Abstract
This review explores eco-friendly methods for extracting bioactive natural products from diverse sources. The introductory exploration emphasizes the increasing demand for sustainable extraction methods, with a focus on the environmental impact of conventional approaches. Addressing existing knowledge gaps, this review outlines the key objectives of evaluating various green extraction technologies, including supercritical fluid extraction, pressurized liquid extraction, ultrasound-assisted extraction, enzyme-assisted extraction, and others. The primary findings underscore the remarkable potential and advancements achieved with green solvents, specifically deep eutectic solvents and bio-based solvents. This review elucidates the synergistic effects achieved by combining different extraction techniques, exemplified by ultrasound-microwave-assisted extraction and sequential supercritical fluid and pressurized liquid extraction, among others. Notwithstanding the promising results, this review emphasizes the importance of acknowledging and addressing challenges such as standardization, selectivity, scalability, and economic viability.
1. Introduction
Natural products (NPs) such as “proteins, dietary fibers, fats and oils, sugars, and antioxidants, etc.” play a pivotal role in daily life, contributing to various aspects of human health, nutrition, and well-being [1]. These compounds, derived from plants, animals, and microorganisms, have been harnessed for centuries for their therapeutic properties and nutritional benefits. The diversity of NPs provides a rich source of bioactive compounds, including antioxidants, antimicrobials, and anti-inflammatory agents, which have been integral to the development of pharmaceuticals and nutraceuticals. Additionally, NPs form the basis of traditional medicine systems across cultures, highlighting their historical significance in healthcare. Incorporating NPs into daily routines, such as consuming fruits, vegetables, and herbal supplements, can positively impact health and contribute to disease prevention [1,2].
The production of NPs involves a diverse array of sources, including plants, microorganisms, and animals. Plants are a primary reservoir, yielding an extensive variety of phytochemicals with medicinal and nutritional value [3]. Microorganisms, such as bacteria and fungi, contribute significantly to the production of antibiotics and enzymes [4,5]. Animal-derived NPs, such as collagen and certain peptides, find applications in cosmetics and pharmaceuticals [6]. However, the sustainable production of NPs faces several challenges. Overharvesting wild plant and animal populations poses a threat to biodiversity, and habitat destruction further exacerbates this issue [7]. Additionally, climate change can impact the availability and composition of NPs, affecting their yield and quality. Balancing the demand for NPs with conservation efforts and implementing sustainable harvesting practices are critical for ensuring the long-term availability of these valuable resources [8,9].
The exploration of alternative sources in the production of NPs aligns with sustainability goals, and an increasingly noteworthy aspect of this trend involves the harnessing of waste products. Amidst the challenges posed by climate change and the overexploitation of traditional sources, waste streams, including food waste and agricultural by-products, emerge as valuable reservoirs for bioactive compounds [1]. Repurposing organic waste not only mitigates environmental concerns related to disposal but also aligns with the principles of the circular economy. Food waste, comprising discarded fruit peels, seeds, and vegetable scraps, has become a focus for extracting antioxidants, dietary fibers, and natural colorants, transforming what was once considered waste into valuable resources [10,11]. Similarly, agricultural residues, such as corn husks and sugarcane bagasse, are being repurposed for the extraction of biofuels, enzymes, and other high-value compounds, illustrating the potential of waste-derived products in contributing to a sustainable natural product supply chain [12,13].
Global waste production, exceeding 2 billion metric tons annually according to the World Bank, includes a significant portion of biowastes (approximately 44% [14]), particularly from the food and beverage sectors. The vast quantities of discarded organic materials, such as fruit peels, vegetable scraps, and expired products, represent a substantial resource for sustainable NPs production [15]. The extraction of NPs from waste streams involves a multifaceted process wherein bioactive compounds are isolated from organic materials, such as food waste and agricultural residues. Conventional extraction techniques, such as solid–liquid extraction and organic solvent-based methods, have historically been used for this purpose [16,17]. While effective in obtaining target compounds, these methods pose challenges related to the use of large quantities of solvents, energy-intensive processes, and the generation of potentially hazardous waste. Additionally, the reliance on conventional techniques may hinder the attainment of sustainability goals in NP production. Recognizing these challenges, the field has shifted toward sustainable and green extraction practices. Recent and emerging approaches, including “green solvent-, supercritical fluid-, subcritical water-, ultrasonic-, and enzyme-assisted- extraction, etc.”, minimize environmental impact by reducing solvent usage and energy consumption [1,3,18,19]. While these green technologies show promise, challenges persist in optimizing their efficiency, ensuring cost-effectiveness, and implementing a holistic life cycle approach to waste management. Overcoming these challenges is critical for the widespread adoption of green extraction practices in the quest for sustainable and eco-friendly NP production [18,19,20].
In light of the evolving landscape of green extraction, this review paper aims to provide a comprehensive overview of current methodologies, challenges, and advancements in sustainable NP extraction. The objective is to critically analyze the effectiveness of green extraction techniques and highlight potential solutions to existing challenges. By synthesizing the current literature, this review seeks to contribute to the understanding of green extraction’s role in sustainable NP production and inspire further research directions in this crucial area.
2. Bioactive Natural Products and Sources
Several investigations have delved into estimating a diverse array of bioactive NPs within bio-waste streams [21,22,23]. It is worth mentioning that the foodstuffs with the highest rates of spoilage include “cereals, roots, tubers, fruits, and vegetables”. “Citrus, watermelons, bananas, apples, grapes, tomatoes, onions, cucumbers, cabbages, carrots, and potatoes dominate fruit and vegetable category” [24]. The bioactive NPs that have been isolated consist of a variety of substances, such as “pigments, fatty acids, volatiles, anthocyanins, vitamins (A and E), minerals, and tannins” [21]. The animal industry’s waste products, which include meat and components derived from fish, are excellent resources for bioactive peptides and proteins [25]. The dairy industry, especially cheese production, stands out as another valuable source [26]. Alternatives for the large volumes of produced wastes include biomass utilization as a source of energy or for feeding animals. Nevertheless, there are additional beneficial substances that can be extracted from these wastes and used as food additives or supplements, such as “fibers, pigments, sugars, flavors, phytochemicals, organic acids, enzymes, and antimicrobial compounds” [27].
Similarly, growing and processing a wide variety of crops also results in massive amounts of waste for the agricultural sector. Significant remnants are produced by various crops and fruits, including “rice, corn, soybeans, sugarcane, potatoes, tomatoes, cucumbers, oranges, grapes, and apples” [23]. These residues are rich in bioactive NPs and have the potential to become essential building blocks for the production of beneficial phytochemicals. Beneficial phytochemicals found in the by-products of processing vegetables and fruits have many uses, such as protecting the “cardiovascular system, fighting inflammation, cancer, and microbes” [28,29,30]. These residues and their bioactive NPs have been investigated in many studies for use in the manufacturing of functional foods and cosmetics [31,32,33]. Additionally, the rising concern about potential health risks associated with synthetic antioxidants like BHA has fueled interest in identifying and isolating natural antioxidants from agricultural waste, adding further allure to this sustainable pursuit [29]. Figure 1 encapsulates a concise overview of potential classes of bioactive NPs derived from agro-food and agricultural waste streams [21,30,34], serving as a quick reference for the extensive range of valuable compounds that can be harnessed from these sources.
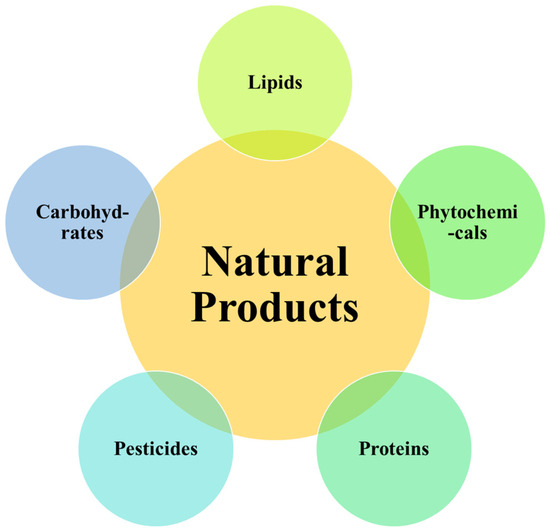
Figure 1.
Bioactive NP classification of food and agriculture wastes.
These abovementioned studies collectively affirm the substantial presence of bioactive compounds, opening avenues for commercialization. In addition, they highlight the bioactive NPs’ multipurpose uses in the chemical, food, and pharmaceutical sectors, which help reduce environmental impacts and advance sustainability initiatives [35,36]. The literature has extensively explored numerous conventional methods for extracting NPs, including “Maceration, Digestion, Infusion, Percolation, Decoction, and Soxhlet extraction” [37,38,39].
To summarize, “maceration is a simple extraction method, entails soaking coarse or powdered plant material in a solvent of choice for at least three days at room temperature, stirring occasionally, in order to extract valuable compounds. Digestive processes, similar to maceration, use gentle heating to facilitate extraction. In contrast, infusion involves soaking plant material in a boiling solvent, usually water, for about 15 min, and then filtering out the marc to get an extract. This method works for phytochemicals that aren’t impacted by rising temperatures. A decoction typically involves steeping plant materials in water for thirty to sixty minutes. Soxhlet extraction is a continuous method, employing hot solvent for the extraction of phytochemicals” [37,39]. Traditional extraction methods come with inherent limitations, including low yields and the substantial use of solvents, posing environmental risks. High-temperature extraction procedures in traditional methods may cause the degradation of heat-sensitive compounds, leading to the loss of valuable elements [40,41]. Moreover, these conventional techniques may struggle to effectively separate compounds of similar chemical properties, resulting in impure extracts. The extensive use of expensive solvents exacerbates environmental concerns, presenting a significant obstacle [42]. Overcoming these challenges necessitates the adoption of alternative extraction methods that are not only more efficient but also aligned with sustainability goals and environmentally friendly practices
3. Green Extraction Techniques
Green extraction techniques represent a paradigm shift in the field of NP extraction, emphasizing sustainability, environmental consciousness, and efficiency. These methods prioritize minimizing the environmental impact associated with traditional extraction processes. Their importance lies in addressing the limitations of conventional techniques, such as low yields, substantial solvent usage, and the risk of degrading heat-sensitive compounds. The benefits of green extraction include enhanced efficiency, reduced solvent consumption, and the preservation of valuable bioactive compounds. Examples of green extraction techniques encompass “supercritical fluid extraction (SFE), subcritical water extraction (SWE), ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE)”, among others. The significance and advantages of each method contribute to a more sustainable and eco-friendly approach to NP extraction. Detailed discussions on each green extraction technique are provided below, highlighting their unique principles, applications, and advantages in obtaining bioactive natural products.
3.1. Supercritical Fluid Extraction (SFE)
SFE is notable for being an eco-friendly method that is used to extract NPs [43]. This technique utilizes supercritical fluids, which are substances that operate at temperatures and pressures above their critical points, to selectively extract desired compounds of interest from the initial material. Notably, carbon dioxide (CO2) takes the forefront as the most widely used supercritical fluid in SFE [44]. SFE offers distinct advantages compared to traditional extraction methods, because it uses non-flammable and non-toxic solvents, guaranteeing both safety and environmental friendliness. Since SFE is very selective, it can preserve the purity of NPs during extraction by allowing for lower temperatures [45]. Additionally, SFE minimizes residue production during extraction, a notable improvement over the conventional method. The recyclability of supercritical fluids further reduces residue, contributing to a reduced environmental footprint in the NP industry [20].
