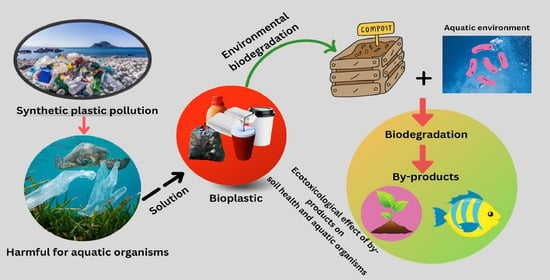
Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review
Abstract
:1. Introduction
2. Synthesis of Bioplastics
| Type of Bioplastic | Source | Use | Production Cost | Toxicity | Reference |
|---|---|---|---|---|---|
| Polyhydroxyalkanoate (PHA) | Wastewater, whey | Food packaging | 1.40–11.80 USD/kg | Baseline toxicity | [17,27,28] |
| Polylactic acid (PLA) | Cassava starch | Bottles, cups, tea bags | 2.71–2.82 USD/kg | Oxidative stress, baseline toxicity | [17,27,29] |
| Polybutylene succinate (PBS) | Food waste, fossil fuel | Food and cosmetics packaging | 3.5–5.21 USD/Kg | Baseline toxicity | [17,27,30] |
| Polyhydroxybutyrate (PHB) | Methan | Medical purposes | 4.1–6.8 USD/kg | Baseline toxicity | [17,27,31] |
| Cellulose-based | Cellulose | Thermoplastics | 3558 USD/t | Baseline toxicity | [17,27,32] |
| Starch-based | Starch | Cell phone cases | 1496 USD/t | Baseline toxicity Phytotoxicity | [17,27,33] |
| Biopolyethylene terephthalate Bio-PET | Bioethanol | Transparent packaging | Not given | Oxidative stress, baseline toxicity | [17,27] |
| Poly (butylene adipate-co- terephthalate (PBAT) | Polycondensation | Shopping bags | 3.6 USD/Kg | Baseline toxicity | [17,27,34,35] |
| of butanediol (BDO) | |||||
| adipic acid (AA) and | |||||
| terephthalic acid (PTA) | |||||
3. Biodegradability of Bioplastic in Complex Environmental Conditions
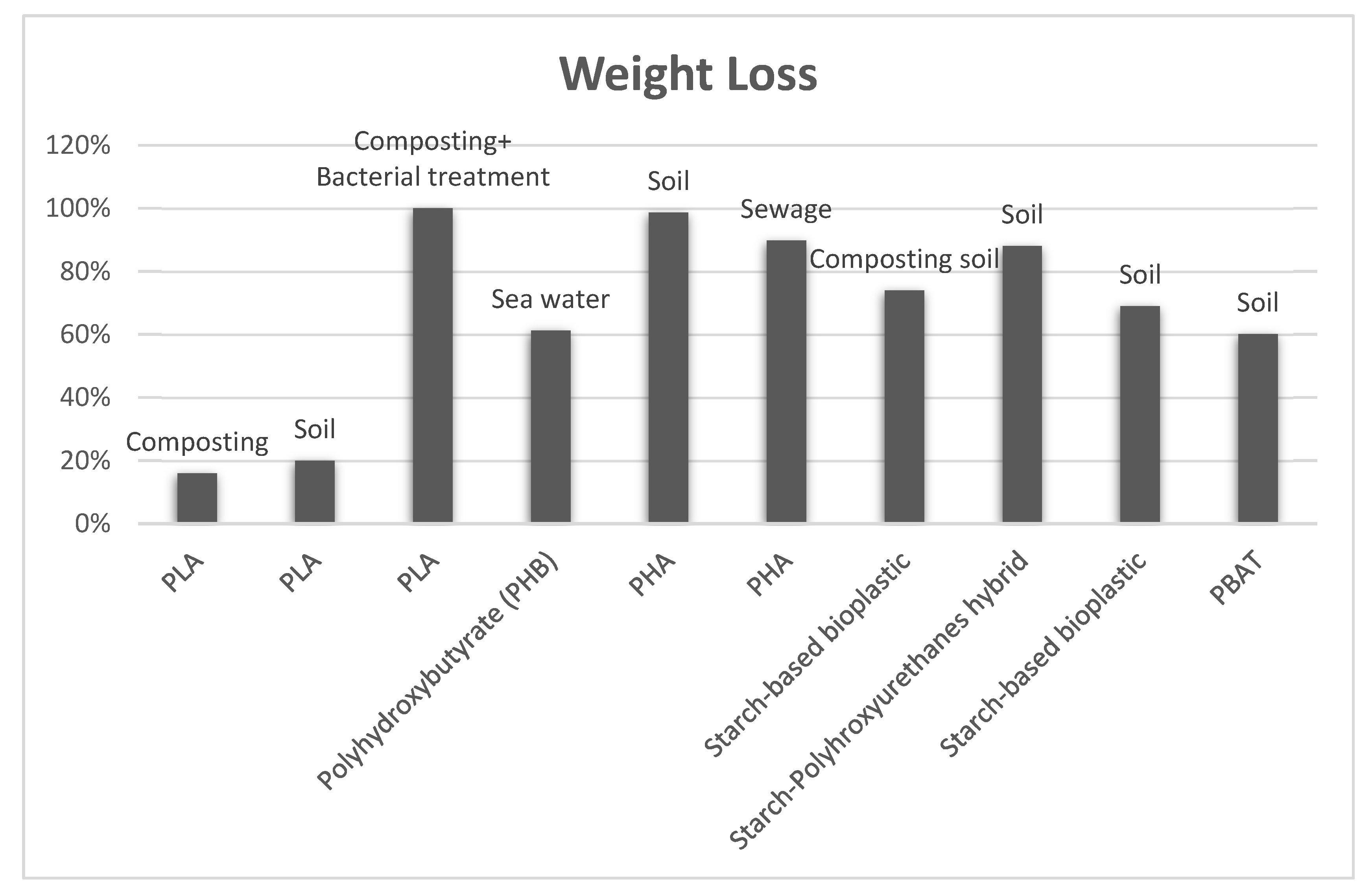
| Plastic Type | Biodegradation Environment | Factors Affecting the Rate of Biodegradation | Weight Loss | Time/Days | Reference |
|---|---|---|---|---|---|
| PLA | Freshwater | Temperature (25 °C) | <2% | 365 | [38] |
| PCL | Controlled | Aerobic, (30 °C), PH = 7 | 8% | 28 | [39] |
| PLA | Sea water | Temperature (25 °C) | <2% | 365 | [38] |
| PLA | Soil | 30% moisture | 10% | 98 | [40] |
| PLA | Sludge | Anaerobic, 37 °C | 29–49% | 277 | [41] |
| PLA | Compost | 58 °C | 13% | 60 | [42] |
| Polyhydroxybutyrate (PHB) | Freshwater | Temperature (25 °C) | 9% | 365 | [38] |
| PHB | Compost | 55 °C, 70% humidity | 80% | 28 | [43] |
| PHB | Sludge | Anaerobic, 37 °C | 90% | 9 | [41] |
| PHB | Microbial culture from Soil | Aerobic | 18% | 18 | [44] |
| Polyhydroxybutyrate (PHB) | Sea water | Temperature (21 °C) | 99.00% | 49 | [45] |
| PHA | Soil | 35% moisture | 35.00% | 60 | [46] |
| PHA | Soil/compost (90/10%) | 25 °C, 65% humidity | 40–50% | 15 | [47] |
| PHBV | Soil | Natural conditions | 8.00% | 365 | [48] |
| Starch-based bioplastic | AD | Anaerobic, 37 °C | 26.40% | 50 | [49] |
| Starch-based bioplastic | Soil | Soil burial test | 96.00% | 28 | [50] |
| Starch-based bioplastic | Compost | Aerobic, 58 °C | 85.00% | 90 | [51] |
| Starch-based bioplastic | Aerobic | Aspergillus niger culture | 20% | 10 | [52] |
| Cellulose-based | Compost | 1 m depth—15.7 °C average outside temperature | 100% | 84 | [53] |
| Cellulose-based | Synthetic soil containing compost | Aerobic, 58 °C | 80% | 154 | [54] |
| PCL | Sludge | Anaerobic, 37 °C | 3–22% | 277 | [41] |
| PCL | Compost | Aerobic, 50 °C, pH = 7–8.5 | 38% | 6 | [55] |
| PCL | Soil and leachate | 28 °C, 60% humidity | 22% | 60 | [56] |
| PBS | Compost | Aerobic, 58–65 °C, pH = 7–8, 50–55% moisture | 90% | 160 | [57] |
| PBS | Landfill | Anaerobic, 25 °C | 2% | 100 | [58] |
3.1. Bioplastic Biodegradation in Ocean
3.2. Bioplastic Biodegradation Soil
3.3. Anaerobic Biodegradation of Bioplastic
3.4. Bioplastic Biodegradation by Composting
3.5. Bioplastic-Degrading Microbial Community in Different Environmental Conditions
3.6. Biodegradation of Conventional Plastic and Bioplastic via Microbes
3.6.1. Biodegradation of Conventional Plastics by Microorganisms
3.6.2. Biodegradation of Bioplastic by Microorganisms
| Plastic Type | Microorganism | Weight Loss | Time/Days | Reference | |
|---|---|---|---|---|---|
| Polyhydroxybutyrate (PHB) | Bacillus sp. JY14 | 98% | 14 | [134] | |
| Polybutylene succinate (PBS) | Terribacillus sp. JY49 | 31.40% | 10 | [135] | |
| Poly(butylene succinate-co-butylene adipate) (PBSA) | Sclerotinia sp. B11IV | 49.68% | 28 | [132] | |
| Poly(ε-caprolactone) (PCL) | Sclerotinia sp. B11IV | 33.70% | 28 | [132] | |
| Bioplastics | Poly(butylene succinate-co-butylene adipate) (PBSA) | Fusarium sp. B3′M | 45.99% | 28 | [132] |
| Poly(ε-caprolactone) (PCL) | Fusarium sp. B3′M | 49.65% | 28 | [132] | |
| Poly(butylene adipate-co-terephthalate) (PBAT) | Bacillus sp. JY35 | 50% | 21 | [131] | |
| Polylactic acid/polybutylene adipate-co-terephthalate (PLA/PBAT) | Pseudomonas mendocina | 12.94% | 5 | [133] | |
| Polylactic acid/polybutylene adipate-co-terephthalate (PLA/PBAT) | A. elegans | 9.27% | 5 | [133] | |
| Low-density polyethylene (LDPE) | Enterobacter and Pseudomonas spp. | 64% | 160 | [104] | |
| Polyethylene terephthalate (PET) | Streptomyces sp. | 68.80% | 18 | [107] | |
| Low-density polyethylene (LDPE) | Acinetobacter pitti | 26.80% | 28 | [110] | |
| Conventional plastics | Low-density polyethylene (LDPE) | Bacillus siamensis | 8.46% | 90 | [105] |
| LDPE | Bacillus amylolyticus | 32% | 30 | [110] | |
| Polyethylene | Pseudomonas | 51.50% | 90 | [111] | |
