Analysis of Asphaltene Precipitation Models from Solubility and Thermodynamic-Colloidal Theories
Abstract
1. Introduction
2. Description of Models for Asphaltene Precipitation
2.1. Solubility Approach Models
2.1.1. Regular Solution Theory
2.1.2. Cubic Equation-of-State (C-EoS)
2.1.3. Statistical Association Fluid Theory Equation of State (SAFT-EoS)
2.1.4. Cubic Plus Association Equation of State (CPA-EoS)
2.1.5. Scott-Magat Theory
2.1.6. Flory-Huggins Theory
2.2. Colloidal Approach Models
2.2.1. Chemical Potential
2.2.2. Micellization
2.2.3. Reverse Micellization
3. Results and Discussion
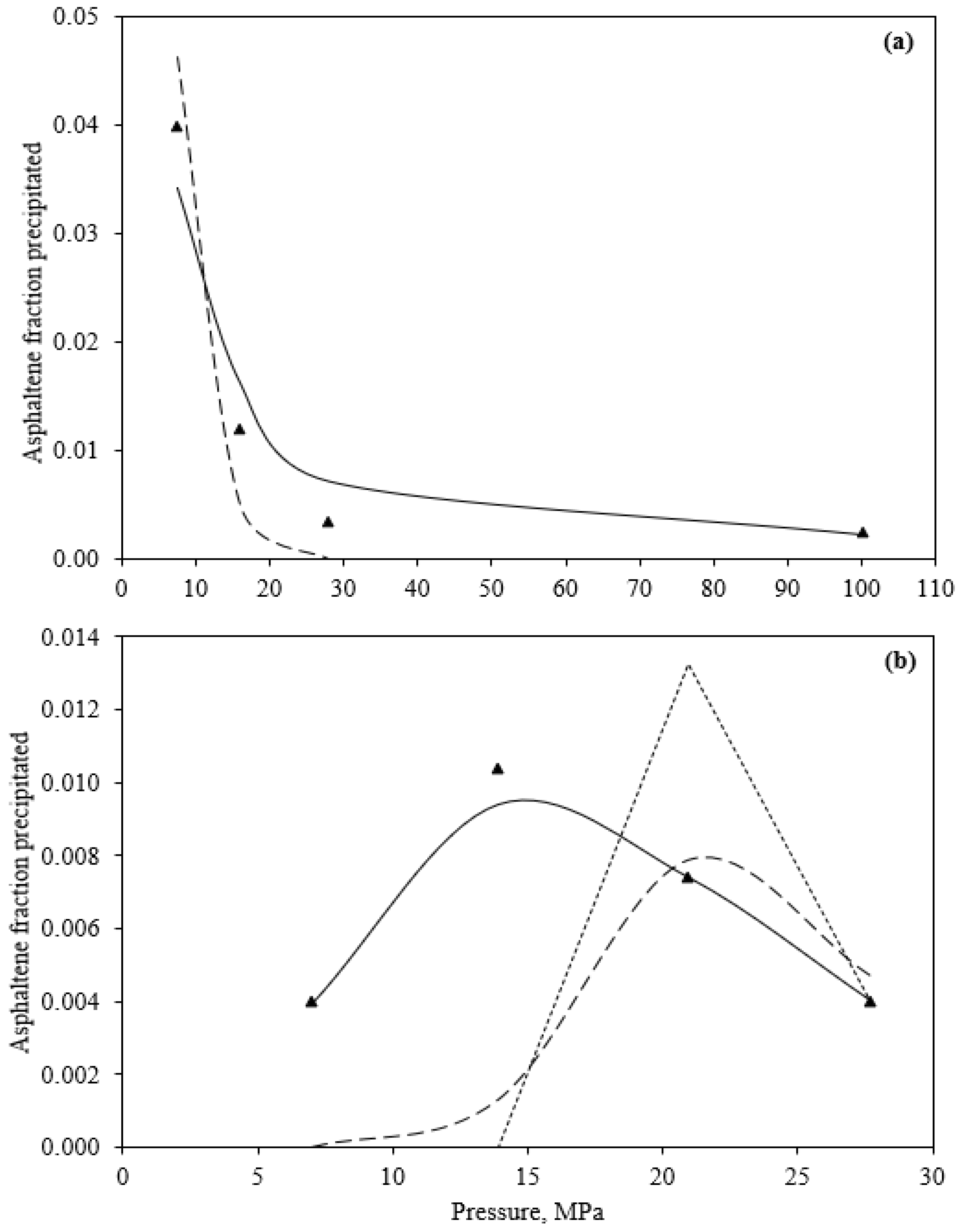
3.1. Experimental Titrations
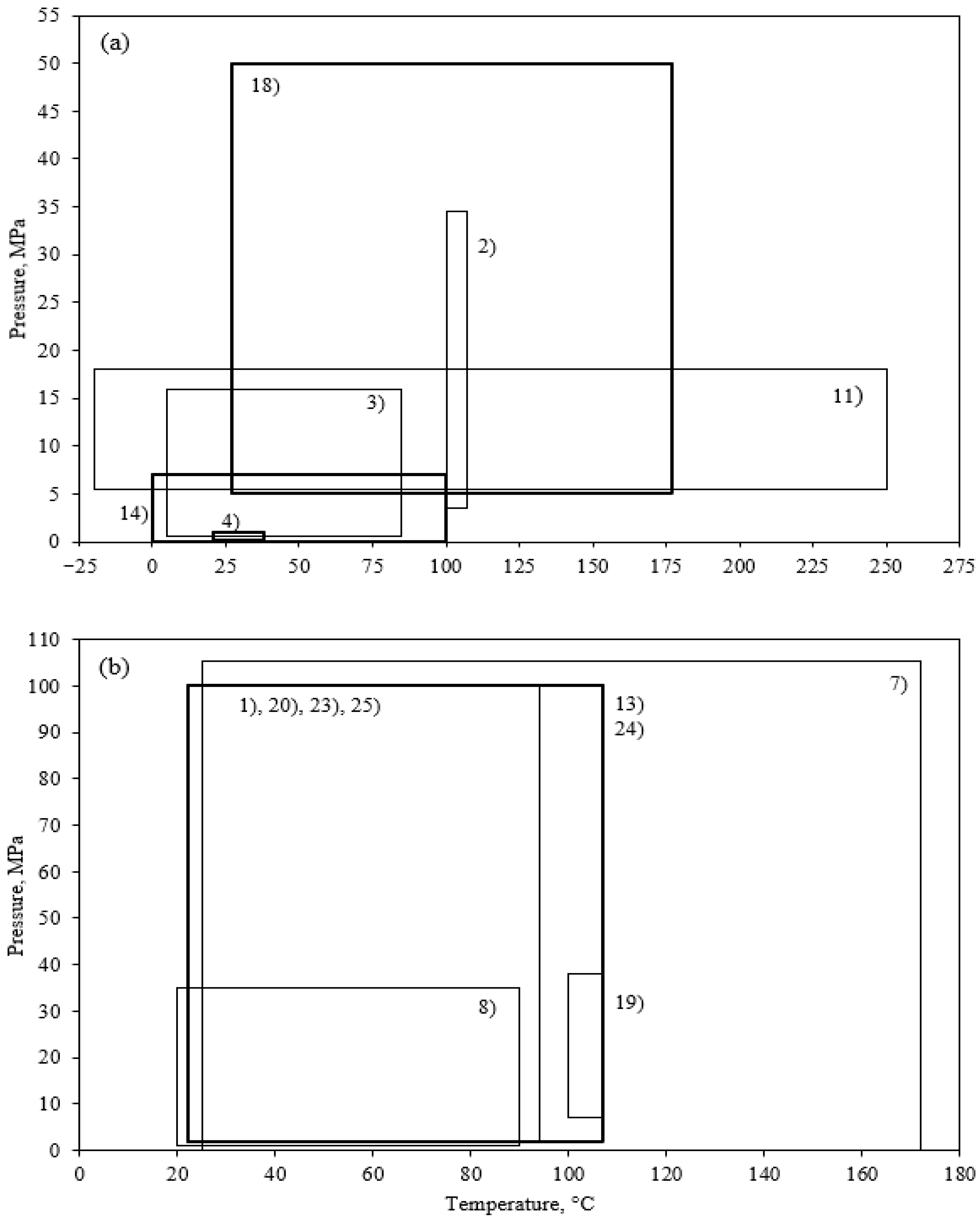
3.2. Pressure and Temperature Conditions for Each Model
3.3. Models Excluded from the Analysis
3.4. Analysis of Models
3.4.1. Effects of Pressure and Temperature
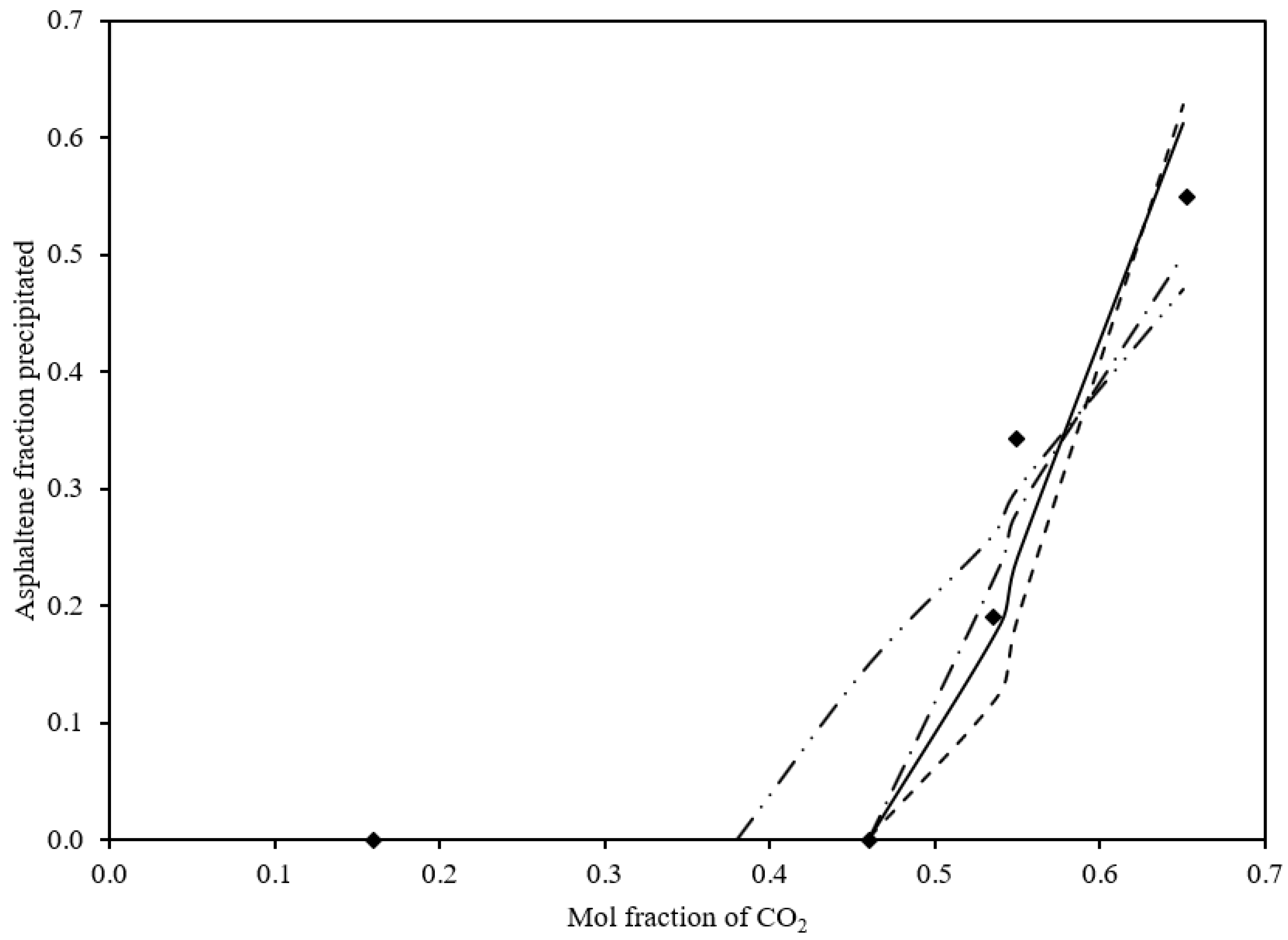
3.4.2. Effects of Injected CO2
3.4.3. Effects of the Addition of n-Alkanes as Solvents
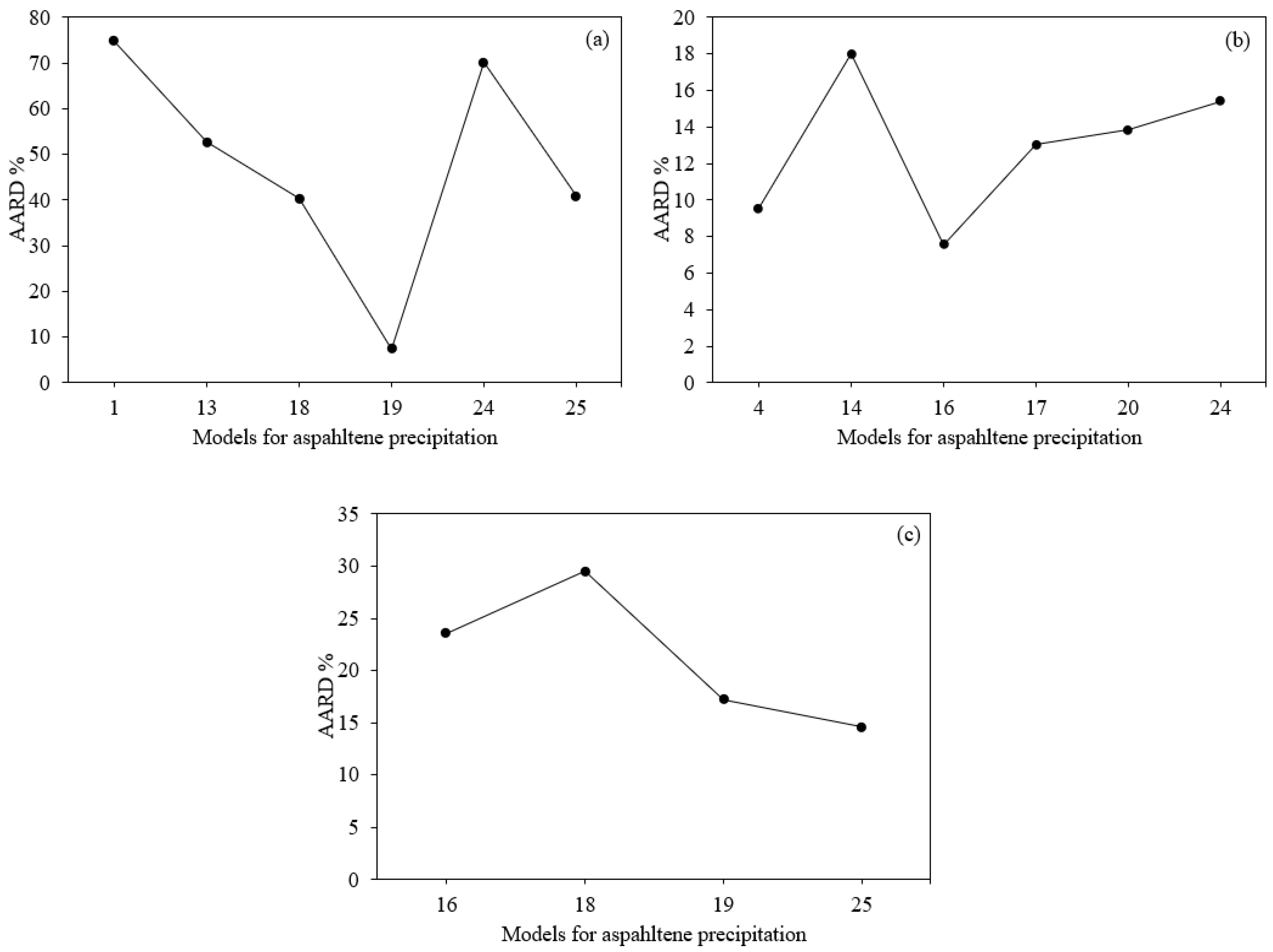
3.5. Statistical Analysis
Statistical Analysis for the Best Model to Calculate Asphaltene Precipitation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez, S.; Ancheyta, J.; Guzmán, R.; Trejo, F. Experimental setups for studying the compatibility of crude oil blends under dynamic conditions. Energy Fuels 2016, 30, 8216–8225. [Google Scholar] [CrossRef]
- Du, J.L.; Zhang, D.A. Thermodynamic model for the prediction of asphaltene precipitation. Pet. Sci. Technol. 2004, 22, 1023–1033. [Google Scholar] [CrossRef]
- Vargas, F.M.; Gonzalez, D.L.; Creek, J.L.; Wang, J.; Buckley, J.; Hirasaki, G.J.; Chapman, W.G. Development of a general method for modeling asphaltene stability. Energy Fuels 2009, 23, 1147–1154. [Google Scholar] [CrossRef]
- Vafaie-Sefti, M.; Mousavi-Dehghani, S.A.; Mohammad-Zadeh, M. A simple model for asphaltene deposition in petroleum mixtures. Fluid Phase Equilibria 2003, 206, 1–11. [Google Scholar] [CrossRef]
