Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nitrogen Adsorption
2.2. X-ray Powder Diffraction
2.3. Band Gap Determination
2.4. FTIR Spectroscopy for the Adsorption of Pyridine
2.5. Fluorescence Technique
2.6. High-Resolution Transmission Electron Microscopy
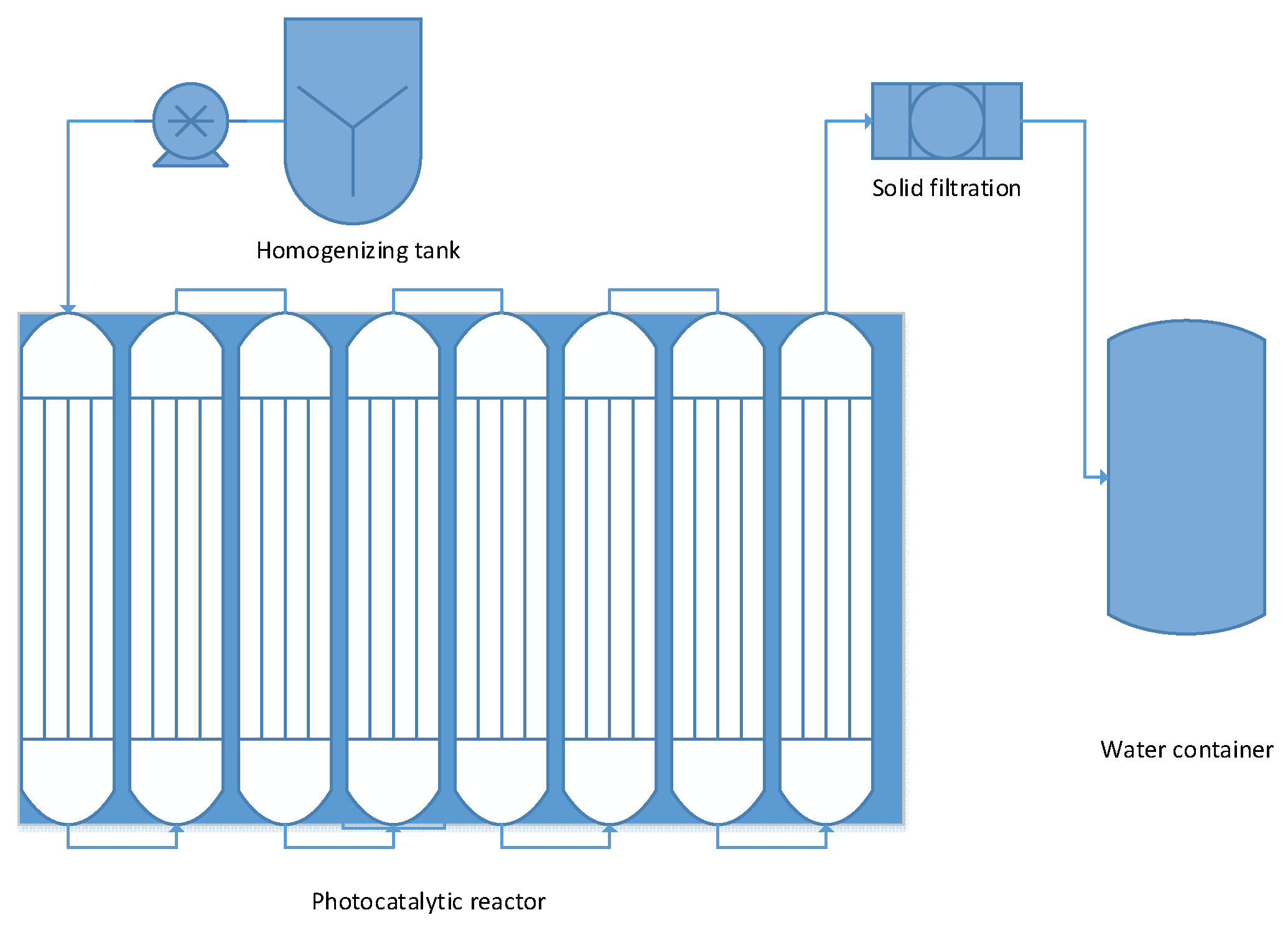
2.7. Photocatalytic Evaluation
3. Results and Discussion
4. Photocatalytic Evaluation
5. Operating Cost
6. Technical Feasibility and Applicability
- Photocatalytic rates and the stability of γ-Al2O3-based catalysts can be further improved. In spite of the high degradation rates achieved so far, the percentage of mineralization can be increased. Furthermore, the stability levels of some catalysts based on γ-Al2O3 are still unsatisfactory due to the loss of active sites and the leaching of metal dopants. Therefore, it is important to optimize catalyst synthesis methods and to develop new types of catalysts based on γ-Al2O3 that are more active and stable.
- The mechanism involved in the degradation of organic pollutants and the toxicity of the reaction intermediates should be thoroughly investigated. A theoretical study based on DFT could reveal the most preferential sites to be attacked (and the possible reaction intermediates) for target contaminants. In addition, it is necessary to evaluate the ecotoxicity and human toxicity of such intermediates since they can result in even more toxicity than the original moieties.
- The performances of photocatalysts must be evaluated using actual wastewater. There are important differences between simulated and actual wastewater that make results obtained for simulated wastewater less accurate.