As illustrated in Figure 2, the SFE system is made up of an extraction vessel, heat exchangers, pressure release valve, pump, and extract recovery, among other necessary parts [46]. In the initial stage of the extraction process, waste material is introduced into the extraction vessel. A pressure release valve and temperature controllers are attached to the extraction vessel to ensure that the conditions are always optimal. Usually, the main vessel is filled with CO2 that is pumped under specific conditions (Pc = 5.7 MPa and Tc < 5 °C). Heat exchangers cool the CO2 at both the inlet and exit of the extraction vessel. Following this, a combination of pressure and temperature is applied, decreasing the solvation properties of the used SFE fluid. The desired bioactive NPs dissolve in the fluid, and separation occurs in the extract separator, facilitated by an outlet valve [47]. This SFE process persists until the highest recovery rates of the desired bioactive NPs are achieved from the waste material sample.
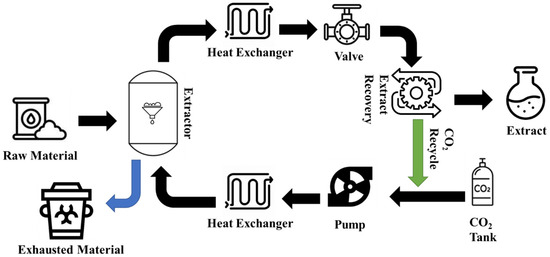
Figure 2.
The schematic flow of the supercritical fluid extraction system.
The efficacy of supercritical CO2 (scCO2) in extraction is particularly pronounced for nonpolar phytochemicals due to its robust solvation capabilities. Polarized phytochemicals, on the other hand, are problematic because of their poor solubility in scCO2. The addition of small quantities of co-solvents, such as “ethyl alcohol, water, methanol, ethyl acetate, acetone, or acetonitrile”, is effective in passing this limitation. This adjustment enhances the solubility of polar phytochemicals, leading to an increased yield of phytochemicals in the extract. An impressive 30% increase in the extraction yield of primary cannabinoids from industrial hemp by-products was observed when ethanol was added to scCO2 as a co-solvent [48]. Furthermore, the phenolic and flavonoid yields were significantly increased, and the antioxidant capacity was significantly enhanced with ethanol as a co-solvent [49]. Studies have also highlighted the positive correlation between ethanol-modified scCO2 and the recovery yield of extracts, along with their antioxidant activity [50]. To further improve scCO2 extraction, a co-solvent consisting of ethanol and water was also used [51]. Table 1 is summarizing a comprehensive list of recent studies on bioactive NP extraction using SFE methods. The table includes information on operating conditions, target compounds, and other relevant details.

Table 1.
Recent studies on bioactive NPs extraction with the SFE technique.
In contrast to traditional extraction methods, SFE boasts several distinct advantages. Firstly, it consistently yields higher quantities of extracts while maintaining their biological activities, outperforming conventional techniques like Soxhlet [55]. For example, SFE not only extracts more oils from apple seeds compared with Soxhlet but also produces extracts with superior oxidative stability. Additionally, SFE is characterized by its simplicity, rapid operation, and exceptional efficiency, resulting in impressive yields. Secondly, the dissolution characteristics of the supercritical fluid can be easily enhanced by adjusting the pressure to a particular temperature. Thirdly, SFE stands out as a non-flammable and non-explosive green extraction technique, ensuring minimal environmental impact. Lastly, adding trace quantities of entrainers to SFE can enhance the polar phytochemicals’ solubility, altering the polarity of the extraction medium. In addition, the cost-effective recyclability of the extraction medium further underscores the economic and environmental benefits of SFE [3].
3.2. Subcritical Water Extraction (SWE)
The term “subcritical water” describes heated water that is subjected to a pressure high enough to keep it in a liquid state at a critical pressure, between the boiling point of 100 °C and the critical point of 374 °C [65]. Its diffusivity properties improve with increasing temperature and its “surface tension, viscosity, and dielectric constant” all decrease. It is interesting to note that at 250 °C and 2.5 MPa, the dielectric constant of water drops to 25, which is similar to organic solvents such as methanol (ε = 33) and ethanol (ε = 24) at 25 °C. In this state, water dissolves a wide range of compounds with medium to low polarity, similar to organic solvents [66,67,68].
SWE, also known as “Pressurized Hot Water Extraction (PHWE)”, is a novel method for extracting bioactive NPs from matrices that include food, agricultural products, and by-products. The utilization of heated water as the solvent for extraction is the defining characteristic of this technique [69,70]. The SWE procedure is carried out in an extractor chamber that is pre-loaded with the sample matrices and a certain quantity of inert material, most often sand, to avoid sample aggregation. The four sequential steps of the extraction mechanism are depicted in the schematic flow diagram of SWE (Figure 3). The first step is to pressurize and heat the sample matrix to a high temperature in order to desorb the solute at different active sites. Extracts are diffused into the matrix in the second step. Step three relies on the sample matrix; in this stage, solutes may separate from the matrix and dissolve in the extraction solvent. Filling the extraction cell with the sample solution and eluting it is the last step [71].
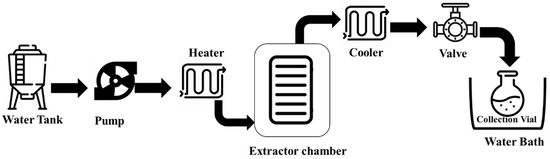
Figure 3.
The schematic flow of the subcritical water extraction system.
SWE stands out as a versatile method due to water’s unique ability to act as a universal solvent, accommodating both polar and nonpolar phytochemicals. Leveraging its capability to dissolve both types of phytochemicals, SWE was applied to extract steviol glycosides and antioxidants (both polar and nonpolar) from Bertoni leaves. Higher yields were obtained from this extraction when carried out at a higher temperature of 160 °C as opposed to the lower temperature of 100 °C [72]. Moreover, SWE makes it easier to selectively extract phytochemicals with particular biological activities. For example, its ability to selectively extract phenolic compounds from kānuka leaves with a high antioxidant capacity is particularly noteworthy, as it outperforms conventional ethanol extraction [73]. Table 2 summarizes a comprehensive list of recent studies on bioactive NP extraction using SWE methods. The table includes information on operating conditions, target compounds, and other relevant details.

Table 2.
Recent studies on bioactive NPs extraction with the SWE technique.
Water, when used in SWE, has demonstrated superiority in recovering phytochemicals compared with other solvents. A number of studies have compared SWE to other extraction methods, such as maceration, Soxhlet, reflux extraction, and others, to determine its extraction efficiency and activities. These works, as referenced in [88,89,90,91], consistently demonstrate that SWE is superior to other extraction techniques or, at the very least, comparable to them, when only one solvent is used. Water was determined to be the most effective solvent for subsequent extractions after a study compared its efficiency to that of “acetone, n-hexane, 96% ethanol, ethyl acetate, and methylene chloride”. The SWE that used water had the highest levels of polyphenolics, flavonoids, and antioxidant activity [90]. Table 2 shows that pressure has less of an impact on extraction efficiency than temperature and time. The optimal extraction temperature for flavonoids falls within the range of 150 to 200 °C, with an extraction time between 10 and 50 min.
On the other hand, polyphenols, which are also referred to as polyhydroxyphenols, constitute a structural class predominantly found in nature, characterized by the presence of multiple phenolic units and the absence of nitrogen-based functions. These compounds have the remarkable ability to resist oxidation, and are commonly found in a wide variety of foods, including herbs, nuts, tea, algae, leaves, fruits, and vegetables [92]. The majority of experiments have consistently demonstrated the superiority of SWE over maceration, Soxhlet, and others [93,94]. On the other hand, a recent study offered an opposing viewpoint, arguing that pomegranate peel is better valued at SWE’s lower temperatures. According to the study’s findings, MAE is more effective than SWE in this situation for phenolic extraction from pomegranate peel, especially when trying to produce an extract devoid of 5-hydroxymethylfurfural [94].
Furthermore, organic acids (e.g., fatty acids) in NPs are ubiquitous in plant tissues, including stems, roots, and fruits. All these acids are water- or ethanol-soluble and manifest acidic properties, yet they pose challenges in dissolving in other organic solvents. The use of SWE was investigated for the extraction of organic acids from diverse matrices, such as ferulic acid, gallic acid, and chlorogenic acid. Nevertheless, it unavoidably coextracted additional active ingredients, such as “proteins and phenolics [95], flavonoids [96], lipids, peptides, amino acids”, and other organic compounds. Recent research on sweet and sour cherry stems used SWE to extract sugars, organic acids, alcohols, and other organic compounds. Remarkably, the two samples’ chemical compositions were discovered to be similar [97].
In general, SWE offers several advantages over steam distillation, providing superior products in less time and with lower energy consumption. Water’s unique properties, such as its eco-friendliness, universal solvent capabilities, suitability for recovering thermally labile phytocompounds, and widespread availability, make it the preferred extractant in SWE.
3.3. Ultrasound-Assisted Extraction (UAE)
UAE is commonly performed with an ultrasonic probe or bath, both of which, as illustrated in Figure 4, depend on a piezoelectric transducer to provide ultrasonic power. The solid matrix is suspended in a solvent in an ultrasonic bath apparatus, which consists of a stainless steel tank linked to the transducer. The ultrasonic probe, in contrast, is a horn or probe attached to a transducer that, when submerged in the extraction vessel, transmits ultrasound waves into the medium with little energy loss. Because of the greater ultrasonic intensity at the probe tip, probe-based systems are often considered to be a more effective tool for bioactive NP extraction than bath systems [98,99].
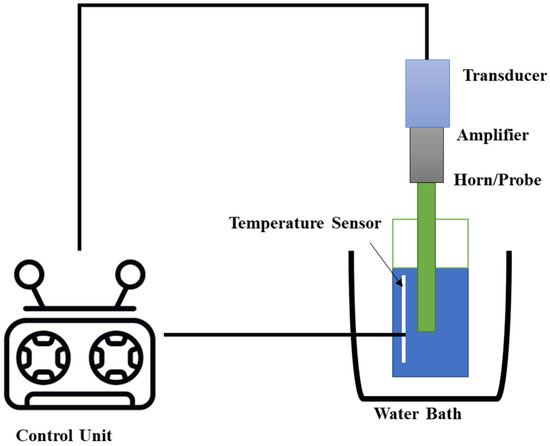
Figure 4.
The ultrasound-assisted extraction (UAE) technique setup or system.
UAE uses solvent extraction and ultrasonic waves to extract desired chemicals from a wide range of materials. “Acidified water, alcohols, ethanol, acetone, and water” are some of the solvents that have been used to extract various compounds during UAE [100]. Ultrasound, with frequencies higher than what humans can hear (>20 kHz), consists of cycles of expansions and contractions of molecules, effectively extracting them from a source material [101]. Increased solvent-to-waste sample contact area and cell wall permeability are both mechanical effects of ultrasonic cavitation [3].
