Bio-Innovative Pretreatment of Coarse Wool Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment Procedures
2.3. Dyeing
2.4. Characterization Methods
3. Results and Discussion
3.1. Coarse Wool Changes in Scouring Process
3.2. Coarse Wool Changes in Bleaching and Dyeing Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vujasinović, E.; Andrassy, M. Croatian wool—High performance fibre instead of waste. In Proceedings Natural Fibres in Australia; Wilson, C.A., Laing, R.M., Eds.; University of Otago: Dunedin, New Zealand, 2009; pp. 10–15. [Google Scholar]
- Erlač, E.; Soljačić, I.; Luktić, A. Struktura vunenog vlakna (Wool fibre structure). Tekstil 1996, 45, 255–262. [Google Scholar]
- Simpson, W.S.; Crawshaw, G.H. Wool: Science and Technology; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Ganci, M.; Biondi, L.; Parlato, M.C.M.; Porto, S.M.C. Methodology for the Localization of Wool Collecting Centers: The Case Study of Sicily. Sustainability 2022, 14, 10378. [Google Scholar] [CrossRef]
- Brown, J.C.; Stevens, C.B.; Whewell, C.S. Promjene u mokro obrađenoj vuni (The changes in wet treated wool). Tekstil 1970, 19, 569–584. [Google Scholar]
- Došen-Šver, D.; Pernar, E.; Bujević, I. Wastewater Treatment after Scourings of Raw Wool. Kem. Ind. 2007, 56, 575–581. [Google Scholar]
- Vujasinović, E.; Anić Vučinić, A.; Ljubas, D. Scouring of Wool and its Impacts on the Environment. Kem. Ind. 2007, 56, 569–574. [Google Scholar]
- Lewis, D.M.; Rippon, J.A. The Coloration of Wool and Other Keratin Fibres; John and Wiley and Sons in association with the Society of Dyers and Colourists: Bradford, UK, 2013. [Google Scholar]
- Vujasinović, E.; Andrassy, M. Istraživanje utjecaja medulacije na gustoću vune (Investigation of the impact of wool medullation on its density). Tekstil 2000, 49, 277–286. [Google Scholar]