| Low-density polyethylene (LDPE) | Streptomyces coelicoflavus | 30% | 28 | [118] | |
| Enterobacter sp. | |||||
| Polypropylene | Enterobacter cloacae | 63% | 160 | [104] | |
| and Pseudomonas aeruginosa | |||||
| Polyethylene | Pseudomonas aeruginosa SH6B | 25% | 120 | [93] | |
| Low-density polyethylene (LDPE) | Rhodococcus UCC0018 | 8.69% | 120 | [106] |
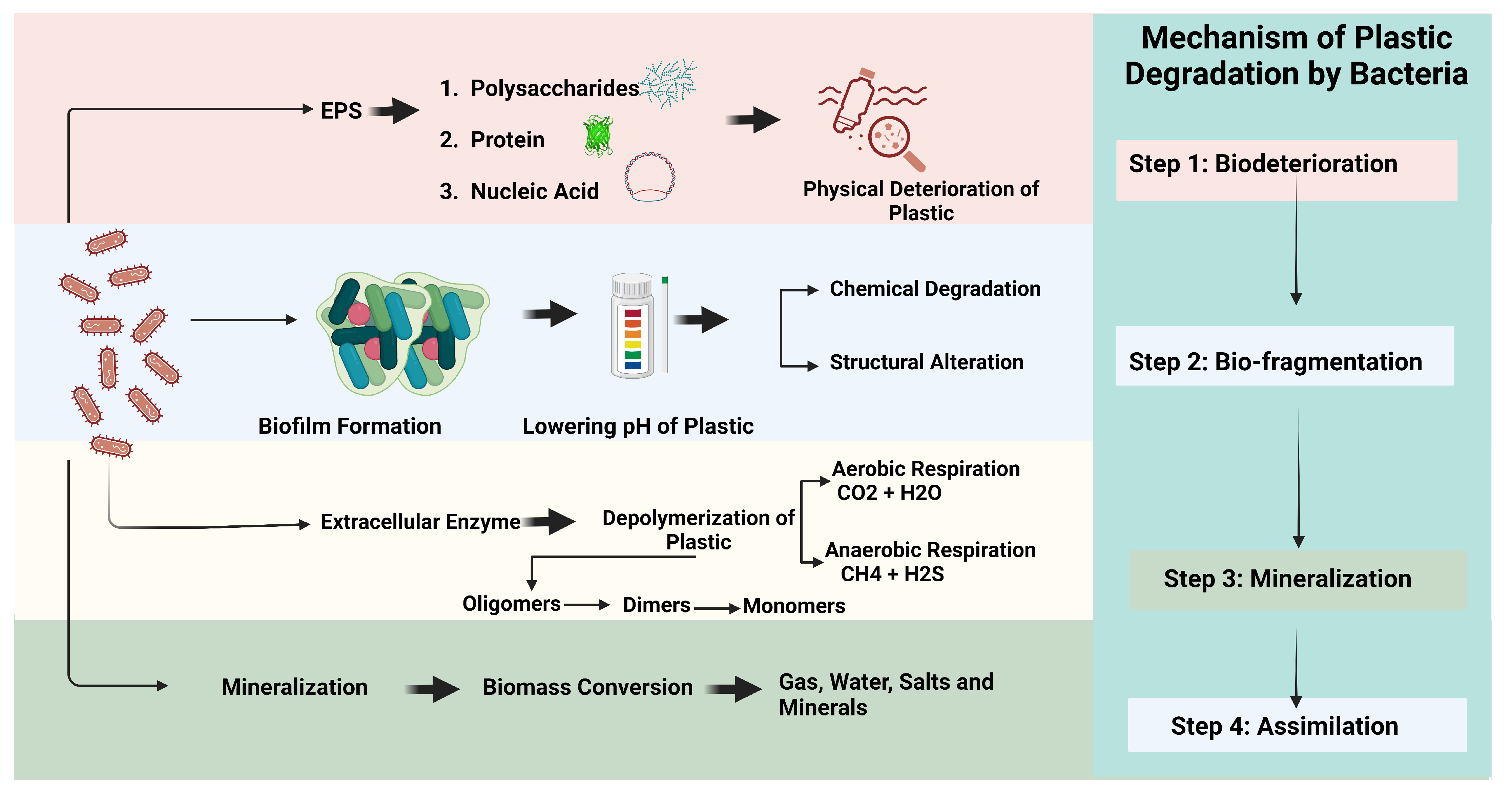
3.6.3. Mechanism of Biodegradation of Plastics by Microorganisms
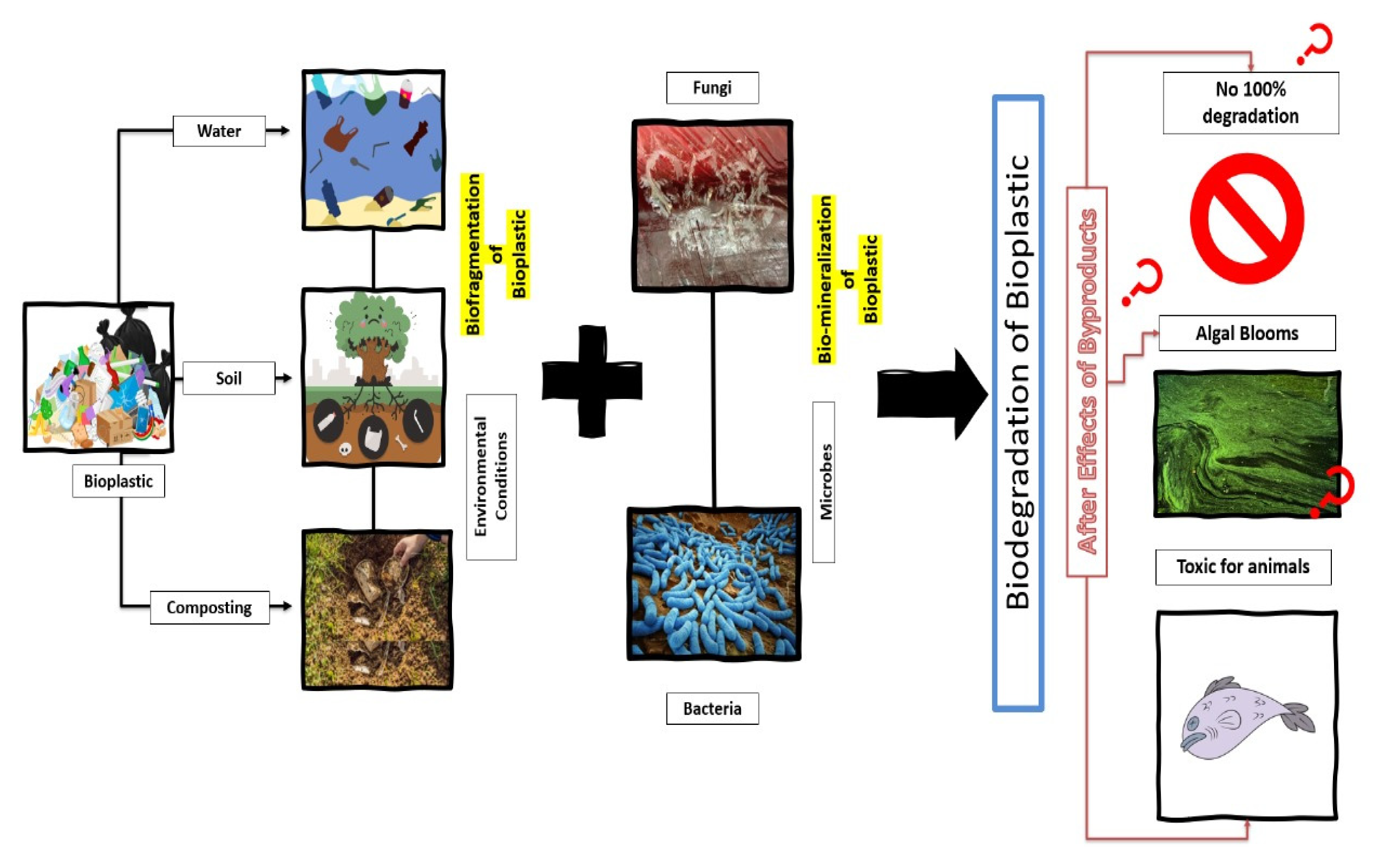
4. Environmental Consequences of Biodegradation
4.1. Bioplastics Contain a Complex Mixture of Chemicals
4.2. Bioplastic Toxicity for the Aquatic Environment
4.3. Bioplastic Toxicity for the Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nanni, A.; Parisi, M.; Colonna, M. Wine by-products as raw materials for the production of biopolymers and of natural reinforcing fillers: A critical review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and consolidated review of plastic waste management and bio-based biodegradable plastics: Challenges and opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly (lactic acid)(PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Agate, S.; Velev, O.D.; Lucia, L.; Pal, L. A critical review of the performance and soil biodegradability profiles of biobased natural and chemically synthesized polymers in industrial applications. Environ. Sci. Technol. 2022, 56, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Kochanska, E.; Wozniak, K.; Nowaczyk, A.; Piedade, P.J.; de Almeida Lavorato, M.L.; Almeida, A.M.; Morais, A.R.C.; Lukasik, R.M. Global Ban on Plastic and What Next? Are Consumers Ready to Replace Plastic with the Second-Generation Bioplastic? Results of the Snowball Sample Consumer Research in China, Western and Eastern Europe, North America and Brazil. Int. J. Environ. Res. Public Health 2022, 19, 13970. [Google Scholar] [CrossRef]
- Goel, V.; Luthra, P.; Kapur, G.S.; Ramakumar, S.S.V. Biodegradable/bio-plastics: Myths and realities. J. Polym. Environ. 2021, 29, 3079–3104. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Shafiei, N.; Nezafat, Z. Application of Biopolymers in Bioplastics; Elsevier EBooks: Amsterdam, The Netherlands, 2021; pp. 1–44. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro) plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef]
- Gricajeva, A.; Nadda, A.K.; Gudiukaite, R. Insights into polyester plastic biodegradation by carboxyl ester hydrolases. J. Chem. Technol. Biotechnol. 2022, 97, 359–380. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of wasted bioplastics in natural and industrial environments: A review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do poly (lactic acid) microplastics instigate a threat? A perception for their dynamic towards environmental pollution and toxicity. Sci. Total Environ. 2022, 832, 155014. [Google Scholar] [CrossRef]
- Gioia, C.; Giacobazzi, G.; Vannini, M.; Totaro, G.; Sisti, L.; Colonna, M.; Marchese, P.; Celli, A. End of life of biodegradable plastics: Composting versus Re/upcycling. ChemSusChem 2021, 14, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.; Lee, C.H. A review on properties and application of bio-based poly (butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Polman, E.M.; Gruter, G.J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, L. Standards and Guidelines for Testing Biodegradability of Bioplastic. In Biodegradable Polymer-Based Food Packaging; Springer Nature: Singapore, 2022; pp. 297–325. [Google Scholar]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic products leach chemicals that induce in vitro toxicity under realistic use conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef]
- Choudhury, B.K.; Haloi, R.; Bharadwaj, K.K.; Rajkhowa, S.; Sarma, J. Bio-Based and Biodegradable Plastics as Alternatives to Conventional Plastics. In Plastic and Microplastic in the Environment: Management and Health Risks; Wiley: Hoboken, NJ, USA, 2022; pp. 170–186. [Google Scholar]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Samadhiya, K.; Sangtani, R.; Nogueira, R.; Bala, K. Insightful advancement and opportunities for microbial bioplastic production. Front. Microbiol. 2022, 12, 674864. [Google Scholar] [CrossRef]
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of algal biomass to value-added metabolites: Emerging trends and opportunities. Phytochem. Rev. 2023, 22, 1015–1040. [Google Scholar] [CrossRef]
- Ferreira-Filipe, D.A.; Paço, A.; Duarte, A.C.; Rocha-Santos, T.; Patrício Silva, A.L. Are biobased plastics green alternatives?—A critical review. Int. J. Environ. Res. Public Health 2021, 18, 7729. [Google Scholar] [CrossRef]
- García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Bordel, S.; Santos-Beneit, F.; Martínez-Mendoza, L.J.; Börner, R.A.; Börner, T.; Munoz, R. Biodegradation of bioplastics under aerobic and anaerobic aqueous conditions: Kinetics, carbon fate and particle size effect. Bioresour. Technol. 2022, 344, 126265. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Yew, G.Y.; Khoo, K.S.; Ho, S.H.; Show, P.L. Recent advances on food waste pretreatment technology via microalgae for source of polyhydroxyalkanoates. J. Environ. Manag. 2021, 293, 112782. [Google Scholar] [CrossRef] [PubMed]