- Experiments should be conducted on a major scale. Until now, most research on γ-Al2O3-based catalysts for the degradation of organic pollutants has been performed at lab-scale reactors. More attention should be paid to the reactor design, as well as to scaling Figure 12.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Li, D.; Sun, F.; Weng, Y.; You, S.; Shou, Y. Temperature-induced phase changes in bismuth oxides and efficient photodegradation of phenol and p-chlorophenol. J. Hazard. Mater. 2016, 301, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Baun, A.; Mikkelsen, P.; Ledin, A. Risk assessment of xenobiotics in stormwater discharged to Harrestrup Å, Denmark. Desalination 2007, 2151, 187–197. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Mantilla, A.; Tzompantzi, F.; Fernández, J.L.; Góngora, J.D.; Gómez, R. Photodegradation of phenol in aqueous medium by using Zn/Al+Fe mixed oxides obtained from layered double hydroxides materials. Catal. Today 2010, 150, 353–357. [Google Scholar] [CrossRef]
- Mahamuni, N.; Pandit, A.B. Effect of additives on ultrasonic degradation of phenol. Ultrason. Sonochem. 2006, 13, 165–174. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R. Semiconductor-catalyzed degradation of phenols with sunlight. Sol. Energy Mater. Sol. Cells 2008, 92, 1315–1321. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2020, 701, 651–669. [Google Scholar] [CrossRef]
- Dewil, R.; Mantzavinos, D.; Poulios, I.; Rodrigo, M.A. New perspectives for Advanced Oxidation Processes. J. Environ. Manag. 2017, 195, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Ahmed, L.M. Bulk and nanocatalysts applications in Advanced Oxidation Processes. In Oxidoreductase; BoD–Books on Demand: Norderstedt, Germany, 2021; Volume 7, pp. 107–111. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment e A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, I.R.; del Angel, G.; Bertin, V. Synthesis and characterization of Sn doped TiO2 photocatalysts: Effect of Sn concentration on the textural properties and on the photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. J. Alloys Compd. 2015, 643, S144–S149. [Google Scholar] [CrossRef]