The number of bioactive NPs produced with UAE is greatly affected by the pretreatment of food scraps. Blanching, drying, and milling are some of the pretreatment processes, that raw materials go through when trying to extract pectin and polysaccharides from fruit and vegetable by-products. Blanching is a process that eliminates enzymes by quickly cooling the by-products in an ice bath after being immersed in hot water for 3 to 5 min at temperatures between 80 °C and 100 °C [102]. The drying process involves placing the sample in a hot air oven set at a temperature range of 45 °C to 60 °C for 24 to 72 h until a constant weight is achieved. Then, the dried peels are ground into particles smaller than 0.25 mm in diameter using an electric grinder [103,104].
UAE has emerged as a versatile and efficient method for extracting bioactive NPs from various sources. This technique has been widely investigated for the extraction of compounds such as pectin [105], polyphenols [106], oils [107], and many others from fruits, vegetables, and agriculture residues. Recent studies have extensively tested UAE for its efficacy in extracting bioactive NPs, and a summary of these findings is compiled in Table 3, providing a comprehensive overview of the diverse compounds successfully extracted using this extraction approach.

Table 3.
Recent studies on bioactive NPs extraction with the UAE technique.
UAE stands out as a pivotal technique for extracting bioactive compounds, showcasing its broad applicability. Noteworthy achievements include the successful extraction of phenolic compounds from strawberries [117], yielding favorable results. Additionally, this technique has been used to extract phenolic derivatives and anthocyanines from grape peels, as documented in another study [118]. In contrast to the 120 min needed for the maceration method, which produced lower quantities, a comparative study showed that UAE significantly reduced extraction time to 30 min, highlighting its efficiency. In addition, UAE is great at increasing the rate of extraction while using a smaller amount of solvent [119,120]. Consequently, UAE emerges as a preferred and efficient extraction method.
However, recent studies have noted that the frequency used in UAE can actively modify and positively impact the extraction of chemicals from a material [121]. Intriguingly, a study found that phenolic yields were higher at 40 kHz compared with 120 kHz [122]. This observation emphasizes the need for a thorough exploration of ultrasonic parameters, encouraging researchers to simultaneously evaluate and optimize these factors to enhance extraction efficiency. The dynamic interplay between frequency and extraction outcomes underscores the importance of meticulous parameter adjustment for effective and tailored UAE processes.
3.4. Microwave-Assisted Extraction (MAE)
MAE is a modern technique for NPs extraction that combines the use of microwaves with solvents. It is extremely useful for enhancing extraction kinetics by heating the solvent and material at frequencies between 300 MHz and 300 GHz. This heating process is achieved with the direct impact of microwaves on polar molecules, leading to energy conversion through dipolar rotations. MAE is a new extraction method that uses microwave energy to quickly and efficiently remove bioactive NPs from a variety of sources (see Figure 5 for a schematic diagram). The heating in MAE is directly correlated with the dielectric constant of the solvents [123].
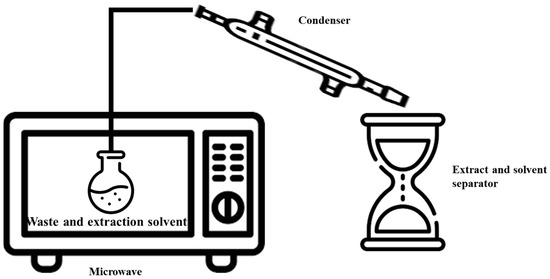
Figure 5.
Microwave-assisted extraction system.
The MAE process unfolds through a series of systematic steps to ensure the efficient extraction of phytochemicals from diverse sources. Commencing with the generation of electromagnetic (EM) waves, this process effectively segregates targeted compounds from the waste matrix (e.g., peel) by creating heightened pressure and temperature conditions. Afterward, bioactive cellular components in the waste matrix (sample) interact with the waves, which transport photon energy and cause the bound moisture in the waste to be heated. The resultant cellular-level pressure buildup causes rapid cellular swelling and rupture, which allows the chosen solvent to diffuse into the waste matrix. In the end, targeted phytochemical solutes are leached from the waste matrix using this orchestrated sequence [39].
The extraction process in MAE encompasses the diffusion of solvents into the sample, followed by the separation of the solute from the functional site and, ultimately, the release of solutes into the solvents. This technique excels in preserving the biological activities of the extracts. An illustrative instance is the improvement in microwave-assisted extraction (MAE) for extracting green tea, demonstrating its ability to boost the antioxidant activity of phytochemicals. Additionally, this optimization has shown gains in the extracts’ intended color quality and total phenolic content. The efficacy of MAE extends beyond extraction, contributing to an enhancement in the key properties in the obtained extracts [124].
The recent studies on the extraction of bioactive NPs using MAE are comprehensively summarized in Table 4, presenting essential information on various aspects of the extraction process.

Table 4.
Recent studies on bioactive NPs extraction with the MAE technique.
The MAE technique has proven to be versatile in obtaining a range of phytochemicals, including saponins, polyphenols, sterols, and flavonoids, from a variety of plant sources [137,138,139]. The direct impact of microwaves on these compounds contributes to the effectiveness of the extraction process. This highlights the adaptability of MAE in acquiring diverse phytochemicals across different plant sources [140].
Furthermore, operating parameters such as microwave power, extraction time, and temperature play crucial roles in influencing the extraction efficiency in MAE. An example that demonstrates this point is the successful carotenoids extracted from carrot waste. The results showed that the extraction time and microwave power played a significant role in this process. The optimization of these parameters becomes imperative for achieving desired outcomes in MAE [141]. It has also been observed that the high dielectric constant of polar solvents, such as water or ethanol, can increase the heating of the solvent–sample mixture in MAE. Therefore, it is recommended to utilize solvents with lower dielectric constants when extracting thermolabile compounds that require relatively lower temperatures. One way to keep thermolabile phytochemicals intact during extraction is to immerse plant material in a solvent that is transparent to microwaves, like n-hexane [142]. This strategic solvent selection contributes to the efficient and targeted extraction of bioactive compounds.
Compared to conventional solvent extraction methods, MAE offers numerous advantages over traditional solvent extraction techniques, such as being rapid and cost-effective, requiring less solvent, and exhibiting a higher extraction rate. However, certain limitations exist, primarily related to its suitability for relatively smaller phenolic molecules such as quercetins and isoflavones, which demonstrate stability within the microwave temperature range. It is noteworthy that MAE is most beneficial for capturing phytochemicals that are substantially lost using conventional methods. For instance, in food processing, conventional methods result in significant flavonoid loss; hence, MAE proves invaluable for extracting flavonoids intended as food additives for supplementation [143].
3.5. Pressurized Liquid Extraction (PLE)
PLE is a fast extraction method that achieves targeted compound extraction by directly interacting liquid solvent with waste matrix particles (e.g., plants) under subcritical temperature and high pressure. The effectiveness of this extraction process is intricately tied to three key aspects: “the nature of the matrix, mass transfer, and solubility”. Important factors in the extraction efficiency are the matrix’s properties, the compound that needs to be extracted, and the distribution of the compound within the matrix [144]. High temperatures are essential for improving the mass transfer and solubility characteristics between the extraction solvent and the plant matrix. This improvement in temperature leads to more efficient extraction kinetics, ultimately contributing to enhanced extraction processes [145].
The solvent is circulated through the extraction cell or column in the PLE process, usually with the help of an HPLC (high-performance liquid chromatography) pump (see in Figure 6) to efficiently remove the bioactive NPs of interest from the waste matrix. This process maintains constant pressure and temperature initially to initiate static extraction, stabilizing the system and facilitating solvent diffusion through the plant matrix. Subsequently, dynamic extraction begins with the maintenance of the required pressure and solvent flow rate.
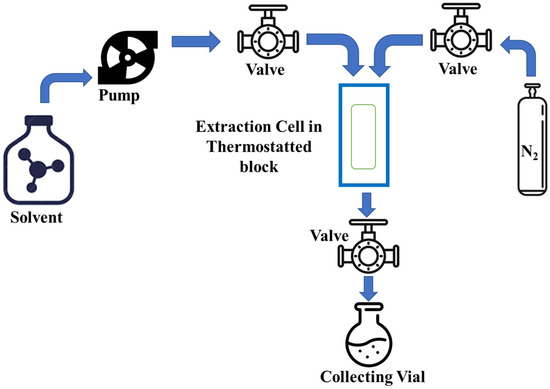
Figure 6.
The pressurized liquid extraction system.
Recent years have seen a number of studies that have used PLE to extract bioactive NPs from various agricultural by-products or agricultural products derived from a wide variety of food matrices, such as cereals, microalgae, fruits, herbs, spices, and vegetables. Table 5 offers a comprehensive compilation of recent studies focusing on the extraction of bioactive NPs using PLE, highlighting the diversity of sources and the bioactive compounds obtained with these advanced extraction techniques.

Table 5.
Recent studies on bioactive NPs extraction with the PLE technique.
PLE is a new, environmentally friendly way to extract valuable compounds from raw materials. Because it makes use of high pressure and temperature, PLE has a high extraction efficiency and is therefore widely used for a variety of NPs, including medicinal plants and fruits. Notably, PLE’s versatility extends to conducting extractions at lower temperatures, protecting target compounds’ bioactivity and stability. This technique is not only highly efficient but also environmentally friendly, generating less waste compared with conventional extraction methods, thereby positioning itself as a sustainable and preferable alternative.
The primary bioactive components of Centella asiatica, triterpenoids, have been successfully extracted using PLE. These triterpenoids have anti-inflammatory and neuroprotective characteristics, which means they could be used to treat inflammatory and neurological disorders [20]. Additionally, Melastoma malabathricum, a medicinal plant known for its traditional use in treating inflammatory and diabetic conditions, has had its bioactive compounds extracted using PLE [154]. This showcases the versatility of PLE in accessing bioactive constituents with therapeutic potential from diverse botanical sources.
Nevertheless, despite its advantages, PLE does encounter some constraints. The substantial cost of equipment, especially for small-scale producers [155], and high energy consumption [156] stand out as a notable limitation. Furthermore, the elevated pressure and temperature integral to the PLE process may impact the stability and quality of specific compounds, leading to degradation and potential loss of bioactivity [157]. These challenges highlight the need for a balanced consideration of factors when opting for PLE in various extraction scenarios.
3.6. Enzyme-Assisted Extraction (EAE)
In certain plant matrices, the extraction of phytochemicals is challenged by the intricate network of polysaccharides and lignin, secured through hydrogen bonding and hydrophobic interactions like van der Waals forces. These phytochemicals, enmeshed in the cell cytoplasm, pose accessibility issues with conventional solvent extraction methods. However, a breakthrough is achieved by pretreating the plant material with specific enzymes [158], releasing bound phytochemicals at high yields. These enzymes, including cellulase, pectinase, and amylase, are introduced during extraction and break down cellular walls and hydrolyze carbohydrates like cellulose and lipid bodies. Two types of extraction methods that make use of enzymes are known as “enzyme-assisted aqueous extraction (EAAE) and enzyme-assisted cold pressing (EACP)”. The former is primarily applied for extracting oils from diverse seeds, while the latter effectively hydrolyzes the cellular wall of plant seeds [159]. The latest research findings on bioactive NPs using EAE are compiled and organized in Table 6.

Table 6.
Recent studies on bioactive NPs extraction with the EAE technique.
