Vapor–Liquid Equilibria of Quaternary Systems of Interest for the Supercritical Antisolvent Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
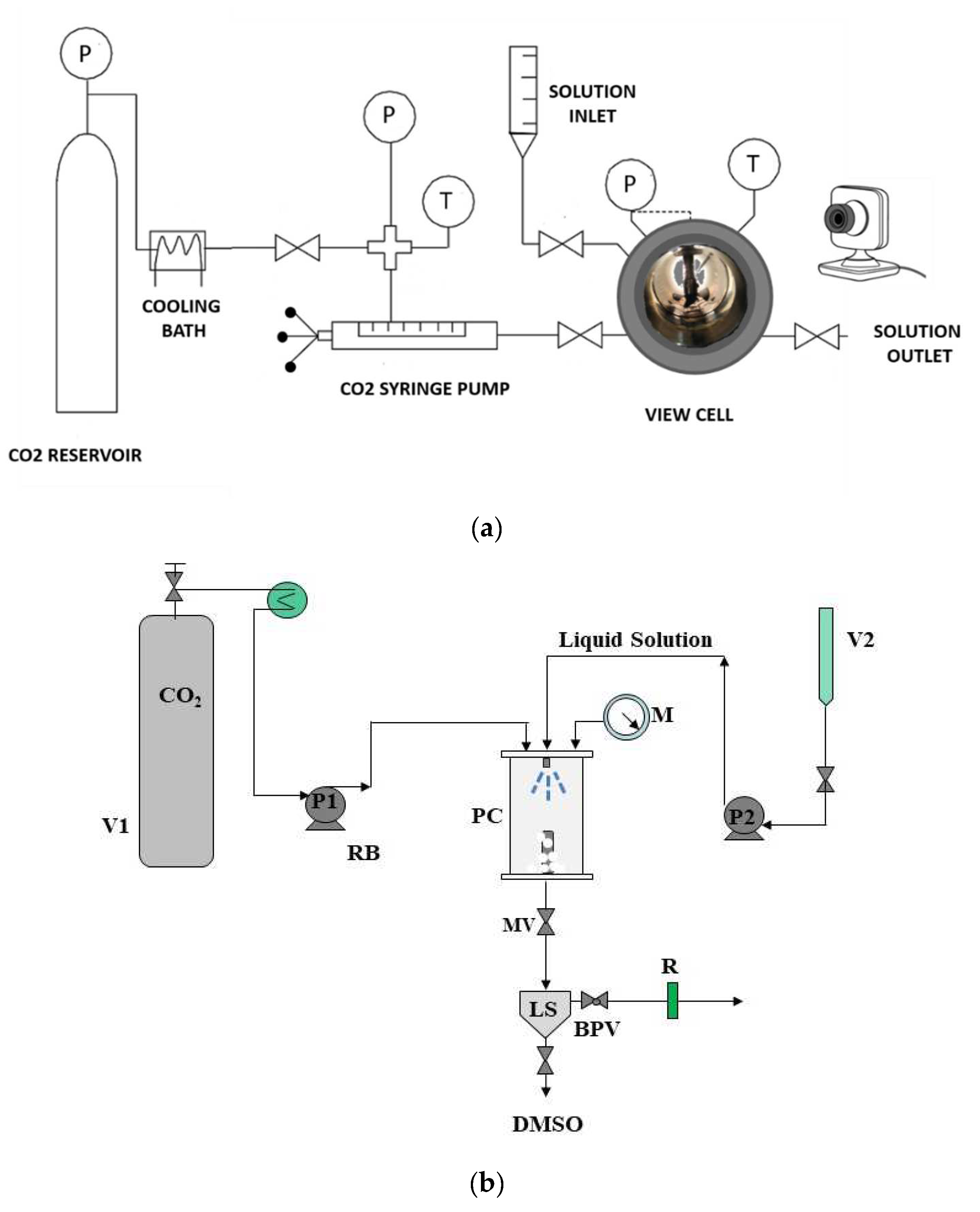
2.2.1. VLE Equipment and Procedure
2.2.2. SAS Apparatus and Procedure
2.3. Analytical Methods
3. Results
3.1. Thermodynamic Analysis of the DMSO/CO2/PVP, DMSO/CO2/MEN, and DMSO/CO2/TOC Ternary Systems
3.2. Thermodynamic Analysis of DMSO/CO2/PVP/Vitamin Quaternary Systems
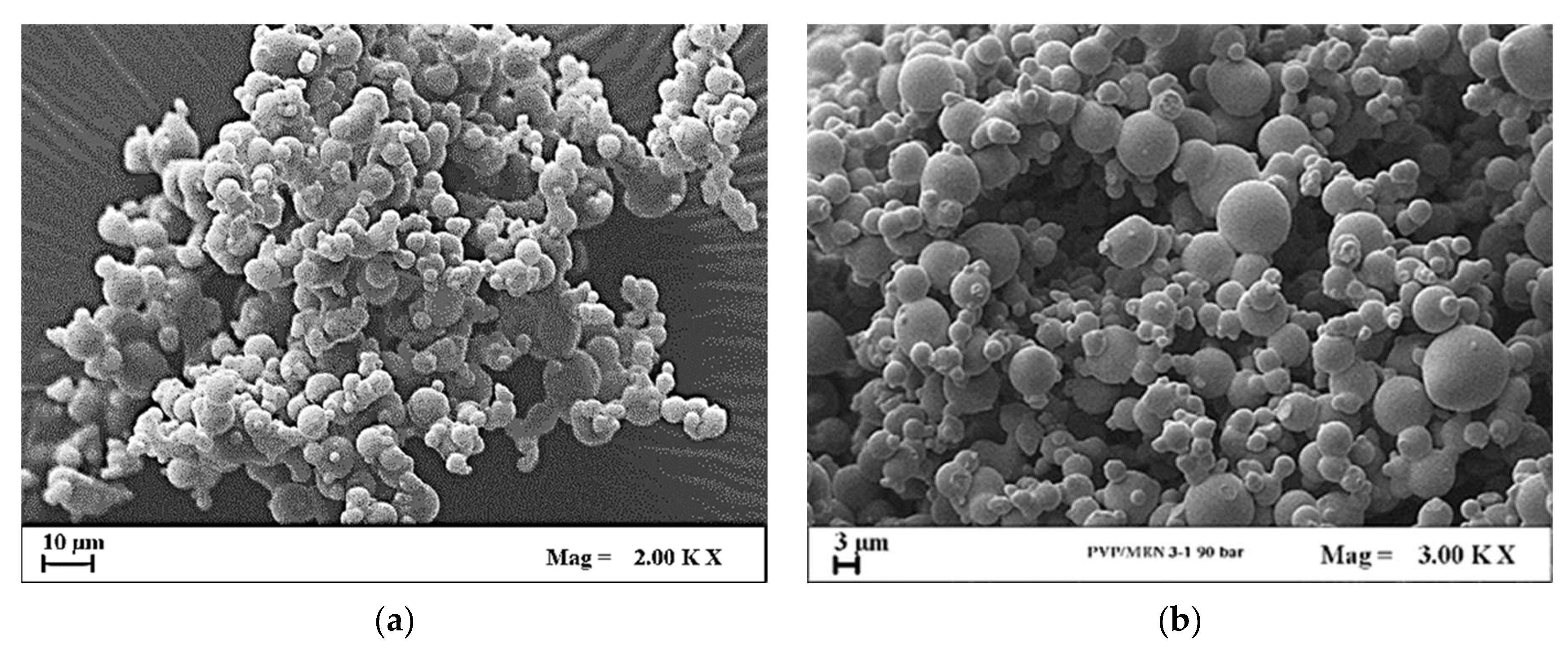
4. SAS Precipitation Experiment of PVP/Vitamin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| DMSO | dimethylsulfoxide |
| EMP | expanded microparticles |
| FESEM | field emission scanning electron microscope |
| MCP | mixture critical point |
| MEN | menadione |
| MP | microparticles |
| NP | nanoparticles |
| PSD | particle size distribution |
| PVP | polyvinylpirrolidone |
| SAS | supercritical antisolvent process |
| scCO2 | supercritical carbon dioxide |
| TOC | α-tocopherol |
| VLE | vapor liquid equilibria |
References
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Machmudah, S.; Winardi, S.; Wahyudiono; Kanda, H.; Goto, M. Formation of Fine Particles from Curcumin/PVP by the Supercritical Antisolvent Process with a Coaxial Nozzle. ACS Omega 2020, 5, 6705–6714. [Google Scholar] [CrossRef]
- Vallejo, R.; Gonzalez-Valdivieso, J.; Santos, M.; Rodriguez-Rojo, S.; Arias, F.J. Production of elastin-like recombinamer-based nanoparticles for docetaxel encapsulation and use as smart drug-delivery systems using a supercritical anti-solvent process. J. Ind. Eng. Chem. 2021, 93, 361–374. [Google Scholar] [CrossRef]
