Abstract
The aim of this study was to evaluate the influence of sous vide technology on the chemical properties of fruit jams produced with medlar fruit (Mespilus germanica L.). The fruit jams were produced using sous vide technology at different temperatures: 60 °C, 70 °C, and 80 °C. The fruit jams were also produced at 100 °C using the traditional cooking method. Experimentally produced samples were evaluated by the following methods: total polyphenols content, ferric reducing antioxidant power assay (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and organic acid determination. Among sous vide-processed samples, the samples with the highest (p < 0.05) antioxidant activity were processed at 80 °C, containing pectin; the results were the following: phenols content 0.56 ± 0.01 mg gallic acid/kg; FRAP antioxidant activity 0.32 ± 0.01 µmol Trolox/g; DPPH antioxidant activity 21.39 ± 0.33%. The obtained results showed that fruit jam production with the use of sous vide technology can affect antioxidant capacity of this food commodity. The research also provided important information about non-traditional fruit, such as medlar fruit (Mespilus germanica L.), and its processing by sous vide technology. Certainly, more analyses are necessary to give a clearer picture of the changes in medlar fruit during processing at different temperatures, and processing technologies, especially because there are presently no publications about the use of medlar fruit as a raw material in fruit jam preparation.
1. Introduction
Fruits are an integral part of the human diet and represent an irreplaceable source of nutrients. The most sought-after and most consumed is fresh fruit, which is also the most valuable for its share of vitamins and other ingredients. When stored improperly, however, it is easily subject to decay or undesirable changes. These processes can cause the loss of nutrients. Therefore, fruit is often processed by preservation. Food preservation means extending its shelf life for the purpose of later consumption. One of the best-known methods of fruit preservation is the production of jams. This method preserves the health benefits as well as the taste of the fruit [1].
A method of food preparation that has become popular in recent years is sous vide, which is a technique of cooking food using vacuum bags at strictly controlled temperatures. The French name sous vide means “in a vacuum”. Vacuum cooking is gaining attention as an alternative cooking technique, thanks to its ability to preserve or even improve the nutritional and organoleptic properties of food [2].
The principle of the method is the hermetic sealing of the raw material in a plastic bag and subsequent cooking in a water bath with a constant temperature, which rarely exceeds 85 °C [3]. The main reason for vacuum-sealing is the removal of oxygen from the food environment, as the absence of oxygen significantly reduces the activity of many bacteria, which are responsible for food degradation [4]. During the treatment of foodstuffs of plant origin by the sous vide method, the walls of the plant cells remain intact in most cases, and the fineness of the fruit is increased by dissolving the pectin, which has the task of holding the cells together [5].
The advantage of this method is the transfer of heat from water to food and the elimination of the risk of re-contamination during storage, thus prolonging its shelf life. There are minimal changes in the processed food, and it helps ensure maximum natural taste and prevent the degradation processes of nutritionally important substances, such as minerals and vitamins. As the food is prepared vacuum-packed, there is no loss of juiciness or taste like when in contact with the surface of the heat source [4,6]. The use of this method is advantageous for fruit which is particularly sensitive to rapid oxidation and colour change and is not subject to the Maillard reaction when using the sous vide method [7].
Common medlar (Mespilus germanica L.) comes from Asia Minor, the Caucasus, and northern Iran, and it is one of the non-traditional types of pip fruit that is very popular for its appearance, juicy taste, and nutrient content. According to the literature data, common medlar fruits represent good sources of soluble fibre, vitamins, carotenoids, antioxidants, and minerals, such as calcium, potassium, phosphorus, and magnesium [8,9]. Medlar can be eaten raw, or its shelf life can be prolonged with the preparation of fruit jams that can be defined as non-traditional jams [10].
This study was focused on assessing the antioxidant properties and total polyphenolic content of common medlar (Mespilus germanica L.) jam produced using sous vide technology.
2. Materials and Methods
The medlar (Mespilus germanica L.) fruits used for the analysis were harvested in November 2019 in Zlín (city in eastern Czech Republic). The fruits were cleaned, weighed, and blended. The resulting mass was weighed (150 g) into vacuum bags (the vacuuming was done by the following vacuum device: Gorenje VS120W) and 120 g of sugar (granulated sugar, manufacturer: Korunní, Česká republika) was added to each sample. The experiment consisted of 8 treatments based on the addition of pectin to samples; 4 samples contained added citrus pectin (1.5 g, produced by Grešík Valdemar) and the remaining 4 were prepared without pectin. The prepared samples were sealed and homogenized by hand. This was followed by cooking in a sous vide device (Sous-Vide Garer, Steba, Strullendorf, Germany) two samples at a time, one pectin-free and one containing pectin, according to the manufacturer’s instructions at temperatures of 60, 70, and 80 °C for 35 min.