EAE is used in tandem with other extraction methods to render non-extractable phytochemicals accessible to solvents, thereby enhancing their susceptibility to extraction. An example highlighting the effectiveness involves the integration of enzymes into microwave processing. This synergistic approach substantially improved the phenolic compound extraction yield, surpassing the limited recovery yields achieved using conventional solvent extraction with water due to the application of higher extraction temperatures and a rapid heating strategy [164]. Furthermore, when compared with alternative extraction methods that did not incorporate enzymes, the pectin yields from different wastes sequentially with enzymes and then administering ultrasound were significantly higher [165]. However, the selection of enzymes poses a challenge and depends on various parameters such as the plant matrix, target compounds, and desired extraction conditions
4. Recent Trends and Developments in Green Extraction
Recent trends in green extraction methods signify a paradigm shift in the field of NP extraction, emphasizing sustainable practices and environmental consciousness. The recent trends in green extraction techniques involve the combination of different extraction techniques and the use of environmentally friendly green solvents, steering away from conventional approaches that often use large quantities of solvents with adverse environmental impacts.
4.1. Combination of Extraction Techniques
With the rising focus on eco-friendly practices, different sectors aim to reduce production costs by enhancing efficiency or increasing yields. While there is no flawless extraction process solely based on technology, a harmonious balance can be achieved among solvent consumption, production costs, and product quality. The combination of different extraction techniques in a single process can yield superior outcomes.
4.1.1. Ultrasound-Microwave-Assisted Extraction (UMAE)
UMAE, also known as “ultrasonic-microwave synergistic extraction (UMSE)”, is an innovative method that synergizes ultrasound and microwaves, incorporating the vibrational–cavitation mechanism of ultrasound and the high energy of microwaves [166]. UMAE provides several advantages, including rapid sample preparation, accelerated extraction processes, cost-effectiveness, short extraction times, and high extraction yields [167,168]. Ultrasound facilitates solvent movement into the sample, enhances solubility, and improves mass transfer by disrupting cell membranes. Simultaneously, microwaves rapidly elevate the sample temperature, increasing solubility, mass transfer, and the desorption of targeted molecules from the matrix, resulting in improved extraction efficiency [169]. One study examined the impact of ultrasonic pretreatment on microwave extraction for extracting pectin from grapefruit peel. The results demonstrated that the pectin yield using the UMAE technique (31.88%) surpassed conventional (19.26%), MAE (27.81%), and UAE (17.92%) methods. Additionally, the qualitative properties of the pectin obtained with UMAE were superior to those obtained with ultrasound, microwave, and traditional methods [170].
4.1.2. Microwave-Assisted Enzymatic Extraction (MAEE)
EAE and MAE techniques are emerging as promising approaches for phytochemical compound extraction. These methods offer advantages such as enhanced extraction efficiency, simplified handling, reduced energy consumption, and minimized solvent usage. In MAEE, the synergy of microwave treatment and enzymolysis effectively breaks down cell walls, improving cell wall permeability. This, in turn, facilitates the efficient transfer of desired molecules from targeted cells into the solvent [171]. In the process of obtaining grapefruit peel soluble dietary fiber, a combined MAEE approach, involving the synergy of microwave and cellulase enzyme (at a ratio of 3000 µg/g), was used. The dietary fiber’s structural and functional properties were both improved with this method. Specifically, the dietary fiber showed enhanced cholesterol, water, oil, and nitrite ion binding capabilities. These advancements highlight the significant potential of MAEE for the extraction of functional dietary fiber from grape peel, with promising applications in the food industry [172].
4.1.3. Ultrasound-Assisted Enzymatic Extraction (UAEE)
To date, researchers have explored the combination of EAE and UAE methods, commonly known as UAEE, for the extraction of phytochemicals [173]. UAEE is considered a synergistic approach that combines two extraction techniques to enhance overall efficiency. UAEE has demonstrated the accelerated extraction of phytochemicals from various plant tissues, as reported by several researchers. Its use has expanded to include the extraction of bioactive NPs from a variety of plant materials, such as wheat bran, fruits, peels, and leaves [174,175,176]. UAEE was proven to enhance extract yields in these studies. For example, a recent study used UAEE to extract a water-soluble polysaccharide from the peel of dragon fruit. The results indicated significantly higher recovery when ultrasound and enzymes were applied simultaneously or sequentially compared with EAE or UAE alone [177].
4.1.4. Supercritical Fluid Extraction and Pressurized Fluid Extraction (SFE-PLE)
The application of innovative extraction strategies, specifically SFE and PLE, holds significant promise when used in an extraction configuration that is sequential or in situ. A notable advancement in this regard is the development of home-built equipment in 2014 that is capable of performing both SFE and PLE. A significant improvement in extraction yield was achieved by using this apparatus to sequentially extract curcuminoids from Curcuma longa L. rhizomes. Moreover, this extraction was completed in half the time or 2.5 times faster compared with traditional methods. The study highlights the cost-effectiveness and practicality of this approach for SFE and PLE [178].
4.1.5. Supercritical Fluid Extraction Assisted with Ultrasound (SFE–UAE)
A recent study reported the effectiveness of an integrated approach to extract capsaicinoids and phenolic compounds from Capsicum frutescens L. In this study, the extraction process involved both ultrasound and supercritical fluid. A study was conducted to compare SFE with and without ultrasonic irradiation, focusing at the global yield and SFE kinetics of the extraction. Compared with SFE alone, the extraction yield of SFE-UAE increased by 35% due to the marked improvement in kinetics brought about by ultrasound treatment. The enhanced permeability was attributed to increased matrix permeability induced with ultrasound irradiation, along with the acoustic streaming and mechanical vibration effects [179].
4.1.6. Ultrasonic Assisted Extraction and Pressurized Liquid Extraction (UAE-PLE)
To extract phenolic compounds from pomegranate peels, ultrasound and PLE were used in a synergistic approach, resulting in improved extraction efficiency. The utilization of ultrasonic enhanced extraction in conjunction with these two techniques made it easier to extract particles larger than a certain size range. Notably, the efficient use of UAE-PLE combined with the shortened extraction period allowed water to be used as the extraction solvent, which increased the extraction yield [180].
In summary, the application of innovative and combined technologies enhances extractability, leading to higher extraction rates, reduced impurities in the final extract, preservation of thermo-sensitive compounds, utilization of various inorganic solvents, and overall lower energy consumption [181].
4.2. Green Extraction Solvents
There has been a notable shift in the extraction processes field toward the use of green solvents in an effort to pursue environmentally friendly and sustainable practices. Green extraction solvents are characterized by their minimal environmental impact, low toxicity, and potential for efficient extraction of bioactive NPs. These solvents aim to replace traditional, often harmful, extraction agents with alternatives that align with the principles of sustainability. Among the notable green extraction solvents are supercritical fluids, deep eutectic solvents, and biobased solvents. Supercritical solvents, such as supercritical CO2 (already discussed in Section 3.1), offer unique properties at specific temperature and pressure conditions. Deep eutectic solvents (DESs), composed of natural components, provide a sustainable alternative. Biobased solvents, derived from renewable resources, further contribute to the eco-friendly landscape of extraction processes [182].
4.2.1. Deep Eutectic Solvents (DESs)
DESs are typically created by combining two solid components capable of interacting through hydrogen bonding [183]. The standard process for producing a DES involves precisely mixing specific amounts of salt and a Hydrogen Bond Donor (HBD) in a moisture-free environment. The mixture is then heated to the desired temperature until the solid components liquefy, resulting in the formation of a homogeneous liquid [184]. Choline chloride (ChCl) stands out as the favored salt for crafting DESs, particularly in combination with carboxylic acids, glycerol, or urea as the Hydrogen Bond Donor (HBD) [185]. The beneficial properties of ChCl, including its biodegradability, cost-effectiveness, non-toxicity, and widespread availability, make it a popular choice for DES formulations [182,183].
DESs have proven to be more effective than traditional solvents because of their remarkable qualities and adaptable interactions with bioactive analytes, such as “hydrogen bonding, electrostatic forces, and π-π interactions” [186,187]. Bioactive molecules are more easily soluble and transferred in large quantities when exposed to DES because of their increased efficacy in disrupting the organized structures of biomass. As an example, DES-assisted MAE with ChCl/glycerol (1:2) and 20% water was able to extract phenolic compounds from mulberry leaves at a higher yield (0.83 wt.%) than both ethanol-based MAE (0.78 wt.%) and methanol-based MAE (0.71 wt.%) [187]. A review of current research on the use of DES for bioactive NP extraction is presented in Table 7.
4.2.2. Bio-Based Solvents
Bio-based solvents, derived from renewable sources such as plant biomass, are commonly produced with methods like the fermentation of vegetable oils and carbohydrates and the steam distillation of wood [182,188]. Various crops, agroforestry, agricultural residues, and waste materials like sugar cane bagasse, wheat straw, corn cubs, and timbers serve as feedstock for bio-derived solvents. Notable features of these solvents include low toxicity, renewability, and biodegradability [182,189]. Bio-based solvents, such as “2-methyl tetrahydrofuran (2-MeTFH), glycerol, ethanol, γ-valerolactone (GVL), p-cymene, and D-limonene”, are commonly used in various applications. These solvents are sourced from renewable materials like cellulose, hemicellulose, lignin, and starch, highlighting their sustainable and eco-friendly nature [182].
Recent studies have tested bio-based solvents in the extraction of bioactive NPs from various sources, revealing notable improvements in the extraction process. To illustrate this point, a recent study showed that by combining bio-based solvents, especially GVL, with other extraction methods, phenolic compounds from kiwifruit by-products could be effectively recovered. This approach showed promise in extracting phenolic compounds, and the results demonstrated the synergy between bio-based solvents and alternative extraction methods [190]. The other studies on bio-based solvent usage in NP extraction have been summarized in Table 7.

Table 7.
Recent studies on bioactive NP extraction with green extraction solvents.
Table 7.
Recent studies on bioactive NP extraction with green extraction solvents.
| Feedstock | Green Extraction | Final Products and Classifications | Remarks | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Source | Type | Technique | Solvent | Target Bioactive NPs | Class | Yield (%, w/w) | ||
| Tea | Seeds | Solid Liquid Extraction (SLE) | ChCl-Gly | Phenolic compounds | Phytochemicals | 0.01 | 93.2% higher than those extracted with methanol/water | [191] |
| Morus alba L. | Leaves | UAE | ChCl-Ca | Phenolic compounds | Phytochemicals | 2.26 | 48.20% higher than those extracted with conventional extraction | [192] |
| Allium cepa L. | Onion peel | MAE | ChCl:U | Phenolic compounds | Phytochemicals | 22.29 | Similar results with the methanol extraction solvent | [193] |
| Grape | Pomace | UMAE | ChCl-Ca | Anthocyanins | Phytochemicals | 0.177 | --- | [194] |
| Actinidia deliciosa | Fruit peel | MAE | GVL | Phenolic compounds | Phytochemicals | 2.97 | The extraction yield followed by GVL:ethanol > acetone > ethanol:water. | [190] |
| Aqueous matrices | -- | Liquid -Liquid Extraction (LLE) | 2-MeTFH | Phenolic compounds | Phytochemicals | 100 | ---- | [195] |
The information summarized in Table 7 underscores the impactful role of DESs and bio-based solvents in the extraction of bioactive NPs. The results showcase a substantial increase in extraction yields, ranging from 48% to 98%, when compared with conventional extraction solvents. This notable enhancement highlights the potential of DES and bio-based solvents as effective alternatives, contributing significantly to the field of green extraction techniques for NPs [196]. While these solvents exhibit promising results, it is crucial to acknowledge that all green extraction techniques and methods, including DES and bio-based solvents, are not without limitations and challenges. These limitations are thoughtfully detailed below, emphasizing the need for continued research and refinement in the quest for sustainable and efficient extraction processes.
