From Foods to Chemotherapeutics: The Antioxidant Potential of Dietary Phytochemicals
Abstract
1. Introduction
2. Oxidants and Endogenous Antioxidants
3. Our Exogenous Antioxidants: Vitamin C/Ascorbic Acid
3.1. Dietary and Supplemental Vitamin C and Health
3.2. Vitamin C as a Chemotherapeutic Agent
4. Our Exogenous Antioxidants: Vitamin E, Tocopherols, and Tocotrienols
4.1. Molecular and Cellular Impacts of Tocopherols
4.2. Health Impacts of Tocopherols
4.3. Tocotrienols
4.4. Vitamin E Supplementation Studies
5. Plant Antioxidants beyond Vitamins
5.1. Chlorophyll and Its Metabolites
Health Impacts of Consuming Leafy, Green Vegetables
5.2. Carotenoids and Their Metabolites
5.2.1. Carotenoid Bioavailability—Impacts on Diet
5.2.2. Carotenoids as Chemotherapeutic Agents
5.2.3. Molecular and Cellular Impacts of Carotenoids
5.2.4. Beneficial Health Impacts of Dietary Carotenoids
Eye and Brain Health
Cancer and Heart Disease
5.2.5. Negative Health Impacts of High Carotenoid Concentrations
5.3. Flavonoids
5.3.1. Flavonoid Bioavailability
5.3.2. Health Impacts of Flavonoids
5.3.3. Impacts of Dietary Flavonoids on Health
5.3.4. Flavonoids as Therapeutic Agents
5.3.5. Health Impacts of Dietary Isoflavonoids
5.3.6. Impacts of Gender, Age, Population, and Hormone Status
5.3.7. Isoflavonoid Use as Chemotherapeutic Agents
5.4. Alkaloids
5.4.1. Dietary Alkaloids
5.4.2. Health Impacts of Dietary Alkaloids—Parkinson’s Disease
5.5. Glucosinolates and Isothiocyanates
5.5.1. Health Impacts of Cruciferous Vegetables
5.5.2. Molecular and Cellular Impacts of Isothiocyanates
6. Synergism
7. Impacts on Subject Health
8. Concluding Thoughts
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, B.P.; Chandran, R.; Abrahamse, H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian dietary patterns and cardiovascular disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Qian, F.; Liu, G.; Hu, F.B.; Bhupathiraju, S.N.; Sun, Q. Association between plant-based dietary patterns and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA Intern. Med. 2019, 179, 1335–1344. [Google Scholar] [CrossRef]
- Jabri, A.; Kumar, A.; Verghese, E.; Alameh, A.; Kumar, A.; Khan, M.S.; Khan, S.U.; Michos, E.D.; Kapadia, S.R.; Reed, G.W.; et al. Meta-analysis of effect of vegetarian diet on ischemic heart disease and all-cause mortality. Am. J. Prev. Cardiol. 2021, 7, 100182. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019, 63, 1800636. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Kuo, H.C.; Hudlikar, R.; Sargsyan, D.; Li, S.; Wang, L.; Wu, R.; Kong, A.N. Gut microbiota, dietary phytochemicals, and benefits to human health. Curr. Pharmacol. Rep. 2019, 5, 332–344. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 854–857. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated fatty acids and cardiovascular disease: Replacements for saturated fat to reduce cardiovascular risk. Healthcare 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Saito, I.; Iso, H.; Yamagishi, K.; Yatsuya, H.; Ishihara, J.; Maruyama, K.; Inoue, M.; Sawada, N.; Tsugane, S.; et al. Dietary magnesium intake and risk of incident coronary heart disease in men: A prospective cohort study. Clin. Nutr. 2018, 37, 1602–1608. [Google Scholar] [CrossRef]
- Doyle, L.; Cashman, K.D. The effect of nutrient profiles of the Dietary Approaches to Stop Hypertension (DASH) diets on blood pressure and bone metabolism and composition in normotensive and hypertensive rats. Br. J. Nutr. 2003, 89, 713–724. [Google Scholar] [CrossRef][Green Version]
- Seymour, E.M.; Singer, A.A.; Bennink, M.R.; Parikh, R.V.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Chronic intake of a phytochemical-enriched diet reduces cardiac fibrosis and diastolic dysfunction caused by prolonged salt-sensitive hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Bennink, M.R.; Bolling, S.F. Diet-relevant phytochemical intake affects the cardiac AhR and nrf2 transcriptome and reduces heart failure in hypertensive rats. J. Nutr. Biochem. 2013, 24, 1580–1586. [Google Scholar] [CrossRef]
- Sharma, J.; Maity, A. Pomegranate phytochemicals: Nutraceutical and therapeutic values. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 56–76. [Google Scholar]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflam. 2011, 2011, 514623. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Chattopadhyay, A.; Ghosh, G.; Datta, A.G. Oxidative stress-induced ischemic heart disease: Protection by antioxidants. Curr. Med. Chem. 2004, 11, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Krumova, K.; Gonzalo, C. Overview of Reactive Oxygen Species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: London, UK, 2016; Volume 1, pp. 1–21. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Zavadskiy, S.P.; Kuz’menko, A.N.; Terentiev, A.A. Dual character of reactive oxygen, nitrogen, and halogen species: Endogenous sources, interconversions and neutralization. Biochemistry 2020, 85, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef]
- Ziech, D.; Franco, R.; Georgakilas, A.G.; Georgakila, S.; Malamou-Mitsi, V.; Schoneveld, O.; Pappa, A.; Panayiotidis, M.I. The role of reactive oxygen species and oxidative stress in environmental carcinogenesis and biomarker development. Chem. Biol. Interact. 2010, 188, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Ślesak, I.; Ślesak, H.; Zimak-Piekarczyk, P.; Rozpądek, P. Enzymatic Antioxidant Systems in Early Anaerobes: Theoretical Considerations. Astrobiology 2016, 16, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical roles of antioxidant enzymes: Basic mechanisms and health implications. Physiol. Rev. 2016, 96, 307–364. [Google Scholar] [CrossRef]
- Rehman, S.; Khan, H. Advances in antioxidant potential of natural alkaloids. Curr. Bioact. Compd. 2017, 13, 101–108. [Google Scholar] [CrossRef]
- Arif, N.; Yadav, V.; Singh, S.; Kumar Kushwaha, B.; Singh, S.; Kumar Tripathi, D.; Vishwakarma, K.; Sharma, S.; Dubey, N.K.; Chauhan, D.K. Assessment of Antioxidant Potential of Plants in Response to Heavy Metals. In Plant Responses to Xenobiotics; Singh, A., Prasad, S., Singh, R., Eds.; Springer: Singapore, 2016; pp. 97–125. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Wibowo, J.P.; Elachouri, M.; Masumi, R.; Salehifard-Jouneghani, A.; Abolhasanzadeh, Z.; Lorigooini, Z. Battle between plants as antioxidants with free radicals in human body. J. Herbmed Pharmacol. 2020, 9, 191–199. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Vissers, M.C.; Das, A.B. Ascorbate as an enzyme cofactor. In Vitamin C: New Biochemical and Functional Insights; Chen, Q., Vissers, M.C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 71–98. [Google Scholar]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Oh, S.W.; Myung, S.K. Efficacy of vitamin C supplements in prevention of cancer: A meta-analysis of randomized controlled trials. Korean J. Fam. Med. 2015, 36, 278. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary antioxidants, circulating antioxidant concentrations, total antioxidant capacity, and risk of all-cause mortality: A systematic review and dose-response meta-analysis of prospective observational studies. Adv. Nutr. 2018, 9, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Raźny, U.; Paweł, K. Association between health risk factors and dietary flavonoid intake in cohort studies. Int. J. Food Sci. Nutr. 2021, 72, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Johnson, E.J.; Hammond, B.R. Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain. Curr. Dev. Nutr. 2019, 3, nzz066. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Carr, A.C.; Cook, J. Intravenous vitamin C for cancer therapy–identifying the current gaps in our knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Leekha, A.; Gurjar, B.S.; Tyagi, A.; Rizvi, M.A.; Verma, A.K. Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J. Cancer Res. Clin. Oncol. 2016, 142, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Bonuccelli, G.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P. Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 2017, 8, 67269. [Google Scholar] [CrossRef] [PubMed]
- NIH. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 18 January 2022).