The last two samples (7, 8) were processed in the traditional way at a temperature of 100 °C. First, the mass was boiled for 3 min, then 120 g of sugar was added, as well as the above amount of pectin to the second sample, and boiled again for 3 min. This was followed by placing into sealable glass containers. The samples were stored in the freezer. They were left at room temperature for about an hour before each analysis. The description of samples preparation is given in Table 1.

Table 1.
The description of samples preparation.
The following parameters (in at least triplicates) were determined for individual samples: total polyphenol content spectrophotometrically, antioxidant activity by FRAP method, antioxidant activity by DPPH method, content of organic acids by HPLC.
2.1. Determination of Total Polyphenols Content Spectrophotometrically
The total polyphenol content was determined on the Cecil Instruments CECIL CE 7210 spectrophotometer (Cecil instruments, CE7210 DIET-QUEST, Cambridge, UK) at a wavelength of 765 nm. The concentration was measured using the Folin–Ciocalteu test. The FC test (Folin–Ciocalteu) is generally used to measure the total concentration of polyphenols in natural products, but its mechanism is the redox reaction, so it can be considered as another way of measuring the antioxidant activity.
Samples for analysis were prepared from 1 g of jam sample and 10 mL of water, then filtered, and 1 mL of the sample was transferred to a 25 mL volumetric flask. Subsequently, 5 mL of FC reagent was added, which was diluted 1:10 with water. Then, 4 mL of sodium carbonate was added. The samples were incubated in the dark for 30 min at room temperature. After incubation, the samples were supplemented with distilled water and then the absorbance was measured on a UV spectrophotometer at a wavelength of 765 nm against a blank. The results are expressed as mg/g of gallic acid equivalent (GAE), since gallic acid was used to obtain the calibration curve [11].
2.2. Determination of Antioxidant Activity by the FRAP Method (Ferric Reducing Antioxidant Power Assay)
This method is based on the principle of redox reaction which uses the ability of antioxidants to reduce iron complexes. The measured parameter is the reduction rate of the TPTZ iron complex (2,4,6-tripyridyl-S-triazine), which has no colour, but is measured by reduction to the iron form producing a blue colour. The increase in absorbance at 593 nm corresponds to the amount of Fe2+ complex [12,13].
Samples for the analysis were prepared from 0.1 g of jam sample with the addition of 20 mL of 75% methanol, then extracted for 30 min in an ultrasonic bath in the dark. A blank was prepared from 960 µL H2O + 7.2 mL of the working solution (composed of 50 mL of acetate buffer + 5 mL of the TPTZ solution + 5 mL of the FeCl3·6H2O solution). The blank was then incubated for 8 min without access to light and then used to zero the spectrophotometer at 593 nm.
An amount of 180 µL of the sample + 300 µL H2O + 3.6 mL of the working solution was used for sample preparation. After incubating the samples for 8 min without access to light, the absorbance at 593 nm was measured. The results are expressed as µmol Trolox/g since Trolox was used to obtain the calibration curve.
2.3. Determination of Antioxidant Activity Using DPPH (2,2-Diphenyl-1-picrylhydrazyl)
The presence of antioxidants with antiradical activity causes the reduction of the coloured (λmax = 517 nm) DPPH stable radical to a colourless neutral molecule. The rate and extent of discoloration (decrease in absorbance) are proportional to the antioxidant activity of the analyte.
An amount of 20 mL of ethanol was added to 0.1 g of the homogenized sample; the sample was extracted for 30 min in an ultrasonic bath and filtered.
An amount of 0.1 mM ethanolic solution of DPPH was added to the prepared extract. Next, a reference solution was prepared from ethanol + 0.1 mM DPPH. Subsequently, the values were read on a spectrophotometer using ethanol as a blank [12,14].
Calculation:
DPPHscavenging activity (%) = [(ADPPH − Asample)/ADPPH] × 100
2.4. Determination of Organic Acids by HPLC
Samples for the determination of organic acids were prepared from 10 g of mass and 50 mL of distilled water. After centrifugation, the samples were filtered through a 0.45 μm nylon filter, and the supernatant was used for the analysis. Organic acids were analysed using a Razex ROA-Organic Acid Column H + (300 × 7.8 mm; BioRad, Agilent, Santa Clara, CA, USA). The column with 8 µM particles was maintained at a temperature of 65 °C, with a flow rate 0.6 mL per min. The injection volume was 20 µL. A UV detector with a wavelength set to λ = 210 nm was used. The mobile phase consisted of 4 mM sulfuric acid, and the elution method was isocratic. Agilent ChemStation software (Agilent, Santa Clara, CA, USA, revision: B.04.02) was used for results’ analysis. The following type of HPLC was used: HPLC 1260 Infinity (Agilent Technologies, Santa Clara, CA, USA). A modified method was used in the test, according to the study of Akagić et al. (2019) [15].