5. Challenges and Future Perspectives
Green extraction techniques for bioactive NPs present numerous advantages, but they are not exempt from challenges and limitations. Some of the key challenges include:
- Standardization and reproducibility: Achieving consistent and reproducible results across different studies and laboratories remains a challenge. The standardization of extraction protocols is essential to ensure the reliability and comparability of results.
- Selectivity: Green extraction techniques may not always provide sufficient selectivity, leading to the co-extraction of unwanted compounds. Enhancing the selectivity of these techniques for specific bioactive NPs is an ongoing challenge.
- Optimization: There is a need for further optimization of extraction parameters, including temperature, pressure, time, and solvent composition. Fine-tuning these parameters is essential for maximizing yield and maintaining the integrity of bioactive NPs.
- Scalability: While these techniques show promise at the laboratory scale, translating them to larger industrial scales may pose challenges. Scaling up without compromising efficiency and sustainability is a critical consideration.
- Solvent compatibility: The compatibility of green solvents with specific bioactive compounds needs careful assessment. Some bio-based and deep eutectic solvents may not be suitable for the extraction of certain classes of NPs.
- Economic viability: The cost-effectiveness of green extraction methods compared with traditional techniques is a significant consideration. Developing economically viable and sustainable processes is crucial for widespread adoption.
- Understanding the mechanisms: A deeper understanding of the mechanisms involved in green extraction processes is needed. This includes elucidating the interactions between solvents and bioactive NPs to optimize extraction efficiency.
- Waste management: Addressing the issue of waste generated during the extraction process is vital. Ensuring that the by-products or waste are environmentally friendly and can be appropriately managed is essential for the overall sustainability of the process.
Future Research
Despite these challenges, the future of green extraction techniques for bioactive NPs is promising. Continued research efforts should focus on overcoming these limitations with innovative approaches, advanced technologies, and interdisciplinary collaborations. Integrating artificial intelligence and machine learning for process optimization, exploring new green solvents, and developing modular and versatile extraction systems are potential avenues for future advancements in the field. Additionally, fostering dialogue between researchers, industry stakeholders, and policymakers will contribute to the successful integration of green extraction techniques into diverse applications, ensuring sustainable and eco-friendly practices in the extraction of bioactive NPs.
6. Conclusions
In conclusion, this comprehensive review highlights the significant advancements and potential of green extraction techniques for obtaining bioactive NPs from various natural sources. The exploration of methods such as SFE, SWE, PLE, UAE, MAE, and EAE, among others, underscores the evolving landscape of sustainable and eco-friendly approaches in the field of nanotechnology.
The use of green solvents, including DESs and bio-based solvents, has emerged as a key component in enhancing the sustainability of extraction processes. These solvents, with their low toxicity, renewability, and biodegradability, contribute to the environmentally conscious ethos of green extraction. Additionally, the combination of different techniques, such as UMAE, MAEE, UAEE, and sequential SFE-PLE, demonstrates the potential for synergistic effects and improved extraction efficiencies.
While the results presented in various studies showcase the promise of green extraction techniques, it is crucial to acknowledge the existing challenges and limitations. Standardization, selectivity, optimization, scalability, solvent compatibility, economic viability, and waste management are critical areas that demand ongoing attention and research efforts. Overcoming these challenges will be instrumental in realizing the full potential of green extraction for bioactive NPs.
Looking forward, future research directions should focus on refining extraction protocols, developing novel green solvents, and advancing the understanding of the underlying mechanisms governing these techniques. The integration of cutting-edge technologies, such as artificial intelligence and machine learning, holds promise for optimizing extraction parameters and enhancing reproducibility.
Author Contributions
Conceptualization, M.U.; methodology, M.U.; validation, M.U.; writing—original draft preparation, M.U.; writing—review and editing, S.C. and M.N. All authors have read and agreed to the published version of this manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors gratefully acknowledge the Japanese Government (Ministry of Education, Culture, Sports, Science, and Technology (Monbukagakusho): MEXT) Scholarship for providing financial support and the Tokyo Institute of Technology (Tokyo Tech) for funding and providing research facilities. The first author also thanks the Cross Lab at the Tokyo Institute of Technology for supporting this research and helping to refine their writing skills.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, Y.H.; Verpoorte, R. Green Solvents for the Extraction of Bioactive Compounds from Natural Products Using Ionic Liquids and Deep Eutectic Solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bitwell, C.; Sen, S.I.; Chimuka, L.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- MartíN, J.F.; García-Estrada, C.; Liras, P. Insights into the Molecular Mechanisms of β-Lactam Antibiotic Synthesizing and Modifying Enzymes in Fungi. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–228. [Google Scholar]
- Chen, Z.-H.; Guo, Y. Recent Advances on Marine Mollusk-Derived Natural Products: Chemistry, Chemical Ecology and Therapeutical Potential. Nat. Prod. Rep. 2023, 40, 509–556. [Google Scholar] [CrossRef] [PubMed]
- CITES. Convention on International Trade in Endangered Species of Wild Fauna and Flora. 2019. Available online: https://www.cites.org/ (accessed on 12 October 2023).
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.V.; Bock, N.A.; Busta, L.; Pearce, M.; Roston, R.; Shomo, Z.D.; Terrell, C.R. Introducing Climate Change into the Biochemistry and Molecular Biology Curriculum. Biochem. Mol. Biol. Educ. 2020, 49, 167–188. [Google Scholar] [CrossRef]
- Liu, Z.; De Souza, T.S.P.; Holland, B.J.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Valorization of Food Waste to Produce Value-Added Products Based on Its Bioactive Compounds. Processes 2023, 11, 840. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of Agro-Waste into Value-Added Bioproducts and Bioactive Compounds: Micro/Nano Formulations and Application in the Agri-Food-Pharma Sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef]
- Rasool, K.; Hussain, S.; Shahzad, A.; Miran, W.; Mahmoud, K.A.; Ali, N.; Almomani, F. Comprehensive Insights into Sustainable Conversion of Agricultural and Food Waste into Microbial Protein for Animal Feed Production. Rev. Environ. Sci. Bio/Technol. 2023, 22, 527–562. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- World Bank. Trends in Solid Waste Management. Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 12 October 2023).
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Bhawani, S.A.; Khan, A.; Ahmad, F.B. Extraction of Natural Products from Agro-Industrial Wastes: A Green and Sustainable Approach; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Chémat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjević, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Chémat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Yustisia, Y.; Heryanto, B.; Asmaliyah, A.; Miswarti, M.; Rizkiyah, D.N.; Yunus, M.A.C.; Irianto, I.; Qomariyah, L.; Rohman, G.A.N. Advancements and Challenges in Green Extraction Techniques for Indonesian Natural Products: A Review. S. Afr. J. Chem. Eng. 2023, 46, 88–98. [Google Scholar] [CrossRef]
- Othman, S.B.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Deo, S.K. A Review on Potential of Bioactive Compounds Obtained from Processing Waste of Various Fruits and Vegetables. Int. J. Pure Appl. Biosci. 2018, 6, 680–686. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, A.; Shevkani, K.; Singh, N. Composition, Bioactive Compounds and Antioxidant Activity of Common Indian Fruits and Vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Food and Agriculture Organization of the United States. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 November 2023).
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of Sheep Cheese Whey as a Source of Bioactive Peptides with Antioxidant and Angiotensin-Converting Enzyme Inhibitory Activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Fleuri, L.F. Orange and Mango By-Products: Agro-Industrial Waste as Source of Bioactive Compounds and Botanical versus Commercial Description—A Review. Food Rev. Int. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Gołębiewska, E.; Kalinowska, M. Agricultural Residues as a Source of Bioactive Substances—Waste Management with the Idea of Circular Economy. Environ. Sci. Proc. 2021, 9, 2. [Google Scholar] [CrossRef]
- Oleszek, M.; Kowalska, I.; Bertuzzi, T.; Oleszek, W. Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications. Molecules 2023, 28, 342. [Google Scholar] [CrossRef] [PubMed]
- Casas-Godoy, L.; Campos-Valdez, A.R.; Alcázar-Valle, M.; Barrera-Martínez, I. Comparison of Extraction Techniques for the Recovery of Sugars, Antioxidant and Antimicrobial Compounds from Agro-Industrial Wastes. Sustainability 2022, 14, 5956. [Google Scholar] [CrossRef]
- Ngwasiri, P.N.; Ambindei, W.A.; Adanmengwi, V.A.; Ngwi, P.; Mah, A.T.; Ngangmou, N.T.; Fonmboh, D.J.; Ngwabie, N.M.; Ngassoum, M.B.; Richard, E. A Review Paper on Agro-Food Waste and Food by-Product Valorization into Value Added Products for Application in the Food Industry: Opportunities and Challenges for Cameroon Bioeconomy. Asian J. Biotechnol. Bioresour. Techonol. 2022, 8, 32–61. [Google Scholar] [CrossRef]
- Rodrigues, F.; Nunes, M.A.; Alves, R.C.; Oliveira, M.B.P. Applications of recovered bioactive compounds in cosmetics and other products. In Handbook of Coffee Processing By-Products; Academic Press: London, UK, 2017; pp. 195–220. [Google Scholar]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.-H.; Al-Sayed, H.M.A.; Yasin, N.M.N.; Afifi, E. Effect of Different Extraction Methods on Stablity of Anthocyanins Extracted from Red Onion Peels (Allium cepa) and Its Uses as Food Colorants. Egypt. J. Nutr. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1000196. [Google Scholar] [CrossRef]