- Lengsfeld, C.S.; Delplanque, J.P.; Barocas, V.H.; Randolph, T.W. Mechanism Governing Microparticle Morphology during Precipitation by a Compressed Antisolvent: Atomization vs Nucleation and Growth. J. Phys. Chem. B 2000, 104, 2725–2735. [Google Scholar] [CrossRef]
- De Marco, I.; Prosapio, V.; Cice, F.; Reverchon, E. Use of solvent mixtures in supercritical antisolvent process to modify precipitates morphology: Cellulose acetate microparticles. J. Supercrit. Fluids 2013, 83, 153–160. [Google Scholar] [CrossRef]
- Yeo, S.D.; Kiran, E. Formation of polymer particles with supercritical fluids: A review. J. Supercrit. Fluids 2005, 34, 287–308. [Google Scholar] [CrossRef]
- Byrappa, K.; Ohara, S.; Adschiri, T. Nanoparticles synthesis using supercritical fluid technology—Towards biomedical applications. Adv. Drug Deliv. Rev. 2008, 60, 299–327. [Google Scholar] [CrossRef]
- Martín, A.; Cocero, M.J. Micronization processes with supercritical fluids: Fundamentals and mechanisms. Adv. Drug Deliv. Rev. 2008, 60, 339–350. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Rodriguez-Meizoso, I. Purification of Natural Products by Selective Precipitation Using Supercritical/Gas Antisolvent Techniques (SAS/GAS). Sep. Purif. Rev. 2021, 50, 32–52. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/flavonoid coprecipitation by supercritical antisolvent process. Chem. Eng. Process. 2019, 146, 1–10. [Google Scholar] [CrossRef]