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Dworski, R.; Han, W.; Blackwell, T.S.; Hoskins, A.; Freeman, M.L. Vitamin E prevents NRF2 suppression by allergens in asthmatic alveolar macrophages in vivo. Free Radic. Biol. Med. 2011, 51, 516–521. [Google Scholar] [CrossRef]
- Hegazy, A.M.; El-Sayed, E.M.; Ibrahim, K.S.; Abdel-Azeem, A.S. Dietary antioxidant for disease prevention corroborated by the Nrf2 pathway. J. Complementary Integr. Med. 2019, 16, 20180161. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The role of vitamin E in preventing and treating osteoarthritis—A review of the current evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell. Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef]
- Rigotti, A. Absorption, transport, and tissue delivery of vitamin E. Mol. Aspects Med. 2007, 28, 423–436. [Google Scholar] [CrossRef]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef]
- Wolf, G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr. Rev. 2006, 64, 295–299. [Google Scholar] [CrossRef]
- Christen, S.; Woodall, A.A.; Shigenaga, M.K.; Southwell-Keely, P.T.; Duncan, M.W.; Ames, B.N. γ-Tocopherol traps mutagenic electrophiles such as NOx and complements α-tocopherol: Physiological implications. Proc. Natl. Acad. Sci. USA 1997, 94, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Suh, N.; Kong, A.N.T. Does vitamin E prevent or promote cancer? Cancer Prev. Res. 2012, 5, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Sheikh, J. How Much Omega 3 Is in Olive Oil? Available online: https://keevs.com/healthguide/how-much-omega3-in-olive-oil/ (accessed on 13 June 2022).
- Waterman, E.; Lockwood, B. Active components and clinical applications of olive oil. Altern. Med. Rev. 2007, 12, 331–342. [Google Scholar]
- Marcelino, G.; Hiane, P.A.; Freitas, K.D.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.D.C.A. Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of α-tocopherol and α-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and tocotrienols—Bioactive dietary compounds; what is certain, what is doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative study on the action of tocopherols and tocotrienols as antioxidant: Chemical and physical effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular aspects of α-tocotrienol antioxidant action and cell signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Nor Azman, N.H.E.; Goon, J.A.; Abdul Ghani, S.M.; Hamid, Z.; Wan Ngah, W.Z. Comparing palm oil, tocotrienol-rich fraction and α-tocopherol supplementation on the antioxidant levels of older adults. Antioxidants 2018, 7, 74. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its antioxidant label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, L.; Perri, L.; Di Castelnuovo, A.; Iacoviello, L.; De Gaetano, G.; Violi, F. Supplementation with vitamin E alone is associated with reduced myocardial infarction: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Zheng, H.; Hu, X.; Liao, X.; Zhang, Y. Transformation pathways and metabolic activity of free chlorophyll compounds from chloroplast thylakoid membrane under in vitro gastrointestinal digestion and colonic fermentation in early life. Food Biosci. 2021, 42, 101196. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Moser, S.; Kräutler, B. In search of bioactivity—Phyllobilins, an unexplored class of abundant heterocyclic plant metabolites from breakdown of chlorophyll. Isr. J. Chem. 2019, 59, 420–431. [Google Scholar] [CrossRef]
- Egner, P.A.; Muñoz, A.; Kensler, T.W. Chemoprevention with chlorophyllin in individuals exposed to dietary aflatoxin. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523, 209–216. [Google Scholar] [CrossRef]
- Qu, J.; Ma, L.; Zhang, J.; Jockusch, S.; Washington, I. Dietary chlorophyll metabolites catalyze the photoreduction of plasma ubiquinone. Photochem. Photobiol. 2013, 89, 310–313. [Google Scholar] [CrossRef]
- Mukherjee, R.; Davies, P.J.A.; Crombie, D.L.; Bischoff, E.D.; Cesario, R.M.; Jow, L.; Hamann, L.G.; Boehm, M.F.; Mondon, C.E.; Nadzan, A.M.; et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 1997, 386, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Palacios, N.; Pixley, K.V. Provitamin A carotenoid bioavailability: What really matters? Int. J. Vitam. Nutr. Res. 2010, 80, 336. [Google Scholar] [CrossRef]
- Törmä, H. Regulation of keratin expression by retinoids. Dermato-Endocrinology 2011, 3, 136–140. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of vitamin A in the immune system. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Maiani, G.; Caston, M.J.; Catasta, G.; Toti, E.; Cambrodon, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- De Pee, S.; West, C.E.; Permaesih, D.; Martuti, S.; Hautvast, J.G. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am. J. Clin. Nutr. 1998, 68, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, J.; Holzenburg, A.; King, S. Physical barriers to carotenoid bioaccessibility. Ultrastructure survey of chromoplast and cell wall morphology in nine carotenoid-containing fruits and vegetables. J. Sci. Food Agric. 2012, 92, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.; Linseisen, J.; Hoffmann, J.; Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 1999, 129, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Schönfeldt, H.C.; Pretorius, B.; Hall, N. Bioavailability of nutrients. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 401–406. [Google Scholar]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Platel, K.; Srinivasan, K. Bioavailability of micronutrients from plant foods: An update. Crit. Rev. Food Sci. Nutr. 2016, 56, 1608–1619. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Badmaev, V.; Majeed, M.; Norkus, E.P. Piperine, an alkaloid derived from black pepper increases serum response of beta-carotene during 14-days of oral beta-carotene supplementation. Nutr. Res. 1999, 19, 381–388. [Google Scholar] [CrossRef]