- Bastos Lima, M.G. Toward multipurpose agriculture: Food, fuels, flex crops, and prospects for a bioeconomy. Glob. Environ. Politics 2018, 18, 143–150. [Google Scholar] [CrossRef]
- Ritzen, L.; Sprecher, B.; Bakker, C.; Balkenende, R. Bio-based plastics in a circular economy: A review of recovery pathways and implications for product design. Resour. Conserv. Recycl. 2023, 199, 107268. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in vitro toxicity and chemical composition of plastic consumer products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Reddy, M.V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost Structure of Bio-Based Plastics: A Monte-Carlo-Analysis for PLA; No. 197; HWWI Research Paper: Hamburg, Germany, 2021. [Google Scholar]
- Rajendran, N.; Han, J. Techno-economic analysis and life cycle assessment of poly (butylene succinate) production using food waste. Waste Manag. 2023, 156, 168–176. [Google Scholar] [CrossRef]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Manandhar, A.; Shah, A. Techno-economic analysis of bio-based lactic acid production utilizing corn grain as feedstock. Processes 2020, 8, 199. [Google Scholar] [CrossRef]
- Liard, G.; Lesage, P.; Samson, R.; Stuart, P.R. Systematic assessment of triticale-based biorefinery strategies: Environmental evaluation using life cycle assessment. Biofuels Bioprod. Biorefining 2018, 12, S60–S72. [Google Scholar] [CrossRef]
- Xiong, S.J.; Pang, B.; Zhou, S.J.; Li, M.K.; Yang, S.; Wang, Y.Y.; Shi, Q.; Wang, S.-F.; Yuan, T.-Q.; Sun, R.C. Economically competitive biodegradable PBAT/lignin composites: Effect of lignin methylation and compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly (butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Kumar, M.; Kumar, V.; Sarsaiya, S.; Anerao, P.; Ghosh, P.; Singh, L.; Liu, H.; Zhang, Z.; Awasthi, M.K. A comprehensive review on recent advancements in biodegradation and sustainable management of biopolymers. Environ. Pollut. 2022, 307, 119600. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.K.; Sarmah, A.; Bhasney, S.M.; Kalamdhad, A.; Katiyar, V. Demonstrating an ideal compostable plastic using biodegradability kinetics of poly (lactic acid)(PLA) based green biocomposite films under aerobic composting conditions. Environ. Chall. 2021, 3, 100030. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of so-called biodegradable polymers in seawater and freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym. Degrad. Stab. 2006, 91, 620–627. [Google Scholar] [CrossRef]
- Wu, C.S. Preparation, characterization, and biodegradability of renewable resource-based composites from recycled polylactide bioplastic and sisal fibers. J. Appl. Polym. Sci. 2012, 123, 347–355. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Mesophilic anaerobic biodegradation test and analysis of eubacteria and archaea involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 2014, 110, 278–283. [Google Scholar] [CrossRef]
- Ahn, H.K.; Huda, M.S.; Smith, M.C.; Mulbry, W.; Schmidt, W.F.; Reeves III, J.B. Biodegradability of injection molded bioplastic pots containing polylactic acid and poultry feather fiber. Bioresour. Technol. 2011, 102, 4930–4933. [Google Scholar] [CrossRef]
- Tabasi, R.Y.; Ajji, A. Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 2015, 120, 435–442. [Google Scholar] [CrossRef]
- Woolnough, C.A.; Charlton, T.; Yee, L.H.; Sarris, M.; Foster, L.J.R. Surface changes in polyhydroxyalkanoate films during biodegradation and biofouling. Polym. Int. 2008, 57, 1042–1051. [Google Scholar] [CrossRef]
- Thellen, C.; Coyne, M.; Froio, D.; Auerbach, M.; Wirsen, C.; Ratto, J.A. A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J. Polym. Environ. 2008, 16, 1–11. [Google Scholar] [CrossRef]
- Wu, C.S. Preparation and characterization of polyhydroxyalkanoate bioplastic-based green renewable composites from rice husk. J. Polym. Environ. 2014, 22, 384–392. [Google Scholar] [CrossRef]
- Arcos-Hernandez, M.V.; Laycock, B.; Pratt, S.; Donose, B.C.; Nikolić, M.A.; Luckman, P.; Werker, A.; Lant, P.A. Biodegradation in a soil environment of activated sludge derived polyhydroxyalkanoate (PHBV). Polym. Degrad. Stab. 2012, 97, 2301–2312. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Prudnikova, S.V.; Karpov, V.A.; Ivonin, V.N.; Đỗ, N.L.; Nguyễn, T.H.; Lê, T.M.H.; Filichev, N.L.; Levin, A.L.; Filipenko, M.L.; et al. Microbial degradation of polyhydroxyalkanoates in tropical soils. Int. Biodeterior. Biodegrad. 2013, 83, 77–84. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C., Jr. Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Jangong, O.S.; Gareso, P.L.; Mutmainna, I.; Tahir, D. Fabrication and characterization starch/chitosan reinforced polypropylene as biodegradable. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1341, p. 082022. [Google Scholar]
- Javierre, C.A.R.L.O.S.; Sarasa, J.; Claveria, I.S.A.B.E.L.; Fernandez, A. Study of the biodisintegration on a painted bioplastic material waste. Mater. Plast. 2015, 52, 116–121. [Google Scholar]