- Thompson, T.L., Jr.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2, New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Xi-yao, Y.; Kai, Z.; Luo, X.; He, J. Inlfuence of TiO2 on surface properties of Al2O3. React. Kinet. Catal. Lett. 1992, 46, 179–186. [Google Scholar] [CrossRef]
- González, V.; Zanella, R.; del Angel, G.; Gómez, R. MTBE visible-light photocatalytic decomposition over Au/TiO2 and Au/TiO2-Al2O3 sol-gel prepared catalysts. J. Mol. Catal. Chem. 2008, 281, 93–98. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Shih, M.; Chang, Y.; Hon, C. Photocatalytic performance of alumina-incorporated titania composite nanoparticles: Surface area and crystallinity. Appl. Catal. A 2009, 364, 182–190. [Google Scholar] [CrossRef]
- Gomez, C.M.; del Angel, G.; Ramos-Ramirez, E.; Rangel-Vazquez, I.; Gonzalez, F.; Arrieta, A.; Vazquez-Zavala, A.; Bonilla-Sanchez, A.; Cantu, M.S. Alumina coating with TiO2 and its effect on catalytic photodegradation of phenol and p-cresol. J. Chem. Technol. Biotechnol. 2016, 91, 2211–2220. [Google Scholar] [CrossRef]
- Mechiakh, R.; Meriche, F.; Kremer, R. TiO2 thin films prepared by sol–gel method for waveguiding applications: Correlation between the structural and optical properties. Opt. Mater. 2007, 30, 645–651. [Google Scholar] [CrossRef]
- Svagelj, Z.; Mandic, V.; Curkovic, L.; Biosic, M.; Zmak, I.; Gaborardi, M. Titania-Coated Alumina Foam Photocatalyst for Memantine Degradation Derived by Replica Method and Sol-Gel Reaction. Materials 2020, 13, 227. [Google Scholar] [CrossRef] [Green Version]