- Obando, G.B.; Mejía, L.R.; Bustamante, F.A.C.; Gutiérrez, J.A.; Bonilla, M.D.Q.; Peña, E.C.Q. Evaluation of wool and luxury fiber medullation of some animal species. Rev. Investig. Vet. Peru 2021, 32, e17639. [Google Scholar]
- Vujasinović, E.; Andrassy, M. Istraživanje sposobnosti pustenja grube vune (The investigation of coarse wool felting property). Tekstil 2003, 52, 268–277. [Google Scholar]
- Sienra, I.; Neimaur, K.; Kremer, R.; Urioste, J.I. Medullated fibres and fleece characteristics in Corriedale hoggets from two flocks in Uruguay. Anim. Prod. Sci. 2011, 51, 1034–1038. [Google Scholar] [CrossRef]
- Bahi, A.; Jones, J.T.; Carr, C.M.; Ulijn, R.V.; Shao, J. Surface Characterization of Chemically Modified Wool. Text. Res. J. 2007, 77, 937–945. [Google Scholar] [CrossRef]
- Erlač, E.; Soljačić, I. Kemijske reakcije u procesima bijeljenja i apreture vune (Chemical reactions during the bleaching and finishing of wool. Tekstil 1993, 42, 379–393. [Google Scholar]
- Asquith, R.S. Chemistry of Natural Protein Fibres; John and Willey and Sons: Chichester, UK, 1977. [Google Scholar]
- Raffaelli, D.; Došen-Šver, D.; Vujasinović, E. Domaća vuna i pročišćavanje otpadnih voda nakon pranja sirove vune (Domestic wool and purification of waste waters after raw wool scouring). Kem. Ind. 1999, 48, 189–195. [Google Scholar]
- Mangovska, B.; Jordanov, I. Enzyme treatments of wool tops. Tekstil 2000, 49, 370–376. [Google Scholar]
- Jovančić, P.; Jocić, D.; Molina, R.; Manich, A.; Sauri, R.; Julia, M.R.; Erra, P. A comparative study of two wool enzyme treatments. Indian J. Fibre Text. Res. 2002, 27, 408–416. [Google Scholar]
- Arami, M.; Mazaheri, F.; Beglou, M.J. Proteolytic enzymes reactions on wool yarn and surfactants effects on the enzyme treatment. Fibers Polym. 2009, 10, 611–616. [Google Scholar] [CrossRef]