2.5. Statistical Evaluation
The resulting values of the individual analyses (all samples were analysed at least in triplicates) were shown in tables as the mean ± standard deviation. IBM SPSS software (version 23.0, SPSS, Chicago, IL, USA) was utilized. One-way ANOVA was used for the analysis, where the homogeneity of the results was determined using the Levene test. At p < 0.05, the Gamel–Howell test was used, and the difference was statistically significant. Otherwise, the TUKEY test was used at p > 0.05, and this was a statistically insignificant difference. Overall differences between samples were assessed according to PCA analysis.
3. Results and Discussion
3.1. Antioxidant Activity
It is not appropriate to use only one method of assessment to evaluate the antioxidant capacity of vegetables, fruits, and plants, because different antioxidant compounds may act by different mechanisms, such as hydrogen atom transfer, single electron transfer, power reduction, and metal chelation [16]. For this reason, multiple antioxidant tests with different mechanisms are used to determine the antioxidant capacity of samples [17].
Many studies have also focused on the role of pectin in the investigated matrix. Basic knowledge of the interactions between pectin and phenolic acids, and of antioxidant capacity, is very important because they are commonly consumed together [18].
FRAP is one of the reliable methods for studying the antioxidant activity of various compounds. This method is often used to quickly evaluate the overall antioxidant capacity of various foods and beverages, as well as various plant extracts [19]. The results of the antioxidant capacity determination using the FRAP method in samples of medlar jam prepared by the sous vide method at 60, 70, and 80 °C, and in the traditional way at 100 °C, are shown in Table 2.

Table 2.
Influence of cooking on the value of antioxidant activity of medlar jam prepared by the sous vide method and the classic cooking method using the FRAP and DPPH methods.
An exponential increase of antioxidant activity with increasing temperature was observed for samples with added pectin, for both processing methods. On the other hand, not adding pectin for the sous vide method increased antioxidant activity (measured by the DPPH method) by a significant amount. The addition of pectin significantly decreased antioxidant activity. This leads to the conclusion that, for the traditional method, the addition of the pectin is essential, whereas for sous vide, it is not necessary for improving antioxidant activity, according to the DPPH measurements. On the contrary, antioxidant activity reported according to the FRAP assay yielded the opposite observation. The study conducted by Araya et al. (2009) stated that the sous vide method is a promising strategy for maintaining a number of parameters (colour stability, flexibility, more fibres, and crunchiness) in carrots [20].
Another suitable method for determining antioxidant activity is the method using DPPH radical. It is a relatively simple and inexpensive test, with low reagent and sample consumption and high-performance antioxidant activity analysis [21].
The results of the analysis (Table 2) indicate that the highest antioxidant activity was demonstrated in sample No. 7, namely 28.68 ± 0.24%; on the other hand, the lowest antioxidant activity was recorded in sample No. 1, namely 7.49 ± 0.01%.
Pectin is an important component in jam production technology, and recent studies have also highlighted that pectin has various biological properties, such as antitumor, anti-inflammatory, and antioxidant effects [22]. Higher values of antioxidant activity could be caused by the Maillard reaction, in which the level of natural antioxidants decreases, but it creates new products with antioxidant properties. In our study, the samples without pectin showed higher antioxidant properties, and only the samples without pectin prepared at 100 °C (antioxidant capacity measured with the DPPH method) showed lower antioxidant capacity. This is probably because samples prepared by the traditional method are more exposed to the air. However, this statement should be supported by further experiments [16].
In a study by Kopjar et al. (2009), the highest content of anthocyanins and the total content of phenols in the samples of strawberry jam with a low content of methoxy amid pectin were found [23]. Kopjar et al. (2007) also achieved the same results in a study with raspberry jam [24].
On the other hand, Poiana et al. (2013) reported that the retention of bioactive compounds stability was strongly dependent on the pectin type and dosage in blackberry jam. The losses recorded in response to processing and storage could be limited by a proper selection of pectin type and dose [25].
In their study, Isbilir et al. (2019) reported values of antioxidant activity in fruit extract from medlar at 39.3 ± 3.9% [17]. Nabavi et al. (2011) studied antioxidant activity in Mespilus germanica L. in methanol and aqueous extracts obtained from leaves, fruits, and bark. Analyses were performed using the DPPH•, NO, H2O2, and ferric thyocyanate methods. The highest values were recorded in tests with methanol and aqueous bark extracts determined by the DPPH method (10.7 ± 0.6 and 11.4 ± 0.8 μg/mL). The methanol extract from the fruit showed higher values in the nitric oxide capture model (247 ± 12.2 μg/mL) [26]. In their study, Ercisli et al. (2012) determined the antioxidant properties of eleven medlar genotypes using the β-carotenic linoleic acid test, the DPPH test, and the overall phenolic test. In the β-carotene linoleic acid test, the antioxidant activity in all eleven genotypes was about 80.8% or 46.6 µg/mL FW DPPH [8]. As argued by Miser-Salihoglu et al. (2013), in general, the antioxidant activity of plants is related to the content of phenolic substances. As a result, he found that medlar extract with a high content of phenols and flavonoids has a higher antioxidant capacity [27].
