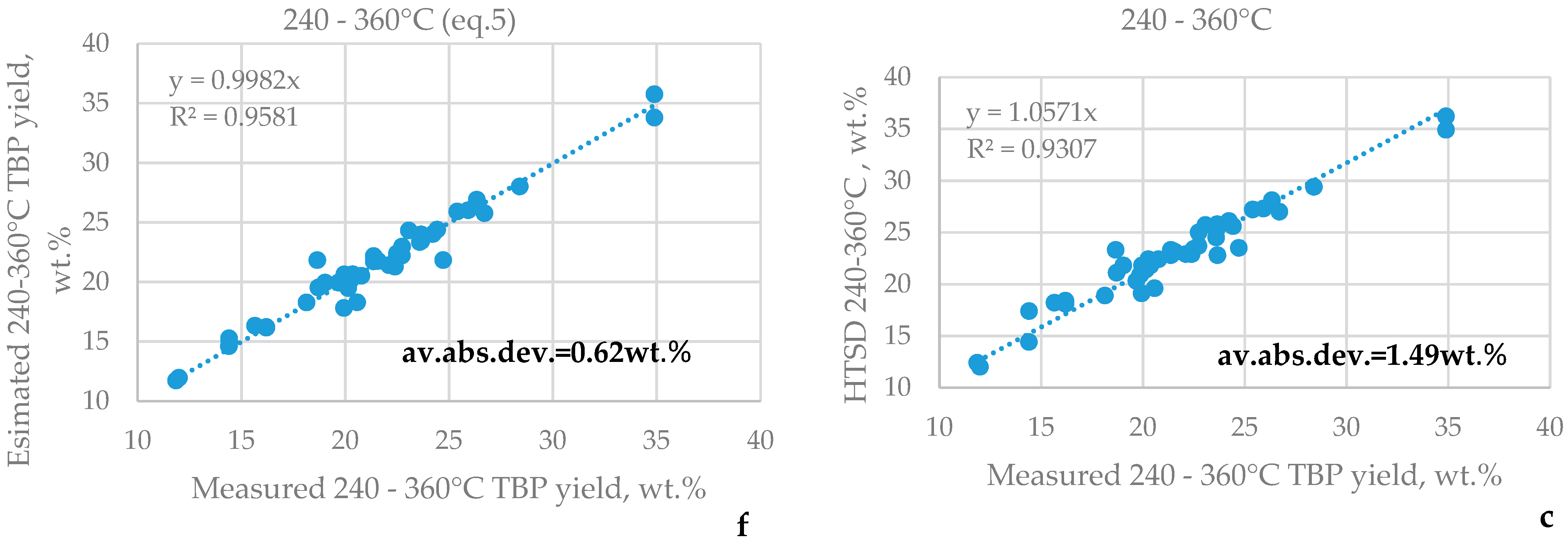


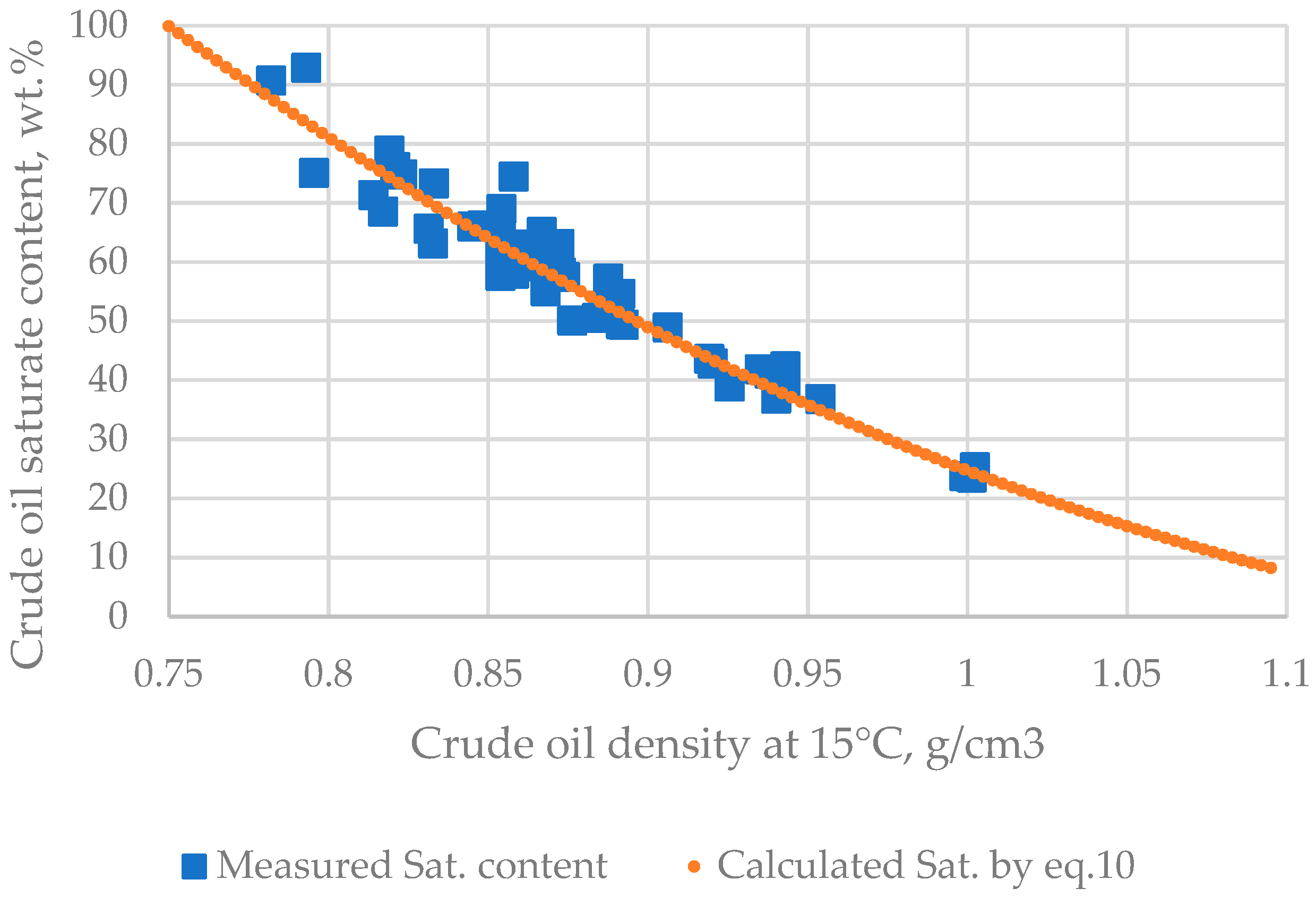
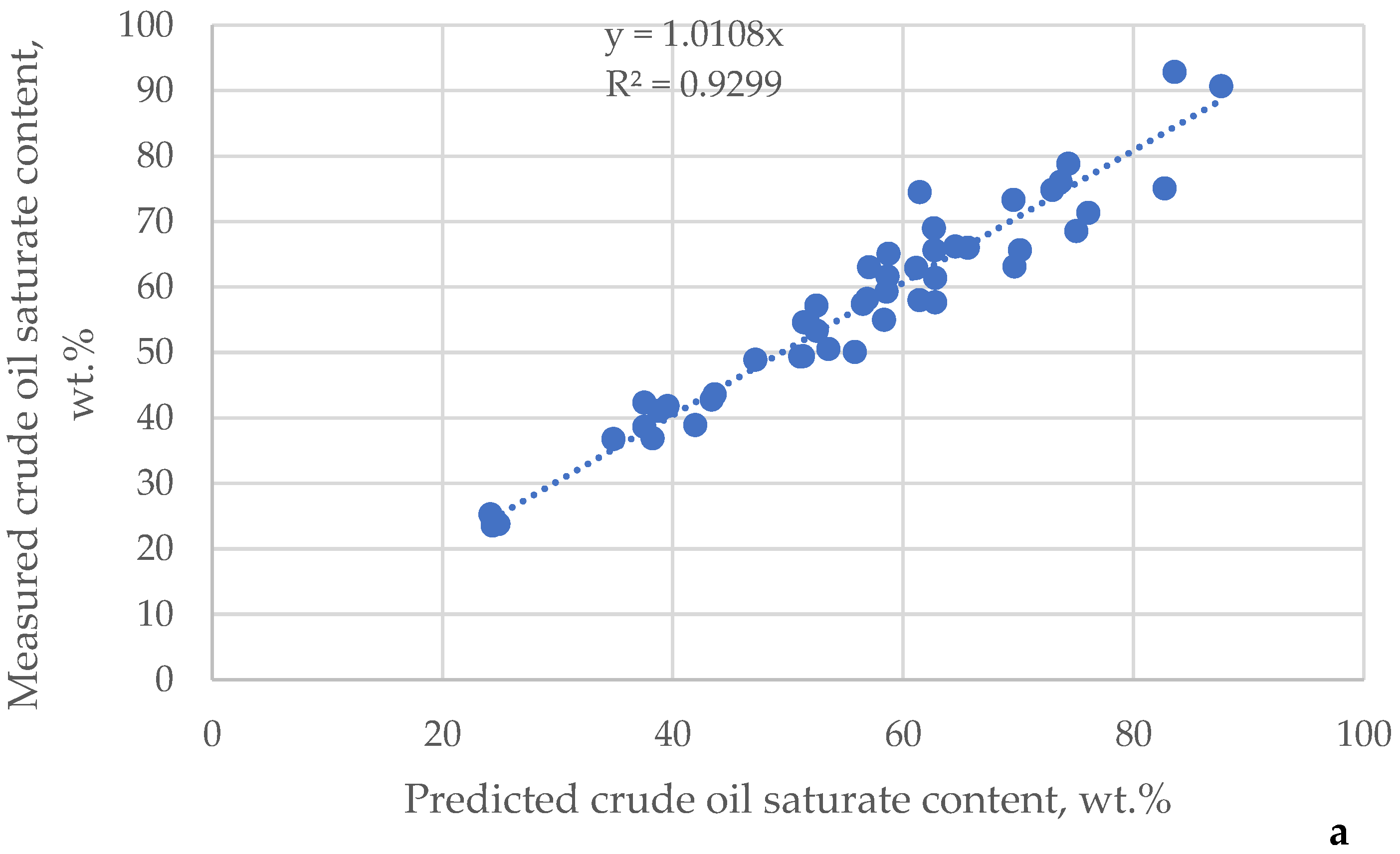
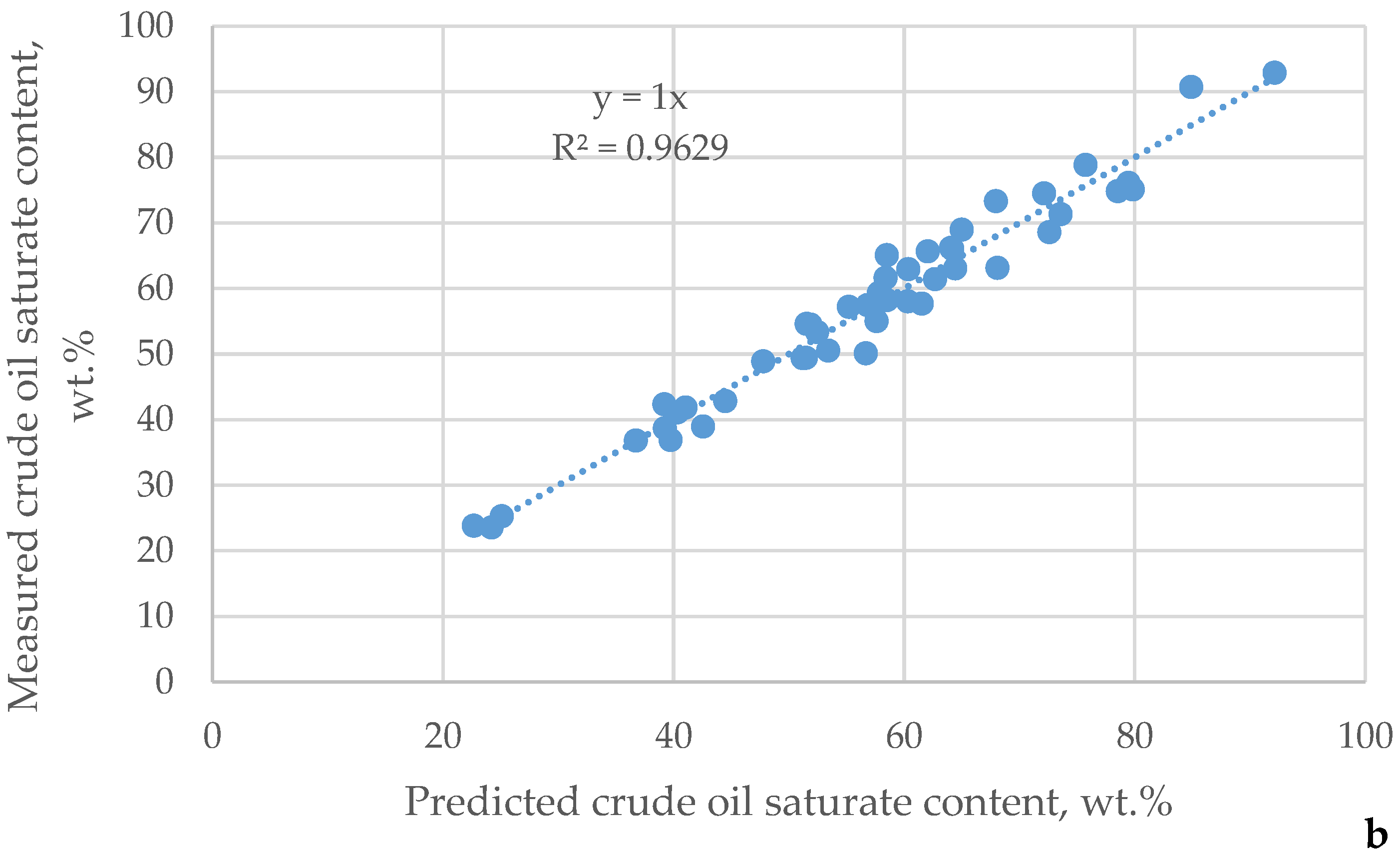
| μ | Urals | Arab M | Arab H | Vald’Agri | Basrah L | Basrah H | Kirkuk | Iranian H | KEB | El Bouri |
|---|---|---|---|---|---|---|---|---|---|---|
| Urals | 1 | 0.5628 | 0.5714 | 0.4372 | 0.5411 | 0.4502 | 0.6017 | 0.7489 | 0.5541 | 0.8398 |
| Arab M | 0.5628 | 1 | 0.71 | 0.7619 | 0.7965 | 0.7013 | 0.8268 | 0.7056 | 0.7619 | 0.5671 |
| Arab H | 0.5714 | 0.71 | 1 | 0.8052 | 0.8485 | 0.8658 | 0.7879 | 0.6104 | 0.7359 | 0.5628 |
| Vald’Agri | 0.4372 | 0.7619 | 0.8052 | 1 | 0.8701 | 0.8312 | 0.7965 | 0.6061 | 0.697 | 0.4632 |
| Basrah L | 0.5411 | 0.7965 | 0.8485 | 0.8701 | 1 | 0.8095 | 0.8701 | 0.658 | 0.7749 | 0.5455 |
| Basrah H | 0.4502 | 0.7013 | 0.8658 | 0.8312 | 0.8095 | 1 | 0.7576 | 0.5498 | 0.7446 | 0.4719 |
| Kirkuk | 0.6017 | 0.8268 | 0.7879 | 0.7965 | 0.8701 | 0.7576 | 1 | 0.7273 | 0.7835 | 0.5844 |
| Iranian H | 0.7489 | 0.7056 | 0.6104 | 0.6061 | 0.658 | 0.5498 | 0.7273 | 1 | 0.6797 | 0.7619 |
| KEB | 0.5541 | 0.7619 | 0.7359 | 0.697 | 0.7749 | 0.7446 | 0.7835 | 0.6797 | 1 | 0.5671 |
| El Bouri | 0.8398 | 0.5671 | 0.5628 | 0.4632 | 0.5455 | 0.4719 | 0.5844 | 0.7619 | 0.5671 | 1 |
| Kazakh | 0.8961 | 0.5584 | 0.5758 | 0.4545 | 0.5498 | 0.4719 | 0.6061 | 0.7489 | 0.6104 | 0.7965 |