- Tambun, R.; Alexander, V.; Ginting, Y. Performance Comparison of Maceration Method, Soxhletation Method, and Microwave-Assisted Extraction in Extracting Active Compounds from Soursop Leaves (Annona muricata): A Review. IOP Conf. Ser. 2021, 1122, 012095. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent Advances in Extraction Technologies for Recovery of Bioactive Compounds Derived from Fruit and Vegetable Waste Peels: A Review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Zuleta, M.; Osorio-Tobón, J.F. Techno-Economic Evaluation of the Extraction of Anthocyanins from Purple Yam (Dioscorea alata) Using Ultrasound-Assisted Extraction and Conventional Extraction Processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Phong, W.N.; Gibberd, M.; Payne, A.D.; Dykes, G.A.; Coorey, R. Methods Used for Extraction of Plant Volatiles Have Potential to Preserve Truffle Aroma: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1677–1701. [Google Scholar] [CrossRef] [PubMed]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.; Yu, K.; Mahlia, T.M.I. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Easmin, S.; Sarker, Z.I.; Ferdosh, S.; Shamsudin, S.H.; Yunus, K.; Uddin, A.; Sarker, M.R.; Jahurul, M.H.A.; Hossain, S.; Khalil, H.A. Bioactive Compounds and Advanced Processing Technology: Phaleria macrocarpa (Sheff.) Boerl, a Review. J. Chem. Technol. Biotechnol. 2014, 90, 981–991. [Google Scholar] [CrossRef]
- Aziz, A.H.A.; Idrus, N.F.M.; Putra, N.R.; Awang, M.A.; Idham, Z.; Mamat, H.; Yunus, M.A.C. Solubility of Rosmarinic Acid in Supercritical Carbon Dioxide Extraction from Orthosiphon stamineus Leaves. ChemEngineering 2022, 6, 59. [Google Scholar] [CrossRef]
- Argun, M.E.; Argun, M.Ş.; Arslan, F.N.; Nas, B.; Ateş, H.; Tongur, S.; Çakmakcı, Ö. Recovery of Valuable Compounds from Orange Processing Wastes Using Supercritical Carbon Dioxide Extraction. J. Clean. Prod. 2022, 375, 134169. [Google Scholar] [CrossRef]
- King, J.W. Modern Supercritical Fluid Technology for Food Applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Khan, M.S.; Mohamed, A.A.A.; Ferdosh, S.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Vági, E.; Balázs, M.; Komóczi, A.; Mihalovits, M.; Székely, E. Fractionation of Phytocannabinoids from Industrial Hemp Residues with High-Pressure Technologies. J. Supercrit. Fluids 2020, 164, 104898. [Google Scholar] [CrossRef]
- Goyeneche, R.; Fanovich, A.; Rodrígues, C.R.; Nicolao, M.C.; Di Scala, K. Supercritical CO2 Extraction of Bioactive Compounds from Radish Leaves: Yield, Antioxidant Capacity and Cytotoxicity. J. Supercrit. Fluids 2018, 135, 78–83. [Google Scholar] [CrossRef]
- Uquiche, E.; Campos, C.J.R.; Marillán, C. Assessment of the Bioactive Capacity of Extracts from Leptocarpha Rivularis Stalks Using Ethanol-Modified Supercritical CO2. J. Supercrit. Fluids 2019, 147, 1–8. [Google Scholar] [CrossRef]
- Hassim, N.; Markom, M.; Rosli, M.I.; Harun, S. Scale-up Approach for Supercritical Fluid Extraction with Ethanol–Water Modified Carbon Dioxide on Phyllanthus Niruri for Safe Enriched Herbal Extracts. Sci. Rep. 2021, 11, 15818. [Google Scholar] [CrossRef] [PubMed]
- Nuapia, Y.; Al-Hamimi, S.; Chimuka, L.; Turner, C. Ultrahigh-Pressure Supercritical Fluid Extraction and Chromatography of Moringa oleifera and Moringa peregrina Seed Lipids. Anal. Bioanal. Chem. 2019, 411, 3685–3693. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Q.; Qiao, G.; Qiu, Z.; Zhuang, W.; Wen, X. Optimizing the Supercritical Carbon Dioxide Extraction of Sweet Cherry (Prunus avium L.) Leaves and UPLC-MS/MS Analysis. Anal. Methods 2020, 12, 3004–3013. [Google Scholar] [CrossRef] [PubMed]
- De Andrade Lima, M.; Andreou, R.; Charalampopoulos, D.; Chatzifragkou, A. Supercritical Carbon Dioxide Extraction of Phenolic Compounds from Potato (Solanum tuberosum) Peels. Appl. Sci. 2021, 11, 3410. [Google Scholar] [CrossRef]
- Trentini, C.P.; Cuco, R.P.; Cardozo-Filho, L.; Da Silva, C. Extraction of Macauba Kernel Oil Using Supercritical Carbon Dioxide and Compressed Propane. Can. J. Chem. Eng. 2018, 97, 785–792. [Google Scholar] [CrossRef]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical Fluid Extraction of Oils from Apple Seeds: Process Optimization, Chemical Characterization and Comparison with a Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- De Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimisation and Modelling of Supercritical CO2 Extraction Process of Carotenoids from Carrot Peels. J. Supercrit. Fluids 2018, 133, 94–102. [Google Scholar] [CrossRef]
- Rosas-Quina, Y.E.; Mejía-Nova, F.C. Supercritical Fluid Extraction with Cosolvent of Alkaloids from Lupinus mutabilis Sweet and Comparison with Conventional Method. J. Food Process Eng. 2021, 44, e13657. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus Pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.P.; Balducchi, R.; Mehariya, S.; Martino, M.; Larocca, V.; Di Sanzo, G.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; et al. Selective Extraction of ω-3 Fatty Acids from Nannochloropsis sp. Using Supercritical CO2 Extraction. Molecules 2019, 24, 2406. [Google Scholar] [CrossRef] [PubMed]
- Jaime, L.; Vázquez, E.S.; Fornari, T.; Del Carmen López-Hazas, M.; García-Risco, M.R.; Santoyo, S.; Reglero, G. Extraction of Functional Ingredients from Spinach (Spinacia oleracea L.) Using Liquid Solvent and Supercritical CO2 Extraction. J. Sci. Food Agric. 2014, 95, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Porcedda, S.; Falconieri, D.; Fais, A.; Era, B.; Carta, G.; Rosa, A. Supercritical Extraction of Volatile and Fixed Oils from Petroselinum crispum L. Seeds: Chemical Composition and Biological Activity. Nat. Prod. Res. 2020, 36, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Topiař, M.; Sajfrtová, M.; Karban, J. Fractionation of Turmerones from Turmeric SFE Isolate Using Semi-Preparative Supercritical Chromatography Technique. J. Ind. Eng. Chem. 2019, 77, 223–229. [Google Scholar] [CrossRef]
- Pellicanò, T.M.; Sicari, V.; Loizzo, M.R.; Leporini, M.; Falco, T.; Poiana, M. Optimizing the Supercritical Fluid Extraction Process of Bioactive Compounds from Processed Tomato Skin By-Products. Food Sci. Technol. 2020, 40, 692–697. [Google Scholar] [CrossRef]
- Todd, R.I.; Baroutian, S. A Techno-Economic Comparison of Subcritical Water, Supercritical CO2 and Organic Solvent Extraction of Bioactives from Grape Marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Hassas-Roudsari, M.; Chang, P.R.; Pegg, R.B.; Tyler, R.T. Antioxidant Capacity of Bioactives Extracted from Canola Meal by Subcritical Water, Ethanolic and Hot Water Extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Kim, W.J.; Kim, J.-H.; Veriansyah, B.; Kim, J.D.; Lee, Y.W.; Oh, S.; Tjandrawinata, R.R. Extraction of Bioactive Components from Centella asiatica Using Subcritical Water. J. Supercrit. Fluids 2009, 48, 211–216. [Google Scholar] [CrossRef]
- Zaibunnisa, A.H.; Saim, N.; Said, M.; Osman, H. An Experimental Design Approach for the Extraction of Volatile Compounds from Turmeric Leaves (Curcuma domestica) Using Pressurised Liquid Extraction (PLE). LWT 2009, 42, 233–238. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M. Subcritical Water Extraction of Bioactive Compounds from Plants and Algae: Applications in Pharmaceutical and Food Ingredients. Food Eng. Rev. 2015, 8, 23–34. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of Polyphenols from Grape Skins and Defatted Grape Seeds Using Subcritical Water: Experiments and Modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Ong, E.S.; Cheong, J.S.H.; Goh, D. Pressurized Hot Water Extraction of Bioactive or Marker Compounds in Botanicals and Medicinal Plant Materials. J. Chromatogr. A 2006, 1112, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović–Uzelac, V. Pressurized Hot Water Extraction (PHWE) for the Green Recovery of Bioactive Compounds and Steviol Glycosides from Stevia Rebaudiana Bertoni Leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Essien, S.; Young, B.R.; Baroutian, S. Subcritical Water Extraction for Selective Recovery of Phenolic Bioactives from Kānuka Leaves. J. Supercrit. Fluids 2020, 158, 104721. [Google Scholar] [CrossRef]
- Nuapia, Y.; Maraba, K.; Tutu, H.; Chimuka, L.; Cukrowska, E. In Situ Decarboxylation-Pressurized Hot Water Extraction for Selective Extraction of Cannabinoids from Cannabis sativa. Chemometric Approach. Molecules 2021, 26, 3343. [Google Scholar] [CrossRef] [PubMed]
- Nastić, N.; Švarc-Gajić, J.; Delerue-Matos, C.; Morais, S.; Barroso, M.F.; Moreira, M.M. Subcritical Water Extraction of Antioxidants from Mountain Germander (Teucrium montanum L.). J. Supercrit. Fluids 2018, 138, 200–206. [Google Scholar] [CrossRef]
- Essien, S.; Young, B.R.; Baroutian, S. The Antibacterial and Antiproliferative Ability of Kānuka, Kunzea Ericoides, Leaf Extracts Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2020, 96, 1308–1315. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Maróstica, M.R.; Rostagno, M.A.; Forster-Carneiro, T. Subcritical Water Extraction of Flavanones from Defatted Orange Peel. J. Supercrit. Fluids 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Kim, S.W.; Ko, M.J.; Chung, M.S. Extraction of the Flavonol Quercetin from Onion Waste by Combined Treatment with Intense Pulsed Light and Subcritical Water Extraction. J. Clean. Prod. 2019, 231, 1192–1199. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Correa, J.R.P. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-N.; Saravana, P.S.; Nkurunziza, D.; Chun, B. Biofunctional Properties of Wild Cultivated and Cultivated Ginseng (Panax ginseng Meyer) Extracts Obtained Using Subcritical Water Extraction. Sep. Sci. Technol. 2020, 56, 1370–1382. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Li, Z.; Liu, R.; Zhang, A.; Xiao, Z.; Ma, L.; Li, J.; Deng, S. Simultaneous Extraction of Oil and Tea Saponin from Camellia Oleifera Abel. Seeds under Subcritical Water Conditions. Fuel Process. Technol. 2018, 174, 88–94. [Google Scholar] [CrossRef]
- Gagić, T.; Knez, Ž.; ŠKerget, M. Hydrothermal Hydrolysis of Sweet Chestnut (Castanea sativa) Tannins. J. Serbian Chem. Soc. 2020, 85, 869–883. [Google Scholar] [CrossRef]
- Cha, J.; Kim, C.-T.; Kim, T.; Cho, Y.-J. Optimization of Subcritical Extraction Process for Cinnamon (Cinnamomum cassia Blume) Using Response Surface Methodology. Food Sci. Biotechnol. 2019, 28, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.G.G.; Mazzutti, S.; Vitali, L.; Micke, G.A.; Ferreira, S.R.S. Recovery of Bioactive Phenolic Compounds from Papaya Seeds Agroindustrial Residue Using Subcritical Water Extraction. Biocatal. Agric. Biotechnol. 2019, 22, 101367. [Google Scholar] [CrossRef]
- Koyu, H.; Kazan, A.; Öztürk, T.K.; Yeşil-Çeliktaş, Ö.; Haznedaroğlu, M.Z. Optimizing Subcritical Water Extraction of Morus nigra L. Fruits for Maximization of Tyrosinase Inhibitory Activity. J. Supercrit. Fluids 2017, 127, 15–22. [Google Scholar] [CrossRef]
- Chikari, F.; Han, J.; Wang, Y.; Ao, W. Synergized Subcritical-Ultrasound-Assisted Aqueous Two-Phase Extraction, Purification, and Characterization of Lentinus Edodes Polysaccharides. Process Biochem. 2020, 95, 297–306. [Google Scholar] [CrossRef]