In a study by Gruz et al. (2011), a decrease in phenols and antioxidant capacity of medlar fruits during ripening was reported, and therefore it is recommended to pay attention to different storage methods in order to minimize the loss of quality and nutritional value of fruit [28].
In a study by Kosewski et al. (2018), the authors measured antioxidant activity in 22 different vegetable samples after conventional and sous vide cooking, not every sample had a higher antioxidant activity after the sous vide method, which is present as a gentler technology [29]. On the other hand, a study by Rinaldi et al. (2021) confirmed the significant extraction of polyphenols, especially gallic acid and naringenin, using the sous vide cooking method.
According to these findings, together with observed findings presented in this study, the determining factor is the type of matrix used for the conventional or sous vide method, and in some cases the conventional method can show a higher antioxidant activity than the sous vide method. These results can be also caused by longer exposure to lower temperature, which can also lead to the reduction of bioactive compounds [28].
Another reason why the antioxidant capacity can be higher in samples prepared by traditional cooking is that during this cooking method the water can be evaporated and then the antioxidant compounds can be more concentrated in the samples [28].
Manach et al. (2004) have reported that the content of polyphenols in foodstuffs is affected by their storage, during which oxidative reactions can occur, which lead to the formation of polymerized substances that cause a change in food quality, especially colour and organoleptic properties. Such changes can be beneficial or harmful (browning of the fruit) [30].
Many studies have indicated that polyphenols in fruits and vegetables play an important role in preventing and alleviating diseases, due to their antioxidant, antidiabetic, and anti-inflammatory activity. These compounds also act significantly in the gastrointestinal tract, where bioavailability is an important task. It has been shown that the bioavailability of polyphenols from homogenised plant matrices can range from 30 to 100% [30,31].
In a study by Kedzierska-Matysek et al. (2021), the measurement results showed the relationships between antioxidant activity and the content of phenolic compounds. The samples showed the strongest antioxidant activity, probably because they had the highest concentrations of total phenols, total flavonoids, and acids [32].
In a study by Molaveisi et al. (2019), Iranian jujube honey was heated at a high temperature for 10 days, and antioxidant activity and total phenol content were evaluated. In general, the increase in the values of all parameters was more pronounced at the highest temperature, indicating a strong dependence of all parameters on temperature [33].
Rinaldi et al. (2021) confirmed that using the sous vide method of food preparation is significant for extraction of polyphenols, especially gallic acid and naringenin [2].
When determining the total content of polyphenols in the samples (Table 3), significant (p < 0.05) differences were observed between all samples, with and without added pectin. In addition, it seems that the main driver of changes in polyphenol content is not the processing method, but temperature.

Table 3.
Influence of cooking on the total polyphenol content in medlar jam prepared by the sous vide method and the classic cooking method, spectrophotometrically.
In a study by Ercisli et al. (2012), the authors noted a total phenolic content in a sample of medlar in the range of 1.14 to 2.93 g GAE/kg fresh weight [8].
In their study, Gülçin et al. (2011) reported the content of phenolic compounds (mg/g) in lyophilized aqueous medlar fruit extract as milligrams of gallic acid equivalents (GAE), and the total of phenolic compounds amounted to 25.08 mg GAE [19].
In their work, Kamiloglu et al. (2015) presented the results of the influence of different processing and storage conditions on the content of polyphenols in red carrot jams. According to these results, the decrease in polyphenols was caused by the disruption of the cell structure during the processing of the raw material into jam, and thus they became more prone to non-enzymatic oxidation, which could be one of the reasons for the loss of total polyphenols. In our study heated samples showed higher polyphenolic content, but they were not exposed after the heating to the possible oxidation processes [30].
In a work by Rababah et al. (2011), the authors found the total content of polyphenols in apricot jams from 20.14 ± 0.12 mg/100 g to 51.48 ± 5.12 mg/100 g. They also noted a higher total content of polyphenols in cherry jams, where the amount of polyphenols was around 50 mg/100 g [34].
In a study by Amakura et al. (2000), the total polyphenol content of jams made from nine types of berries (blackcurrants, blackberries, blueberries, cranberries, raspberries, redcurrants, and strawberries) changed negligibly during cooking [35].
In contrast, Scibisz et al. (2009) reported a loss of total polyphenols after heat treatment of blueberries of 7–10%, compared to fresh fruit. Blackcurrant and blackberry jams were found to lose 26, 49, and 80% compared to fresh fruit. This suggests that cooking has an adverse effect on the total polyphenol content. These findings are also not in accordance with our results, though our experimentally produced jams were not exposed to the oxidation processes [36].