| CPC | 0.7835 | 0.5065 | 0.4242 | 0.3939 | 0.4978 | 0.355 | 0.5238 | 0.6883 | 0.5325 | 0.7532 |
| LSCO | 0.8701 | 0.5195 | 0.4719 | 0.3896 | 0.4978 | 0.3766 | 0.5455 | 0.6883 | 0.5152 | 0.7922 |
| Rhem. | 0.7576 | 0.4286 | 0.3896 | 0.3203 | 0.4286 | 0.3074 | 0.4719 | 0.6061 | 0.4805 | 0.7013 |
| Prinos | 0.5541 | 0.5974 | 0.5455 | 0.6277 | 0.6537 | 0.5974 | 0.6364 | 0.6364 | 0.5931 | 0.5411 |
| Azeri L | 0.7186 | 0.4113 | 0.329 | 0.29 | 0.3896 | 0.2597 | 0.4199 | 0.6277 | 0.4372 | 0.7056 |
| SGC | 0.697 | 0.5368 | 0.5411 | 0.4935 | 0.5931 | 0.4545 | 0.619 | 0.7273 | 0.5455 | 0.71 |
| Oryx | 0.4199 | 0.6883 | 0.7922 | 0.8398 | 0.7706 | 0.8658 | 0.7229 | 0.5152 | 0.684 | 0.4242 |
| Okwuib | 0.6537 | 0.6883 | 0.8268 | 0.658 | 0.7359 | 0.7186 | 0.7273 | 0.6667 | 0.7403 | 0.6494 |
| RasGharib | 0.8355 | 0.6147 | 0.632 | 0.5152 | 0.5974 | 0.5368 | 0.6537 | 0.7273 | 0.645 | 0.8139 |
| Varandey | 0.7403 | 0.4762 | 0.3939 | 0.3463 | 0.4242 | 0.2857 | 0.4632 | 0.6667 | 0.4762 | 0.7446 |
| Arab L | 0.5498 | 0.7446 | 0.8442 | 0.8182 | 0.8442 | 0.8139 | 0.8268 | 0.645 | 0.7619 | 0.5541 |
| Tempa Rossa | 0.3853 | 0.671 | 0.7835 | 0.8182 | 0.7143 | 0.8485 | 0.6667 | 0.4762 | 0.645 | 0.3896 |
| μ | Tempa Rossa | Forties | Kuwait Light | Arabian light | Kumkol | Arabian Heavy | Alban Crude | Ras Gharib |
|---|---|---|---|---|---|---|---|---|
| Tempa Rossa | 1 | 0.8261 | 0.3852 | 0.916 | 0.6025 | 0.9683 | 0.9717 | 0.9076 |
