Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Synthesized Materials
Characterization of Mo/SynTiO2 and SynTiO2
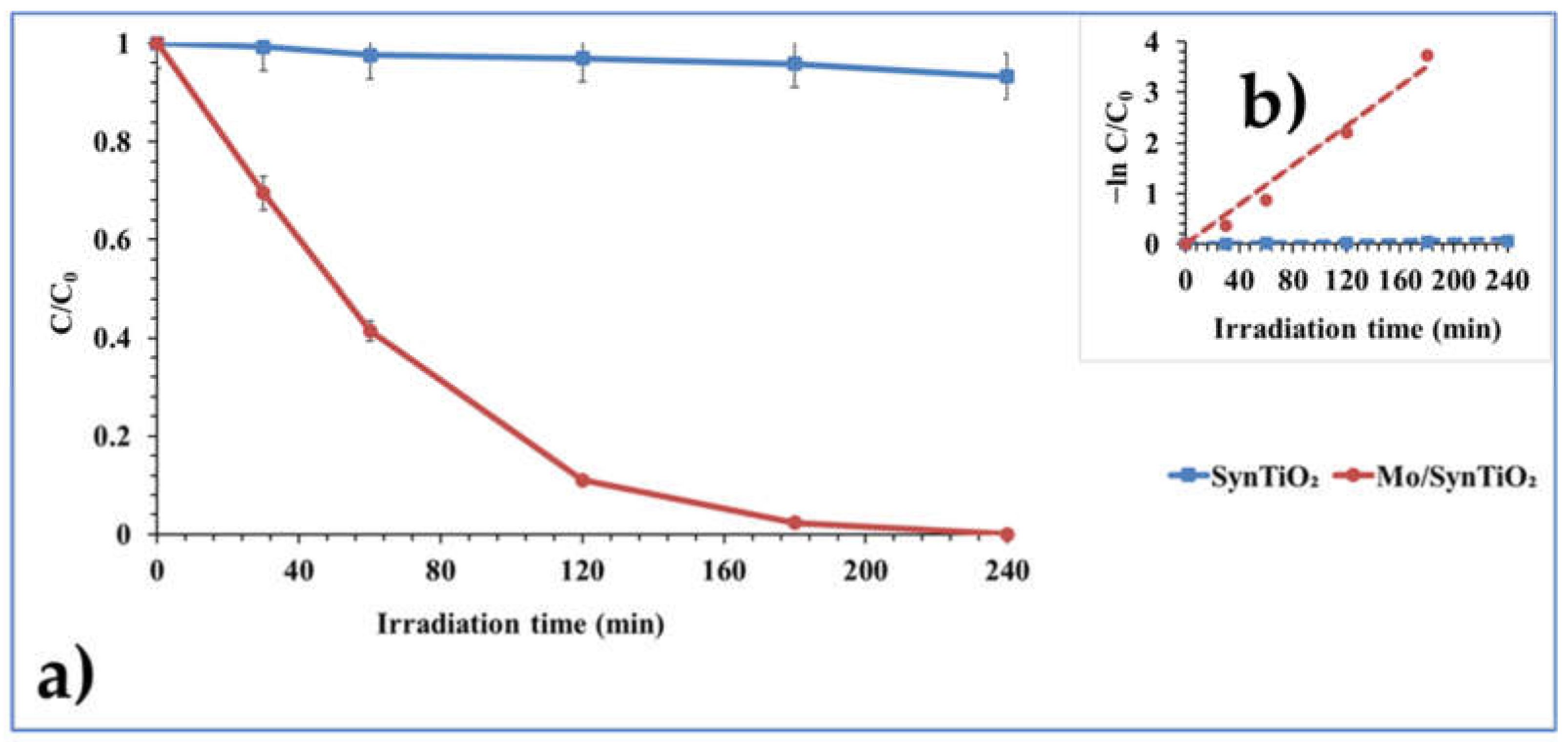