- Nissa, R.C.; Fikriyyah, A.K.; Abdullah, A.H.D.; Pudjiraharti, S. Preliminary study of biodegradability of starch-based bioplastics using ASTM G21-70, dip-hanging, and Soil Burial Test methods. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 277, p. 012007. [Google Scholar]
- Adamcová, D.; Elbl, J.; Zloch, J.; Vaverková, M.D.; Kintl, A.; Juřička, D.; Hladký, J.; Brtnický, M. Study on the (bio) degradation process of bioplastic materials under industrial composting conditions. Acta Univ. Agric. Et Silvic. Mendel. Brun. 2017, 65, 791–798. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Adamcová, D. Biodegrability of bioplastic materials in a controlled composting environment. J. Ecol. Eng. 2015, 16, 155–160. [Google Scholar] [CrossRef]
- Nakasaki, K.; Matsuura, H.; Tanaka, H.; Sakai, T. Synergy of two thermophiles enables decomposition of poly-ε-caprolactone under composting conditions. FEMS Microbiol. Ecol. 2006, 58, 373–383. [Google Scholar] [CrossRef]
- Campos, A.D.; Marconato, J.C.; Martins-Franchetti, S.M. The influence of soil and landfill leachate microorganisms in the degradation of PVC/PCL films cast from DMF. Polímeros 2012, 22, 220–227. [Google Scholar] [CrossRef]
- Anstey, A.; Muniyasamy, S.; Reddy, M.M.; Misra, M.; Mohanty, A. Processability and biodegradability evaluation of composites from poly (butylene succinate)(PBS) bioplastic and biofuel co-products from Ontario. J. Polym. Environ. 2014, 22, 209–218. [Google Scholar] [CrossRef]
- Cho, H.S.; Moon, H.S.; Kim, M.; Nam, K.; Kim, J.Y. Biodegradability and biodegradation rate of poly (caprolactone)-starch blend and poly (butylene succinate) biodegradable polymer under aerobic and anaerobic environment. Waste Manag. 2011, 31, 475–480. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Pattanasupong, A. Evaluation of Biodegradabilities of Biosynthetic Polyhydroxyalkanoates in Thailand Seawater and Toxicity Assessment of Environmental Safety Levels. Polymers 2022, 14, 428. [Google Scholar] [CrossRef]
- Patil, P.B.; Sarkar, D.; Poddar, K.; Gu, J.D.; Sarkar, A. Degradation profiling of in-vitro-produced polyhydroxyalkanoate synthesized by the soil bacterium Bacillus sp. PhNs9 under different microenvironments. Int. Biodeterior. Biodegrad. 2023, 181, 105615. [Google Scholar] [CrossRef]
- Wicaksono, J.A.; Purwadaria, T.; Yulandi, A.; Tan, W.A. Bacterial dynamics during the burial of starch-based bioplastic and oxo-low-density-polyethylene in compost soil. BMC Microbiol. 2022, 22, 309. [Google Scholar] [CrossRef]
- Abe, M.M.; Branciforti, M.C.; Brienzo, M. Biodegradation of hemicellulose-cellulose-starch-based bioplastics and microbial polyesters. Recycling 2021, 6, 22. [Google Scholar] [CrossRef]
- Brdlík, P.; Borůvka, M.; Běhálek, L.; Lenfeld, P. Biodegradation of poly (lactic acid) biocomposites under controlled composting conditions and freshwater biotope. Polymers 2021, 13, 594. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly (lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Boonluksiri, Y.; Prapagdee, B.; Sombatsompop, N. Promotion of polylactic acid biodegradation by adding PLA-degrading bacterium and nitrogen source under submerged and soil burial conditions. Polym. Degrad. Stab. 2021, 188, 109562. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Mo, A.; Jiang, J.; He, D. Degradation of polylactic acid/polybutylene adipate films in different ratios and bacterial community response in soil environments. Environ. Pollut. 2022, 313, 120167. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, B.; Cai, Q.; Zhu, Z.; Liu, B.; Dong, G.; Greer, C.W.; Lee, K.; Chen, B. Responses of Alcanivorax species to marine alkanes and polyhydroxybutyrate plastic pollution: Importance of the ocean hydrocarbon cycles. Environ. Pollut. 2022, 313, 120177. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Microbial degradation of plastics and its biotechnological advancement. Environ. Biotechnol. 2021, 3, 1–30. [Google Scholar]
- Engler, L.G.; Farias, N.C.; Crespo, J.S.; Gately, N.M.; Major, I.; Pezzoli, R.; Devine, D.M. Designing sustainable polymer blends: Tailoring mechanical properties and degradation behaviour in PHB/PLA/PCL blends in a seawater environment. Polymers 2023, 15, 2874. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, Q.; Wang, L.; Zhang, C.; Zhang, Y. Are biodegradable mulch films a sustainable solution to microplastic mulch film pollution? A biogeochemical perspective. J. Hazard. Mater. 2023, 459, 132024. [Google Scholar] [CrossRef] [PubMed]
- Mosquera Rodríguez, F.S.; Quintero Vélez, A.; Córdoba Urrutia, E.; Ramírez-Malule, H.; Mina Hernandez, J.H. Study of the Degradation of a TPS/PCL/Fique Biocomposite Material in Soil, Compost, and Water. Polymers 2023, 15, 3952. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Chadwick, D.R.; Zang, H.; Graf, M.; Liu, X.; Wang, K.; Greenfield, L.M.; Jones, D.L. Bioplastic (PHBV) addition to soil alters microbial community structure and negatively affects plant-microbial metabolic functioning in maize. J. Hazard. Mater. 2023, 441, 129959. [Google Scholar] [CrossRef]