In their work, Kovacevic et al. (2009) studied the effect of heat treatment on the total content of polyphenols in low-calorie jam prepared from three different strawberry cultivars, and the results show a loss of 45, 49, and 63% [37]. Likewise, in the work by Rababah et al. (2011), the authors reported that the cooking of strawberry, cherry, apricot, fig, and orange jams caused a significant loss of total polyphenol content of 93, 88, 72, 76, and 69% (p < 0.05), respectively, which could be due to cellular structure disruption during processing, possibly by reducing the content of ellagic acid [34]. Selcuk and Erkan (2015) stated that the total phenolic content in the medlar was affected by the storage time. With longer storage time, the initial value of 763.03 mg GAE 100 g−1 decreased to 81.15 mg GAE 100 g−1 after 60 days [38]. The study suggests that the total phenol content of fruit can vary depending on harvest time and storage time [39].
3.2. Organic Acid Content
An important component of many foods are naturally occurring organic acids, which greatly affect the acidity of the fruit. Phenolic acids, along with other phenolics, are associated with the colour, taste, and nutritional properties of the fruit. They can also help protect them from animals and microbes [40,41]. Studies show that consumption of foods fortified with phenolic acids is associated with a reduced risk of several diseases, such as dyslipidemia, atherosclerosis, and diabetes [42]. The possibility of increasing the intake of phenolic acids is reason for the preparation of concentrated fruit products. According to Cheng et al. (2014), some of the phenolic acid compounds showed very minor decomposition at 200 °C. The results showed that these compounds are relatively thermostable, and by adjusting the temperature, the extraction of phenolic compounds can be controlled [43].
The most common organic acids in fruit are malic, citric, oxalic, and quinic acids. Their content is known to decrease during maturation [30].
Organic acids are important in the food industry, especially in the process of the solidification of pectin. The COOH groups in pectin are usually ionized, and the negative charges on the molecules caused by this ionization can cause repellence and prevent pectin chains from forming a gel network. To prevent this, the pH of the mixture needs to be in the range of 2.8–3.3. At such a pH, COOH groups are not ionized, which reduces the magnitude of repulsive forces [40,41]. The oxalic and malic acid contents are shown in Table 4.

Table 4.
Influence of cooking on the total oxalic acid and malic acid content in medlar jam prepared by the sous vide method and the classic cooking method.
The oxalic acid content determined in samples of medlar fruit jam after various methods of cooking is described in Table 4. The amount of oxalic acid in samples 1, 3, 5, and 7 indicates that the addition of pectin is extremely beneficial for the preservation of this compound. A local minimum of oxalic acid content was observed for processing at a temperature of 70 °C, independently of added pectin, which indicates that this acid is most unstable at this temperature.
Silva et al. (2002) have stated that quince jam (Cydonia oblonga), belonging to the Rosaceae family like medlar, also contained a small amount of oxalic acid [44]. The oxalic acid content of the fruit may also be affected by the conditions under which the fruit was grown, stored, or processed after being harvested. For example, oxalic acid has been found to delay banana ripening and maintain the post-harvest quality of peaches and plums [45].
The content of malic acid in the jam samples after different cooking methods is given in Table 4.
The addition of pectin again introduces a local minimum for a temperature of 70 °C. On the other hand, the absence of pectin shows the direct dependence of the preserved content of acid on increasing temperature. In addition, the data indicate that the processing method has no significant impact on acid content when no pectin is added.
Out of the samples without added pectin, the highest values were reported in sample No. 8, which was cooked in the classic way at a temperature of 100 °C (13.6771 ± 0.01 mg/kg). The lowest values of malic acid were determined for sample No. 2, which was cooked at 60 °C in a sous vide device.
The main organic acids that have been identified in medlar fruit are malic acid and oxalic acid. The presence of these antioxidant compounds can be considered as a quality parameter for edible medlar fruits [19]. Romero-Rodriguer et al. (1992) report malic, quinic, and tannic acids as the main acids during the ripening of medlar fruit, with malic acid being the highest during ripening [46]. Similarly, according to Gülbahar Cevahir and Saim Zeki Bostan (2021), the malic, succinic, and citric acid contents of the medlar genotypes were between 590.5 and 1074.5 mg 100 g−1, 127.0–419.0 mg 100 g−1, and 2.0–32.0 mg 100 g−1, respectively [39].
In their work, Ozturk et al. (2019) pointed out that the most abundant organic acid in medlar fruit was also malic acid, which corresponds to the results of measurements in the present study [47].
3.3. Principal Components Analysis-PCA
A principal components analysis (PCA) was conducted, and it is shown in Figure 1. The values of all determined parameters of medlar jam produced at different temperatures and technologies were used to create Figure 1. The PCA revealed three clusters (according to Eigen value): the first cluster (Samples 2 and 8; 83.03% of cumulative variance) contained medlar jams processed at 100 °C and 60 °C; the second cluster (Samples 5, 6, and 7; 98.73% of cumulative variance), the medlar jams processed at 80 °C and 100 °C; the third cluster (Samples 1, 3, and 4; 99.99% of cumulative variance), the medlar jams processed at 60 °C and 70 °C. It is notable that clusters were formed according to temperature treatments, and differences (p < 0.05) are observable. However, no highly observable differences between sous vide preparation and traditional preparation were observed (Figure 1).