- Kotlińska, A.; Lipp-Symonowicz, B. Research on the Enzymatic Treatment of Wool Fibres and Changes in Selected Properties of Wool. Fibres Text. East. Eur. 2011, 19, 88–93. [Google Scholar]
- Mojsov, K. Enzymatic treatment of wool fabrics—opportunity of the improvement on some physical and chemical properties of the fabrics. J. Text. Inst. 2017, 108, 1136–1143. [Google Scholar]
- Das, T.; Ramaswamy, G.N. Enzyme Treatment of Wool and Specialty Hair Fibers. Text. Res. J. 2006, 76, 126–133. [Google Scholar] [CrossRef]
- Gomaa, S.K.; Zaki, R.A.; Wahba, M.I.; Taleb, M.A.; El-Refai, H.A.; El-Fiky, A.F.; El-Sayed, H. Green method for improving performance attributes of wool fibres using immobilized proteolytic thermozyme. Biotech 2022, 12, 254. [Google Scholar] [CrossRef]
- Demirkan, E.; Kut, D.; Sevgi, T.; Dogan, M.; Baygin, E. Investigation of effects of protease enzyme produced by Bacillus subtilis 168 E6–5 and commercial enzyme on physical properties of woolen fabric. J. Text. Inst. 2019, 111, 26–35. [Google Scholar] [CrossRef]
- Radetić, M.M.; Jocić, D.M.; Jovančić, P.M.; Petrović, Z.L. Low-temperature plasma modification of wool. Tekstil 2005, 54, 266–278. [Google Scholar]
- Parveen, S.; Rana, S.; Goswami, P. Developing Super-Hydrophobic and Abrasion-Resistant Wool Fabrics Using Low-PressureHexafluoroethane Plasma Treatment. Materials 2021, 14, 3228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Wang, A.Y. Modification of wool by air plasma and enzymes as a cleaner and environmentally friendly process. J. Clean. Prod. 2015, 87, 961–965. [Google Scholar] [CrossRef]
- Čunko, R.; Ercegović, S.; Gordoš, D.; Pezelj, E. Impact of ultrasound on physical properties of wool fibres. Tekstil 2006, 55, 1–10. [Google Scholar]