- Alimohamadi, S.; Zendehboudi, S.; James, L. A comprehensive review of asphaltene deposition in petroleum reservoirs: Theory, challenges, and tips. Fuel 2019, 252, 753–791. [Google Scholar] [CrossRef]
- Ancheyta, J.; Trejo, F.; Rana, M.S. Asphaltenes: Chemical Transformations during Hydroprocessing of Heavy Oils, 1st ed.; CRC Press, Taylor and Francis Group: Abingdon-on-Thames, UK, 2009. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Moud, A.A. Asphaltene induced changes in rheological properties: A review. Fuel 2022, 316, 123372. [Google Scholar] [CrossRef]
- Guzmán, R.; Ancheyta, J.; Trejo, F.; Rodríguez, S. Methods for determining asphaltene stability in crude oils. Fuel 2017, 188, 530–543. [Google Scholar] [CrossRef]
- Guzmán, R.; Rodríguez, S.; Torres-Mancera, P.; Ancheyta, J. Evaluation of asphaltene stability of a wide range of Mexican crude oils. Energy Fuels 2021, 35, 408–418. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Gnanasundaram, N.; Arunagiri, A.; Murugesan, T. A comprehensive review on the recent advances on the petroleum asphaltene aggregation. J. Pet. Sci. Eng. 2019, 176, 249–268. [Google Scholar] [CrossRef]
- Vargas, F.M.; Tavakkoli, M. Asphaltene Deposition: Fundamentals, Prediction, Prevention, and Remediation, 1st ed.; CRC Press, Taylor and Francis Group: Abingdon-on-Thames, UK, 2018. [Google Scholar] [CrossRef]
- Hirschberg, A.; de Jong, L.N.J.; Schipper, B.A.; Meijer, J.G. Influence of temperature and pressure on asphaltene flocculation. Soc. Pet. Eng. 1984, 24, 283–293. [Google Scholar] [CrossRef]
- Burke, N.E.; Hobbs, R.E.; Kashou, S.F. Measurement and modeling of asphaltene precipitation. JPT J. Pet. Technol. 1990, 42, 1440–1446. [Google Scholar] [CrossRef]
- Novosad, Z.; Costain, T.G. Experimental and modeling studies of asphaltene equilibria for a reservoir under CO2 injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, Gamma, New Orleans, LA, USA, 23–26 September 1990; pp. 599–607. [Google Scholar] [CrossRef]
