Low-Pressure Hydrothermal Processing of Disposable Face Masks into Oils
Abstract
:1. Introduction
2. Materials and Methods
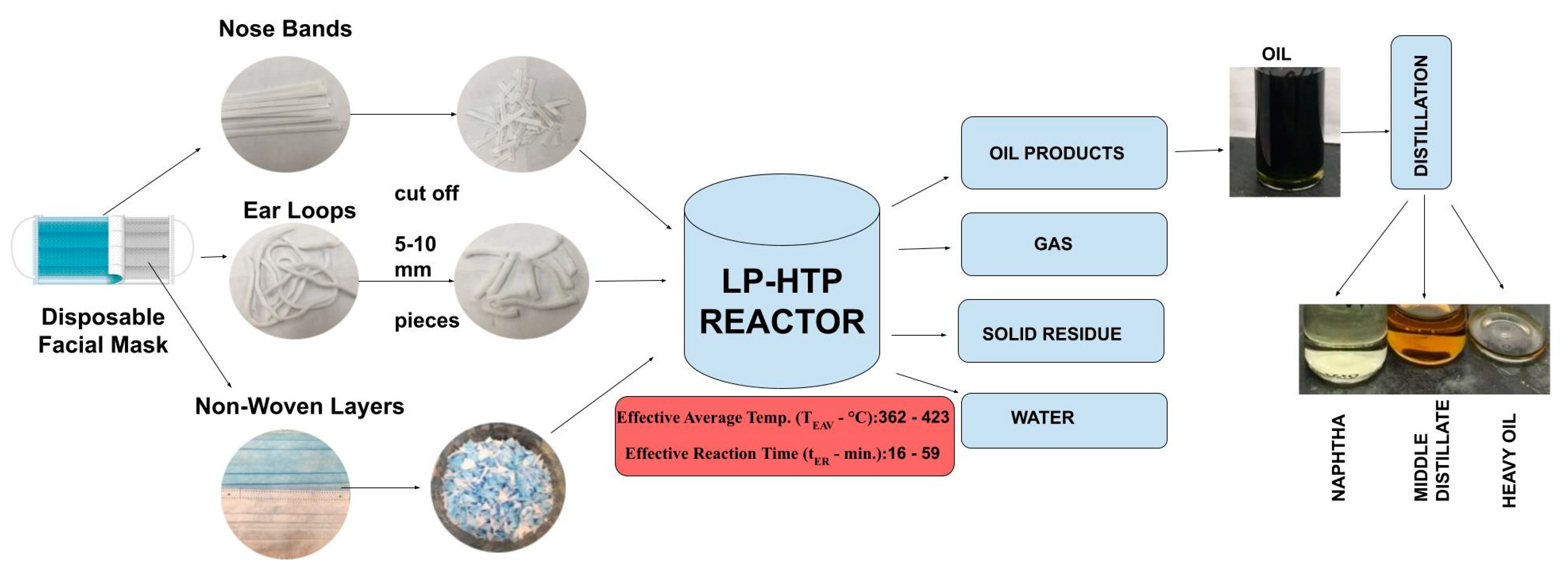
2.1. Feedstocks
2.2. Reactor Equipment
2.3. Experimental Methodology
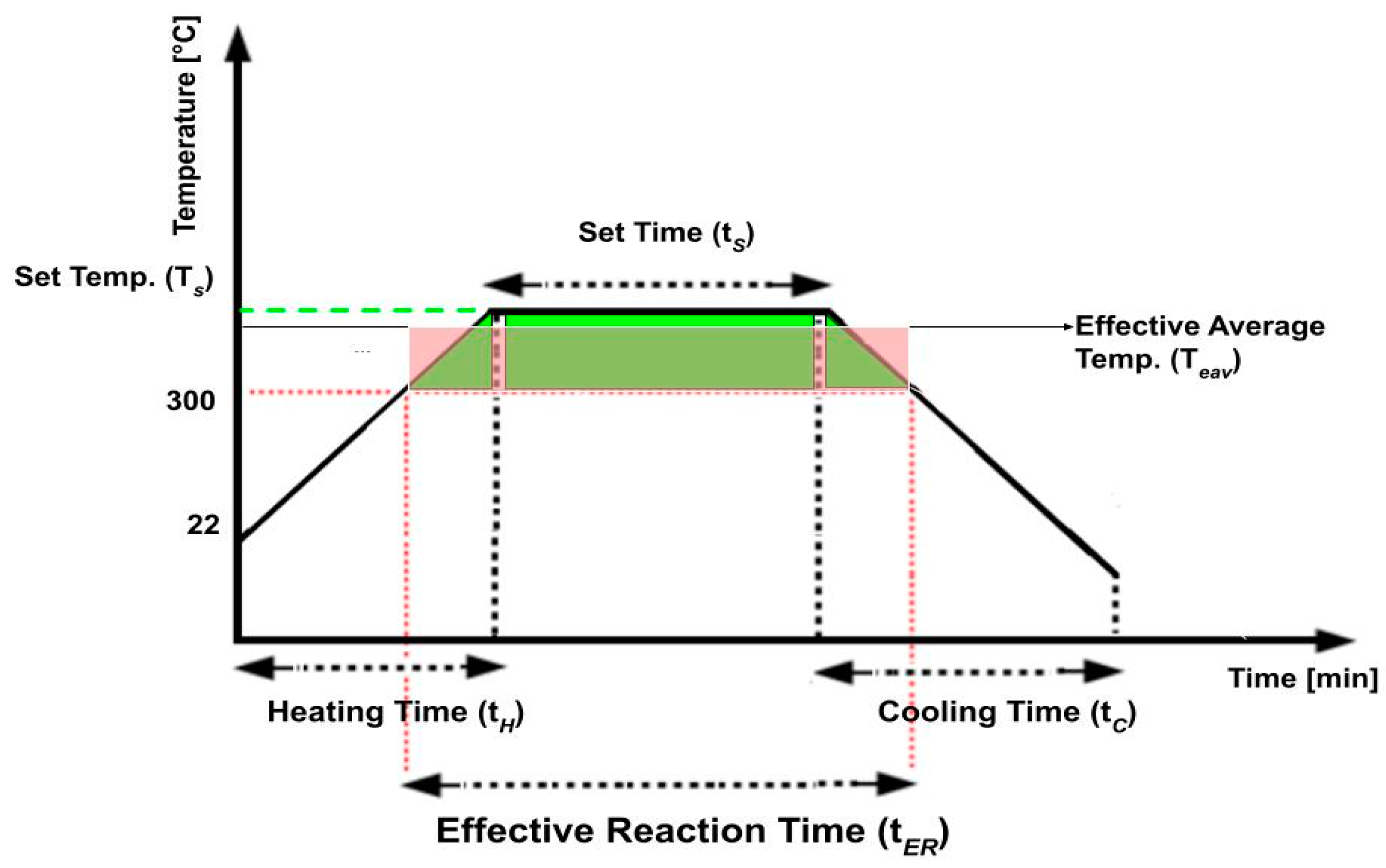
- Set Temperature (Ts): The temperature to which the feedstocks will be heated to for depolymerization.
- Set Time (tS): The duration for which the process is operated at the designated set temperature.
- Heating Time (tH): The time period during which the material is actively heated to reach the desired set temperature.
- Cooling Time (tC): The time period during which the material is allowed to cool down after the desired set time.
- Effective Reaction Time (tER): The time period during which the material depolymerized and conversion occurred. Based on preliminary experiments, no reaction was observed below 300 °C. Therefore, 300 °C was specified as the minimum temperature for conversion.
- Effective Average Temperature (TEAV): The average temperature for conversion throughout the entire effective reaction time (tER). This average temperature is calculated by dividing the area under the temperature profile (shown in green) by the effective reaction time (Figure 2), such that the area under the average temperature profile (shown in red) is equal to the green area.