- Nkurunziza, D.; Pendleton, P.; Chun, B.S. Optimization and Kinetics Modeling of Okara Isoflavones Extraction Using Subcritical Water. Food Chem. 2019, 295, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Wu, W.; Zhang, G.; Zheng, Y.; Ma, C.; Li, W.; Yan, Y.; Xu, Z. Green Extraction of Active Ingredients from Finger Citron Using Subcritical Water and Assessment of Antioxidant Activity. Ind. Crops Prod. 2023, 200, 116821. [Google Scholar] [CrossRef]
- Zeković, Z.; Cvetanović, A.; Švarc-Gajić, J.; Gorjanović, S.; Sužnjević, D.Ž.; Mašković, P.; Savić, S.; Radojković, M.; Đurović, S. Chemical and Biological Screening of Stinging Nettle Leaves Extracts Obtained by Modern Extraction Techniques. Ind. Crops Prod. 2017, 108, 423–430. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Cindrić, M.; Jovanov, P.; Sakač, M.; Mandić, A.; Vidović, S. Subcritical Water Extraction of Wild Garlic (Allium ursinum L.) and Process Optimization by Response Surface Methodology. J. Supercrit. Fluids 2017, 128, 79–88. [Google Scholar] [CrossRef]
- Vladić, J.; Nastić, N.; Stanojkoivć, T.; Žižak, Ž.; Čakarević, J.; Popović, L.; Vidović, S. Subcritical Water for Recovery of Polyphenols from Comfrey Root and Biological Activities of Extracts. Acta Chim. Slov. 2019, 66, 473–783. [Google Scholar] [CrossRef] [PubMed]
- Vladić, J.; Janković, T.; Živković, J.; Tomić, M.; Zdunić, G.; Šavikin, K.; Vidović, S. Comparative Study of Subcritical Water and Microwave-Assisted Extraction Techniques Impact on the Phenolic Compounds and 5-Hydroxymethylfurfural Content in Pomegranate Peel. Plant Foods Hum. Nutr. 2020, 75, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of Subcritical Water Scale-up from Laboratory to Pilot System for Brewer’s Spent Grain Valorization. Ind. Crops Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Khoza, B.S.; Dubery, I.A.; Byth-Illing, H.-A.; Steenkamp, P.A.; Chimuka, L.; Madala, N.E. Optimization of Pressurized Hot Water Extraction of Flavonoids from Momordica Foetida Using UHPLC-qTOF-MS and Multivariate Chemometric Approaches. Food Anal. Methods 2015, 9, 1480–1489. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Cerdà, V.; Clavijo, S.; Suárez, R.; Mašković, P.; Cvetanović, A.; Delerue-Matos, C.; Carvalho, A.P.; Novakov, V. Bioactive Compounds of Sweet and Sour Cherry Stems Obtained by Subcritical Water Extraction. J. Chem. Technol. Biotechnol. 2018, 93, 1627–1635. [Google Scholar] [CrossRef]
- Chémat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Périno, S.; Fabiano-Tixier, A.; Vian, M.A. Review of Green Food Processing Techniques. Preservation, Transformation, and Extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Li, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Sengar, A.S.; Rawson, A.; Muthiah, M.; Kalakandan, S.K. Comparison of Different Ultrasound Assisted Extraction Techniques for Pectin from Tomato Processing Waste. Ultrason. Sonochem. 2020, 61, 104812. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Karuppiah, P.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound Assisted Citric Acid Mediated Pectin Extraction from Industrial Waste of Musa Balbisiana. Ultrason. Sonochem. 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Patience, N.A.; Schieppati, D.; Boffito, D.C. Continuous and Pulsed Ultrasound Pectin Extraction from Navel Orange Peels. Ultrason. Sonochem. 2021, 73, 105480. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Karuppiah, P.; Maran, J.P. Development and Validation of Ultrasound-Assisted Solid-Liquid Extraction of Phenolic Compounds from Waste Spent Coffee Grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of Ultrasound-Assisted Extraction of Oil from Papaya Seed by Response Surface Methodology: Oil Recovery, Radical Scavenging Antioxidant Activity, and Oxidation Stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Moorthy, I.G.; Maran, J.P.; Surya, S.; Naganyashree, S.; Shivamathi, C.S. Response Surface Optimization of Ultrasound Assisted Extraction of Pectin from Pomegranate Peel. Int. J. Biol. Macromol. 2015, 72, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, B.B.V.; Rodrigues, N.P.; Marczak, L.D.F. Sequential Extraction of Phenolics and Pectin from Mango Peel Assisted by Ultrasound. Food Res. Int. 2019, 119, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Khodaiyan, F. Optimization and Characterization of Pectin Extracted from Sour Orange Peel by Ultrasound Assisted Method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F. Eggplant Peel as a High Potential Source of High Methylated Pectin: Ultrasonic Extraction Optimization and Characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; De Rosso, V.V. Ionic Liquid Associated with Ultrasonic-Assisted Extraction: A New Approach to Obtain Carotenoids from Orange Peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Thakker, M.R.; Parikh, J.; Desai, M.A. Ultrasound Assisted Hydrotropic Extraction: A Greener Approach for the Isolation of Geraniol from the Leaves of Cymbopogon Martinii. ACS Sustain. Chem. Eng. 2018, 6, 3215–3224. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Rai, D.K.; Sun, D.; Tiwari, B.K. Ultrasound-assisted Extraction (UAE) of Bioactive Compounds from Coffee Silverskin: Impact on Phenolic Content, Antioxidant Activity, and Morphological Characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Garièpy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Ummat, V.; Tiwari, B.K.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme- and Ultrasound-assisted Extractions of Polyphenols from Citrus sinensis (Cv. Malta) Peel: A Comparative Study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Petigny, L.; Périno-Issartier, S.; Wajsman, J.; Chémat, F. Batch and Continuous Ultrasound Assisted Extraction of Boldo Leaves (Peumus boldus Mol.). Int. J. Mol. Sci. 2013, 14, 5750–5764. [Google Scholar] [CrossRef] [PubMed]
- Del Hierro, J.N.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martín, D. Ultrasound-Assisted Extraction and Bioaccessibility of Saponins from Edible Seeds: Quinoa, Lentil, Fenugreek, Soybean and Lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Podgórski, R. Influence of Different Extraction Methods on the Quantification of Selected Flavonoids and Phenolic Acids from Tilia Cordata Inflorescence. Ind. Crops Prod. 2015, 76, 509–514. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Sumere, B.R.; Mekaru, C.; Martínez, J.; Bezerra, R.M.N.; Rostagno, M.A. Extraction of Polyphenols and Antioxidants from Pomegranate Peel Using Ultrasound: Influence of Temperature, Frequency and Operation Mode. Int. J. Food Sci. Technol. 2019, 54, 2792–2801. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of Acoustic Frequency and Power Density on the Aqueous Ultrasonic-Assisted Extraction of Grape Pomace (Vitis vinifera L.)—A Response Surface Approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2019, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Tram, N.N. Optimizing the Extraction Conditions of Phenolic Compounds from Fresh Tea Shoot. J. Food Nutr. Sci. 2015, 3, 106. [Google Scholar] [CrossRef][Green Version]
- Taşkın, B.; Özbek, Z. Optimisation of Microwave Effect on Bioactives Contents and Colour Attributes of Aqueous Green Tea Extracts by Central Composite Design. J. Food Meas. Charact. 2020, 14, 2240–2252. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S.; et al. A Comparison of Extraction Yield, Quality and Thermal Properties from Sapindus mukorossi Seed Oil between Microwave Assisted Extraction and Soxhlet Extraction. Ind. Crops Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Elik, A.; Yanık, D.K.; Göğüş, F. Microwave-Assisted Extraction of Carotenoids from Carrot Juice Processing Waste Using Flaxseed Oil as a Solvent. LWT 2020, 123, 109100. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.M.; Andrés, M.Á.; Labidi, J. Microwave-Assisted Extraction of Curcuma longa L. Oil: Optimization, Chemical Structure and Composition, Antioxidant Activity and Comparison with Conventional Soxhlet Extraction. Molecules 2021, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, X.; Chi, M.; Chen, K.; Zhang, Y.; Kong, W.; Zi-Ying, L.; Huang, S.; Kunming, Q. Microwave-Assisted Extraction Coupled with Mass Spectrometry for Determining Five Volatile Compounds from Soy Sauces. J. Anal. Methods Chem. 2021, 2021, 6625929. [Google Scholar] [CrossRef] [PubMed]
- Rahmawati, A.; Fachri, B.A.; Oktavia, S.; Abrori, F. Extraction Bioactive Compound of Pegagan (Centella asiatica L.) Using Solvent-Free Microwave-Assisted Extraction. IOP Conf. Ser. 2021, 1053, 012125. [Google Scholar] [CrossRef]
- Doulabi, M.; Golmakani, M.; Ansari, S. Evaluation and Optimization of Microwave-assisted Extraction of Bioactive Compounds from Eggplant Peel By-product. J. Food Process. Preserv. 2020, 44, e14853. [Google Scholar] [CrossRef]
- Jiménez-Amezcua, I.; González-Prada, A.; Díez-Municio, M.; Soria, A.C.; Ruiz-Matute, A.I.; Sanz, M.L. Simultaneous Microwave-Assisted Extraction of Bioactive Compounds from Aged Garlic. J. Chromatogr. A 2023, 1704, 464128. [Google Scholar] [CrossRef] [PubMed]
- Nisoa, M.; Plodkaew, A.; Sirisathitkul, C.; Wattanasit, K.; Somjit, B.; Pacdeepin, P.; Sirisathitkul, Y. Simulation and Experimentation on Parameters Influencing Microwave-Assisted Extraction of Bioactive Compounds from Kaempferia parviflora Rhizomes. Alex. Eng. J. 2023, 65, 357–366. [Google Scholar] [CrossRef]
- Mali, P.S.; Kumar, P. Simulation and Experimentation on Parameters Influencing Microwave-Assisted Extraction of Bioactive Compounds from Punica granatum Waste and Its Preliminary Analysis. Food Chem. Adv. 2023, 3, 100344. [Google Scholar] [CrossRef]
- Wong, J.C.J.; Nillian, E. Microwave-assisted Extraction of Bioactive Compounds from Sarawak liberica sp. Coffee Pulp: Statistical Optimization and Comparison with Conventional Methods. Food Sci. Nutr. 2023, 11, 5364–5378. [Google Scholar] [CrossRef]
- Bansod, S.P.; Parikh, J.; Sarangi, P.K. Pineapple Peel Waste Valorization for Extraction of Bio-Active Compounds and Protein: Microwave Assisted Method and Box Behnken Design Optimization. Environ. Res. 2023, 221, 115237. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, M.; Sun, Q.; Mujumdar, A.S.; Yu, D. Extraction of Functional Extracts from Berries and Their High Quality Processing: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 63, 7108–7125. [Google Scholar] [CrossRef]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-Assisted Extraction (MAE) Conditions Using Polynomial Design for Improving Antioxidant Phytochemicals in Berberis asiatica Roxb. Ex DC. Leaves. Ind. Crops Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.E.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of Microwave-Assisted Extraction of Ergosterol from Agaricus bisporus L. by-Products Using Response Surface Methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing Conditions for Microwave-Assisted Extraction of Polyphenolic Content and Antioxidant Activity of Barleria lupulina Lindl. Plants 2021, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Rizwan, D.; Bakshi, R.A.; Wani, S.M.; Masoodi, F.A. Extraction of Carotenoids from Agro-Industrial Waste. In Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2023; pp. 157–178. [Google Scholar]