- Vílchez, S.; Manich, A.M.; Jovančić, P.; Erra, P. Chitosan contribution on wool treatments with enzyme. Carbohydr. Polym. 2008, 71, 515–523. [Google Scholar] [CrossRef]
- Dragčević, Z.; Soljačić, I.; Erlač, E. Kinetika reakcije vodik-peroksida u procesu bijeljenja vune (Kinetics of the reaction of hydrogen peroxide in the process of wool bleaching). Tekstil 1993, 42, 145–150. [Google Scholar]
- Karunditu, A.W.; Carr, C.M.; Dodd, K.; Mallinson, P.; Fleet, I.A.; Tetler, L.W. Activated Hydrogen Peroxide Bleaching of Wool. Text. Res. J. 1994, 64, 570–572. [Google Scholar]
- Gacen, J.; Cayuela, D. Comparison of wool bleaching with hydrogen peroxide in alkaline and acidic media. Color. Technol. 2000, 116, 13–15. [Google Scholar] [CrossRef]
- Cegarra, J.; Gacén, J.; Cayuela, D.; Riva, M.C. Comparison of stabilisers for wool bleaching with hydrogen peroxide. J. SDC 2008, 110, 308–310. [Google Scholar] [CrossRef]
- Millington, K.R. Bleaching and Whitening of Wool: Photostability of Whites. Chapter 5. In The Coloration of Wool and other Keratin Fibres, 1st ed.; Lewis, D.M., Rippon, J.A., Eds.; SDC (Society of Dyers and Colourists); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 131–155. [Google Scholar] [CrossRef]
- Yilmazer, D.; Kanik, M. Bleaching of Wool with Sodium Borohydride. J. Eng. Fibers Fabr. 2009, 4, 45–50. [Google Scholar] [CrossRef]
- Chen, W.; Chen, D.; Wang, X. Surface Modification and Bleaching of Pigmented Wool. Text. Res. J. 2001, 71, 441–445. [Google Scholar] [CrossRef]
- Tarbuk, A.; Dekanić, T.; Pušić, T. Oxidative bleaching of woolen fabrics. In Proceedings of the 9th International Textile, Clothing & Design Conference, Zagreb, Croatia, 7–10 October 2018; pp. 151–156. [Google Scholar]
- Khanmohammadi, M.; Mashkuri, N.; Rostami, M.; Garmarudi, A.B. Quantitative Determination of Sodium Perborate and Sodium Percarbonate in Detergent Powders by Infrared Spectrometry. J. Anal. Chem. 2012, 67, 330–334. [Google Scholar] [CrossRef]