2.4. Analysis of Liquid Products
2.4.1. Yield Calculation
2.4.2. Distillation
2.4.3. GC-FID
2.4.4. GCxGC-FID
3. Results and Discussions
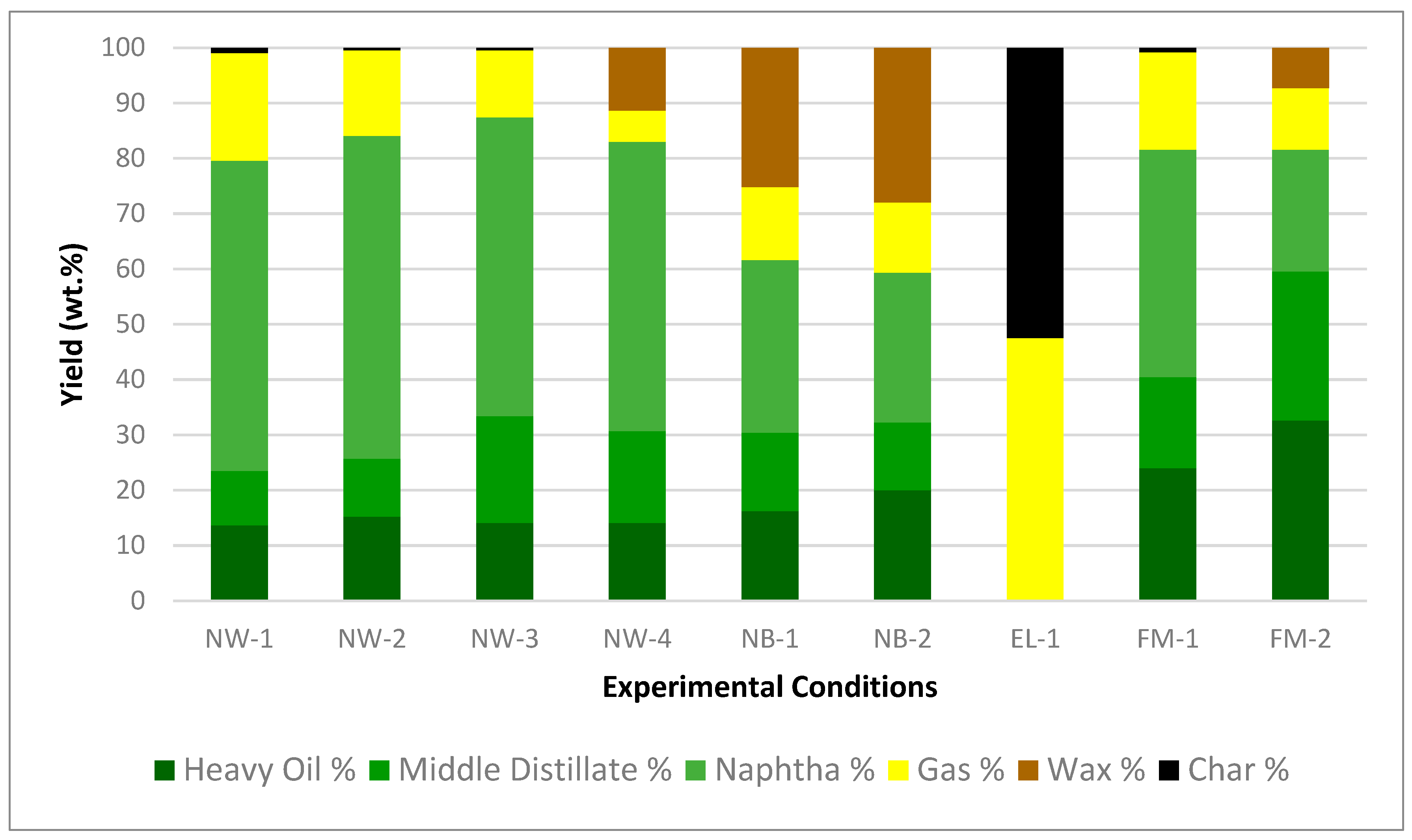
3.1. Product Yields
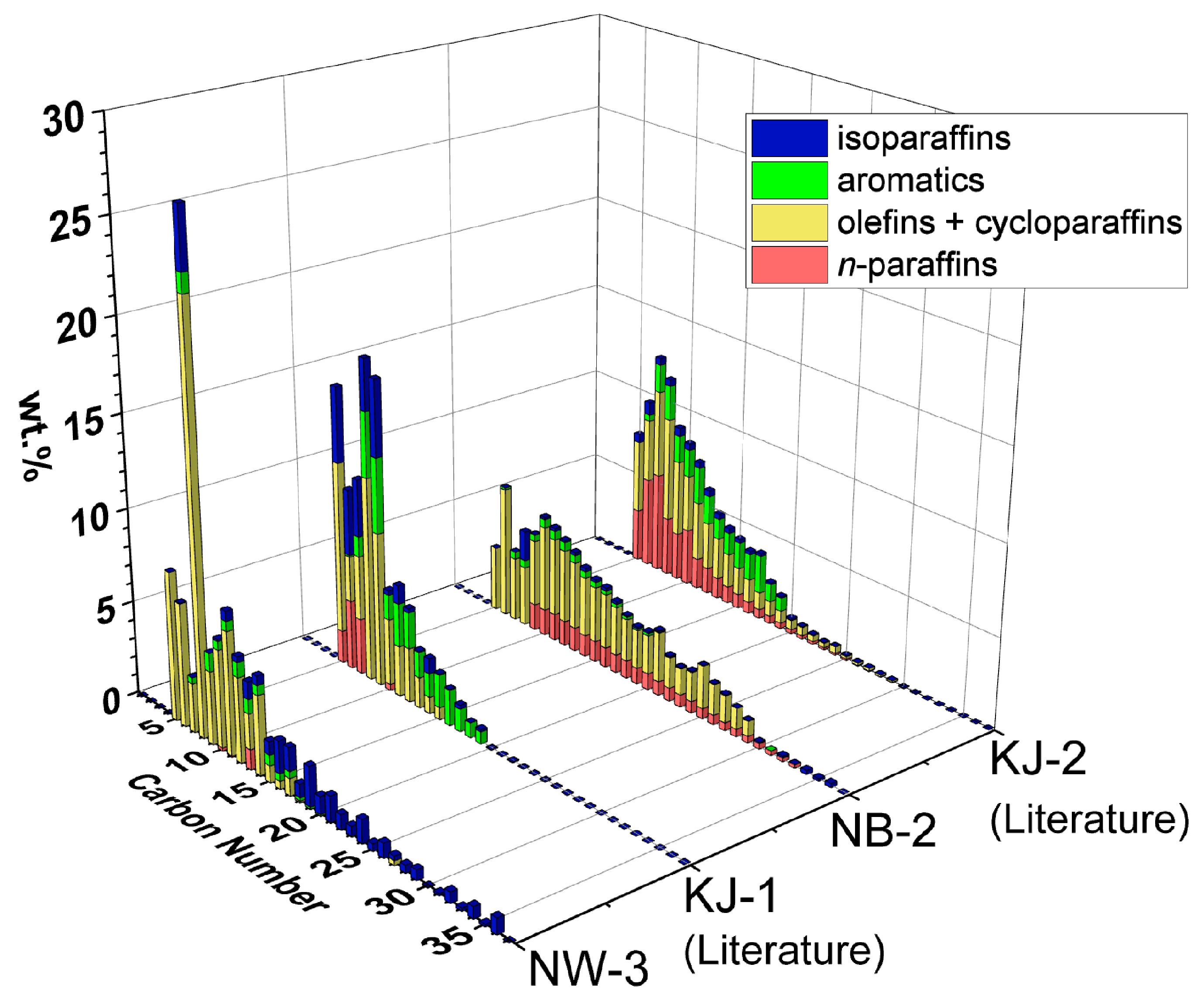
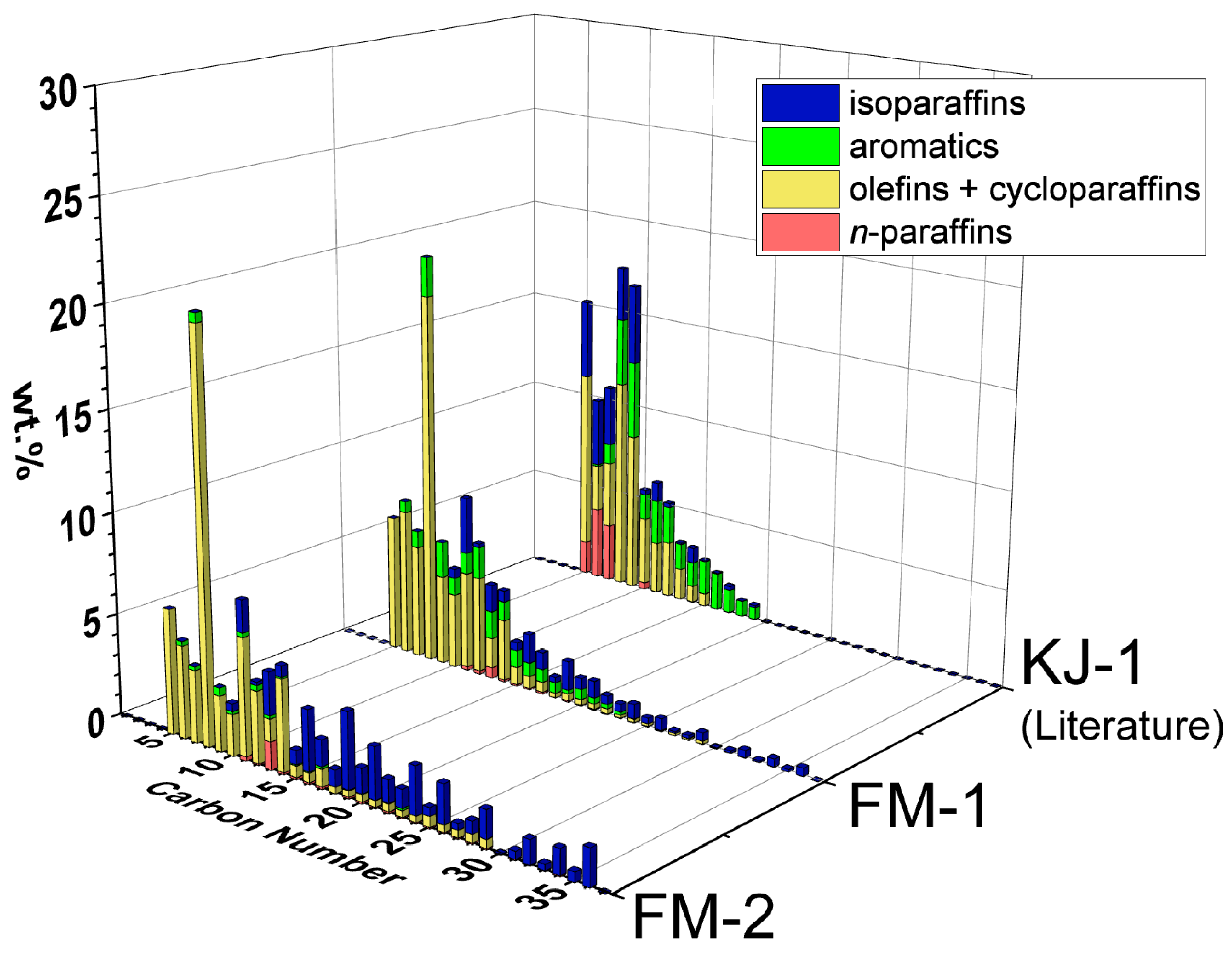
3.2. Chemical Compositions
3.3. Energy Consumption, GHG Emissions, and Potential Impacts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Jung, S.; Lee, S.; Dou, X.; Kwon, E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021, 405, 126658. [Google Scholar] [CrossRef] [PubMed]
- Phelps Bondaroff, T.; Cooke, S. Masks on the Beach: The Impact of COVID-19 on Marine Plastic Pollution; OceansAsia: Hong Kong, China, 2020. [Google Scholar]
- Plastics Pandemic: The COVID-19 Pandemic is Adding to the Glut of Plastic Waste, United Nations Environment Programme. Available online: https://news.un.org/en/story/2020/07/1069151 (accessed on 30 August 2023).