- Merle, B.M.; Cougnard-Grégoire, A.; Korobelnik, J.F.; Schalch, W.; Etheve, S.; Rougier, M.B.; Féart, C.; Samieri, C.; Delyfer, M.N.; Delcourt, C. Plasma Lutein, a Nutritional Biomarker for Development of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2021, 13, 2047. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Roshan, Z.; Assadpour, E.; Jafari, S.M. Improving the cancer prevention / treatment role of carotenoids through various nano-delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 61, 522–534. [Google Scholar] [CrossRef]
- Maghsoudi, S.; Taghavi Shahraki, B.; Rabiee, N.; Fatahi, Y.; Bagherzadeh, M.; Dinarvand, R.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Hamblin, M.R.; et al. The colorful world of carotenoids: A profound insight on therapeutics and recent trends in nano delivery systems. Crit. Rev. Food Sci. Nutr. 2021, 62, 3658–3697. [Google Scholar] [CrossRef]
- Veda, S.; Srinivasan, K. Influence of dietary spices–Black pepper, red pepper and ginger on the uptake of β-carotene by rat intestines. J. Funct. Foods 2009, 1, 394–398. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Yeum, K.J. Carotenoid–radical interactions. Biochem. Biophys. Res. Commun. 2003, 305, 754–760. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Norat, T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2012, 96, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.A.; Otton, R. Impact of the carotenoid astaxanthin on phagocytic capacity and ROS/RNS production of human neutrophils treated with free fatty acids and high glucose. Int. Immunopharmacol. 2011, 11, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Ghoreishi, S.; Baharvand, P. Astaxanthin induces apoptosis and increases activity of antioxidant enzymes in LS-180 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 891–895. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of lutein and astaxanthin intake on the improvement of cognitive functions among healthy adults: A systematic review of randomized controlled trials. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J.; et al. Relationship between serum and brain carotenoids, α-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 951786. [Google Scholar] [CrossRef]
- Wisniewska, A.; Subczynski, W.K. Accumulation of macular xanthophylls in unsaturated membrane domains. Free Radic. Biol. Med. 2006, 40, 1820–1826. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.H.; Oh, J.W.; Keum, Y.S.; Saini, R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: A review of emerging evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J. The alpha-tocopherol, beta-carotene cancer prevention study in Finland. Nutr. Rev. 1994, 52, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Haider, C.; Ferk, F.; Bojaxhi, E.; Martano, G.; Stutz, H.; Bresgen, N.; Knasmüller, S.; Alija, A.; Eckl, P. Effects of β-Carotene and Its Cleavage Products in Primary Pneumocyte Type II Cells. Antioxidants 2017, 6, 37. [Google Scholar] [CrossRef]
- Karak, P. Biological activities of flavonoids: An overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Samanta, A.; Das, G.; Das, S.K. Roles of flavonoids in plants. Carbon 2011, 100, 12–35. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- DuPont, M.S.; Day, A.J.; Bennett, R.N.; Mellon, F.A.; Kroon, P.A. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur. J. Clin. Nutr. 2004, 58, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Milbury, P.E.; Kalt, W. Xenobiotic metabolism and berry flavonoid transport across the blood−brain barrier. J. Agric. Food Chem. 2010, 58, 3950–3956. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.; Spencer, J.; Schroeter, H.; Rice-Evans, C. Dietary Flavonoids as Potential Neuroprotectants. Biol. Chem. 2002, 383, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Kicinska, A.; Jarmuszkiewicz, W. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways? Molecules 2020, 25, 3060. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef]
- Shishtar, E.; Rogers, G.T.; Blumberg, J.B.; Au, R.; Jacques, P.F. Long-term dietary flavonoid intake and risk of Alzheimer disease and related dementias in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2020, 112, 343–353. [Google Scholar] [CrossRef]
- Konishi, K.; Wada, K.; Yamakawa, M.; Goto, Y.; Mizuta, F.; Koda, S.; Uji, T.; Tsuji, M.; Nagata, C. Dietary soy intake is inversely associated with risk of type 2 diabetes in Japanese women but not in men. J. Nutr. 2019, 149, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone intake and the risk of coronary heart disease in US men and women: Results from 3 prospective cohort studies. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose–Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S.K. Synergistic anti-tumor actions of luteolin and silibinin prevented cell migration and invasion and induced apoptosis in glioblastoma SNB19 cells and glioblastoma stem cells. Brain Res. 2015, 1629, 85–93. [Google Scholar] [CrossRef]