- Cizmic, M.; Ljubas, D.; Rozman, M.; Asperger, D.; Curkovic, L.; Babic, S. Photocatalytic degradation of azithromycin by nanostructured TiO2 film: Kinetics, degradation products, and toxicity. Materials 2019, 12, 873. [Google Scholar] [CrossRef] [Green Version]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M.A. Román-Martínez, Effect of the Preparation Method (Sol-Gel or Hydrothermal) and Conditions on the TiO2 Properties and Activity for Propene Oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Matsubara, T.; Numata, D.; Serizawa, A. Facile Synthesis of Potassium-Doped Titanium Oxide Nanostructure (KTiOxs)/AlO(OH) Composites for Enhanced Photocatalytic. Catalysts 2021, 11, 548. [Google Scholar] [CrossRef]
- Xu, X.; Zu, X.; Ao, D.; Yu, J.; Xiang, X.; Xie, W.; Tang, Y.; Li, S.; Fu, Y. NH3-Sensing Mechanism Using Surface Acoustic Wave Sensor with AlO(OH) Film. Nanomaterials 2019, 9, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-W.; Jang, Y.-I.; Park, W.-S.; Kim, S.-W.; Lee, B.-J. Photocatalytic and Pozzolanic Properties of Nano-SiO2/Al2O3-TiO2 Powder for Functional Mortar. Materials 2019, 12, 1037. [Google Scholar] [CrossRef] [Green Version]
- Roongraung, K.; Chuangchote, S.; Laosiripojana, N. Enhancement of Photocatalytic Oxidation of Glucose to Value-Added Chemicals on TiO2 Photocatalysts by A Zeolite (Type Y) Support and Metal Loading. Catalysts 2020, 10, 423. [Google Scholar] [CrossRef]
- Park, Y.; Kim, S.; Kim, J.; Khan, S.; Han, C. UV/TiO2 Photocatalysis as an Efficient Livestock Wastewater Quaternary Treatment for Antibiotics Removal. Water 2022, 14, 958. [Google Scholar] [CrossRef]
- Marizcal-Barba, A.; Sanchez-Burgos, J.A.; Zamora-Gasga, V.; Perez Larios, A. Study of the Response Surface in the Photocatalytic Degradation of Acetaminophen Using TiO2. Photochem 2022, 2, 225–236. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdelfattah, I.; Atitar, M.F.; Robben, L.; Bouzid, H.; Al-Sayari, S.A.; Bahnemann, D.W. Photocatalytic degradation of imazapyr using mesoporous Al2O3–TiO2 nanocomposites. Sep. Purif. Technol. 2015, 145, 147–153. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, H.; Ma, M.; Yang, Z. Facile synthesis of magnetically recoverable Fe3O4/Al2O3/molecularly imprinted TiO2 nanocomposites and its molecular recognitive photocatalytic degradation of target contaminant. J. Mol. Catal. A Chem. 2015, 402, 10–16. [Google Scholar] [CrossRef]