- Yasin, N.M.; Akkermans, S.; Van Impe, J.F. Enhancing the biodegradation of (bio) plastic through pretreatments: A critical review. Waste Manag. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Adhikari, D.; Mukai, M.; Kubota, K.; Kai, T.; Kaneko, N.; Araki, K.S.; Kubo, M. Degradation of bioplastics in soil and their degradation effects on environmental microorganisms. J. Agric. Chem. Environ. 2016, 5, 23. [Google Scholar] [CrossRef]
- Han, Y.; Teng, Y.; Wang, X.; Ren, W.; Wang, X.; Luo, Y.; Zhang, H.; Christie, P. Soil type driven change in microbial community affects poly (butylene adipate-co-terephthalate) degradation potential. Environ. Sci. Technol. 2021, 55, 4648–4657. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Murdoch, B.J.; Ball, A.S.; Ivanova, E.P.; Adhikari, B. Biodegradation of novel bioplastics made of starch, polyhydroxyurethanes and cellulose nanocrystals in soil environment. Sci. Total Environ. 2022, 815, 152684. [Google Scholar] [CrossRef]
- Hobbs, S.R.; Parameswaran, P.; Astmann, B.; Devkota, J.P.; Landis, A.E. Anaerobic codigestion of food waste and polylactic acid: Effect of pretreatment on methane yield and solid reduction. Adv. Mater. Sci. Eng. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Üveges, Z.; Damak, M.; Klátyik, S.; Ramay, M.W.; Fekete, G.; Varga, Z.; Gyuricza, C.; Székács, A.; Aleksza, L. Biomethane Potential in Anaerobic Biodegradation of Commercial Bioplastic Materials. Fermentation 2023, 9, 261. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.H.; Fu, J.; Dai, Y.; Tong, Y.W. Valorization of poly-β-hydroxybutyrate (PHB)-based bioplastic waste in anaerobic digesters of food waste for bioenergy generation: Reactor performance, microbial community analysis, and bioplastic biodegradation. Carbon Neutrality 2022, 1, 8. [Google Scholar] [CrossRef]
- Ebrahimzade, I.; Ebrahimi-Nik, M.; Rohani, A.; Tedesco, S. Towards monitoring biodegradation of starch-based bioplastic in anaerobic condition: Finding a proper kinetic model. Bioresour. Technol. 2022, 347, 126661. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Bordel, S.; Lebrero, R.; Santos-Beneit, F.; Börner, R.A.; Börner, T.; Muñoz, R. Inspired by nature: Microbial production, degradation and valorization of biodegradable bioplastics for life-cycle-engineered products. Biotechnol. Adv. 2021, 53, 107772. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzade, I.; Ebrahimi-Nik, M.; Rohani, A.; Tedesco, S. Higher energy conversion efficiency in anaerobic degradation of bioplastic by response surface methodology. J. Clean. Prod. 2021, 290, 125840. [Google Scholar] [CrossRef]
- Álvarez-Méndez, S.J.; Ramos-Suárez, J.L.; Ritter, A.; González, J.M.; Pérez, Á.C. Anaerobic digestion of commercial PLA and PBAT biodegradable plastic bags: Potential biogas production and 1H NMR and ATR-FTIR assessed biodegradation. Heliyon 2023, 9, e16691. [Google Scholar] [CrossRef]
- Bracciale, M.P.; De Gioannis, G.; Falzarano, M.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Sarasini, F.; Tirillò, J.; Zonfa, T. Anaerobic biodegradation of disposable PLA-based products: Assessing the correlation with physical, chemical and microstructural properties. J. Hazard. Mater. 2023, 452, 131244. [Google Scholar] [CrossRef]
- Shrestha, A.; van-Eerten Jansen, M.C.; Acharya, B. Biodegradation of bioplastic using anaerobic digestion at retention time as per industrial biogas plant and international norms. Sustainability 2020, 12, 4231. [Google Scholar] [CrossRef]
- Raunhan, R.; Jantharadej, K.; Mhuantong, W.; Napathorn, S.C.; Suwannasilp, B.B. Valorization of food waste derived anaerobic digestate into polyhydroxyalkanoate (PHA) using Thauera mechernichensis TL1. Waste Manag. 2023, 171, 248–258. [Google Scholar] [CrossRef]
- Jeon, Y.; Jin, H.; Kong, Y.; Cha, H.G.; Lee, B.W.; Yu, K.; Yi, B.; Kim, H.T.; Joo, J.C.; Yang, Y.H.; et al. Poly (3-hydroxybutyrate) Degradation by Bacillus infantis sp. Isolated from Soil and Identification of phaZ and bdhA Expressing PHB Depolymerase. J. Microbiol. Biotechnol. 2023, 33, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Dell, E.; Lewis, C.; Trabold, T.A.; Diaz, C.A. Anaerobic biodegradation of bioplastic packaging materials. In Proceedings of the 21st IAPRI World Conference on Packaging, Zhuhai, China, 19–22 June 2018; Destech Publications: Lancaster, PA, USA, 2018. [Google Scholar]
- Folino, A.; Pangallo, D.; Calabrò, P.S. Assessing bioplastics biodegradability by standard and research methods: Current trends and open issues. J. Environ. Chem. Eng. 2023, 11, 109424. [Google Scholar] [CrossRef]
- Dahdah, K.; Charchar, N.; Bouchaala, L.; Nourine, H.; Belkabla, N.; Melo, J.; Nabti, E.H. Isolation, in vitro evaluation and construction of Versatile Microbial Consortia. Cell. Mol. Biol. 2022, 68, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Binti Jalani, J.C.; Arshad, Z.I.M. PLA Degradation and PLA-Degrading Bacteria: A Mini-Review. Key Eng. Mater. 2022, 932, 103–110. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gupta, A. Current trends in applicability of thermophiles and thermozymes in bioremediation of environmental pollutants. In Microbial Extremozymes; Academic Press: Cambridge, MA, USA, 2022; pp. 161–176. [Google Scholar]