- Tauchen, J.; Huml, L.; Rimpelova, S.; Jurášek, M. Flavonoids and related members of the aromatic polyketide group in human health and disease: Do they really work? Molecules 2020, 25, 3846. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- dos Santos Lima, B.; Shanmugam, S.; Quintans, J.D.S.S.; Quintans-Junior, L.J.; de Souza Araujo, A.A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion delivery system of tea polyphenols enhanced the bioavailability of catechins in rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.H.; Han, N.S.; Kim, D.O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Kwak, H.S.; Lim, H.J.; Lee, S.H.; Kang, Y.S.; Choe, T.B.; Hur, H.G.; Han, K.O. Isoflavone metabolites and their in vitro dual functions: They can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J. Steroid Biochem. Mol. Biol. 2006, 101, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Sathyapalan, T.; Aye, M.; Rigby, A.S.; Thatcher, N.J.; Dargham, S.R.; Kilpatrick, E.S.; Atkin, S.L. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 691–697. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Yi, M.; Wan, Q.; Du, L.; Zhou, Y. Soy and isoflavone consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Mol. Nutr. Food Res. 2020, 64, 1900751. [Google Scholar] [CrossRef]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.A.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Shafiee, G.; Saidijam, M.; Tavilani, H.; Ghasemkhani, N.; Khodadadi, I. Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int. J. Mol. Cell. Med. 2016, 5, 178–191. [Google Scholar]
- Rampino, A.; Annese, T.; Margari, A.; Tamma, R.; Ribatti, D. Nutraceuticals and their role in tumor angiogenesis. Exp. Cell Res. 2021, 408, 112859. [Google Scholar] [CrossRef]
- Hodis, H.N.; Mack, W.J.; Kono, N.; Azen, S.P.; Shoupe, D.; Hwang-Levine, J.; Petitti, D.; Whitfield-Maxwell, L.; Yan, M.; Franke, A.A.; et al. Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women: A randomized controlled trial. Stroke 2011, 42, 3168–3175. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, M.L.; Qin, Y.; Zhang, Q.Y.; Xu, H.X.; Zhou, Y.; Mi, M.T.; Zhu, J.D. Isoflavone consumption and risk of breast cancer: A dose-response meta-analysis of observational studies. Asia Pac. J. Clin. Nutr. 2013, 22, 118–127. [Google Scholar] [PubMed]
- Sekikawa, A.; Ihara, M.; Lopez, O.; Kakuta, C.; Lopresti, B.; Higashiyama, A.; Aizenstein, H.; Chang, Y.F.; Mathis, C.; Miyamoto, Y.; et al. Effect of S-equol and soy isoflavones on heart and brain. Curr. Cardiol. Rev. 2019, 15, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.L.; Tang, X.Y.; Deng, Y.Y.; Zhong, Q.W.; Wang, C.; Zhang, Z.Q.; Chen, Y.M. Urinary equol, but not daidzein and genistein, was inversely associated with the risk of type 2 diabetes in Chinese adults. Eur. J. Nutr. 2020, 59, 719–728. [Google Scholar] [CrossRef]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy isoflavones in integrative oncology: Increased efficacy and decreased toxicity of cancer therapy. Integr. Cancer Ther. 2019, 18, 1534735419835310. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Antonelli, A.; Guidi, P.; Bernardeschi, M.; Scarcelli, V.; Fallahi, P.; Frenzilli, G. Genotoxicity evaluation of the soybean isoflavone genistein in human papillary thyroid cancer cells. Study of its potential use in thyroid cancer therapy. Nutr. Cancer 2019, 71, 1335–1344. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A.K. Dietary isoflavone daidzein synergizes centchroman action via induction of apoptosis and inhibition of PI3K/Akt pathway in MCF-7/MDA MB-231 human breast cancer cells. Phytomedicine 2018, 40, 116–124. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Adibah, K.Z.M.; Azzreena, M.A. Plant toxins: Alkaloids and their toxicities. GSC Biol. Pharm. Sci. 2019, 6, 021–029. [Google Scholar] [CrossRef]
- Munir, S.; Shahid, A.; Aslam, B.; Ashfaq, U.A.; Akash, M.S.H.; Ali, M.A.; Almatroudi, A.; Allemailem, K.S.; Rajoka, M.S.R.; Khurshid, M. The therapeutic prospects of naturally occurring and synthetic indole alkaloids for depression and anxiety disorders. J. Evid. Based Complementary Altern. Med. 2020, 2020, 8836983. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, N.; Tian, M.; Ma, L.; Yang, J.; Lan, X.; Ma, H.; Niu, J.; Yu, J. Effects of alkaloids on peripheral neuropathic pain: A review. Chin. Med. 2020, 15, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Wennström, P.; Gry, O.J. Nicotine Alkaloids in Solanaceous Food Plants; Nordic Council of Ministers: Copenhagen, Denmark, 2003; pp. 17–23. [Google Scholar]
- Appendino, G.; Taglialatela-Scafati, O. Drug-like compounds from food plants and spices. In Dietary Supplements of Plant Origin: A Nutrition and Health Approach; Maffei, M., Ed.; Taylor & Francis: London, UK, 2003; pp. 43–74. [Google Scholar]
- Koleva, I.I.; van Beek, T.A.; Soffers, A.E.; Dusemund, B.; Rietjens, I.M. Alkaloids in the human food chain–natural occurrence and possible adverse effects. Mol. Nutr. Food Res. 2012, 56, 30–52. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Pereboom-de Fauw, D.P.; Mulder, P.P.; Spanjer, M.; de Stoppelaar, J.; Mol, H.G.; de Nijs, M. Straightforward analytical method to determine opium alkaloids in poppy seeds and bakery products. Food Chem. 2018, 242, 443–450. [Google Scholar] [CrossRef]
- Selmar, D.; Engelhardt, U.H.; Hänsel, S.; Thräne, C.; Nowak, M.; Kleinwächter, M. Nicotine uptake by peppermint plants as a possible source of nicotine in plant-derived products. Agron. Sustain. Dev. 2015, 35, 1185–1190. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Sun, F.; Zhang, J.; Jin, Y.; Li, Y.; Zhou, J.; Li, Y.; Zhu, K. Gelsedine-type alkaloids: Discovery of natural neurotoxins presented in toxic honey. J. Hazard. Mater. 2020, 381, 120999. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Franklin, G.M.; Longstreth, W.T.; Swanson, P.D.; Checkoway, H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann. Neurol. 2013, 74, 472–477. [Google Scholar] [CrossRef]
- Ma, C.; Molsberry, S.; Li, Y.; Schwarzschild, M.; Ascherio, A.; Gao, X. Dietary nicotine intake and risk of Parkinson disease: A prospective study. Am. J. Clin. Nutr. 2020, 112, 1080–1087. [Google Scholar] [CrossRef]