- 30. Rodríguez-Barajas, N.; Becerra-Solano, L.; Gutiérrez-Mercado, Y.K.; Macías-Carballo, M.; Gómez, C.M.; Pérez-Larios, A. Study of the Interaction of Ti–Zn as a Mixed Oxide at Different pH Values Synthesized by the Sol–Gel Method and Its Antibacterial Properties. Nanomaterials 2022, 12, 1948. [Google Scholar] [CrossRef]
- Magnone, E.; Kim, M.K.; Lee, H.J.; Park, J.H. Testing and substantial improvement of TiO2/UV photocatalysts in the degradation of Methylene Blue. Ceram. Int. 2019, 45, 3359–3367. [Google Scholar] [CrossRef]
- Vargova, M.; Plesch, G.; Vogt, U.F.; Zahoran, M.; Gorbar, M.; Jesenak, K. TiO2 thick films supported on reticulated macroporous Al2O3 foams and their photoactivity in phenol mineralization. Appl. Surf. Sci. 2011, 257, 4678–4684. [Google Scholar] [CrossRef]
- Shtyka, O.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Dubkov, S.; Gromov, D.; Maniecki, T. Photocatalytic reduction of CO2 Over Me (Pt, Pd, Ni, Cu)/TiO2 Catalysts. Top. Catal. 2020, 63, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cui, J.; Zhang, N.; Song, J.; Fan, X.; Zhao, Z.; Kong, L.; Xiao, X.; Xie, Z. Propane Dehydrogenation over PtSn/Al2O3 Catalysts: Influence of Urea to Al(NO3)3_9H2O Ratio. Catalysts 2022, 12, 157. [Google Scholar] [CrossRef]
- Murillo-Acevedo, Y.; Giraldo, L.; Moreno-Pirajan, J.C. Nanoparticles size distribution and phenol photodegradation with TiO2/C support obtained by phosphoric acid activation of palm kernel shell. Microporous Mesoporous Mater. 2020, 304, 109325. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). J. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Gómez, C.M.; del Angel, G.; Tzompantzi, F.; Gómez, R.; Torres-Martínez, L. Photocatalytic degradation of p-cresol on Pt/γAl2O3-TiO2 mixed oxides: Effect of oxidizing and reducing pre-treatments. J. Photochem. Photobiol. A Chem. 2012, 236, 21–25. [Google Scholar] [CrossRef]