| Forties | 0.8261 | 1 | 0.4168 | 0.7899 | 0.4725 | 0.8255 | 0.805 | 0.7588 |
| Kuwait Light | 0.3852 | 0.4168 | 1 | 0.4401 | 0.5745 | 0.3854 | 0.3908 | 0.3908 |
| Arabian light | 0.916 | 0.7899 | 0.4401 | 1 | 0.656 | 0.923 | 0.9115 | 0.8863 |
| Kumkol | 0.6025 | 0.4725 | 0.5745 | 0.656 | 0.9765 | 0.6221 | 0.6112 | 0.6711 |
| Arabian heavy | 0.9683 | 0.8255 | 0.3854 | 0.923 | 0.6221 | 1 | 0.9507 | 0.9092 |
| Alban crude | 0.9717 | 0.805 | 0.3908 | 0.9115 | 0.6112 | 0.9507 | 1 | 0.8992 |
| Ras Gharib | 0.9076 | 0.7588 | 0.3908 | 0.8863 | 0.6711 | 0.9092 | 0.8992 | 1 |
| Boscan | 0.0034 | 0.1006 | 0.012 | 0.0104 | 0.021 | 0.0157 | 0.0008 | 0.0381 |
| Aseng | 0.2297 | 0.1448 | 0.5605 | 0.3 | 0.5499 | 0.2443 | 0.2389 | 0.2793 |
| El Sharara | 0.4126 | 0.3485 | 0.7759 | 0.4849 | 0.6994 | 0.428 | 0.4202 | 0.4619 |
| Helm C.O. | 0.5165 | 0.4667 | 0.7916 | 0.5874 | 0.6661 | 0.5277 | 0.523 | 0.5759 |
| Arab Medium | 0.9683 | 0.8255 | 0.3966 | 0.9272 | 0.6087 | 0.9636 | 0.9549 | 0.9028 |
| Azeri light | 0.5011 | 0.4322 | 0.7952 | 0.5524 | 0.7375 | 0.4983 | 0.5028 | 0.5095 |
| Basrah Light | 0.9549 | 0.8277 | 0.3905 | 0.9137 | 0.6157 | 0.9588 | 0.9465 | 0.9017 |
| Bozachi | 0.4555 | 0.4583 | 0.8165 | 0.5325 | 0.6249 | 0.4745 | 0.4667 | 0.523 |
| Cheleken | 0.2521 | 0.2686 | 0.7941 | 0.3218 | 0.5899 | 0.2644 | 0.2569 | 0.3112 |
| CPC | 0.1403 | 0.188 | 0.7246 | 0.2084 | 0.4936 | 0.1532 | 0.1437 | 0.2034 |