- Metro, D.; Cernaro, V.; Santoro, D.; Papa, M.; Buemi, M.; Benvenga, S.; Manasseri, L. Beneficial effects of oral pure caffeine on oxidative stress. J. Clin. Transl. Endocrinol. 2017, 10, 22–27. [Google Scholar] [CrossRef]
- Ikram, M.; Park, T.J.; Ali, T.; Kim, M.O. Antioxidant and neuroprotective effects of caffeine against Alzheimer’s and Parkinson’s disease: Insight into the role of Nrf-2 and A2AR signaling. Antioxidants 2020, 9, 902. [Google Scholar] [CrossRef]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine may abrogate LPS-induced oxidative stress and neuroinflammation by regulating Nrf2/TLR4 in adult mouse brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef]
- Vieira, A.J.; Gaspar, E.M.; Santos, P.M. Mechanisms of potential antioxidant activity of caffeine. Radiat. Phys. Chem. 2020, 174, 108968. [Google Scholar] [CrossRef]
- Tung, W.C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; et al. Polyphenols bind to low density lipoprotein at biologically relevant concentrations that are protective for heart disease. Arch. Biochem. Biophys. 2020, 694, 108589. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.G.; Li, Z.Y.; Feng, G.S.; Ji, X.W.; Tan, Y.T.; Li, H.L.; Gunter, M.; Xiang, Y.B. Coffee drinking and cancer risk: An umbrella review of meta-analyses of observational studies. BMC Cancer 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Herr, I.; Lozanovski, V.; Houben, P.; Schemmer, P.; Büchler, M.W. Sulforaphane and related mustard oils in focus of cancer prevention and therapy. Wien. Med. Wochenschr. 2013, 163, 80–88. [Google Scholar] [CrossRef]
- Angelino, D.; Dosz, E.B.; Sun, J.; Hoeflinger, J.L.; Van Tassell, M.L.; Chen, P.; Harnly, J.M.; Miller, M.J.; Jeffery, E.H. Myrosinase-dependent and -independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front. Plant Sci. 2015, 6, 831. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Le Ma, G.L.; Zong, G.; Sampson, L.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Rexrode, K.M.; Sun, Q. Intake of glucosinolates and risk of coronary heart disease in three large prospective cohorts of US men and women. Clin. Epidemiol. 2018, 10, 749. [Google Scholar] [CrossRef]
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Becker, T.M.; Juvik, J.A. The role of glucosinolate hydrolysis products from Brassica vegetable consumption in inducing antioxidant activity and reducing cancer incidence. Diseases 2016, 4, 22. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The integrative role of sulforaphane in preventing inflammation, oxidative stress and fatigue: A review of a potential protective phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.N.T. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3, 3′-diindolylmethane: Antioxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Dayalan Naidu, S.; Suzuki, T.; Yamamoto, M.; Fahey, J.W.; Dinkova-Kostova, A.T. Phenethyl isothiocyanate, a dual activator of transcription factors NRF2 and HSF1. Mol. Nutr. Food Res. 2018, 62, 1700908. [Google Scholar] [CrossRef] [PubMed]
- Muzzaffar, S.; Nazir, T.; Bhat, M.M.; Wani, I.A.; Masoodi, F.A. Goitrogens. In Handbook of Plant and Animal Toxins in Food; Nayik, G.A., Kour, J., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 125–154. [Google Scholar]
- Bischoff, K.L. Glucosinolates. In Nutraceuticals: Efficacy, Safety and Toxicity; Gupta, R., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 903–909. [Google Scholar]
- Pacholak, L.M.; Amarante, M.K.; Guembarovski, R.L.; Watanabe, M.A.E.; Panis, C. Polymorphisms in GSTT1 and GSTM1 genes as possible risk factors for susceptibility to breast cancer development and their influence in chemotherapy response: A systematic review. Mol. Biol. Rep. 2020, 47, 5495–5501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Leng, E.; Xiao, Y.; Mo, Z.; Li, Y.; Zhang, Y.; Deng, X.; Zhou, M.; Zhou, C.; He, Z.; He, J.; et al. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complementary Altern. Med. 2018, 18, 122. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Kumar, A.; Doble, M. Combination therapy: A new strategy to manage diabetes and its complications. Phytomedicine 2014, 21, 123–130. [Google Scholar] [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential lead molecules for MDR reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Wink, M. Molecular modes of action of cytotoxic alkaloids: From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 64, pp. 1–47. [Google Scholar] [CrossRef]
- Thompson, H.J.; Heimendinger, J.; Diker, A.; O’Neill, C.; Haegele, A.; Meinecke, B.; Wolfe, P.; Sedlacek, S.; Zhu, Z.; Jiang, W. Dietary botanical diversity affects the reduction of oxidative biomarkers in women due to high vegetable and fruit intake. J. Nutr. 2006, 136, 2207–2212. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Serafini, M.; Estruch, R.; Lamuela-Raventós, R.M.; Martínez-González, M.A.; Salas-Salvadó, J.; Fiol, M.; Lapetra, J.; Arós, F.; Covas, M.I.; et al. Mediterranean diet and non enzymatic antioxidant capacity in the PREDIMED study: Evidence for a mechanism of antioxidant tuning. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
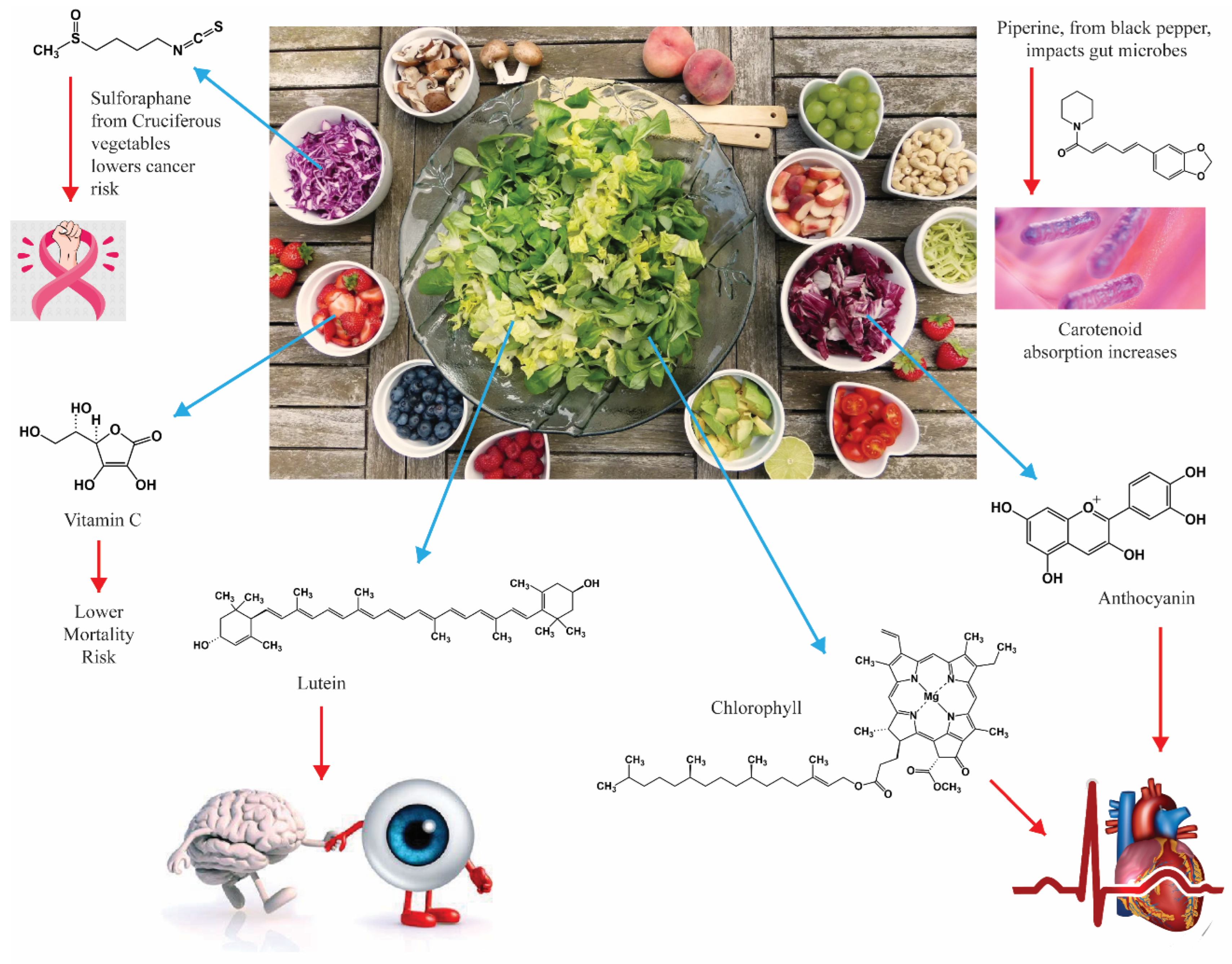
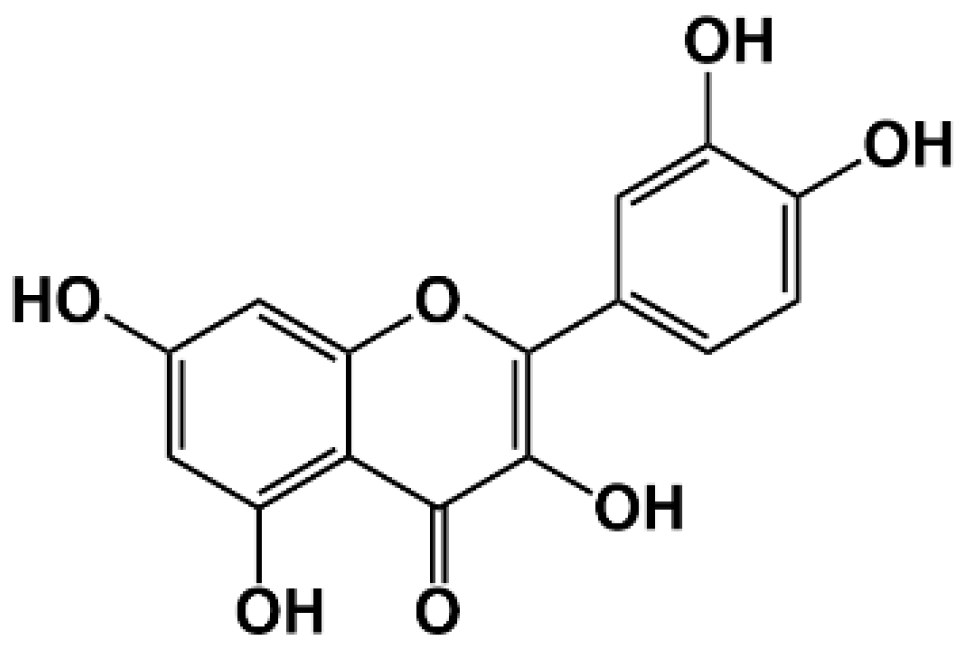
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Vitamin C | Cancer. (RR 1.00; 95%CI 0.95, 1.05). Subgroup meta-analysis by dose or type of cancer also showed vitamin C supplementation did not effectively prevent cancer. | Supplementation; meta-analysis of seven randomized controlled trials investigating vitamin C’s impact on cancer prevention; 62,619 individuals. | [55] |