- Ali, S.; Rehman, A.; Hussain, S.Z.; Bukhari, D.A. Characterization of plastic degrading bacteria isolated from sewage wastewater. Saudi J. Biol. Sci. 2023, 30, 103628. [Google Scholar] [CrossRef] [PubMed]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, V.; Chatterjee, S. Contribution of plastic and microplastic to global climate change and their conjoining impacts on the environment-A review. Sci. Total Environ. 2023, 875, 162627. [Google Scholar] [CrossRef]
- Feijoo, P.; Marín, A.; Samaniego-Aguilar, K.; Sánchez-Safont, E.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Effect of the Presence of Lignin from Woodflour on the Compostability of PHA-Based Biocomposites: Degradation, Biodegradation and Microbial Dynamics. Polymers 2023, 15, 2481. [Google Scholar] [CrossRef]
- Ruggero, F.; Roosa, S.; Onderwater, R.; Delacuvellerie, A.; Lotti, T.; Gori, R.; Lubello, C.; Wattiez, R. Characterization of bacterial communities responsible for bioplastics degradation during the thermophilic and the maturation phases of composting. J. Mater. Cycles Waste Manag. 2023, 25, 3270–3285. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Araneda, N.; Valdebenito, E.; Hansen, F.; Nuti, M. Microbial Community in the Composting Process and Its Positive Impact on the Soil Biota in Sustainable Agriculture. Agronomy 2023, 13, 542. [Google Scholar] [CrossRef]
- Lai, J.; Huang, H.; Lin, M.; Xu, Y.; Li, X.; Sun, B. Enzyme catalyzes ester bond synthesis and hydrolysis: The key step for sustainable usage of plastics. Front. Microbiol. 2023, 13, 1113705. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, Z.; Guo, N.; Zhou, Z.; Liu, Y.; Tang, Q. Microplastics spatiotemporal distribution and plastic-degrading bacteria identification in the sanitary and non-sanitary municipal solid waste landfills. J. Hazard. Mater. 2022, 438, 129452. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Wierckx, N.; Narancic, T.; Eberlein, C.; Wei, R.; Drzyzga, O.; Magnin, A.; Steffan, R. Consequences of microbial interactions with hydrocarbons, oils, and lipids: Biodegradation and bioremediation. In Handbook of Hydrocarbon and Lipid Microbiology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Wei, R.; Tiso, T.; Bertling, J.; O’Connor, K.; Blank, L.M.; Bornscheuer, U.T. Possibilities and limitations of biotechnological plastic degradation and recycling. Nat. Catal. 2020, 3, 867–871. [Google Scholar] [CrossRef]
- Skariyachan, S.; Taskeen, N.; Kishore, A.P.; Krishna, B.V.; Naidu, G. Novel consortia of Enterobacter and Pseudomonas formulated from cow dung exhibited enhanced biodegradation of polyethylene and polypropylene. J. Environ. Manag. 2021, 284, 112030. [Google Scholar] [CrossRef] [PubMed]
- Maroof, L.; Khan, I.; Yoo, H.S.; Kim, S.; Park, H.T.; Ahmad, B.; Azam, S. Identification and characterization of low density polyethylene-degrading bacteria isolated from soils of waste disposal sites. Environ. Eng. Res. 2021, 26, 200167. [Google Scholar] [CrossRef]
- Abdullah, H.; Othman, N.S.; Yaacob, N.S.; Ahmad, M.F.; Ibrahim, M.; Maniyam, M.N.; Azman, H.H. Low-Density Polyethylene (LDPE) Degradation by Malaysian Rhodococcus spp. Using Weight Reduction Test. Selangor Sci. Technol. Rev. (SeSTeR) 2021, 5, 41–47. [Google Scholar]
- Nag, M.; Lahiri, D.; Dutta, B.; Jadav, G.; Ray, R.R. Biodegradation of used polyethylene bags by a new marine strain of Alcaligenes faecalis LNDR-1. Environ. Sci. Pollut. Res. 2021, 28, 41365–41379. [Google Scholar] [CrossRef]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Sangale, M.K.; Ade, A.B. Rhizosphere of Avicennia marina (Forsk.) Vierh. as a landmark for polythene degrading bacteria. Environ. Sci. Pollut. Res. 2016, 23, 14621–14635. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. In vitro degradation of low-density polyethylene by new bacteria from larvae of the greater wax moth, Galleria mellonella. Can. J. Microbiol. 2021, 67, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.C. Screening and characterization of plastic degrading bacteria from garbage soil. Br. J. Environ. Sci. 2018, 6, 33–40. [Google Scholar]
- Agrawal, P.; Singh, R.K. Breaking down of polyethylene by Pseudomonas species. Int. J. Sci. Eng. Res. 2016, 7, 124–127. [Google Scholar]
- Sriningsih, A.; Shovitri, M. Potensi isolat bakteri Pseudomonas sebagai pendegradasi plastik. J. Sains. Dan. Seni. ITS 2016, 4. [Google Scholar]
- Asmita, K.; Shubhamsingh, T.; Tejashree, S. Isolation of plastic degrading micro-organisms from soil samples collected at various locations in Mumbai, India. Int. Res. J. Environ. Sci. 2015, 4, 77–85. [Google Scholar]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Labrenz, M. Marine microbial assemblages on microplastics: Diversity, adaptation, and role in degradation. Annu. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef]
- Jamil, S.U.; Zada, S.; Khan, I.; Sajjad, W.; Rafiq, M.; Shah, A.A.; Hasan, F. Biodegradation of polyethylene by bacterial strains isolated from Kashmir Cave, Buner, Pakistan. J. Cave Karst Stud. 2017, 79, 73–80. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef]