| El Bouri | 0.8591 | 0.7325 | 0.4675 | 0.8731 | 0.709 | 0.8675 | 0.851 | 0.851 |
| Kazakh | 0.2992 | 0.2123 | 0.6669 | 0.3725 | 0.6555 | 0.3157 | 0.3123 | 0.3745 |
| Kirkuk | 0.9669 | 0.8305 | 0.4 | 0.9258 | 0.6039 | 0.9594 | 0.949 | 0.8947 |
| Kuwait Export Blend | 0.9695 | 0.8322 | 0.3815 | 0.9151 | 0.6031 | 0.963 | 0.9476 | 0.9039 |
| Okwibome | 0.1006 | 0.0933 | 0.6053 | 0.1616 | 0.419 | 0.1014 | 0.1112 | 0.1367 |
| Oryx | 0.9863 | 0.83 | 0.3784 | 0.9216 | 0.6059 | 0.9756 | 0.9664 | 0.9134 |
| Urals | 0.8605 | 0.7387 | 0.4672 | 0.8874 | 0.6762 | 0.8695 | 0.8588 | 0.8454 |
| Rhemoura | 0.9014 | 0.8062 | 0.4297 | 0.8863 | 0.5941 | 0.8936 | 0.898 | 0.8723 |
| Sib. Light | 0.5476 | 0.4585 | 0.7073 | 0.6185 | 0.8216 | 0.5627 | 0.5501 | 0.6123 |
| South Green Canyon | 0.97 | 0.8185 | 0.3672 | 0.9109 | 0.6168 | 0.9737 | 0.9485 | 0.9283 |
| Prinos | 0.9597 | 0.8028 | 0.4064 | 0.9221 | 0.6246 | 0.9443 | 0.9574 | 0.9154 |
| ValD’Agri | 0.5966 | 0.5695 | 0.6919 | 0.6199 | 0.5053 | 0.5835 | 0.5983 | 0.5745 |
| μ | Urals | Arab M | Arab H | Vald’Agri | Basrah L | Basrah H | Kirkuk | Iranian H | KEB | El Bouri |
|---|---|---|---|---|---|---|---|---|---|---|
| Urals | 1 | 0.6324 | 0.498 | 0.5573 | 0.5138 | 0.4585 | 0.5613 | 0.6047 | 0.5494 | 0.4269 |
| Arab M | 0.6324 | 1 | 0.8498 | 0.9091 | 0.834 | 0.7787 | 0.8893 | 0.9407 | 0.9091 | 0.7628 |
| Arab H | 0.498 | 0.8498 | 1 | 0.8854 | 0.834 | 0.8735 | 0.8735 | 0.8617 | 0.917 | 0.8656 |