- Rassamdana, H.; Dabir, B.; Nematy, M.; Farhani, M.; Sahimi, M. Asphalt flocculation and deposition: I. The onset of precipitation. AIChE J. 1996, 42, 10–22. [Google Scholar] [CrossRef]
- Buckley, J.S.; Hirasaki, G.J.; Liu, Y.; Von Drasek, S.; Wang, J.X.; Gill, B.S. Asphaltene precipitation and solvent properties of crude oils. Pet. Sci. Technol. 1998, 16, 251–285. [Google Scholar] [CrossRef]
- Chung, T.H. Thermodynamic modeling for organic solid precipitation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Washington, DC, USA, 4–7 October 1992. [Google Scholar] [CrossRef]
- Cimino, R.; Correra, S.; Sacomani, P.A.; Carniani, C. Thermodynamic modelling for prediction of asphaltene deposition in live oils. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar] [CrossRef]
- De Boer, R.B.; Leerlooyer, K.; Eigner, M.R.P.; van Bergen, A.R.D. Screening of crude oils for asphalt precipitation: Theory, practice, and the selection of inhibitors. SPE Prod. Facil. 1995, 10, 55–61. [Google Scholar] [CrossRef]
- Alboudwarej, H.; Akbarzadeh, K.; Beck, J.; Svrcek, W.Y.; Yarranton, H.W. Regular solution model for asphaltene precipitation from bitumens and solvents. AIChE J. 2010, 49, 2948–2956. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Masliyah, J.H. Molar mass distribution and solubility modeling of asphaltenes. AIChE J. 1996, 42, 3533–3543. [Google Scholar] [CrossRef]
- Thomas, F.B.; Bennion, D.B.; Bennion, D.W.; Hunter, B.E. Experimental and theoretical studies of solids precipitation from reservoir fluid. J. Can. Pet. Technol. 1992, 31. [Google Scholar] [CrossRef]
- Wang, J.X.; Buckley, J.S. An experimental approach to prediction of asphaltene flocculation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 13–16 February 2001; pp. 179–187. [Google Scholar] [CrossRef]
- Wang, J.X.; Buckley, J.S. A two-component solubility model of the onset of asphaltene flocculation in crude oils. Energy Fuels 2001, 15, 1004–1012. [Google Scholar] [CrossRef]
- Nghiem, L.X.; Hassam, M.S.; Nutakki, R.; George, A.E.D. Efficient modelling of asphaltene precipitation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Sigma, Houston, TX, USA, 3–6 October 1993; pp. 375–384. [Google Scholar] [CrossRef]