- Wang, H.-L.; Qi, H.-P.; Wei, X.-N.; Liu, X.-Y.; Jiang, W.-F. Photocatalytic activity of TiO2 supported SiO2-Al2O3 aerogels prepared from industrial fly ash. Chin. J. Catal. 2016, 37, 2025–2033. [Google Scholar] [CrossRef]
- Escobar, J.; de los Reyes, J.A.; Viveros, T. Sol–gel Al2O3 structure modification by Ti and Zr addition. A NMR study. Stud. Surf. Sci. Catal. 2000, 143, 547–554. [Google Scholar]
- Kwak, J.H.; Hu, J.Z.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Penta-coordinated Al3+ ions as preferential nucleation sites for BaO on γ -Al2O3: An ultra-high-magnetic field 27Al MAS NMR study. J. Catal. 2007, 251, 189–194. [Google Scholar] [CrossRef]
- Li, L.D.; Xu, C.Z.; Zheng, M.Q.; Chen, X.H. Effect of B2O3 modified Ag/TiO2-Al2O3 adsorbents on the adsorption desulfurization of diesel. J. Fuel Chem. Technol. 2015, 43, 990–997. [Google Scholar] [CrossRef]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.N.; Serpone, N. Visible Light Absorption by Various Titanium Dioxide Specimen. J. Phys. Chem. B 2006, 110, 50. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.H.; Wang, L.-W. Oxygen vacancy and hole conduction in amorphous TiO2. Phys. Chem. Chem. Phys. 2015, 17, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, V.N.; Serpone, N. On the Origin of the Spectral Bands in the Visible Absorption Spectra of Visible-Light-Active TiO2 Specimens Analysis and Assignments. J. Phys. Chem. C 2009, 113, 15110–15123. [Google Scholar] [CrossRef]
- Liu, X.; Truitt, R.E. DRFT-IR Studies of the Surface of γ-Alumina. J. Am. Chem. Soc. 1997, 119, 9856–9860. [Google Scholar] [CrossRef]
- Yang, X.; Song, Y.; Cao, T.; Wang, L.; Song, H.; Lin, W. The double tuning effect of TiO2 on Pt catalyzed dehydrogenation of methylcyclohexane. Mol. Catal. 2020, 492, 110971. [Google Scholar] [CrossRef]
- Liu, X. DRIFTS Study of Surface of α-Alumina and Its Dehydroxylation. J. Phys. Chem. C 2008, 112, 5066–5073. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Robben, L.; Yarovyi, V.; Wark, M. Palladium Doped Porous Titania Photocatalysts: Impact of Mesoporous Order and Crystallinity. Chem. Mater. 2010, 22, 108–116. [Google Scholar] [CrossRef]