| Vald’Agri | 0.5573 | 0.9091 | 0.8854 | 1 | 0.8696 | 0.8221 | 0.8933 | 0.9051 | 0.9289 | 0.7826 |
| Basrah L | 0.5138 | 0.834 | 0.834 | 0.8696 | 1 | 0.8972 | 0.9209 | 0.8458 | 0.8538 | 0.7549 |
| Basrah H | 0.4585 | 0.7787 | 0.8735 | 0.8221 | 0.8972 | 1 | 0.8577 | 0.7984 | 0.8379 | 0.7945 |
| Kirkuk | 0.5613 | 0.8893 | 0.8735 | 0.8933 | 0.9209 | 0.8577 | 1 | 0.917 | 0.9091 | 0.7708 |
| Iranian H | 0.6047 | 0.9407 | 0.8617 | 0.9051 | 0.8458 | 0.7984 | 0.917 | 1 | 0.9051 | 0.751 |
| KEB | 0.5494 | 0.9091 | 0.917 | 0.9289 | 0.8538 | 0.8379 | 0.9091 | 0.9051 | 1 | 0.8063 |
| El Bouri | 0.4269 | 0.7628 | 0.8656 | 0.7826 | 0.7549 | 0.7945 | 0.7708 | 0.751 | 0.8063 | 1 |
| Kazakh | 0.6838 | 0.7115 | 0.6245 | 0.6561 | 0.6206 | 0.5534 | 0.664 | 0.7036 | 0.6798 | 0.5455 |
| CPC | 0.8063 | 0.4545 | 0.3439 | 0.3953 | 0.3992 | 0.3597 | 0.4229 | 0.4506 | 0.3874 | 0.3281 |
| LSCO | 0.8142 | 0.7154 | 0.6206 | 0.664 | 0.6285 | 0.5494 | 0.6917 | 0.7115 | 0.664 | 0.5099 |
| Rhem. | 0.7549 | 0.7747 | 0.664 | 0.7154 | 0.6561 | 0.5929 | 0.7273 | 0.7708 | 0.7312 | 0.5613 |
| Prinos | 0.8261 | 0.5692 | 0.4625 | 0.5178 | 0.4783 | 0.4308 | 0.5494 | 0.585 | 0.5178 | 0.4032 |
| Azeri L | 0.6285 | 0.3004 | 0.1818 | 0.2332 | 0.1818 | 0.1265 | 0.2451 | 0.2964 | 0.249 | 0.2055 |
| SGC | 0.6957 | 0.8735 | 0.7945 | 0.8142 | 0.7549 | 0.7233 | 0.834 | 0.8696 | 0.8221 | 0.6838 |