- Yang, Q.; Yang, S.; Jiao, Y. Assessing disposable masks consumption and littering in the post COVID-19 pandemic in China. Environ. Pollut. 2023, 334, 122190. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, Y.; Ge, W.; Wu, J.; Song, N.; Yin, Z.; Chai, C. Release kinetics of microplastics from disposable face masks into the aqueous environment. Sci. Total Environ. 2022, 816, 151650. [Google Scholar]
- Cao, J.; Shi, Y.; Yan, M.; Zhu, H.; Chen, S.; Xu, K.; Wang, L.; Sun, H. Face Mask: As a Source or Protector of Human Exposure to Microplastics and Phthalate Plasticizers? Toxics 2023, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.L.; Ambrose-Dempster, E.; Aparsi, D.T.; Bawn, M.; Casas Arredondo, M.; Chau, C.; Chandler, K.; Dobrijevic, D.; Hailes, H.; Lettieri, P. The Environmental Dangers of Employing Single-Use Face Masks as Part of a COVID-19 Exit Strategy. UCL Open Environ. 2020. [Google Scholar] [CrossRef]
- Aragaw, T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar]
- Cudjoe, D.; Wang, H. Thermochemical treatment of daily COVID-19 single-use facemask waste: Power generation potential and environmental impact analysis. Energy 2022, 249, 123707. [Google Scholar] [CrossRef]
- Vlasopoulos, A.; Malinauskaite, J.; Żabnieńska-Góra, A.; Jouhara, H. Life cycle assessment of plastic waste and energy recovery. Energy 2023, 277, 127576. [Google Scholar]
- Ilyas, S.; Srivastava, R.R.; Kim, H. Disinfection technology and strategies for COVID-19 hospital and bio-medical waste management. Sci. Total Environ. 2020, 749, 141652. [Google Scholar]
- Nam, J.Y.; Tokmurzin, D.; Yoon, S.M.; Ra, H.W.; Lee, J.G.; Lee, D.H.; Seo, M.W. Carbon dioxide gasification characteristics of disposable COVID-19 masks using bubbling fluidized bed reactor. Environ. Res. 2023, 235, 116669. [Google Scholar] [CrossRef]
- Nam, J.Y.; Lee, T.R.; Tokmurzin, D.; Park, S.J.; Ra, H.W.; Yoon, S.J.; Mun, T.Y.; Yoon, S.M.; Moon, J.H.; Lee, J.G.; et al. Hydrogen-rich gas production from disposable COVID-19 mask by steam gasification. Fuel 2023, 331, 125720. [Google Scholar] [PubMed]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [PubMed]
- Aydin, K.; Ün, Ç. Development of Solid Waste Management System for Adana Metropolitan Municipality. In Exergy for A Better Environment and Improved Sustainability 2; Aloui, F., Dincer, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 115–131. [Google Scholar]
- Aragaw, T.A.; Mekonnen, B.A. Current plastics pollution threats due to COVID-19 and its possible mitigation techniques: A waste-to-energy conversion via Pyrolysis. Environ. Syst. Res. 2021, 10, 8. [Google Scholar]
- Fu, Z.; Zhang, Y.S.; Ji, G.; Li, A. Hydrothermal transformation behavior and degradation pathway analysis of waste surgical masks in supercritical water. Process Saf. Environ. Prot. 2023, 176, 776–785. [Google Scholar] [CrossRef]
- Chen, W.T.; Jin, K.; Wang, N.H.L. Use of Supercritical Water for the Liquefaction of Polypropylene into Oil. ACS Sustain. Chem. Eng. 2019, 7, 3749–3758. [Google Scholar] [CrossRef]
- Jin, K.; Vozka, P.; Gentilcore, C.; Kilaz, G.; Wang, N.H.L. Low-pressure hydrothermal processing of mixed polyolefin wastes into clean fuels. Fuel 2021, 294, 120505. [Google Scholar]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I. Pyrolysis of all layers of surgical mask waste as a mixture and its life-cycle assessment. Sustain. Prod. Consum. 2022, 32, 519–531. [Google Scholar] [CrossRef]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of waste surgical masks into liquid fuel and its life-cycle assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar]
- Park, C.; Choi, H.; Lin, K.Y.A.; Kwon, E.E.; Lee, J. COVID-19 mask waste to energy via thermochemical pathway: Effect of Co-Feeding food waste. Energy 2021, 230, 120876. [Google Scholar]
- Lee, S.B.; Lee, J.; Tsang, Y.F.; Kim, Y.M.; Jae, J.; Jung, S.C.; Park, Y.K. Production of value-added aromatics from wasted COVID-19 mask via catalytic pyrolysis. Environ. Pollut. 2021, 283, 117060. [Google Scholar]
- Sun, X.; Liu, Z.; Shi, L.; Liu, Q. Pyrolysis of COVID-19 disposable masks and catalytic cracking of the volatiles. J. Anal. Appl. Pyrolysis 2022, 163, 105481. [Google Scholar] [CrossRef] [PubMed]
- Full Plastic Nose Wire Specification Report. Available online: https://www.fysensi.com/products/full-plastic-nose-wire/ (accessed on 30 August 2023).