- Nair, S.B.; John, K.A.; Joseph, J.A.; Babu, S.; Shinoj, V.K.; Remillard, S.K.; Shaji, S.; Philip, R.R. Influence of pn junction mechanism and alumina overlayer on the photocatalytic performance of TiO2 nanotubes. Nanotechnology 2020, 31, 275403. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Roy, M.; Kundu, S.; Naskar, M.K. TiO2 hollow microspheres impregnated with biogenic gold nanoparticles for the efficient visible light-induced photodegradation of phenol. J. Phys. Chem. Solids 2019, 129, 329–339. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Castro, L.V.; Ortiz-Islas, E.; Manríquez, M.E.; Albiter, E.; Cabrera-Sierra, R.; Alvarado-Zavala, B. Photocatalytic Degradation of Mixed Dyes in Aqueous Phase by MgAlTi and ZnAlTi Mixed Oxides. Top. Catal. 2020, 64, 97–111. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Castillo-Rodríguez, J.C.; Tzompantzi-Flores, C.; Pérez-Hernández, R.; Gómez, R.; Santolalla-Vargas, C.E.; Che-Galicia, G.; Ramos-Ramírez, E. Addition of SnO2 over an oxygen deficient zirconium oxide (ZrxOy) and its catalytic evaluation for the photodegradation of phenol in water. Catal. Today 2022, 394–396, 376–389. [Google Scholar] [CrossRef]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A. WO3–based photocatalysts: A review on synthesis, performance enhancement and photocatalytic memory for environmental applications. Ceram. Int. 2022, 48, 5845–5875. [Google Scholar] [CrossRef]
- Mengting, Z.; Kurniawan, T.A.; Duan, L.; Song, Y.; Hermanowicz, S.W.; Othman, M.H.D. Advances in BIOX-based ternary photocatalysis for water technology and energy storage applications: Research trends, challenges, solutions and ways forward. Rev. Environ. Sci. Bio/Technol. 2022, 21, 331–370. [Google Scholar] [CrossRef]
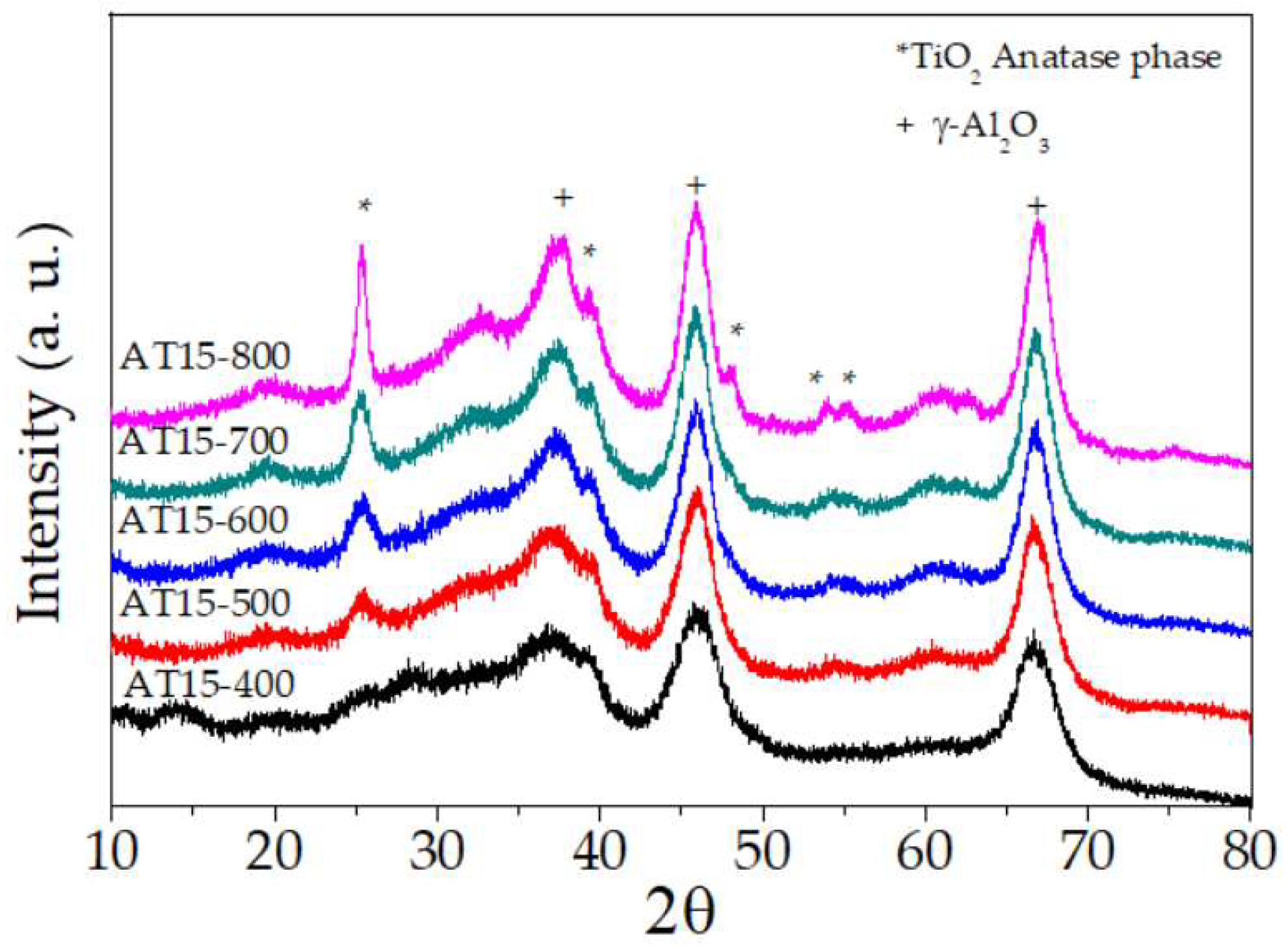

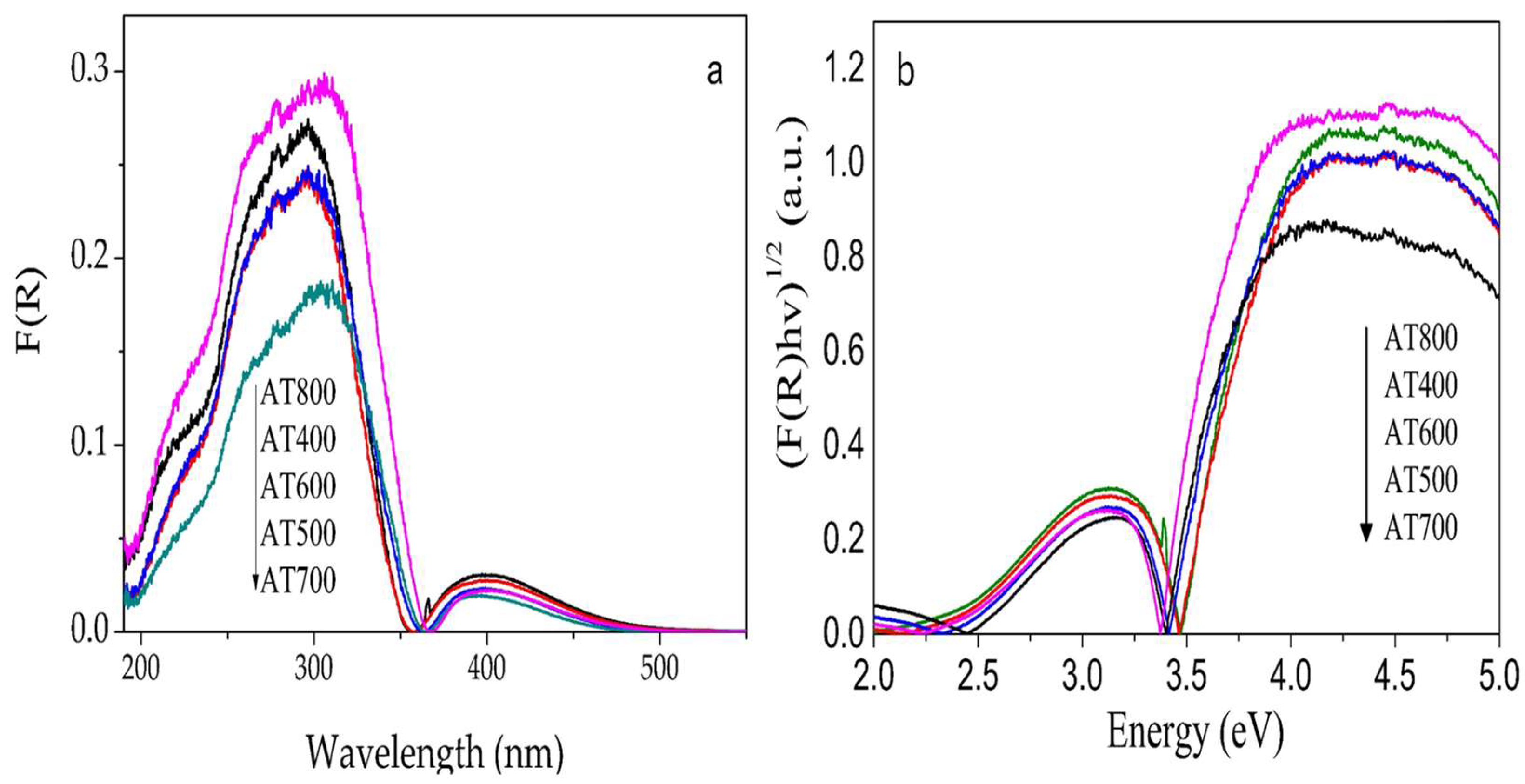

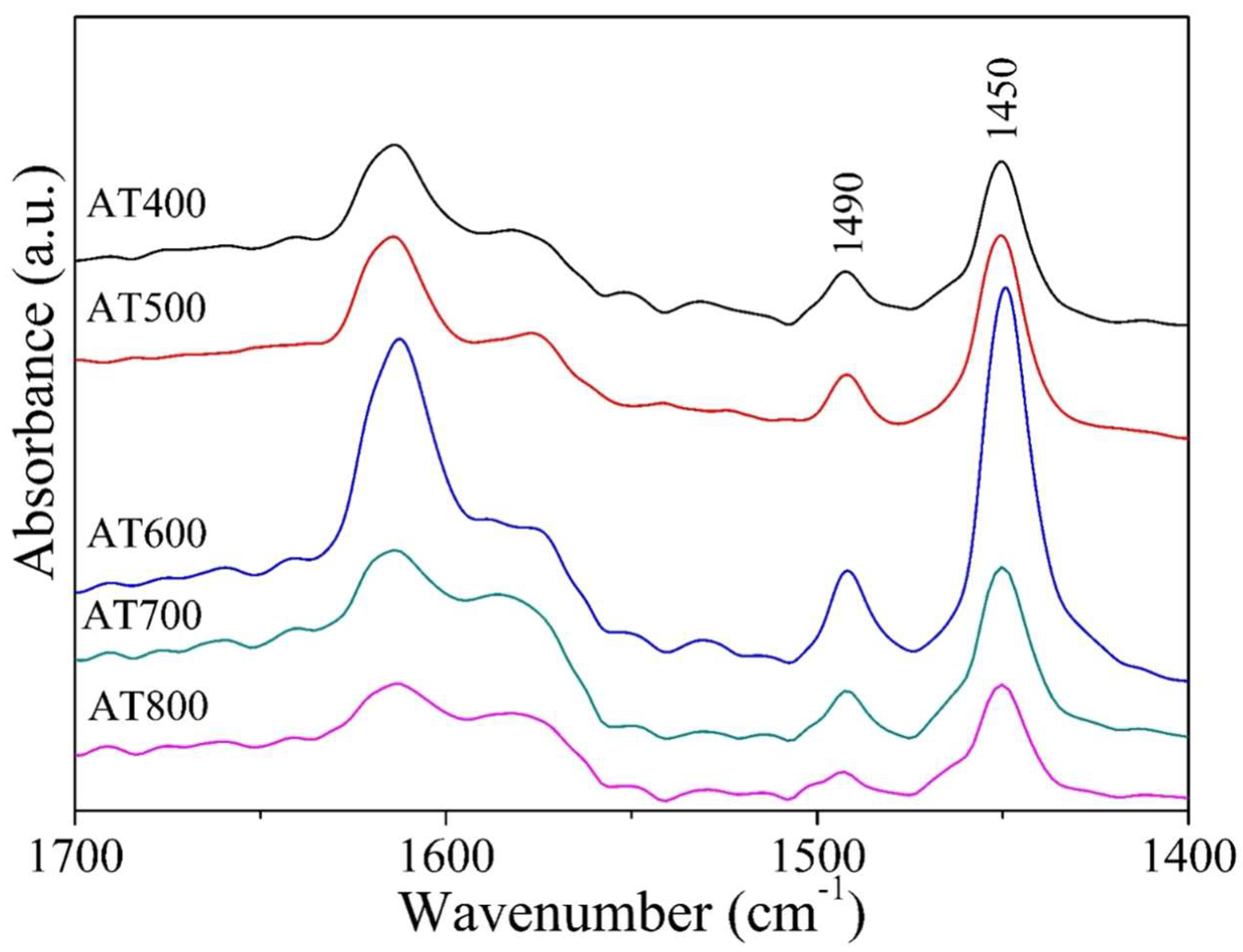
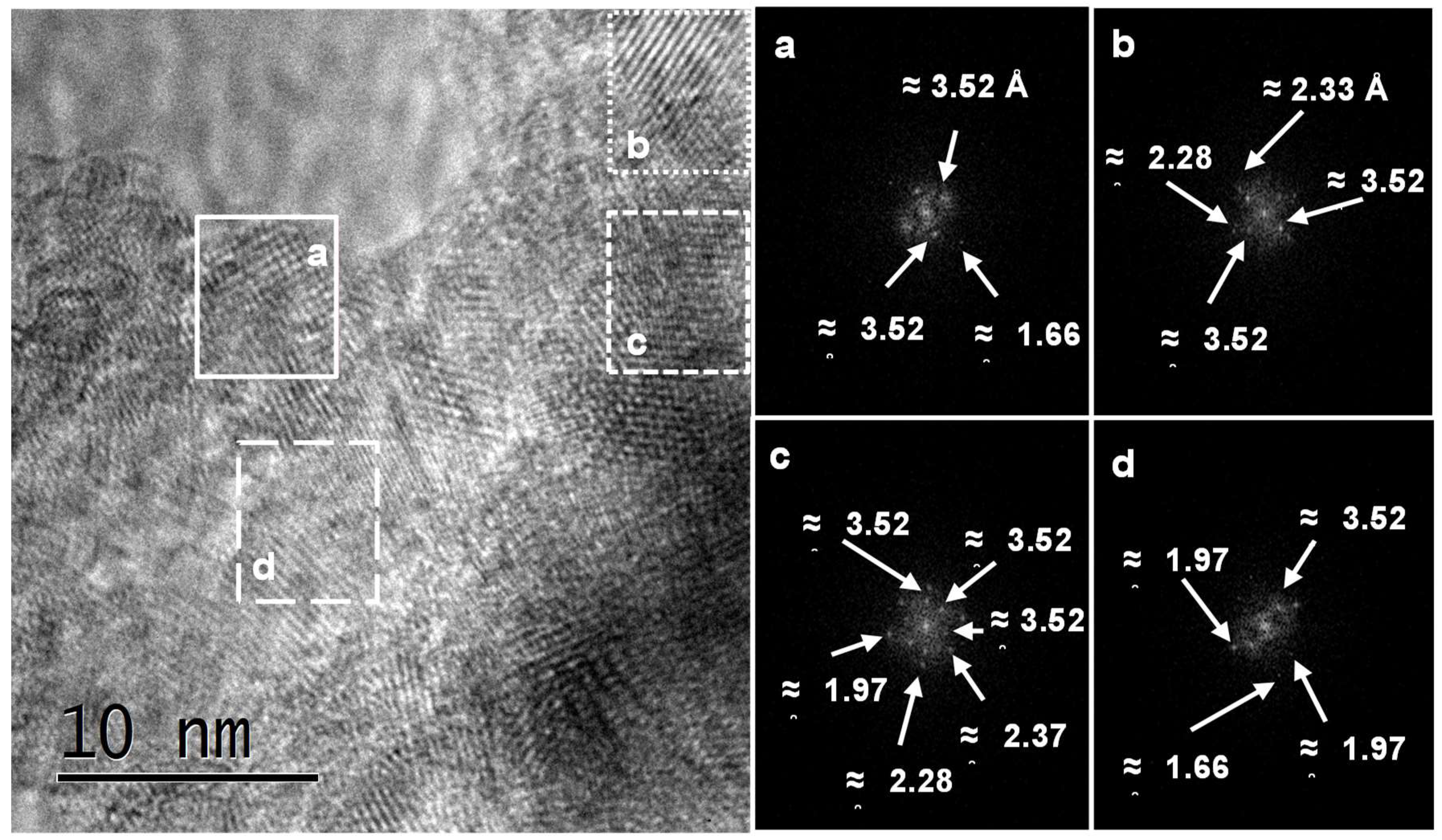