- Sabbagh, O.; Akbarzadeh, K.; Badamchi-Zadeh, A.; Svrcek, W.Y.; Yarranton, H.W. Applying the PR-EoS to asphaltene precipitation from n-alkane diluted heavy oils and bitumens. Energy Fuels 2006, 20, 625–634. [Google Scholar] [CrossRef]
- Ting, P.D.; Hirasaki, G.J.; Chapman, W.G. Modeling of asphaltene phase behavior with the SAFT equation of state. Pet. Sci. Technol. 2003, 21, 647–661. [Google Scholar] [CrossRef]
- Wu, J.; Prausnitz, J.M.; Prausnitz, J.M. Molecular-thermodynamic framework for asphaltene-oil equilibria. AIChE J. 1998, 44, 1188–1199. [Google Scholar] [CrossRef]
- Wu, J.; Prausnitz, J.M.; Firoozabadi, A. Molecular thermodynamics of asphaltene precipitation in reservoir fluids. AIChE J. 2000, 46, 197–209. [Google Scholar] [CrossRef]
- Buenrostro-Gonzalez, E.; Lira-Galeana, C.; Gil-Villegas, A.; Wu, J. Asphaltene precipitation in crude oils: Theory and experiments. AIChE J. 2004, 50, 2552–2570. [Google Scholar] [CrossRef]
- Li, Z.; Firoozabadi, A. Cubic-plus-association equation of state for asphaltene precipitation in live oils. Energy Fuels 2010, 24, 2956–2963. [Google Scholar] [CrossRef]
- Shirani, B.; Nikazar, M.; Naseri, A.; Mousavi-Dehghani, S.A. Modeling of asphaltene precipitation utilizing Association Equation of State. Fuel 2012, 93, 59–66. [Google Scholar] [CrossRef]
- Kawanaka, S.; Park, S.J.; Mansoori, G.A. Organic deposition from reservoir fluids: A thermodynamic predictive technique. SPE Reserv. Eng. 1991, 6, 185–192. [Google Scholar] [CrossRef]
- Huggins, M.L. Solutions of long chain compounds. J. Chem. Phys. 1941, 9, 440. [Google Scholar] [CrossRef]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Zuo, J.Y.; Mullins, O.C.; Freed, D.; Elshahawi, H.; Dong, C.; Seifert, D.J. Advances in the Flory-Huggins-Zuo equation of state for asphaltene gradients and formation evaluation. Energy Fuels 2013, 27, 1722–1735. [Google Scholar] [CrossRef]