- Hubbard, V.M.; Brunson, W.K. Face Mask with Ear Loops and Method for Forming. U.S. Patent 4941470, 17 July 1990. [Google Scholar]
- Kameel, N.I.A.; Daud, W.M.A.W.; Patah, M.F.A.; Zulkifli, N.W.M. Influence of reaction parameters on thermal liquefaction of plastic wastes into oil: A review. Energy Convers. Manag. X 2022, 14, 100196. [Google Scholar]
- ASTM D86-23; ASTM Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2023. Available online: https://www.astm.org/d0086-23.html (accessed on 30 August 2023).
- Vozka, P.; Kilaz, G. How to obtain a detailed chemical composition for middle distillates via GCxGC-FID without the need of GCxGC-TOF/MS. Fuel 2019, 247, 368–377. [Google Scholar] [CrossRef]
- Šindelářová, L.; Luu, E.N.; Vozka, P. Comparison of gas and kerosene oils chemical composition before and after hydrotreating using comprehensive two-dimensional gas chromatography. J. Chromatogr. Open 2022, 2, 100068. [Google Scholar] [CrossRef]
- Ortega, F.; Martín-Lara, M.Á.; Pula, H.J.; Zamorano, M.; Calero, M.; Blázquez, G. Characterization of the Products of the Catalytic Pyrolysis of Discarded COVID-19 Masks over Sepiolite. Appl. Sci. 2023, 13, 3188. [Google Scholar]
- Tenhunen-Lunkka, A.; Rommens, T.; Vanderreydt, I. Greenhouse Gas Emission Reduction Potential of European Union’s Circularity Related Targets for Plastics. Circ. Econ. Sust. 2023, 3, 475–510. [Google Scholar]
| Authors | Year | Material | Method | Temp. (°C) | Pressure (psig) | Time (min.) | Oil Yield % | Gas Yield % | Solid Yield (Char/Wax) % |
|---|---|---|---|---|---|---|---|---|---|
| S. Yousef et al. [21] | 2022 | Non-Woven | Pyrolysis | 500 | - | 60 | 42 | 54 | 4 (char) |
| Chao Li et al. [22] | 2022 | Non-Woven | Slow pyrolysis in fixed-bed reactor | 500 | - | 90 | 81 | 15 | 4 (char) |
| Park C. et al. [23] | 2021 | Non-Woven + Ear Loop | Pyrolysis in split-hinge tube furnace | 500–900 | - | - | 52–59 * | 48–41 | No char |
| Lee et al. [24] | 2021 | Full Mask | Non-catalytic pyrolysis in fixed bed reactor | 550 | - | 30 | 81 | 10 | 9 (char) |
| Sun X. et al. [25] | 2022 | Full Mask | Catalytic cracking pyrolysis in vertical quartz tube reactor | 440 | - | 1 ** | 75 | 23 | 2 (solid) |
| Fu et al. [18] | 2023 | Full Mask | Supercritical water (SWL) | 400 | - | 60 | 67 | 12 | 21 (solid) |
| Chen et al. [19] | 2019 | PP Pellets | Supercritical water (SWL) | 455 | 3336 | 30–60 | 91 | 9 | 0 |
| Jin et al. [20] | 2021 | PP Pellets | LP-HTP | 450 | 225 | 60 | 87 | 12 | 1 (char) |