- Uzun, I.N.; Sipahigil, O.; Dinçer, S. Coprecipitation of Cefuroxime Axetil-PVP composite microparticles by batch supercritical antisolvent process. J. Supercrit. Fluids 2011, 55, 1059–1069. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.; Prosapio, V.; McConville, C.; Leeke, G.; Ingram, A. Coprecipitation of curcumin/PVP with enhanced dissolution properties by the supercritical antisolvent process. J. CO2 Util. 2019, 30, 48–62. [Google Scholar] [CrossRef]
- Taki, S.; Badens, E.; Charbit, G. Controlled release system formed by supercritical anti-solvent coprecipitation of a herbicide and a biodegradable polymer. J. Supercrit. Fluids 2001, 21, 61–70. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; Martínez de la Ossa, E.J. Polymer and ampicillin co-precipitation by supercritical antisolvent process. J. Supercrit. Fluids 2012, 63, 92–98. [Google Scholar] [CrossRef]
- Reverchon, E.; Torino, E.; Dowy, S.; Braeuer, A.; Leipertz, A. Interactions of phase equilibria, jet fluid dynamics and mass transfer during supercritical antisolvent micronization. Chem. Eng. J. 2010, 156, 446–458. [Google Scholar] [CrossRef]
- Werling, J.O.; Debenedetti, P.G. Numerical modeling of mass transfer in the supercritical antisolvent process. J. Supercrit. Fluids 1999, 16, 167–181. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Mechanisms controlling supercritical antisolvent precipitate morphology. Chem. Eng. J. 2011, 169, 358–370. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. Dependence of SAS particle morphologies on the ternary phase equilibria. J. Supercrit. Fluids 2017, 130, 273–281. [Google Scholar] [CrossRef]