| Oryx | 0.419 | 0.7628 | 0.8972 | 0.8142 | 0.8538 | 0.8933 | 0.8379 | 0.7787 | 0.8379 | 0.7866 |
| Okwuib | 0.5652 | 0.2292 | 0.1107 | 0.1621 | 0.1265 | 0.0791 | 0.166 | 0.2174 | 0.17 | 0.2055 |
| RasGharib | 0.3004 | 0.3202 | 0.4071 | 0.3557 | 0.3913 | 0.4625 | 0.3676 | 0.3241 | 0.3557 | 0.4941 |
| Varandey | 0.6838 | 0.5138 | 0.4032 | 0.4466 | 0.4664 | 0.4506 | 0.498 | 0.4862 | 0.4466 | 0.4506 |
| Arab L | 0.7075 | 0.8617 | 0.7352 | 0.8182 | 0.7352 | 0.6561 | 0.7905 | 0.8419 | 0.7866 | 0.6245 |
| KBT | 0.5652 | 0.8458 | 0.8063 | 0.8577 | 0.8854 | 0.7984 | 0.9249 | 0.8577 | 0.8498 | 0.7036 |
| Tempa Rossa | 0.0158 | 0.2213 | 0.3241 | 0.2727 | 0.3202 | 0.3676 | 0.2885 | 0.2332 | 0.2727 | 0.3281 |
| Sat, wt.% | Aro, wt.% | Resins, wt.% | As, wt.% | SG | Sulphur, wt.% | VR Yield, wt.% | Pour Point, °C | VIS at 40 °C, mm2/s | Slope | Kw | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| min | 23.5 | 7.1 | 0.0 | 0.1 | 0.782 | 0.03 | 1.4 | −45.6 | 0.9 | 2.9 | 11.33 |
| max | 92.9 | 62.1 | 7.7 | 22.2 | 1.002 | 5.64 | 60.7 | 37.8 | 19430.0 | 5.2 | 12.67 |
| μ | Sat | Aro | Resins | As | SG | Sulphur | VR Yield | PP | VIS |
|---|---|---|---|---|---|---|---|---|---|
| Sat | 1 | 0.1687 | 0.1404 | 0.1727 | 0.0677 | 0.1525 | 0.101 | 0.4667 | 0.1283 |
| Aro | 0.1687 | 1 | 0.7535 | 0.7566 | 0.7727 | 0.7707 | 0.7626 | 0.4515 | 0.7646 |
| Resins | 0.1404 | 0.7535 | 1 | 0.8202 | 0.797 | 0.8394 | 0.8212 | 0.4566 | 0.7889 |