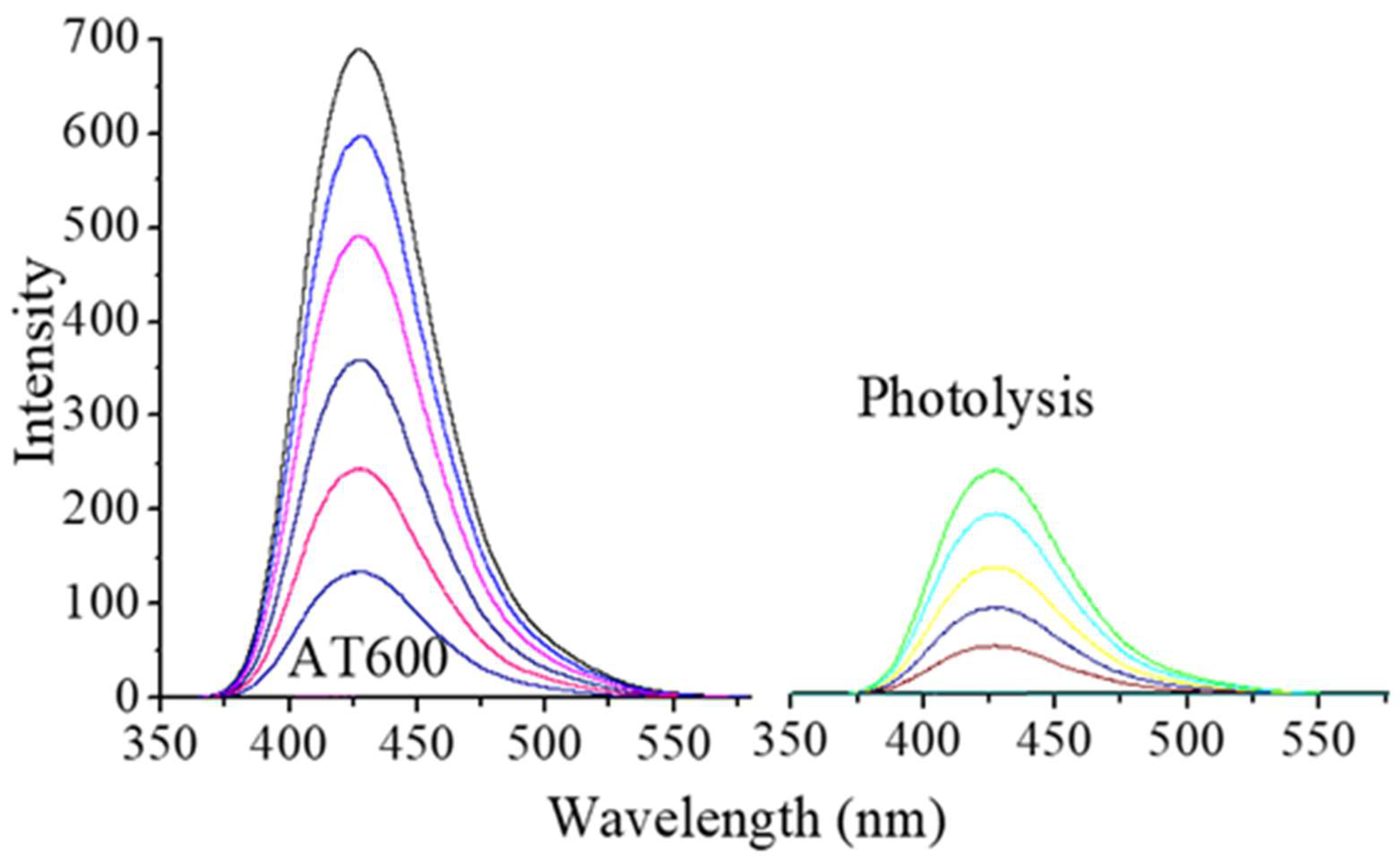
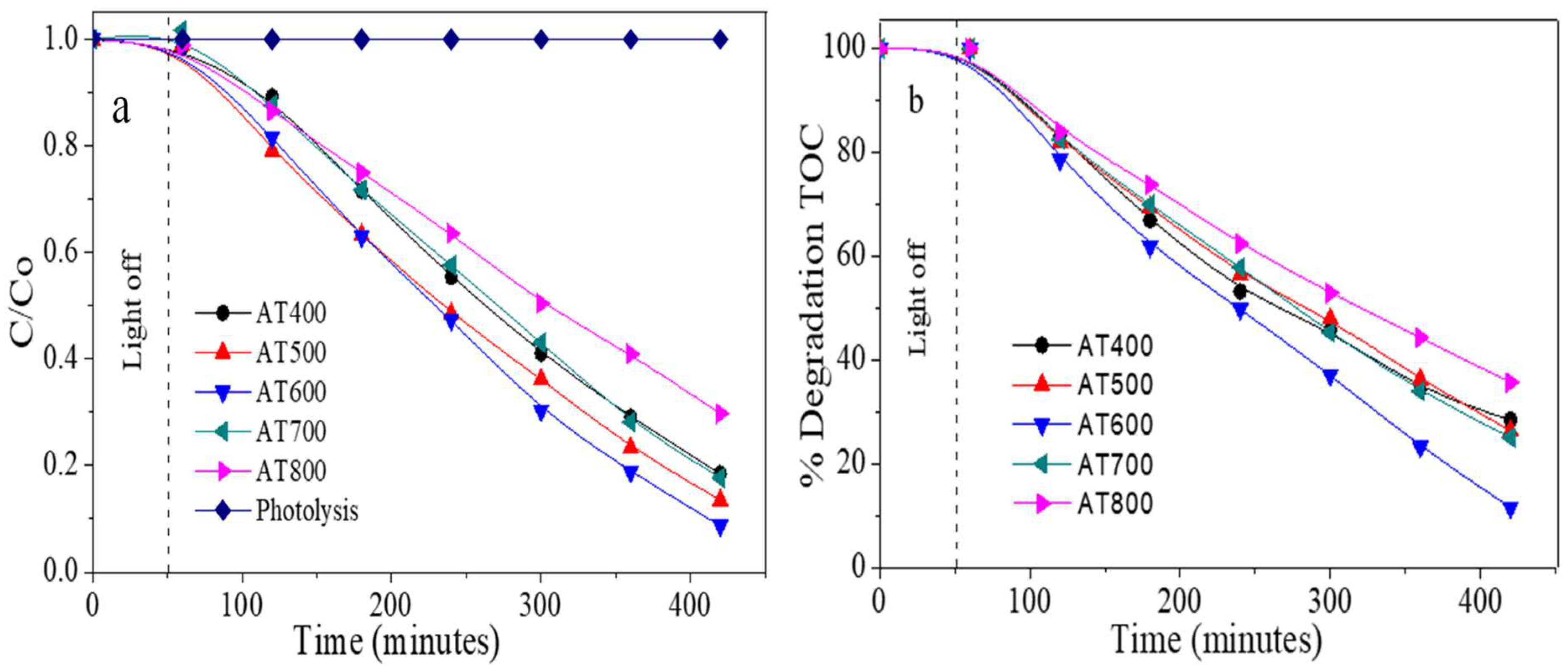

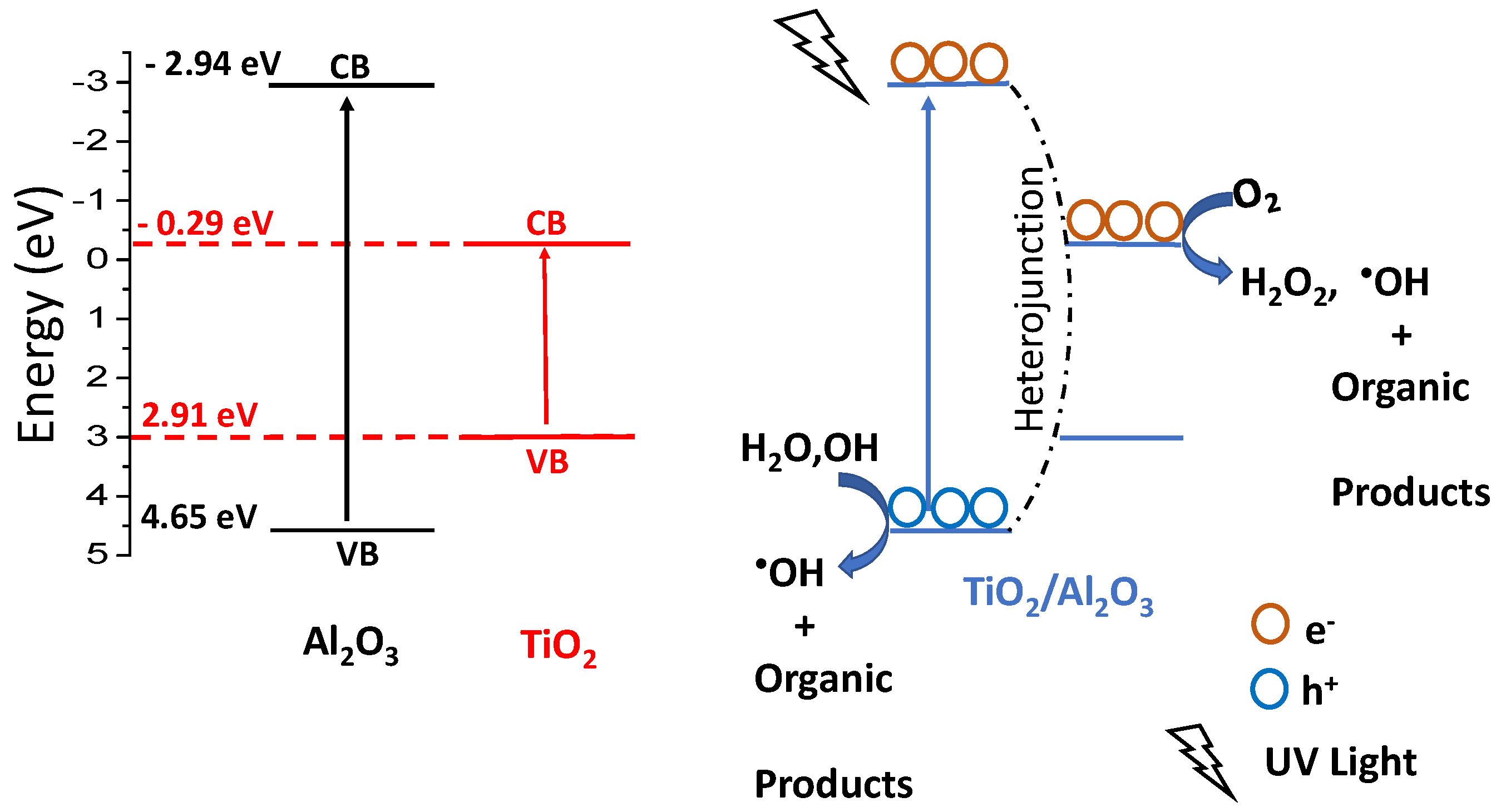

| Catalysts | Surface Area m2/g | Total Acidity (µmol/gcat) | γ-Al2O3 * Crystallite Size nm | Anatase + Crystallite Size nm | Dp (Å) | Vp (cm3/g) |
|---|---|---|---|---|---|---|
| AT400 | 308 | 140 | 4.88 | 3.95 | 50–65 | 0.51 |
| AT500 | 266 | 172 | 4.84 | 3.93 | 60 | 0.46 |
| AT600 | 262 | 314 | 4.82 | 3.95 | 45 | 0.41 |
| AT700 | 232 | 270 | 4.79 | 3.96 | 50 | 0.40 |
| AT800 | 179 | 260 | 4.83 | 3.97 | 65 | 0.39 |
| Catalysts | Band Gap Eg (eV) | kapp ×10−3 | t1/2 (min) | |
|---|---|---|---|---|
| a | b | |||
| AT400 | 3.3 | 2.30 | 2.1 | 270 |
| AT500 | 3.5 | 2.35 | 2.2 | 255 |
| AT600 | 3.4 | 2.40 | 2.5 | 217 |
| AT700 | 3.4 | 2.50 | 2.1 | 270 |
| AT800 | 3.35 | 2.40 | 1.8 | 325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Gómez, C.; Rangel-Vazquez, I.; Zarraga, R.; del Ángel, G.; Ruíz-Camacho, B.; Tzompantzi, F.; Vidal-Robles, E.; Perez-Larios, A. Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment. Processes 2022, 10, 1186. https://doi.org/10.3390/pr10061186
Martinez-Gómez C, Rangel-Vazquez I, Zarraga R, del Ángel G, Ruíz-Camacho B, Tzompantzi F, Vidal-Robles E, Perez-Larios A. Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment. Processes. 2022; 10(6):1186. https://doi.org/10.3390/pr10061186
Chicago/Turabian StyleMartinez-Gómez, Claudia, Israel Rangel-Vazquez, Ramon Zarraga, Gloria del Ángel, Beatriz Ruíz-Camacho, Francisco Tzompantzi, Esmeralda Vidal-Robles, and Alejandro Perez-Larios. 2022. "Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment" Processes 10, no. 6: 1186. https://doi.org/10.3390/pr10061186
APA StyleMartinez-Gómez, C., Rangel-Vazquez, I., Zarraga, R., del Ángel, G., Ruíz-Camacho, B., Tzompantzi, F., Vidal-Robles, E., & Perez-Larios, A. (2022). Photodegradation and Mineralization of Phenol Using TiO2Coated γ-Al2O3: Effect of Thermic Treatment. Processes, 10(6), 1186. https://doi.org/10.3390/pr10061186