- Rossmann, M.; Braeuer, A.; Dowy, S.; Gallinger, T.G.; Leipertz, A.; Schluecker, E. Solute solubility as criterion for the appearance of amorphous particle precipitation or crystallization in the supercritical antisolvent (SAS) process. J. Supercrit. Fluids 2012, 66, 350–358. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. PVP microparticles precipitation from acetone-ethanol mixtures using SAS process: Effect of phase behavior. J. Supercrit. Fluids 2019, 143, 321–329. [Google Scholar] [CrossRef]
- Vitu, S.; Jaubert, J.N.; Pauly, J.; Daridon, J.L.; Barth, D. Phase equilibria measurements of CO2 + methyl cyclopentane and CO2 + isopropyl cyclohexane binary mixtures at elevated pressures. J. Supercrit. Fluids 2008, 44, 155–163. [Google Scholar] [CrossRef]
- Bender, E. Equation of state exactly representing the phase behaviors of pure substances. In Proceedings of the 5th Symposium on Thermophysical Properties, Boston, MA, USA, 30 September–2 October 1970; ASME: New York, NY, USA, 1970; pp. 227–235. [Google Scholar]
- Rajasingam, R.; Lioe, L.; Pham, Q.T.; Lucien, F.P. Solubility of carbon dioxide in dimethylsulfoxide and N-methyl-2- pyrrolidone at elevated pressure. J. Supercrit. Fluids 2004, 31, 227–234. [Google Scholar] [CrossRef]
- Andreatta, A.E.; Florusse, L.J.; Bottini, S.B.; Peters, C.J. Phase equilibria of dimethyl sulfoxide (DMSO) + carbon dioxide, and DMSO + carbon dioxide + water mixtures. J. Supercrit. Fluids 2007, 42, 60–68. [Google Scholar] [CrossRef]
- Vega Gonzalez, A.; Tufeu, R.; Subra, P. High-pressure vapor-liquid equilibrium for the binary systems carbon dioxide + dimethyl sulfoxide and carbon dioxide + dichloromethane. J. Chem. Eng. Data 2002, 47, 492–495. [Google Scholar] [CrossRef]