| As | 0.1727 | 0.7566 | 0.8202 | 1 | 0.7828 | 0.8273 | 0.8081 | 0.4596 | 0.7475 |
| SG | 0.0677 | 0.7727 | 0.797 | 0.7828 | 1 | 0.7919 | 0.9 | 0.4879 | 0.8626 |
| Sulphur | 0.1525 | 0.7707 | 0.8394 | 0.8273 | 0.7919 | 1 | 0.8242 | 0.404 | 0.7778 |
| VR yield | 0.101 | 0.7626 | 0.8212 | 0.8081 | 0.9 | 0.8242 | 1 | 0.5081 | 0.9091 |
| PP | 0.4667 | 0.4515 | 0.4566 | 0.4596 | 0.4879 | 0.404 | 0.5081 | 1 | 0.5141 |
| VIS | 0.1283 | 0.7646 | 0.7889 | 0.7475 | 0.8626 | 0.7778 | 0.9091 | 0.5141 | 1 |
| υ | Sat | Aro | Resins | As | SG | Sulphur | VR Yield | PP | VIS |
|---|---|---|---|---|---|---|---|---|---|
| Sat | 0 | 0.8273 | 0.8394 | 0.8192 | 0.9202 | 0.8394 | 0.897 | 0.4737 | 0.8677 |
| Aro | 0.8273 | 0 | 0.2263 | 0.2354 | 0.2131 | 0.2212 | 0.2354 | 0.4889 | 0.2313 |
| Resins | 0.8394 | 0.2263 | 0 | 0.1556 | 0.1727 | 0.1364 | 0.1606 | 0.4717 | 0.1909 |
| As | 0.8192 | 0.2354 | 0.1556 | 0 | 0.199 | 0.1606 | 0.1859 | 0.4768 | 0.2444 |
| SG | 0.9202 | 0.2131 | 0.1727 | 0.199 | 0 | 0.1899 | 0.0879 | 0.4424 | 0.1232 |
| Sulphur | 0.8394 | 0.2212 | 0.1364 | 0.1606 | 0.1899 | 0 | 0.1697 | 0.5323 | 0.2141 |
| VR yield | 0.897 | 0.2354 | 0.1606 | 0.1859 | 0.0879 | 0.1697 | 0 | 0.4343 | 0.0889 |
| PP | 0.4737 | 0.4889 | 0.4717 | 0.4768 | 0.4424 | 0.5323 | 0.4343 | 0 | 0.4263 |