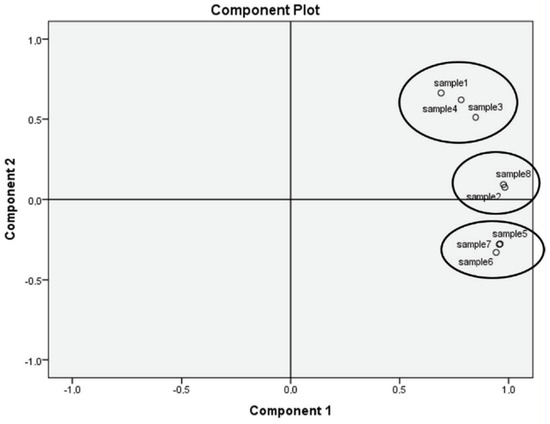
Figure 1.
Principal Components Analysis (PCA) of medlar jam–all determined parameters. Explanatory notes: A70p—cont. pectin, 70 °C; A70—pectin—free, 70 °C; A60p—cont. pectin, 60 °C; A60—pectin—free, 60 °C.
4. Conclusions
This study was focused on monitoring chemical changes in fruit jams, experimentally produced from medlar fruit (Mespilus germanica L.), with the use of sous vide technology. The results of the research showed higher antioxidant properties in samples processed at higher temperatures. From a gastronomic point of view, sous vide represents a new way of making jam that can be attractive to consumers. However, sous vide technology cannot be considered beneficial from the point of antioxidant capacity and phenols content. Certainly, additional studies are necessary to get the full picture on the possibility of applying sous vide technology in the production of fruit jams, especially with the use of non-traditional fruits, such as medlar fruit (Mespilus germanica L.). Above all, the sensory properties of these kinds of products may affect consumers’ acceptance.
Author Contributions
Conceptualization, D.D. and B.T.; methodology, D.D.; formal analysis, D.S., S.D., B.A. and J.Z.; writing—original draft preparation, S.D., H.K.M. and D.D.; writing—review and editing, H.K.M., S.D., D.D. and B.T.; supervision, B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by funds from the Ministry of Education, Youth and Sports for institutional support of long-term conceptual development of research organizations.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shinwari, K.J.; Rao, P.S. Stability of Bioactive Compounds in Fruit Jam and Jelly during Processing and Storage: A Review. Trends Food Sci. Technol. 2018, 75, 181–193. [Google Scholar] [CrossRef]
- Rinaldi, M.; Santi, S.; Paciulli, M.; Ganino, T.; Pellegrini, N.; Visconti, A.; Vitaglione, P.; Barbanti, D.; Chiavaro, E. Comparison of Physical, Microstructural and Antioxidative Properties of Pumpkin Cubes Cooked by Conventional, Vacuum Cooking and Sous Vide Methods. J. Sci. Food Agric. 2021, 101, 2534–2541. [Google Scholar] [CrossRef]
- Aisala, H.; Laaksonen, O.; Manninen, H.; Raittola, A.; Hopia, A.; Sandell, M. Sensory Properties of Nordic Edible Mushrooms. Food Res. Int. 2018, 109, 526–536. [Google Scholar] [CrossRef]
- Keller, T. Under Pressure: Cooking Sous Vide; Artisan: New York, NY, USA, 2008; ISBN 978-1-57965-351-4. [Google Scholar]
- Zavadlav, S.; Blažić, M.; de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous Vide Cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Ruiz-Carrascal, J.; Roldan, M.; Refolio, F.; Perez-Palacios, T.; Antequera, T. Sous-Vide Cooking of Meat: A Maillarized Approach. Int. J. Gastron. Food Sci. 2019, 16, 100138. [Google Scholar] [CrossRef]
- Ercisli, S.; Sengul, M.; Yildiz, H.; Sener, D.; Duralija, B.; Voca, S.; Purgar, D.D. Phytochemical and Antioxidant Characteristics of Medlar Fruits (Mespilus germanica L.). J. Appl. Bot. Food Qual. 2012, 85, 86–90. [Google Scholar]
- Glew, R.H.; Ayaz, F.A.; Sanz, C.; VanderJagt, D.J.; Huang, H.-S.; Chuang, L.-T.; Strnad, M. Changes in Sugars, Organic Acids and Amino Acids in Medlar (Mespilus germanica L.) during Fruit Development and Maturation. Food Chem. 2003, 83, 363–369. [Google Scholar] [CrossRef]
- Solgi, M.; Najib, T.; Ahmadnejad, S.; Nasernejad, B. Synthesis and Characterization of Novel Activated Carbon from Medlar Seed for Chromium Removal: Experimental Analysis and Modeling with Artificial Neural Network and Support Vector Regression. Resour. Technol. 2017, 3, 236–248. [Google Scholar] [CrossRef]