- Van Konynenburg, P.; Scott, R. Critical lines and phase equilibria in binary van der Waals mixtures. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 298, 495–540. [Google Scholar]



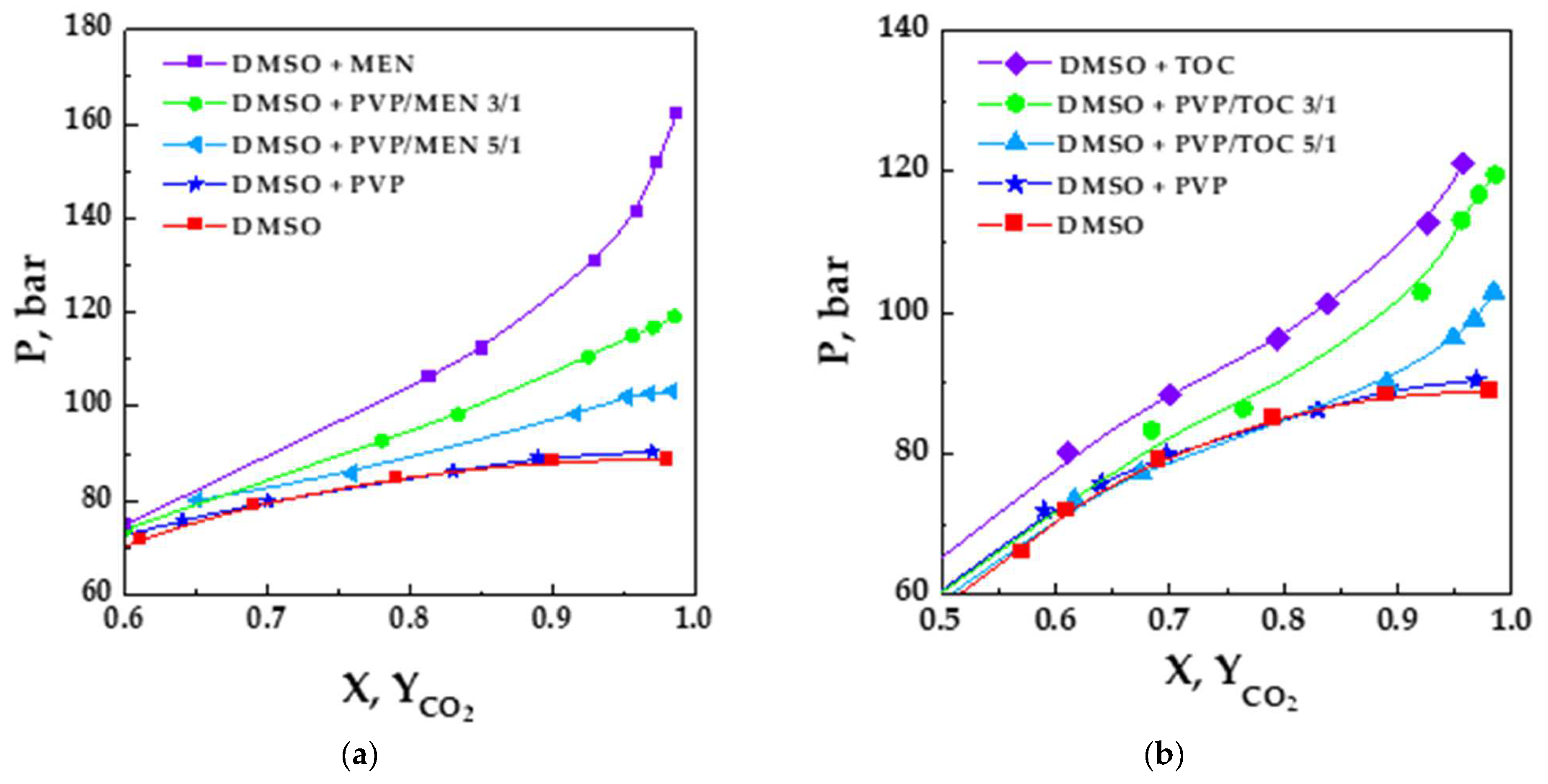

| XCO2 | P, Bar |
|---|---|
| DMSO | |
| 0.98 | 88.8 |
| 0.90 | 88.4 |
| 0.79 | 85.0 |
| 0.69 | 79.1 |
| 0.61 | 72.0 |
| 0.57 | 66.0 |
| DMSO/MEN | |
| 0.99 | 162.1 |
| 0.97 | 151.6 |
| 0.93 | 130.6 |
| 0.85 | 112.3 |
| 0.81 | 106.1 |
| 0.60 | 75 |
| XCO2 | P, Bar |
|---|---|
| DMSO | |
| 0.98 | 88.8 |
| 0.90 | 88.4 |
| 0.79 | 85.0 |
| 0.69 | 79.1 |
| 0.61 | 72.0 |
| 0.57 | 66.0 |
| DMSO/TOC | |
| 0.96 | 121.1 |
| 0.93 | 112.8 |
| 0.84 | 101.3 |
| 0.79 | 96.3 |
| 0.70 | 88.3 |
| 0.61 | 80.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campardelli, R.; Mottola, S.; De Marco, I. Vapor–Liquid Equilibria of Quaternary Systems of Interest for the Supercritical Antisolvent Process. Processes 2022, 10, 2544. https://doi.org/10.3390/pr10122544
Campardelli R, Mottola S, De Marco I. Vapor–Liquid Equilibria of Quaternary Systems of Interest for the Supercritical Antisolvent Process. Processes. 2022; 10(12):2544. https://doi.org/10.3390/pr10122544
Chicago/Turabian StyleCampardelli, Roberta, Stefania Mottola, and Iolanda De Marco. 2022. "Vapor–Liquid Equilibria of Quaternary Systems of Interest for the Supercritical Antisolvent Process" Processes 10, no. 12: 2544. https://doi.org/10.3390/pr10122544
APA StyleCampardelli, R., Mottola, S., & De Marco, I. (2022). Vapor–Liquid Equilibria of Quaternary Systems of Interest for the Supercritical Antisolvent Process. Processes, 10(12), 2544. https://doi.org/10.3390/pr10122544