- Duddu, M.K.; Tripura, K.L.; Guntuku, G.; Divya, D.S. Biodegradation of low density polyethylene (LDPE) by a new biosurfactant-producing thermophilic Streptomyces coelicoflavus NBRC 15399T. Afr. J. Biotechnol. 2015, 14, 327–340. [Google Scholar]
- Soud, S.A. Biodegradation of Polyethylene LDPE plastic waste using Locally Isolated Streptomyces sp. J. Pharm. Sci. Res. 2019, 11, 1333–1339. [Google Scholar]
- Waithaka, P.N.; Gathuru, E.M.; Githaiga, B.M.; Ochieng, E.O.; Linet, L.T. Microbial Degradation of Polythene using Actinomycetes Isolated from Maize Rhizosphere, Forest and Waste Damping sites within Egerton University, Kenya. Int. J. Emerg. Technol. 2017, 8, 5–10. [Google Scholar]
- Riandi, M.I.; Kawuri, R.; Sudirga, S.K. Potential of pseudomonas sp. and Ochrobacterum sp. isolated from various soil sample as degrading bacteria of high density polyethylene (HDPE) and low density polyethylene (LDPE) plastic. SIMBIOSIS J. Biol. Sci. 2017, 5, 58–63. [Google Scholar] [CrossRef]
- Helen, A.S.; Uche, E.C.; Hamid, F.S. Screening for polypropylene degradation potential of bacteria isolated from mangrove ecosystems in Peninsular Malaysia. Int. J. Biosci. Biochem. Bioinform. 2017, 7, 245–251. [Google Scholar] [CrossRef]
- Hayase, N.; Yano, H.; Kudoh, E.; Tsutsumi, C.; Ushio, K.; Miyahara, Y.; Tanaka, S.; Nakagawa, K. Isolation and characterization of poly (butylene succinate-co-butylene adipate)-degrading microorganism. J. Biosci. Bioeng. 2004, 97, 131–133. [Google Scholar] [CrossRef]
- Teeraphatpornchai, T.; Nakajima-Kambe, T.; Shigeno-Akutsu, Y.; Nakayama, M.; Nomura, N.; Nakahara, T.; Uchiyama, H. Isolation and characterization of a bacterium that degrades various polyester-based biodegradable plastics. Biotechnol. Lett. 2003, 25, 23–28. [Google Scholar] [CrossRef]
- Sriyapai, P.; Chansiri, K.; Sriyapai, T. Isolation and characterization of polyester-based plastics-degrading bacteria from compost soils. Microbiology 2018, 87, 290–300. [Google Scholar] [CrossRef]
- Kim, M.Y.; Kim, C.; Moon, J.; Heo, J.; Jung, S.P.; Kim, J.R. Polymer film-based screening and isolation of polylactic acid (PLA)-degrading microorganisms. J. Microbiol. Biotechnol. 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Aly, M.M.; Tork, S.; Qari, H.A.; Al-Seeni, M.N. Poly-A3/4-hydroxy butyrate depolymerase from Streptomyces lydicus MM10, isolated from wastewater sample. Int. J. Agric. Biol. 2015, 17, 891–900. [Google Scholar]
- Aburas, M.M.A. Degradation of poly (3-hydroxybuthyrate) using Aspergillus oryzae obtained from uncultivated soil. Life Sci. J. 2016, 13, 51–56. [Google Scholar]
- Sekiguchi, T.; Sato, T.; Enoki, M.; Kanehiro, H.; Uematsu, K.; Kato, C. Isolation and characterization of biodegradable plastic degrading bacteria from deep-sea environments. JAMSTEC Rep. Res. Dev. 2011, 11, 33–41. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Strzelecki, M.C.; Mirończuk, A.M. The potential of cold-adapted microorganisms for biodegradation of bioplastics. Waste Manag. 2021, 119, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Park, S.L.; Kim, S.H.; Jung, H.J.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Novel Poly (butylene adipate-co-terephthalate)-degrading Bacillus sp. JY35 from wastewater sludge and its broad degradation of various bioplastics. Waste Manag. 2022, 144, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Zhang, M.; Weng, Y.; Li, C. Degradation of polylactic acid/polybutylene adipate-co-terephthalate by coculture of Pseudomonas mendocina and Actinomucor elegans. J. Hazard. Mater. 2021, 403, 123679. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Park, S.L.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Ham, S.; Bhatia, S.K.; Gurav, R.; Park, S.H.; Park, K.; et al. Polyhydroxyalkanoates (PHAs) degradation by the newly isolated marine Bacillus sp. JY14. Chemosphere 2021, 283, 131172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, J.Y.; Cho, D.H.; Jung, H.J.; Kim, B.C.; Bhatia, S.K.; Park, S.H.; Park, K.; Yang, Y.H. Acceleration of Polybutylene Succinate Biodegradation by Terribacillus sp. JY49 Isolated from a Marine Environment. Polymers 2022, 14, 3978. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Crispim, C.A.; Gaylarde, C.C. Cyanobacteria and biodeterioration of cultural heritage: A review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Okshevsky, M.; Gautier, E.; Farner, J.M.; Schreiber, L.; Tufenkji, N. Biofilm formation by marine bacteria is impacted by concentration and surface functionalization of polystyrene nanoparticles in a species-specific manner. Environ. Microbiol. Rep. 2020, 12, 203–213. [Google Scholar] [CrossRef]
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021, 412, 125227. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly (glycolic acid)(PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.M.; Silas, K.; Aji, M.M. An elaborate breakdown of the essentials of biogas production. J. Eng. Res. Sci. 2022, 1, 93–118. [Google Scholar] [CrossRef]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Kasuya, K.I. Biodegradability of poly (3-hydroxyalkanoate) and poly (ε-caprolactone) via biological carbon cycles in marine environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, S.; Ray, S. Study of microbes having potentiality for biodegradation of plastics. Environ. Sci. Pollut. Res. 2013, 20, 4339–4355. [Google Scholar] [CrossRef] [PubMed]