- Li, Z. Bleaching process of cashmere with sodium percarbonate. Wool Text. J. 2014, 42, 45–47. [Google Scholar]
- Levene, R. Enzyme-enhanced bleaching of wool. J. SDC 2008, 113, 206–210. [Google Scholar] [CrossRef]
- Raffaelli, D.; Vujasinović, E. Bubrenje keratinskih vlakana u vodi (Swelling of keratin fibers in water). Tekstil 1990, 39, 515–522. [Google Scholar]
- Merck, E.A.G. Chemish-Technishe Untersuchungs Methoden fur die Textiindustrie; Verlag Chemie Gmbh: Weinheim, Germany, 1961. [Google Scholar]
- Bradbury, J.H.; Leeder, J.D. Keratin Fibres VI. Mechanism of the Allworden Reaction. Aust. J. Biol. Sci. 1972, 25, 133–138. [Google Scholar] [CrossRef]
- IWTO-8–2011; Method of Determining Fibre Diameter Distribution Parameters and Percentage of Medullated Fibres in Wool and other Animal Fibres by the Projection Microscope. International Wool Textile Organisation: Brussels, Belgium.
- Iglesias, M.S.; Sequeiros, C.; García, S.; Olivera, N.L. Eco-friendly anti-felting treatment of wool top based on biosurfactant and enzymes. J. Clean. Prod. 2019, 220, 846–852. [Google Scholar] [CrossRef]
- Rippon, J.A.; Evans, D.J. Improving the Properties of Natural Fibres by Chemical Treatments. Chapter 3 in Handbook of Natural Fibres, Processing and Applications; Woodhead Publishing Series in Textiles: Sawston, Cambridge, UK, 2012; Volume 2, pp. 63–140. [Google Scholar]
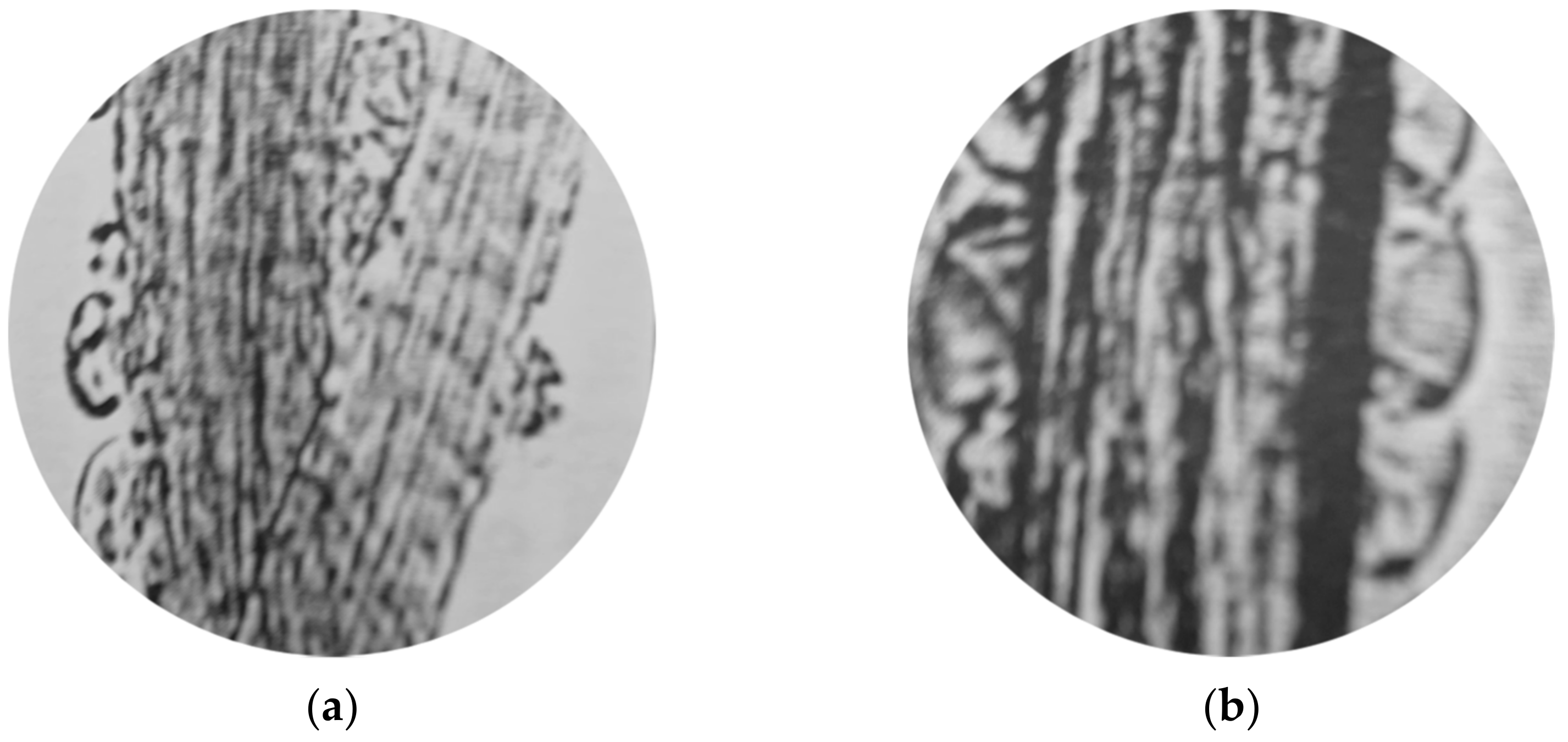
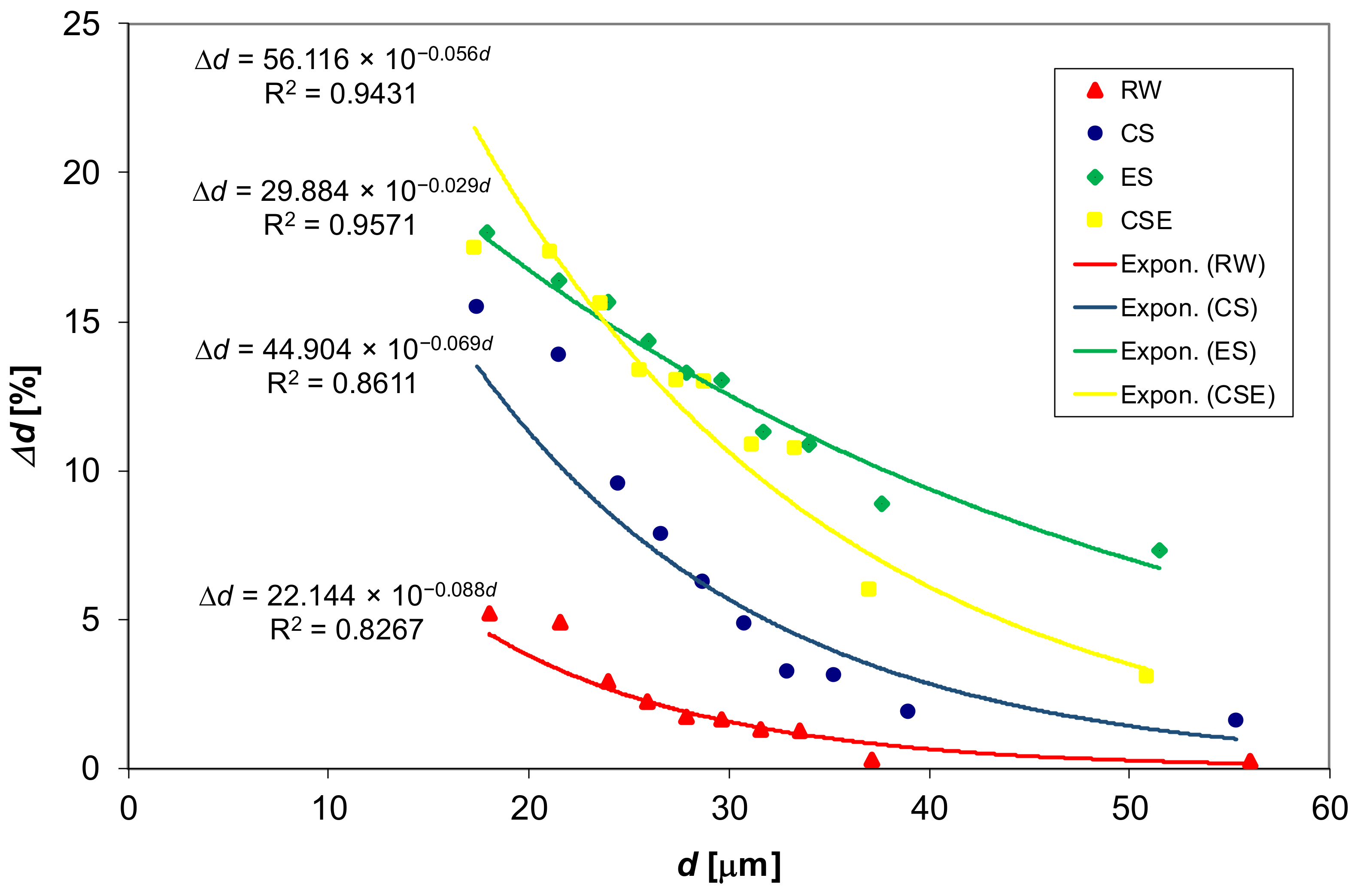
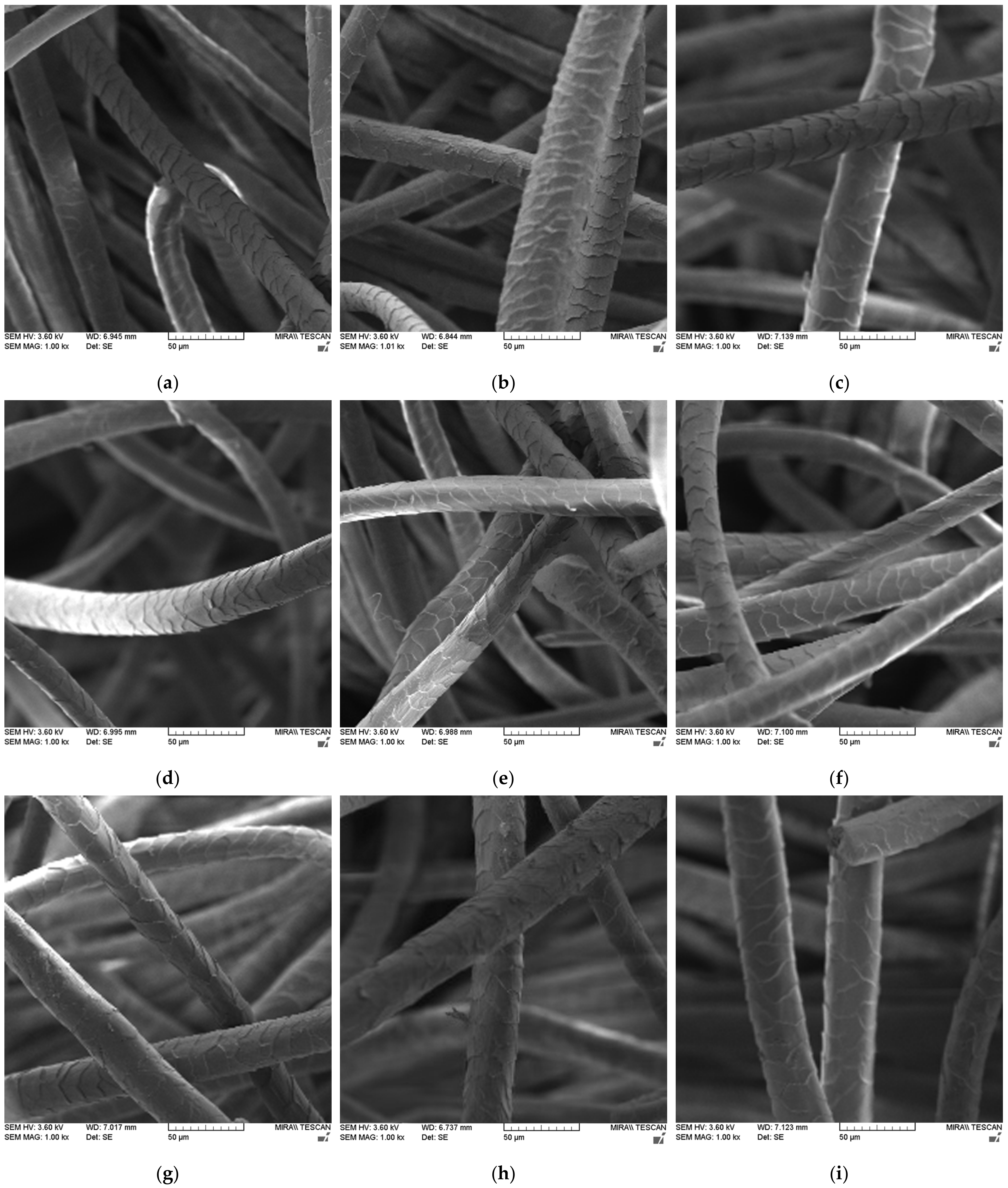
| Wool | Δmscouring [%] | mclean wool [%] | mgrease [%] | mdirt [%] |
|---|---|---|---|---|
| RW | - | - | 4.91 | 14.20 |
| CS | 30.13 | 69.87 | 0.99 a | 12.90 b |
| ES | 27.27 | 72.73 | 0.84 a | 13.10 b |
| CS-E | 29.15 | 70.85 | 0.75 a | 13.80 b |