- Zuo, J.Y.; Mullins, O.C.; Dong, C.; Zhang, D. Modeling of Asphaltene Grading in Oil Reservoirs. Nat. Resour. 2010, 1, 19–27. [Google Scholar] [CrossRef]
- Leontaritis, K.J.; Mansoori, G.A. Asphaltene flocculation during oil production and processing: A thermodynamic colloidal model. In Proceedings of the Society of Petroleum Engineers of AIME, San Antonio, TX, USA, 8 February 1987; pp. 149–158. [Google Scholar]
- Victorov, A.I.; Firoozabadi, A. Thermodynamic micellization model of asphaltene precipitation from petroleum fluids. AIChE J. 1996, 42, 1753–1764. [Google Scholar] [CrossRef]
- Pan, H.Q.; Firoozabadi, A. Thermodynamic micellization model for asphaltene precipitation from reservoir crudes at high pressures and temperatures. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar] [CrossRef]
- Pan, H.Q.; Firoozabadi, A. Thermodynamic micellization model for asphaltene precipitation inhibition. AIChE J. 2000, 46, 416–426. [Google Scholar] [CrossRef]
- Szewczyk, V.; Thomas, M.; Behar, E. Prediction of volumetric properties and (multi-) phase behavior of asphaltenic crudes. Rev. Inst. Fr. Pet. 1998, 53, 51–58. [Google Scholar] [CrossRef]
- Szewczyk, V.; Behar, E. Compositional model for predicting asphaltenes flocculation. Fluid Phase Equilibria 1999, 158–160, 459–469. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Huang, S.S.; Dong, M. Asphaltene deposition during CO2 flooding. SPE Prod. Facil. 1999, 14, 235–245. [Google Scholar] [CrossRef]



| Regular Solution Theory | |||
|---|---|---|---|
| Author | Equation | Author | Equation |
| (1) Hirschberg et al. | (6) Chung | ||
| (2) Burke et al. | (7) Cimino et al. | ||
| (3) Novosad and Constain | (8) de Boer et al. | ||
| (4) Rassamdana et al. | (9) Alboudwarej et al. | ||
| (5) Buckley et al. | (10) Yarranton and Masliyah | ||
| (11) Thomas et al. | |||
| (12) Wang and Buckley | |||
| Equation of State (EoS) | |||
|---|---|---|---|
| Author | Equation | Author | Equation |
| (13) Nghiem et al. | (14) Sabbagh et al. | ||