- Vînătoru, M.; Mason, T.J.; Călinescu, I. Ultrasonically Assisted Extraction (UAE) and Microwave Assisted Extraction (MAE) of Functional Compounds from Plant Materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2011, 5, 409–424. [Google Scholar] [CrossRef]
- Álvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A. Pressurized Liquid Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Fraguela-Meissimilly, H.; Bastías-Montes, J.M.; Vergara, C.E.; Ortiz-Viedma, J.; Lemus-Mondaca, R.; Flores, M.; Toledo-Merma, P.R.; Alcázar-Alay, S.C.; Bedoya, M.G. New Trends in Supercritical Fluid Technology and Pressurized Liquids for the Extraction and Recovery of Bioactive Compounds from Agro-Industrial and Marine Food Waste. Molecules 2023, 28, 4421. [Google Scholar] [CrossRef]
- De Moraes, M.R.; Ryan, S.M.; Godoy, H.T.; Thomas, A.L.; Maia, J.G.S.; Richards, K.M.; Tran, K.; Smith, R.E. Phenolic Compounds and Metals in Some Edible Annonaceae Fruits. Biol. Trace Elem. Res. 2020, 197, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Khaleghipour, L.; Linares-Pastén, J.A.; Rashedi, H.; Siadat, S.O.R.; Jasilionis, A.; Al-Hamimi, S.; Sardari, R.R.R.; Karlsson, E.N. Extraction of Sugarcane Bagasse Arabinoxylan, Integrated with Enzymatic Production of Xylo-Oligosaccharides and Separation of Cellulose. Biotechnol. Biofuels 2021, 14, 153. [Google Scholar] [CrossRef]
- Tacchini, M.; Burlini, I.; Bernardi, T.; De Risi, C.; Massi, A.; Guerrini, A.; Sacchetti, G. Chemical Characterisation, Antioxidant and Antimicrobial Screening for the Revaluation of Wine Supply Chain by-Products Oriented to Circular Economy. Plant Biosyst. 2018, 153, 809–816. [Google Scholar] [CrossRef]
- Ortiz-Viedma, J.; Bastías-Montes, J.M.; Char, C.; Vega, C.; Quintriqueo, A.; Bedoya, M.G.; Flores, M.; Aguilera, J.M.; Miranda, J.M.; Barros-Velázquez, J. Sequential Biorefining of Bioactive Compounds of High Functional Value from Calafate Pomace (Berberis microphylla) Using Supercritical CO2 and Pressurized Liquids. Antioxidants 2023, 12, 323. [Google Scholar] [CrossRef]
- Constantin, O.E.; Milea, A.Ș.; Bolea, C.A.; Mihalcea, L.; Enachi, E.; Copolovici, D.M.; Copolovici, L.; Munteanu, F.; Bahrim, G.; Râpeanu, G. Onion (Allium cepa L.) Peel Extracts Characterization by Conventional and Modern Methods. Int. J. Food Eng. 2020, 17, 485–493. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.F.; De Paula Menezes Barbosa, P.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martínez, J. Recovery of Phenolic Compounds from Citrus By-Products Using Pressurized Liquids—An Application to Orange Peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; De Moraes, L.A.B.; De Melo, M.P.; De Oliveira, A.L.; Da Costa Rodrigues, C.E. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef] [PubMed]
- Khanum, F. Therapeutic Foods: An Overview; CRC Press: London, UK, 2021; pp. 31–70. [Google Scholar]
- Picot-Allain, M.C.N.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Carpena, M.; Barciela, P.; Cassani, L.; Simal-Gándara, J.; Prieto, M.A. Pressurized Liquid Extraction for the Recovery of Bioactive Compounds from Seaweeds for Food Industry Application: A Review. Antioxidants 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.B.; Viganó, J.; Sanches, V.L.; Rostagno, M.A. Extraction of Potential Bioactive Compounds from Industrial Tahiti Lime (Citrus latifólia Tan.) by-Product Using Pressurized Liquids and Ultrasound-Assisted Extraction. Food Res. Int. 2022, 157, 111381. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869. [Google Scholar] [CrossRef]
- Das, S.; Nadar, S.S.; Rathod, V.K. Integrated Strategies for Enzyme Assisted Extraction of Bioactive Molecules: A Review. Int. J. Biol. Macromol. 2021, 191, 899–917. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Enzyme-Assisted Extraction of Bioactive Non-Extractable Polyphenols from Sweet Cherry (Prunus avium L.) Pomace. Food Chem. 2021, 339, 128086. [Google Scholar] [CrossRef]
- Vilet, N.Z.; Gué, E.; Servent, A.; Delalonde, M.; Wisniewski, C. Filtration-Compression Step as Downstream Process for Flavonoids Extraction from Citrus Peels: Performances and Flavonoids Dispersion State in the Filtrate. Food Bioprod. Process. 2020, 120, 104–113. [Google Scholar] [CrossRef]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; De Moura Bell, J.M.L.N. Integrated Microwave- and Enzyme-Assisted Extraction of Phenolic Compounds from Olive Pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J. Efficient Extraction of Pectin from Sisal Waste by Combined Enzymatic and Ultrasonic Process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Núñez, Ó.; Granados, M.; Sentellas, S.; Saurina, J. An Overview of the Extraction and Characterization of Bioactive Phenolic Compounds from Agri-Food Waste within the Framework of Circular Bioeconomy. TrAC Trends Anal. Chem. 2023, 161, 116994. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic Activity of Armillaria Mellea Polysaccharides: Joint Ultrasonic and Enzyme Assisted Extraction. Ultrason. Sonochem. 2023, 95, 106370. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Lin, S.; Jian, Y.; Miao, S.; Zheng, B. Ultrasonic–Microwave Synergistic Extraction (UMSE) and Molecular Weight Distribution of Polysaccharides from Fortunella margarita (Lour.) Swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Sun, H.; Li, C.; Ni, Y.; Yao, L.; Jiang, H.; Ren, X.; Fu, Y.; Zhao, C. Ultrasonic/Microwave-Assisted Extraction of Polysaccharides from Camptotheca acuminata Fruits and Its Antitumor Activity. Carbohydr. Polym. 2019, 206, 557–564. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved Extraction of Vegetable Oils under High-Intensity Ultrasound and/or Microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Milman, B.L.; Журкoвич, И.К. The Chemical Space for Non-Target Analysis. TrAC Trends Anal. Chem. 2017, 97, 179–187. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process.-Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Cheng, Z.; Song, H.; Yang, Y.; Liu, Y.; Liu, Z.; Hu, H.; Zhang, Y. Optimization of Microwave-Assisted Enzymatic Extraction of Polysaccharides from the Fruit of Schisandra chinensis Baill. Int. J. Biol. Macromol. 2015, 76, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Huang, Z.; Yu, Q.; Peng, G.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Microwave Assisted Extraction with Three Modifications on Structural and Functional Properties of Soluble Dietary Fibers from Grapefruit Peel. Food Hydrocoll. 2020, 101, 105549. [Google Scholar] [CrossRef]
- Mehmood, A.; Ishaq, M.; Zhao, L.; Yaqoob, S.; Safdar, B.; Nadeem, M.; Munir, M.; Wang, C. Impact of Ultrasound and Conventional Extraction Techniques on Bioactive Compounds and Biological Activities of Blue Butterfly Pea Flower (Clitoria ternatea L.). Ultrason. Sonochem. 2019, 51, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, S.-S.; Wang, M.; He, L. PEG-Based Ultrasound-Assisted Enzymatic Extraction of Polysaccharides from Ginkgo biloba Leaves. Int. J. Biol. Macromol. 2015, 80, 644–650. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of Ultrasound-Assisted Enzymatic Extraction of Arabinoxylan from Wheat Bran. Food Chem. 2014, 150, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Tian, J.; Wang, J.; Wang, Y.; Xing, L. Enzymolysis-Ultrasonic Assisted Extraction, Chemical Characteristics and Bioactivities of Polysaccharides from Corn Silk. Carbohydr. Polym. 2014, 101, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fang, X.; Dan, D.; Diao, E.; Lu, Z. Ultrasonic-Assisted Enzymatic Extraction of a Water Soluble Polysaccharide from Dragon Fruit Peel and Its Antioxidant Activity. RSC Adv. 2018, 8, 42145–42152. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F.; Carvalho, P.I.N.; Rostagno, M.A.; Petenate, A.J.; Meireles, M.Â.A. Extraction of Curcuminoids from Deflavored Turmeric (Curcuma longa L.) Using Pressurized Liquids: Process Integration and Economic Evaluation. J. Supercrit. Fluids 2014, 95, 167–174. [Google Scholar] [CrossRef]
- Santos, P.D.; De Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsaicinoids from Malagueta Pepper (Capsicum frutescens L.) Assisted by Ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef]
- Sumere, B.R.; Souza, M.C.; Santos, M.P.; Bezerra, R.M.N.; Da Cunha, D.T.; Martínez, J. Combining Pressurized Liquids with Ultrasound to Improve the Extraction of Phenolic Compounds from Pomegranate Peel (Punica granatum L.). Ultrason. Sonochem. 2018, 48, 151–162. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Dehdashtian, S. Application of Green Solvents as Sorbent Modifiers in Sorptive-Based Extraction Techniques for Extraction of Environmental Pollutants. TrAC Trends Anal. Chem. 2018, 109, 50–61. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESS) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.; Canals, A. Magnetic Deep Eutectic Solvents in Microextraction Techniques. TrAC Trends Anal. Chem. 2022, 146, 116500. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A Green Extraction Process for Polyphenols from Elderberry (Sambucus nigra) Flowers Using Deep Eutectic Solvent and Ultrasound-Assisted Pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Cui, Q.; Wang, L.; Meng, Y.; Yu, L.; Li, Y.; Fu, Y. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Tobiszewski, M. Analytical Chemistry with Biosolvents. Anal. Bioanal. Chem. 2019, 411, 4359–4364. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the New Generation of Green Solvents in Extraction Processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Silva, S.S.; Justi, M.; Chagnoleau, J.-B.; Papaïconomou, N.; Fernández, X.; Santos, S.A.O.; Passos, H.; Ferreira, A.M.; Coutinho, J. Using Biobased Solvents for the Extraction of Phenolic Compounds from Kiwifruit Industry Waste. Sep. Purif. Technol. 2023, 304, 122344. [Google Scholar] [CrossRef]
- Wang, X.; Jia, W.; Lai, G.; Wang, L.; Del Mar Contreras, M.; Yang, D. Extraction for Profiling Free and Bound Phenolic Compounds in Tea Seed Oil by Deep Eutectic Solvents. J. Food Sci. 2020, 85, 1450–1461. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced Phenolic Compounds Extraction from Morus alba L. Leaves by Deep Eutectic Solvents Combined with Ultrasonic-Assisted Extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep Eutectic Solvent-based Extraction of Polyphenolic Antioxidants from Onion (Allium cepa L.) Peel. J. Sci. Food Agric. 2018, 99, 1969–1979. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Evaluation of Bio-Based Solvents for Phenolic Acids Extraction from Aqueous Matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Hashemi, B.; Shiri, F.; Švec, F.; Nováková, L. Green Solvents and Approaches Recently Applied for Extraction of Natural Bioactive Compounds. TrAC Trends Anal. Chem. 2022, 157, 116732. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).