| Vitamin C | CVD. Unconvincing evidence that vitamin C supplementation improved CVD risk markers; however, there was weakly significant evidence that vitamin C supplementation improved CVD biomarkers in the elderly, obese, those with low baseline vitamin C status, and those with higher CVD risk. | Supplementation; umbrella review of ten systematic reviews and meta-analyses; 6409 participants. | [56] |
| Vitamin C | All-cause mortality. Comparison of highest vs. lowest subgroupings based on dietary vitamin C intake RR 0.88 (95%CI 0.83, 0.94); response was U-shaped with higher levels less protective. Comparison of highest vs. lowest subgroupings based on circulating plasma levels of vitamin C, RR 0.61 (95%CI 0.53, 0.69); this response was linear, with higher blood levels being more protective. | Meta-analysis of 41 prospective observational studies including 507,251 participants and 73,965 cases of all-cause mortality. | [57] |
| Vitamin C | All-cause mortality, total cancer, CVD. Per 100 mg/d increment of dietary vitamin C intake, all-cause mortality (RR 0.89, 95%CI 0.85, 0.94), total cancer (RR 0.93, 95%CI 0.87, 0.99), and CVD (RR 0.89, 95%CI 0.85, 0.94) all had reduced risk. For measures of blood concentrations: Per 50 μmol/L increase in vitamin C, all-cause mortality (RR 0.72, 95%CI 0.66, 0.79), total cancer (RR 0.74, 95%CI 0.66, 0.82), and CVD (RR 0.76, 95%CI 0.65, 0.87) all had lowered risk. | Meta-analysis included 69 prospective cohort studies from Europe, America, and Asia. | [58] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Vitamin E | Prostate cancer. Compared to the placebo group, Hazard ratios for developing prostate cancer for the vitamin E treatment were HR 1.17 (99%CI 1.004, 1.36, p = 0.008); for selenium HR 1.09 (99%CI 0.93, 1.27; p = 0.18); for selenium plus vitamin E HR 1.05 (99%CI 0.89, 1.22, p = 0.46). | Selenium and Vitamin E Cancer Prevention Trial (SELECT). Analysis of 34,887 men randomly assigned to one of four treatment groups (selenium: 200 μg/d from L-selenomethionine plus vitamin E placebo; vitamin E: 400 IU/d of all rac-α-tocopheryl acetate plus selenium placebo; both selenium and vitamin E; placebo). | [86] |
| Vitamin E | Mortality rates. Supplementation with vitamin E significantly increased overall mortality rates (RR 1.03, 95%CI 1.00, 1.05). | Meta-analysis of 46 supplementation trials with low bias risk; included 171,244 participants. | [87] |
| Vitamin E | CVD. When comparing high vs. low intake groups, there was no significant protective role of vitamin E against CVD (RR 0.90, 95%CI 0.78, 1.03), total cancer (RR 1.01, 95%CI 0.92, 1.10), or total mortality (RR 0.98, 95%CI 0.93, 1.04). | Meta-analysis of dietary vitamin E and risk of CVD (eight studies), total cancer (five studies), total mortality (nine studies). | [58] |
| Vitamin E | Myocardial infarction. Compared to controls, vitamin E given alone significantly decreased myocardial infarction (3.0% vs. 3.4%) (Risk Ratio 0.82, 95%CI 0.70, 0.96; p = 0.01). | Meta-analysis of 16 randomized controlled clinical trials examined vitamin E’s impact on myocardial infarction. Dose ranged from 33–800 IU, with follow-up ranging from 0.5 to 9.4 years. | [89] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Green, leafy vegetables | In high vs. low comparison, green, leafy vegetables were inversely related to development of CHD (RR 0.83, 95%CI 0.75, 0.91), CVD (RR 0.84, 95%CI 0.71, 0.99), total stroke risk (RR 0.88, 95%CI 0.81, 0.95), ischemic stroke (RR 0.88, 95%CI 0.78, 0.99), and all-cause mortality (RR 0.92, 95%CI 0.86, 0.97). In dose-response analyses, green leafy vegetables and salads were only significantly associated with lower all-cause mortality (per 100 g/d RR 0.78, 95%CI 0.71, 0.86). | Meta-analysis of ten prospective studies comparing high vs. low intake groups; analysis of nine studies investigating dose-response, in increments of 100 g/day. | [6] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Carotenoids | Mean changes in serum retinol concentrations after consuming retinol-rich foods 0.23 micromol/L (95%CI 0.18, 0.28), fruit 0.12 micromol/L (95%CI 0.06, 0.18), vegetable 0.07 micromol/L (95%CI 0.03, 0.11), and low-retinol, low-carotene groups 0.00 micromol/L (95%CI −0.06, 0.05). Mean changes in serum beta-carotene concentrations in the vegetable group was 0.14 micromol/L (95%CI 0.12, 0.17) while the increase in the fruit group was 0.52 micromol/L (95%CI 0.43, 0.60). | Anemic school children (7–11 y) in West Java, Indonesia were randomly assigned to 1 of 4 groups. Children consumed two meals/day, six days/week for nine weeks. Group 1 (n = 48) consumed 556 retinol equivalents (RE)/d from retinol-rich food; Group 2 (n = 49) consumed 509 RE/d from fruit; Group 3 (n = 45) consumed 684 RE/d from dark-green, leafy vegetables and carrots; Group 4 (n = 46) consumed 44 RE/d from low-retinol, low-carotene food. | [106] |
| Beta-carotene plus alkaloid piperine | Significantly greater serum beta-carotene increases were observed during supplementation with beta-carotene plus piperine (49.8 ± 9.6 μg/dL) as compared to beta-carotene plus placebo (30.9 ± 5.4 μg/dL), (p < 0.0001). | Double-blind cross-over study with healthy adult males. Examined serum beta-carotene levels after 16 days of oral supplementation with 15 mg beta-carotene either with 5 mg 98% pure piperine, the main alkaloid of Piper nigrum, black pepper, or with a placebo control. | [113] |