| Wool | AS [%] | UBS [%] | tKMV [min] | tA [min] |
|---|---|---|---|---|
| RW | 18.60 | 48.20 | - | - |
| CS | 9.00 | 32.65 | 6–9 | 2 |
| ES | 10.75 | 33.15 | 7–8 | 2 |
| CS-E | 11.05 | 32.70 | 7–8 | 2 |
| Wool/Group | RW | CS | ES | CS-E | |||||
|---|---|---|---|---|---|---|---|---|---|
| d1 | d2 | d1 | d2 | d1 | d2 | d1 | d2 | ||
| Average n = 1000 | drange [μm] | 10–132 | 10–138 | 10–118 | 12–124 | 10–132 | 12–136 | 10–112 | 12–136 |
| d [μm] | 30.33 | 31.09 | 31.18 | 32.82 | 30.17 | 33.80 | 29.61 | 32.74 | |
| CV [%] | 40.75 | 38.79 | 37.62 | 32.81 | 34.17 | 31.75 | 33.72 | 30.15 | |
| Group 1 n = 100 | drange [μm] | 10–20 | 10–20 | 10–20 | 12–22 | 10–20 | 12–24 | 10–20 | 12–22 |
| d [μm] | 18.02 | 18.96 | 17.42 | 20.12 | 17.90 | 21.12 | 17.30 | 20.12 | |
| CV [%] | 12.57 | 12.41 | 14.25 | 11.11 | 12.24 | 11.91 | 21.97 | 11.96 | |
| Group 2 n = 100 | drange [μm] | 20–22 | 22–24 | 20–24 | 22–26 | 20–22 | 24–26 | 20–22 | 22–26 |
| d [μm] | 21.58 | 22.64 | 21.48 | 24.46 | 21.52 | 25.04 | 21.06 | 24.78 | |
| CV [%] | 3.79 | 4.14 | 4.31 | 4.17 | 3.99 | 4.01 | 4.76 | 4.66 | |
| Group 3 n = 100 | drange [μm] | 22–24 | 24–26 | 24–26 | 26–28 | 22–24 | 26–28 | 22–24 | 26–28 |
| d [μm] | 23.94 | 24.64 | 24.46 | 26.8 | 23.98 | 27.74 | 23.58 | 27.26 | |
| CV [%] | 1.43 | 3.80 | 3.46 | 3.67 | 0.83 | 2.44 | 3.47 | 3.56 | |
| Group 4 n = 100 | drange [μm] | 24–26 | 26–28 | 26–28 | 28–30 | 24–26 | 28–30 | 24–26 | 28–30 |
| d [μm] | 25.94 | 26.52 | 26.62 | 28.72 | 25.96 | 29.68 | 25.54 | 28.96 | |
| CV [%] | 1.31 | 3.32 | 3.49 | 3.36 | 1.09 | 2.48 | 3.31 | 3.47 | |
| Group 5 n = 100 | drange [μm] | 26–28 | 28–30 | 28–30 | 30–32 | 26–28 | 30–32 | 26–28 | 30–32 |
| d [μm] | 27.90 | 28.36 | 28.66 | 30.46 | 27.90 | 31.66 | 27.34 | 30.90 | |
| CV [%] | 1.57 | 2.72 | 3.29 | 2.77 | 1.57 | 2.38 | 3.48 | 3.24 | |