- Malafeev, K.V.; Apicella, A.; Incarnato, L.; Scarfato, P. Understanding the Impact of Biodegradable Microplastics on Living Organisms Entering the Food Chain: A Review. Polymers 2023, 15, 3680. [Google Scholar] [CrossRef]
- Horie, Y.; Okamura, H. Ecotoxicity Assessment of Biodegradable Plastics in Marine Environments. In Photo-Switched Biodegradation of Bioplastics in Marine Environments; Springer Nature: Singapore, 2023; pp. 135–152. [Google Scholar]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are bioplastics and plant-based materials safer than conventional plastics? In vitro toxicity and chemical composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Bejgarn, S.; MacLeod, M.; Bogdal, C.; Breitholtz, M. Toxicity of leachate from weathering plastics: An exploratory screening study with Nitocra spinipes. Chemosphere 2015, 132, 114–119. [Google Scholar] [CrossRef]
- Lithner, D.; Damberg, J.; Dave, G.; Larsson, Å. Leachates from plastic consumer products–screening for toxicity with Daphnia magna. Chemosphere 2009, 74, 1195–1200. [Google Scholar] [CrossRef]
- Li, H.X.; Getzinger, G.J.; Ferguson, P.L.; Orihuela, B.; Zhu, M.; Rittschof, D. Effects of toxic leachate from commercial plastics on larval survival and settlement of the barnacle Amphibalanus amphitrite. Environ. Sci. Technol. 2016, 50, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.M.; Grant, J.; Fard, P.S.; Farner, J.M.; Tufenkji, N. Analysis of ultraviolet and thermal degradations of four common microplastics and evidence of nanoparticle release. J. Hazard. Mater. Lett. 2023, 4, 100078. [Google Scholar] [CrossRef]
- Miglioli, A.; Balbi, T.; Besnardeau, L.; Dumollard, R.; Canesi, L. Bisphenol A interferes with first shell formation and development of the serotoninergic system in early larval stages of Mytilus galloprovincialis. Sci. Total Environ. 2021, 758, 144003. [Google Scholar] [CrossRef]
- Charoeythornkhajhornchai, P.; Kunjiek, T.; Chaipayang, S.; Phosri, S. Toxicity assessment of bioplastics on brine shrimp (Artemia franciscana) and cell lines. Emerg. Contam. 2023, 9, 100253. [Google Scholar] [CrossRef]
- Monikh, F.A.; Durão, M.; Kipriianov, P.V.; Huuskonen, H.; Kekäläinen, J.; Uusi-Heikkilä, S.; Uurasjärvi, E.; Akkanen, J.; Kortet, R. Chemical composition and particle size influence the toxicity of nanoscale plastic debris and their co-occurring benzo (α) pyrene in the model aquatic organisms Daphnia magna and Danio rerio. NanoImpact 2022, 25, 100382. [Google Scholar] [CrossRef]
- Li, R.Y.; Liu, Z.G.; Liu, H.Q.; Chen, L.; Liu, J.F.; Pan, Y.H. Evaluation of biocompatibility and toxicity of biodegradable poly (DL-lactic acid) films. Am. J. Transl. Res. 2015, 7, 1357. [Google Scholar]
- Malafaia, G.; Nascimento, Í.F.; Estrela, F.N.; Guimarães, A.T.B.; Ribeiro, F.; da Luz, T.M.; de Lima Rodrigues, A.S. Green toxicology approach involving polylactic acid biomicroplastics and neotropical tadpoles:(Eco) toxicological safety or environmental hazard? Sci. Total Environ. 2021, 783, 146994. [Google Scholar] [CrossRef]
- Fojt, J.; David, J.; Přikryl, R.; Řezáčová, V.; Kučerík, J. A critical review of the overlooked challenge of determining micro-bioplastics in soil. Sci. Total Environ. 2020, 745, 140975. [Google Scholar] [CrossRef]
- Chah, C.N.; Banerjee, A.; Gadi, V.K.; Sekharan, S.; Katiyar, V. A systematic review on bioplastic-soil interaction: Exploring the effects of residual bioplastics on the soil geoenvironment. Sci. Total Environ. 2022, 851, 158311. [Google Scholar] [CrossRef] [PubMed]
- Satti, S.M.; Shah, A.A.; Marsh, T.L.; Auras, R. Biodegradation of poly (lactic acid) in soil microcosms at ambient temperature: Evaluation of natural attenuation, bio-augmentation and bio-stimulation. J. Polym. Environ. 2018, 26, 3848–3857. [Google Scholar] [CrossRef]
- Greenfield, L.M.; Graf, M.; Rengaraj, S.; Bargiela, R.; Williams, G.; Golyshin, P.N.; Chadwick, D.R.; Jones, D.L. Field response of N2O emissions, microbial communities, soil biochemical processes and winter barley growth to the addition of conventional and biodegradable microplastics. Agric. Ecosyst. Environ. 2022, 336, 108023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Isha; Chang, Y.-C. Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes 2023, 11, 3445. https://doi.org/10.3390/pr11123445
Ali S, Isha, Chang Y-C. Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes. 2023; 11(12):3445. https://doi.org/10.3390/pr11123445
Chicago/Turabian StyleAli, Shakir, Isha, and Young-Cheol Chang. 2023. "Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review" Processes 11, no. 12: 3445. https://doi.org/10.3390/pr11123445
APA StyleAli, S., Isha, & Chang, Y.-C. (2023). Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes, 11(12), 3445. https://doi.org/10.3390/pr11123445