| Statistical Association Fluid Theory (SAFT) | Statistical Association Fluid Theory-Hard Sphere (SAFT-HS) | ||
| Author | Equation | Author | Equation |
| (15) Ting et al. | (16) Wu et al. | ||
| Statistical Association Fluid Theory-Variable Range (SAFT-VR) | |||
| Author | Equation | ||
| (17) Buenrostro et al. | |||
| Cubic Plus Association Equation of State | |||
|---|---|---|---|
| Author | Equation | Author | Equation |
| (18) Li and Firoozabadi | (19) Shirani et al. | ||
| Scott-Magat theory | |||
| Author | Equation | ||
| (20) Kawanaka et al. | |||
| Flory-Huggins theory | |||
| Author | Equation | ||
| (21) Flory-Huggins | |||
| (22) Flory-Huggins-Zuo equation of state (FHZ EoS) | |||
| Chemical Potential | Micellization | ||
|---|---|---|---|
| Author | Equation | Author | Equation |
| (23) Leontaritis and Mansoori | (24) Victorov and Firozoobadi | ||
| Reverse Micellization | |||
| Author | Equation | ||
| (25) Pan and Firoozabadi | |||
| Model | Samples | Solvent | Method | Range of Asphaltene Content (wt%) |
|---|---|---|---|---|
| 1 | Two crude oils (30.54–34.97 °API) | C1, C3, n-C5, n-C7, n-C10, CO2 | IP 143 | 0.60 to 3.90 |
| 2 | Six crude oils (19.0–48.0 °API) | n-C7 | ASTM D893-80 with n-C7 | 0.40 to 16.80 |
| 3 | Crude oil (29.0 °API) | n-C6, n-C10, CO2 | n-C6, n-C10, CO2 precipitations | 5.50 |
| 4 | Light tank crude oil (29.7 °API) | n-C5 to n-C10 | IP 143 and Thin Layer Chromatography | 2.20 |
| 5 | Crude oils (19.0–41.0 °API) | n-C7 | n-C7 precipitation and Refractive Index measurements | 1.20 to 10.90 |
| 6 | From the bottom-hole of a production well in San Andres Unit of Seminole oilfield (TX) | n-C5 | n-C5 precipitation | -- |
| 7 | Villafortuna-Trecate oil (41.2 °API) and crude oil (35.6 °API) | n-C5 | IP 143 | 0.10 to 1.40 |
| 8 | Light crude oils (40° API) | n-C7 | n-C7 precipitation | 0.30 |
| 9 | Athabasca Bitumen, Cold Lake, Lloydminster | n-C5, n-C7, n-C10 | ASTM D2007M | 14.60 to 15.30 |
| 10 | Syncrude coker feed Athabasca bitumen | n-C7 | n-C7 precipitation | 14.50 |