| Group 6 n = 100 | drange [μm] | 28–30 | 30–32 | 30–32 | 32–34 | 28–30 | 32–34 | 28–30 | 32–34 |
| d [μm] | 29.62 | 30.14 | 30.74 | 32.24 | 29.64 | 33.50 | 28.78 | 32.52 | |
| CV [%] | 2.66 | 1.70 | 3.16 | 2.03 | 2.61 | 2.59 | 3.41 | 2.71 | |
| Group 7 n = 100 | drange [μm] | 30–32 | 32–32 | 32–34 | 34–34 | 30–32 | 34–36 | 30–32 | 34–36 |
| d [μm] | 31.58 | 32.00 | 32.92 | 34.00 | 31.70 | 35.28 | 31.12 | 34.50 | |
| CV [%] | 2.59 | 0 | 3.04 | 0 | 2.26 | 2.73 | 3.21 | 2.52 | |
| Group 8 n = 100 | drange [μm] | 32–34 | 32–36 | 34–36 | 36–38 | 32–36 | 36–40 | 32–34 | 36–38 |
| d [μm] | 33.52 | 33.94 | 35.22 | 36.32 | 33.98 | 37.66 | 33.26 | 36.84 | |
| CV [%] | 2.56 | 1.56 | 2.78 | 2.03 | 3.50 | 2.62 | 2.92 | 2.69 | |
| Group 9 n = 100 | drange [μm] | 36–40 | 36–40 | 36–42 | 38–44 | 36–40 | 40–44 | 34–40 | 38–42 |
| d [μm] | 37.12 | 37.20 | 38.94 | 39.68 | 37.62 | 40.96 | 37.02 | 39.24 | |
| CV [%] | 4.57 | 4.04 | 3.21 | 5.13 | 3.83 | 2.81 | 4.03 | 2.88 | |
| Group 10 n = 100 | drange [μm] | 40–132 | 40–138 | 42–118 | 44–124 | 40–132 | 44–136 | 40–112 | 42–136 |
| d [μm] | 56.06 | 56.22 | 55.34 | 56.22 | 51.50 | 55.28 | 50.86 | 52.92 | |
| CV [%] | 10.12 | 39.60 | 32.89 | 28.52 | 30.32 | 32.27 | 25.71 | 29.72 | |
| Wool | RRH30 [%] | RRH65 [%] | RRH72 [%] | RRH100 [%] | W | YI |
|---|---|---|---|---|---|---|
| RW | - | - | - | - | −2.5 | 25.9 |
| CS | 6.56 | 13.53 | 13.62 | 27.23 | 2.1 | 17.4 |
| ES | 6.77 | 14.15 | 14.64 | 29.53 | 10.3 | 14.3 |
| CS-E | 6.62 | 13.83 | 13.91 | 27.64 | 4.7 | 16.4 |
| CS-HP 1 | 8.12 | 15.42 | 15.22 | 30.34 | 12.7 | 13.7 |
| CS-PC 2 | 7.98 | 14.88 | 14.77 | 28.12 | 11.8 | 12.5 |
| ES-HP 1 | 8.11 | 15.74 | 15.66 | 32.33 | 17.6 | 10.3 |
| ES-PC 2 | 8.08 | 15.15 | 15.29 | 30.94 | 15.3 | 11.2 |
| CS-E-HP 1 | 8.14 | 15.51 | 15.74 | 32.45 | 14.7 | 12.0 |