| VIS | 0.8677 | 0.2313 | 0.1909 | 0.2444 | 0.1232 | 0.2141 | 0.0889 | 0.4263 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratiev, D.; Dinkov, R.; Tavlieva, M.; Shishkova, I.; Nikolov Palichev, G.; Ribagin, S.; Atanassov, K.; Stratiev, D.D.; Nenov, S.; Pilev, D.; et al. Correlations of HTSD to TBP and Bulk Properties to Saturate Content of a Wide Variety of Crude Oils. Processes 2023, 11, 420. https://doi.org/10.3390/pr11020420
Stratiev D, Dinkov R, Tavlieva M, Shishkova I, Nikolov Palichev G, Ribagin S, Atanassov K, Stratiev DD, Nenov S, Pilev D, et al. Correlations of HTSD to TBP and Bulk Properties to Saturate Content of a Wide Variety of Crude Oils. Processes. 2023; 11(2):420. https://doi.org/10.3390/pr11020420
Chicago/Turabian StyleStratiev, Dicho, Rosen Dinkov, Mariana Tavlieva, Ivelina Shishkova, Georgi Nikolov Palichev, Simeon Ribagin, Krassimir Atanassov, Danail D. Stratiev, Svetoslav Nenov, Dimitar Pilev, and et al. 2023. "Correlations of HTSD to TBP and Bulk Properties to Saturate Content of a Wide Variety of Crude Oils" Processes 11, no. 2: 420. https://doi.org/10.3390/pr11020420
APA StyleStratiev, D., Dinkov, R., Tavlieva, M., Shishkova, I., Nikolov Palichev, G., Ribagin, S., Atanassov, K., Stratiev, D. D., Nenov, S., Pilev, D., Sotirov, S., Sotirova, E., Simeonov, S., & Boyadzhieva, V. (2023). Correlations of HTSD to TBP and Bulk Properties to Saturate Content of a Wide Variety of Crude Oils. Processes, 11(2), 420. https://doi.org/10.3390/pr11020420