| 11 | Keg river crude oil, Nisku crude oil | C2, C3, n-C4 | C2, C3, and n-C4 precipitations | -- |
| 12 | Mars-Pink crude oil | n-C5 to n-C15 | n-C5, n-C15 precipitations and Refractive Index measurements | 4.40 |
| 13 | Crude oils (19.0–48.0 °API) | C1, C3, n-C5, n-C7, n-C10, CO2 | IP 143, ASTM D893-80 with n-C7 | 1.90 to 7.80 |
| 14 | Athabasca Bitumen, Cold Lake, Lloydminster, Venezuelan 1 and 2, Russian, Indonesian | n-C5, n-C6, n-C7, n-C8 n-C10 | ASTM D2007M | 4.70 to 21.80 |
| 18 | Crude oils (24.6–44.2 °API) | n-C7, CO2 | n-C7, CO2 precipitations | 0.40 to 4.90 |
| 19 | Crude oil (19 °API), Iranian oil field, crude oil (29 °API) | CO2 | CO2 precipitation | |
| 20 | Crude oils (30.54 °API) | n-C5, n-C7, and n-C10 | IP 143 | 4.02 |
| 23 | Two crude oils (30.54–34.97 °API) | C1, C3, n-C5, n-C7, n-C10, CO2 | IP 143 | 0.60 to 3.90 |
| 24 | Crude oils (19.0–48.0 °API) | C1, C3, n-C5, n-C7, n-C10, CO2 | IP 143, ASTM D893-80 with n-C7 | 0.40 to 16.80 |
| 25 | Crude oil (34.97 °API), North Sea reservoir, Weyburn reservoir | C3, CO2 | IP 143 | 0.60 to 8.90 |
| Model | Hirschberg et al. 1 | Nghiemet al. 13 | Li and Firoozabadi 18 | Shirani et al. 19 | Victorov and Firoozabadi 24 | Pan and Firoozabadi 25 |
|---|---|---|---|---|---|---|
| AARD, % | 74.8564 | 52.6946 | 40.3938 | 7.3951 | 70.0516 | 40.8937 |
| (+) Residuals | 4 | 2 | 13 | 8 | 3 | 3 |
| (−) Residuals | 1 | 2 | 4 | 7 | 1 | 5 |
| Highest positive residual | 0.0070 | 0.0091 | 0.3588 | 0.0093 | 0.0104 | 0.0057 |
| Lowest negative residual | −0.0064 | −0.0007 | −0.1058 | −0.0034 | −0.0058 | −0.0044 |
| Range | 0.0134 | 0.0097 | 0.4646 | 0.0127 | 0.0162 | 0.0101 |
| R2 | 0.9817 | 0.0014 | 0.7902 | 0.9857 | 0.0028 | 0.9532 |
| Slope | 1.2743 | 0.0434 | 1.0470 | 0.9714 | 0.1092 | 0.8243 |
| Intercept | −0.0057 | 0.0032 | −0.0522 | 0.0000 | 0.0036 | 0.0025 |
| Model | Wu et al. 16 | Li and Firoozabadi 18 | Shirani et al. 19 | Pan and Firoozabadi 25 |
|---|---|---|---|---|
| AARD, % | 23.5848 | 29.4782 | 17.2113 | 14.5783 |
| (+) Residuals | 2 | 2 | 2 | 2 |
| (−) Residuals | 1 | 1 | 1 | 1 |