- Tomadoni, B.; Cassani, L.; Ponce, A.; Moreira, M.R.; Agüero, M. V Optimization of Ultrasound, Vanillin and Pomegranate Extract Treatment for Shelf-Stable Unpasteurized Strawberry Juice. LWT Food Sci. Technol. 2016, 72, 475–484. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago Major Seed Mucilage as a Novel Edible Coating Incorporated with Anethum Graveolens Essential Oil on Shelf Life Extension of Beef in Refrigerated Storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.Y.; Lim, Y.Y.; Yule, C.M. Evaluation of Antioxidant, Antibacterial and Anti-Tyrosinase Activities of Four Macaranga Species. Food Chem. 2009, 114, 594–599. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Akagić, A.; Vranac, A.; Gaši, F.; Drkenda, P.; Spaho, N.; Oručević Žuljević, S.; Kurtović, M.; Musić, O.; Murtić, S.; Hudina, M. Sugars, Acids and Polyphenols Profile of Commercial and Traditional Apple Cultivars for Processing. Acta Agric. Slov. 2019, 113, 239–250. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Isbilir, S.S.; Kabala, S.I.; Yagar, H. Assessment of in Vitro Antioxidant and Antidiabetic Capacities of Medlar (Mespilus germanica). Not. Bot. Horti. Agrobot. Cluj Napoca 2018, 47, 384–389. [Google Scholar] [CrossRef]
- Dominguez Avila, J.A.; Villegas Ochoa, M.A.; Alvarez Parrilla, E.; Montalvo Gonzalez, E.; Gonzalez Aguilar, G.A. Interactions between Four Common Plant-Derived Phenolic Acids and Pectin, and Its Effect on Antioxidant Capacity. J. Food Meas. Charact. 2018, 12, 992–1004. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant Activity of Eugenol: A Structure-Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Araya, X.; Smale, N.; Zabaras, D.; Winley, E.; Forde, C.; Stewart, C.; Mawson, J. Sensory Perception and Quality Attributes of High Pressure Processed Carrots in Comparison to Raw, Sous-Vide and Cooked Carrots. Innov. Food Sci. Emerg. Technol. 2009, 10, 420–433. [Google Scholar] [CrossRef]
- Sirivibulkovit, K.; Nouanthavong, S.; Sameenoi, Y. Paper-Based DPPH Assay for Antioxidant Activity Analysis. Anal. Sci. 2018, 34, 795–800. [Google Scholar] [CrossRef]
- Wathoni, N.; Shan, C.Y.; Shan, W.Y.; Rostinawati, T.; Indradi, R.B.; Pratiwi, R.; Muchtaridi, M. Characterization and Antioxidant Activity of Pectin from Indonesian Mangosteen (Garcinia mangostana L.) Rind. Heliyon 2019, 5, e02299. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Pilizota, V.; Tiban, N.N.; Subaric, D.; Babic, J.; Ackar, D.; Sajdl, M. Strawberry Jams: Influence of Different Pectins on Colour and Textural Properties. Czech J. Food Sci. 2009, 27, 20–28. [Google Scholar] [CrossRef]
- Kopjar, M.; Pilizota, V.; Tiban, N.N.; Subaric, D.; Babic, J.; Ackar, D. Effect of Different Pectin Addition and Its Concentration on Colour and Textural Properties of Raspberry Jam. Dtsch. Leb. 2007, 103, 164–168. [Google Scholar]
- Poiana, M.-A.; Munteanu, M.-F.; Bordean, D.-M.; Gligor, R.; Alexa, E. Assessing the Effects of Different Pectins Addition on Color Quality and Antioxidant Properties of Blackberry Jam. Chem. Cent. J. 2013, 7, 121. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Nabavi, S.M.; Ebrahimzadeh, M.A.; Asgarirad, H. The Antioxidant Activity of Wild Medlar (Mespilus germanica L.) Fruit, Stem Bark and Leaf. Afr. J. Biotechnol. 2011, 10, 283–289. [Google Scholar]
- Miser-Salihoglu, E.; Akaydin, G.; Caliskan-Can, E.; Yardim-Akaydin, S. Evalution of Antioxidant Activity of Various Herbal Folk Medicines. Nutr. Food Sci. 2013, 3, 1–9. [Google Scholar]
- Gruz, J.; Ayaz, F.A.; Torun, H.; Strnad, M. Phenolic Acid Content and Radical Scavenging Activity of Extracts from Medlar (Mespilus germanica L.) Fruit at Different Stages of Ripening. Food Chem. 2011, 124, 271–277. [Google Scholar] [CrossRef]
- Kosewski, G.; Gorna, I.; Boleslawska, I.; Kowalowka, M.; Wieckowska, B.; Glowka, A.K.; Morawska, A.; Jakubowski, K.; Dobrzynska, M.; Miszczuk, P.; et al. Comparison of Antioxidative Properties of Raw Vegetables and Thermally Processed Ones Using the Conventional and Sous-Vide Methods. Food Chem. 2018, 240, 1092–1096. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Van Camp, J.; Capanoglu, E. Influence of Different Processing and Storage Conditions on in Vitro Bioaccessibility of Polyphenols in Black Carrot Jams and Marmalades. Food Chem. 2015, 186, 74–82. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M.; Delosière, M.; Buffière, C.; Hafnaoui, N.; Santé-Lhoutellier, V.; Rémond, D. The Matrix of Fruit & Vegetables Modulates the Gastrointestinal Bioaccessibility of Polyphenols and Their Impact on Dietary Protein Digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar] [CrossRef]
- Kedzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skalecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef] [PubMed]
- Molaveisi, M.; Beigbabaei, A.; Akbari, E.; Noghabi, M.S.; Mohamadi, M. Kinetics of Temperature Effect on Antioxidant Activity, Phenolic Compounds and Color of Iranian Jujube Honey. Heliyon 2019, 5, e01129. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Al-Mahasneh, M.A.; Kilani, I.; Yang, W.; Alhamad, M.N.; Ereifej, K.; Al-u’datt, M. Effect of Jam Processing and Storage on Total Phenolics, Antioxidant Activity, and Anthocyanins of Different Fruits. J. Sci. Food Agric. 2011, 91, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Tonogai, Y. Influence of Jam Processing on the Radical Scavenging Activity and Phenolic Content in Berries. J. Agric. Food Chem. 2000, 48, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- Scibisz, I.; Mitek, M. The changes of antioxidant properties in highbush blueberries (Vaccinium corymbosum L.) During freezing and long-term frozen storage. Acta Sci. Pol. Aliment. 2007, 6, 75–81. [Google Scholar]
- Bursać Kovačević, D.; Levaj, B.; Dagović-Uzelac, V. Free Radical Scavenging Activity and Phenolic Content in Strawberry Fruit and Jam. Agric. Conspec. Sci. 2009, 74, 155–159. [Google Scholar]
- Selcuk, N.; Erkan, M. The Effects of 1-MCP Treatment on Fruit Quality of Medlar Fruit (Mespilus germanica L. Cv. Istanbul) during Long Term Storage in the Palliflex Storage System. Postharvest Biol. Technol. 2015, 100, 81–90. [Google Scholar] [CrossRef]
- Cevahir, G.; Bostan, S.Z. Organic Acids, Sugars and Bioactive Compounds of Promising Medlar (Mespilus germanica L.) Genotypes Selected from Turkey. Int. J. Fruit Sci. 2021, 21, 312–322. [Google Scholar] [CrossRef]
- Vincente, A.R.; Manganaris, G.A.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Chapter 5—Nutritional Quality of Fruits and Vegetables. In Postharvest Handling, 3rd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 69–122. ISBN 978-0-12-408137-6. [Google Scholar]
- Cho, E.-H.; Jung, H.-T.; Lee, B.-H.; Kim, H.-S.; Rhee, J.-K.; Yoo, S.-H. Green Process Development for Apple-Peel Pectin Production by Organic Acid Extraction. Carbohydr. Polym. 2019, 204, 97–103. [Google Scholar] [CrossRef]
- Zolnierczyk, A.K.; Cialek, S.; Styczynska, M.; Oziemblowski, M. Functional Properties of Fruits of Common Medlar (Mespilus germanica L.) Extract. Appl. Sci. 2021, 11, 7528. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. J. Braz. Chem. Soc. 2014, 25, 2102–2107. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Mendes, G.C.; Seabra, R.M.; Ferreira, M.A. Study of the Organic Acids Composition of Quince (Cydonia Oblonga Miller) Fruit and Jam. J. Agric. Food Chem. 2002, 50, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Mir, M.; Ganai, D.; Maqbool, T.; Mir, S.; Shah, M. Postharvest Biology and Technology of Peach. In Postharvest Biology and Technology of Temperate Fruits; Springer: Cham, Switzerland, 2018; pp. 169–199. ISBN 978-3-319-76842-7. [Google Scholar]
- Rodriguez, M.A.R.; Oderiz, M.L.V.; Hernandez, J.L.; Lozano, J.S. Determination of Vitamin C and Organic Acids in Various Fruits by HPLC. J. Chromatogr. Sci. 1992, 30, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Yildiz, K.; Ozturk, B.; Karakaya, O.; Gun, S.; Uzun, S.; Gundogdu, M. Maintaining Postharvest Quality of Medlar (Mespilus germanica) Fruit Using Modified Atmosphere Packaging and Methyl Jasmonate. LWT 2019, 111, 117–124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).