| This Study | 2023 | Non-Woven | LP-HTP | 450 (392 ***) | 350 | ~1 (25 ****) | 87 | 12 | 1 (char) |
| This Study | 2023 | Full Mask | LP-HTP | 450 (396 ***) | 400 | 10 (32 ****) | 82 | 17 | 1 (char) |
| Parts | Weight Percentage (wt.%) | Chemical Compositions | Dimensions L × W × T * (cm) |
|---|---|---|---|
| Non-Woven (NW) Mask Body | 80.7 | Polypropylene (PP) | 1.5 × 1.5 × 0.1 |
| Nose band (NB) | 7.9 | High-Density Polyethylene (HDPE) ** [26] | 1.5 × 0.4 × 0.1 |
| Ear Loops (EL) | 11.4 | Polyester (90.3%) + Spandex (9.7%) [27] | 1.5 × 0.5 × 0.5 |
| Exp. No. | Set Temp., TS (°C) and Set Time, tS(min.) | Effective Average Temp., TEAV (°C) | Effective Reaction Time, tER (min.) | Oil Yield % | Gas Yield % | Wax Yield % | Char Yield % |
|---|---|---|---|---|---|---|---|
| NW-1 | 450–30 | 423 | 59 | 80 | 19 | 0 | 1 |
| NW-2 | 450–15 | 408 | 38 | 84 | 15 | 0 | 1 |
| NW-3 | 450–10 | 392 | 25 | 87 | 12 | 0 | 1 |
| NW-4 | 400–0 | 362 | 16 | 83 | 6 | 11 | 0 |
| KJ-1 (PP) [20] | 450–60 | 426 | 87 | 87 | 12 | 0 | 1 |
| NB-1 | 450–10 | 404 | 31 | 62 | 13 | 25 | 0 |
| NB-2 | 450–0 | 395 | 19 | 59 | 13 | 28 | 0 |
| KJ-2 (HDPE) [20] | 450–60 | 426 | 84 | 86 | 13 | 0 | 1 |
| EL-1 | 450–0 | 385 | 19 | - | 48 | 0 | 52 |
| EL-2 | 275–0 | <300 | 0 | - | 1 | 99 * | 0 |
| FM-1 (3 trials) | 450–10 | 401 ± 4 | 30 ± 2 | 82 ± 0.2 | 17 ± 0.2 | 0 | 1 ± 0.2 |
| FM-2 | 430–0 | 375 | 19 | 82 | 11 | 6 | 1 |
| FM-Theoretical (see Supplementary-Figure S2) | 450–10 | 396 | 32 | 75 | 16 | 2 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Un, C.; Gentilcore, C.; Ault, K.; Gieng, H.; Vozka, P.; Wang, N.-H.L. Low-Pressure Hydrothermal Processing of Disposable Face Masks into Oils. Processes 2023, 11, 2819. https://doi.org/10.3390/pr11102819
Un C, Gentilcore C, Ault K, Gieng H, Vozka P, Wang N-HL. Low-Pressure Hydrothermal Processing of Disposable Face Masks into Oils. Processes. 2023; 11(10):2819. https://doi.org/10.3390/pr11102819
Chicago/Turabian StyleUn, Cagri, Clayton Gentilcore, Kathryn Ault, Hung Gieng, Petr Vozka, and Nien-Hwa Linda Wang. 2023. "Low-Pressure Hydrothermal Processing of Disposable Face Masks into Oils" Processes 11, no. 10: 2819. https://doi.org/10.3390/pr11102819
APA StyleUn, C., Gentilcore, C., Ault, K., Gieng, H., Vozka, P., & Wang, N.-H. L. (2023). Low-Pressure Hydrothermal Processing of Disposable Face Masks into Oils. Processes, 11(10), 2819. https://doi.org/10.3390/pr11102819