| Highest positive residual | 0.0800 | 0.1492 | 0.0622 | 0.1017 |
| Lowest negative residual | −0.0800 | −0.0741 | −0.0463 | −0.0624 |
| Range | 0.1600 | 0.2233 | 0.1085 | 0.1642 |
| R2 | 0.9312 | 0.9036 | 0.9237 | 0.9030 |
| Slope | 0.5799 | 1.3970 | 0.7508 | 1.2222 |
| Intercept | 0.1365 | −0.1863 | 0.0679 | −0.0949 |
| Model | Rassamdana et al. 4 | Sabbagh et al. 14 | Wu et al. 16 | Buenrostro et al. 17 | Kawanaka et al. 20 | Victorov and Firoozabadi 24 |
|---|---|---|---|---|---|---|
| AARD, % | 9.5662 | 18.0057 | 7.5443 | 13.0570 | 13.8824 | 15.4236 |
| (+) Residuals | 15 | 18 | 11 | 11 | 3 | 6 |
| (−) Residuals | 7 | 10 | 9 | 5 | 4 | 1 |
| Highest positive residual | 0.0048 | 0.0371 | 0.1099 | 0.0069 | 0.0014 | 0.0056 |
| Lowest negative residual | −0.0025 | −0.0151 | −0.2413 | −0.0018 | −0.0054 | −0.0005 |
| Range | 0.0073 | 0.0522 | 0.3512 | 0.0086 | 0.0068 | 0.0061 |
| R2 | 0.9728 | 0.8745 | 0.8743 | 0.9307 | 0.3049 | 0.9815 |
| Slope | 1.0417 | 0.8781 | 0.7686 | 1.0427 | 0.3458 | 1.1614 |
| Intercept | −0.0020 | 0.0043 | 0.2098 | −0.0023 | 0.0208 | −0.0072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, E.A.; Lira-Galeana, C.; Ancheyta, J. Analysis of Asphaltene Precipitation Models from Solubility and Thermodynamic-Colloidal Theories. Processes 2023, 11, 765. https://doi.org/10.3390/pr11030765
Hernández EA, Lira-Galeana C, Ancheyta J. Analysis of Asphaltene Precipitation Models from Solubility and Thermodynamic-Colloidal Theories. Processes. 2023; 11(3):765. https://doi.org/10.3390/pr11030765
Chicago/Turabian StyleHernández, Esaú A., Carlos Lira-Galeana, and Jorge Ancheyta. 2023. "Analysis of Asphaltene Precipitation Models from Solubility and Thermodynamic-Colloidal Theories" Processes 11, no. 3: 765. https://doi.org/10.3390/pr11030765
APA StyleHernández, E. A., Lira-Galeana, C., & Ancheyta, J. (2023). Analysis of Asphaltene Precipitation Models from Solubility and Thermodynamic-Colloidal Theories. Processes, 11(3), 765. https://doi.org/10.3390/pr11030765