| Carotenoids—Lutein and Zeaxanthin | The study investigated the association between age-related macular degeneration and lutein. Participants with higher plasma lutein had a significantly lower risk of developing age-related macular degeneration (Hazard Ratio 0.63 per 1-SD increase, 95%CI 0.41, 0.97). Zeaxanthin, however, was not significantly associated with reduced risk. | A prospective, population-based cohort study (Alienor: Antioxydants Lipides Essentiels Nutrition et Maladies Oculaires) followed 963 residents of Bordeaux, France, for eight years. Subjects were 73 years or older at baseline. | [114] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| High flavonoid foods and beverages | CVD, blood pressure, lipoproteins, flow-mediated dilatation. Chocolate intake increased flow-mediated dilatation after both acute (up to 6 h after ingesting; 3.99%; 95%CI 2.86, 5.12) and chronic exposure (greater than two weeks; 1.45%; 95%CI 0.62, 2.28). Chocolate was associated with a significant reduction in systolic (−5.88 mm Hg; 95%CI −9.55, −2.21) and diastolic (−3.30 mm Hg; 95%CI −5.77, −0.83) blood pressure. Green tea reduced LDL cholesterol (−0.23 mmol/L; 95%CI −0.34, −0.12). When measured up to six hours after ingestion, black tea increased both systolic (5.69 mm Hg; 95%CI 1.52, 9.86) and diastolic (2.56 mm Hg; 95%CI 1.03, 4.10) blood pressure. | Meta-analysis of 133 randomized controlled trials. | [144] |
| Anthocyanins | CHD and CVD mortality. Dietary anthocyanins were associated with reduced risk of CHD (RR 0.91, 95%CI 0.83, 0.99) and CVD mortality (RR 0.92, 95%CI 0.87, 0.97). No relationship was observed between intake of high anthocyanin food and a reduced risk of myocardial infarction, stroke, or total CVD. Subgroup analysis indicates reduced risks were more prevalent for anthocyanidin intake, as compared to consumption of anthocyanin or berries. | Meta-analysis of prospective cohort studies. Nineteen studies involving 602,054 participants and more than 22,673 cases of non-fatal or fatal cardiovascular disease were included. | [145] |
| Flavonoids | Type 2 diabetes. Flavonoids were associated with reduced risk of Type 2 diabetes; for total flavonoid intake, RR 0.89 (95%CI 0.82, 0.96). | Meta-analysis of eight prospective cohort studies that included 312,015 participants. Of these, 19,953 developed type 2 diabetes mellitus during the follow-up periods, ranging from 4 to 28 years. | [146] |
| Flavonols, anthocyanins | Alzheimer’s Disease and related dementias. Results indicate participants with the greatest intakes of flavonols, anthocyanins, and flavonoid polymers had the lowest health risk of Alzheimer’s Disease and related dementias, relative to those with the lowest intakes. Significant protective relationships were seen for flavonols (health risk 0.54, 95%CI 0.32, 0.90) and anthocyanins (health risk 0.24, 95%CI 0.15, 0.39). | The Framingham Heart Study Offspring cohort, including more than 2800 participants who were followed for an average of 19.7 y. | [147] |
| Soy iso-flavonoids | Type 2 Diabetes. Women, but not men, in the upper third of soy-based food and isoflavone intake had a significantly lower risk of developing type 2 diabetes (HR 0.45, 95%CI 0.30, 0.68), as compared with women in the lowest third of intake. | Prospective cohort study of 13,521 Japanese subjects (5883 men, 7638 women; 35–69 y old). Subjects completed questionnaires regarding diet and lifestyle during a 10-year follow-up period. | [148] |
| Isoflavone intake | Coronary Heart Disease. Isoflavone intake was inversely associated with CHD when comparing the upper and lower quintiles. Isoflavone intake (HR 0.87, 95%CI 0.81, 0.94; p = 0.008), and tofu (HR 0.82, 95%CI 0.70, 0.95; p = 0.005) were significantly associated with decreased CHD, but this significance was mostly driven by pre-menopausal women and postmenopausal women who were not using hormone therapy. Soy milk was not significantly associated with decreased CHD risk. | Analysis of three cohort studies Nurses’ Health study (n = 74,241 women, data from 1984–2012), Nurses’ Health Study II (n = 94,233 women, data from 1991–2013), and Health Professionals Follow-up Study (n = 42,226 men, data from 1986–2012). Subjects were free of cardiovascular disease and cancer at baseline. | [149] |
| Soy intake | Breast Cancer. Soy intake lowered breast cancer risk in Asian women, both pre- and post-menopause (pre-menopause OR 0.59, 95%CI 0.48, 0.69; post-menopause OR 0.59, 95%CI 0.44, 0.74); in Western countries, this association was marginal, and only in post-menopausal women (OR 0.92, 95%CI 0.83, 1.00). | Meta-analysis of 35 epidemiological studies. | [150] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Nicotine containing foods | Parkinson’s Disease was inversely associated with consumption of all edible Solanaceae combined (RR 0.81, 95%CI 0.65, 1.01 per time per day), but not consumption of all other vegetables combined (RR 1.00, 95%CI 0.92, 1.10). The trend was strengthened by weighting the edible Solanaceae by their nicotine concentration (p = 0.004). Individually, peppers were also inversely associated with developing Parkinson’s Disease (p = 0.005). This protective effect of edible Solanaceae largely occurred in those who had never used tobacco or had smoked cigarettes for less than 10 years. | Population-based study of 490 newly diagnosed with Parkinson’s Disease compared with 644 unrelated, neurologically normal controls. | [184] |