| CS-E-PC 2 | 8.05 | 15.23 | 15.41 | 31.04 | 14.3 | 12.4 |
| Wool | L* | C* | h° | Dex [%] | K/S |
|---|---|---|---|---|---|
| CS | 45.05 | 74.12 | 38.26 | 77.2 | 6.72 |
| ES | 45.53 | 76.93 | 38.54 | 82.4 | 7.34 |
| CS-E | 45.40 | 76.11 | 38.76 | 80.9 | 6.88 |
| CS-HP | 46.11 | 76.39 | 38.30 | 79.8 | 7.75 |
| CS-PC | 44.36 | 75.81 | 38.96 | 78.7 | 7.87 |
| ES-HP | 45.43 | 76.42 | 38.77 | 84.9 | 8.24 |
| ES-PC | 46.69 | 76.17 | 38.42 | 83.8 | 8.68 |
| CS-E-HP | 45.52 | 76.36 | 38.88 | 82.1 | 8.04 |
| CS-E-PC | 46.04 | 76.14 | 38.79 | 82.0 | 8.01 |
| Wool | L* | C* | h° | Dex [%] | K/S |
|---|---|---|---|---|---|
| CS | 18.12 | 12.27 | 277.12 | 82.8 | 3.67 |
| ES | 17.96 | 11.98 | 277.07 | 86.5 | 4.21 |
| CS-E | 18.05 | 12.04 | 277.31 | 85.0 | 3.97 |
| CS-HP | 19.03 | 12.19 | 276.67 | 85.5 | 4.33 |
| CS-PC | 18.61 | 12.60 | 277.36 | 84.9 | 4.07 |
| ES-HP | 19.07 | 13.65 | 276.05 | 88.0 | 5.54 |
| ES-PC | 18.43 | 12.57 | 277.19 | 87.6 | 4.97 |
| CS-E-HP | 18.54 | 12.87 | 277.30 | 87.8 | 5.28 |
| CS-E-PC | 18.71 | 12.62 | 277.26 | 87.6 | 4.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujasinović, E.; Tarbuk, A.; Pušić, T.; Dekanić, T. Bio-Innovative Pretreatment of Coarse Wool Fibers. Processes 2023, 11, 103. https://doi.org/10.3390/pr11010103
Vujasinović E, Tarbuk A, Pušić T, Dekanić T. Bio-Innovative Pretreatment of Coarse Wool Fibers. Processes. 2023; 11(1):103. https://doi.org/10.3390/pr11010103
Chicago/Turabian StyleVujasinović, Edita, Anita Tarbuk, Tanja Pušić, and Tihana Dekanić. 2023. "Bio-Innovative Pretreatment of Coarse Wool Fibers" Processes 11, no. 1: 103. https://doi.org/10.3390/pr11010103
APA StyleVujasinović, E., Tarbuk, A., Pušić, T., & Dekanić, T. (2023). Bio-Innovative Pretreatment of Coarse Wool Fibers. Processes, 11(1), 103. https://doi.org/10.3390/pr11010103