| Nicotine containing foods | Parkinson’s Disease. Participants who had never smoked were followed for 26 years. HR for the highest compared with the lowest quintile of dietary nicotine intake was 0.70 (95%CI 0.51, 0.94). A significant inverse association was only observed in women (adjusted HR 0.64, 95%CI 0.42, 0.96), not in men (adjusted HR 0.77, 95%CI 0.50, 1.20). Greater pepper consumption was associated with lower Parkinson’s Disease risk in women (adjusted HR for consuming peppers ≥5 times/w compared with ≤3 times/mo: 0.49, 95%CI 0.25, 0.94), but not in men (adjusted HR: 1.04, 95%CI 0.57, 1.90). | Dietary nicotine intake was calculated (based on consumption of peppers, tomatoes, processed tomatoes, potatoes, and tea) for two large prospective cohorts: The Nurses’ Health Study (n = 31,615) and the Health Professionals Follow-up Study (n = 19,523) who completed dietary questionnaires. | [185] |
| Caffeine | Markers of oxidative stress, including total antioxidant capacity, glutathione, oxidized glutathione, the ratio of glutathione to oxidized glutathione, lipid hydroperoxide, and malondialdehyde were measured. All measures changed significantly, and favorably after caffeine administration. Oxidized glutathione levels decreased 41% while lipid hydroperoxides levels decreased 70%. Glutathione levels increased 106%, while the ratio of glutathione to oxidized glutathione rose 249%. Changes were uniform across subjects. Caffeine appears to have consistent antioxidant properties. | Fifteen male volunteers (18–25 y with normal body mass index) who regularly consumed coffee were tested. Plasma oxidative stress markers were analyzed before and after caffeine consumption. A room temperature caffeine solution was consumed, at a rate of 5 mg/kg body weight/day, that was given in two daily doses (2.5 mg/kg mornings, 2.5 mg/kg after lunch) for seven days. Blood was drawn in the morning prior to the first dose of caffeine, and the final sample was drawn in the morning of the eighth day. | [186] |
| Antioxidant/Food | Biological Impacts Observed | Type of Study | Reference |
|---|---|---|---|
| Cruciferous vegetables (glucosinolates) | Comparison of high vs. low intake of cruciferous vegetables indicated they were inversely associated with both total cancer (RR 0.84, 95%CI 0.72,0.97) and all-cause mortality (RR 0.88, 95%CI 0.80, 0.97). | Meta-analyses examining coronary heart disease (seven studies), total stroke (four studies), cardiovascular disease (eight studies), total cancer (five studies), all-cause mortality (six studies). | [6] |
| Glucosinolate-containing foods (Brussels sprouts and cabbage) | After adjusting for risk factors, comparison of the top and bottom quintiles found a weak but significant association with increased CHD risk (HR 1.09, 95%CI 1.01, 1.17); those who consumed one or more servings of Brussels sprouts and cabbage per week had a higher CHD risk than those who consumed them less than once a month. | Participants from three prospective longitudinal cohort studies (Nurses’ Health Study, Nurses’ Health Study II, Health Professionals Follow-up Study) who were free of cardiovascular diseases and cancer at baseline completed food-frequency questionnaires. | [195] |
| Biological Impacts Observed | Details of Study | Reference |
|---|---|---|
| Researchers found urinary 8-isoprostane F2α levels, a measure of lipid peroxidation, were significantly lowered in both low and high diversity diets; although, women who consumed the high diversity diet showed a greater attenuation in lipid peroxidation. The more compelling evidence was a measure of DNA oxidation based on 8-hydroxy-2-deoxyguanosine concentration from the DNA of peripheral lymphocytes. This measure indicated that only those consuming the high diversity diet experienced a significant reduction in DNA oxidation. | Two diets were developed, both supplying 8–10 daily servings of fruits and vegetables; the number of servings was based on individual energy intakes; 106 women consumed these diets over the course of two weeks. One diet (low diversity, antioxidant-rich) utilized mostly plants from five botanical families noted to have high antioxidant activity (e.g., spinach and beet from Chenopodiaceae, broccoli, and cabbage from Brassicaceae, garlic, and onion from Liliaceae, grapefruit and orange from Rutaceae, tomato, and peppers from Solanaceae). These same families were represented, albeit in lower concentration, in the high diversity (18 plant families) diet; the additional plant families in the high diversity diet were chosen because they were associated with a reduction in oxidative damage to lipids or DNA. | [211] |
| Changes in plasma non-enzymatic antioxidant capacity (NEAC) were much greater, and more often significant, when studies examined humans with oxidative stress-related risk factors, as opposed to healthy subjects (Standardized mean NEAC difference for beverages: healthy subjects 0.177, p = 0.296; subjects with risk factors 0.765, p < 0.001; for food: healthy subjects 0.502, p < 0.001; subjects with risk factors 1.253, p < 0.001). | Meta-analyses were performed on studies that examined the effects of long-term dietary supplementation with either plant-based foods (chocolate, fruits, vegetables) and/or beverages (tea, fruit juice, red wine) on plasma non-enzymatic antioxidant capacity. | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briggs, M.A. From Foods to Chemotherapeutics: The Antioxidant Potential of Dietary Phytochemicals. Processes 2022, 10, 1222. https://doi.org/10.3390/pr10061222
Briggs MA. From Foods to Chemotherapeutics: The Antioxidant Potential of Dietary Phytochemicals. Processes. 2022; 10(6):1222. https://doi.org/10.3390/pr10061222
Chicago/Turabian StyleBriggs, Michelle A. 2022. "From Foods to Chemotherapeutics: The Antioxidant Potential of Dietary Phytochemicals" Processes 10, no. 6: 1222. https://doi.org/10.3390/pr10061222
APA StyleBriggs, M. A. (2022). From Foods to Chemotherapeutics: The Antioxidant Potential of Dietary Phytochemicals. Processes, 10(6), 1222. https://doi.org/10.3390/pr10061222