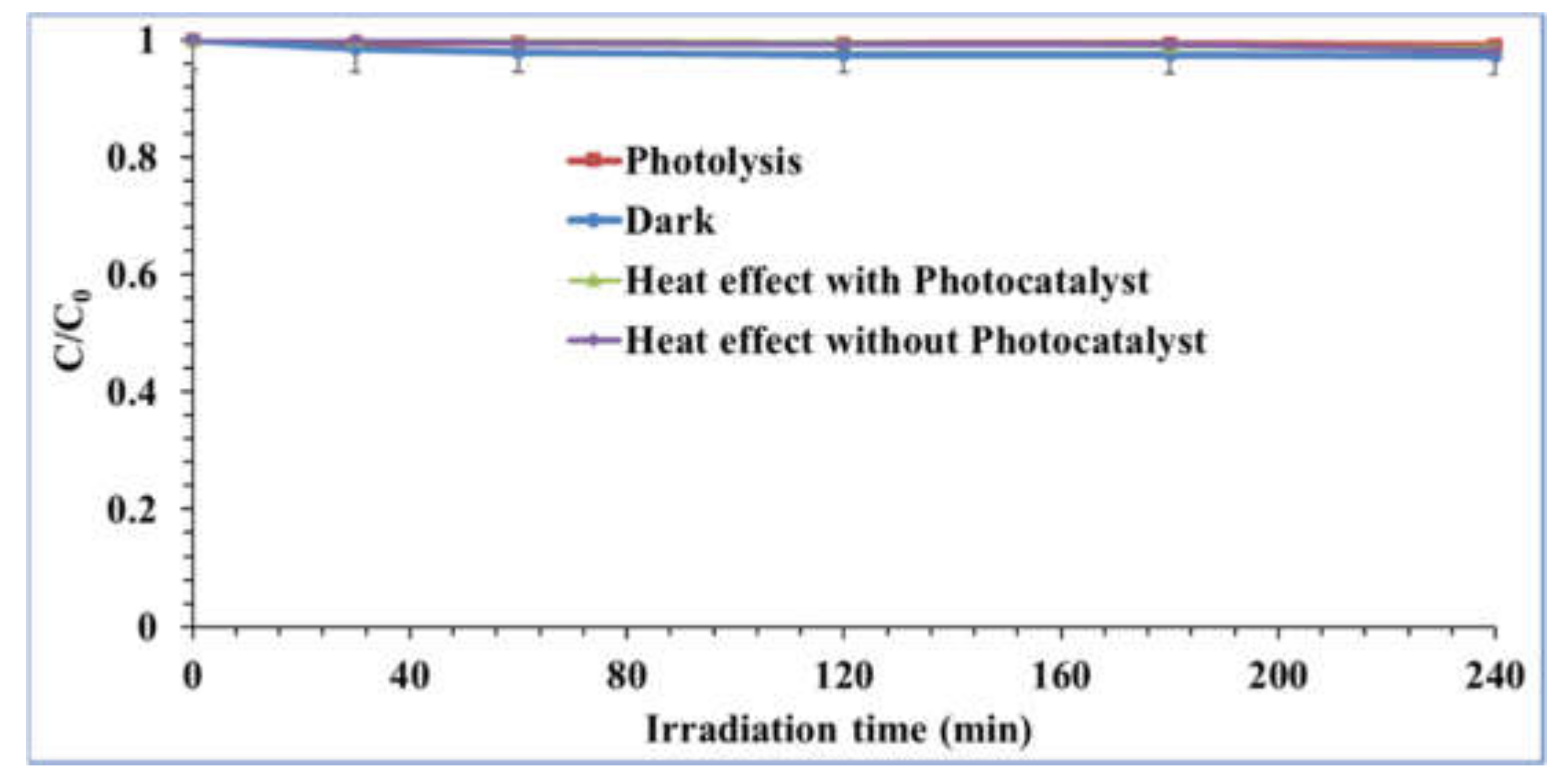
2.2. Photocatalyst Test Evaluation
- Physicochemical Properties of Carbamazepine (CBZ)
2.2.1. Effect of pH
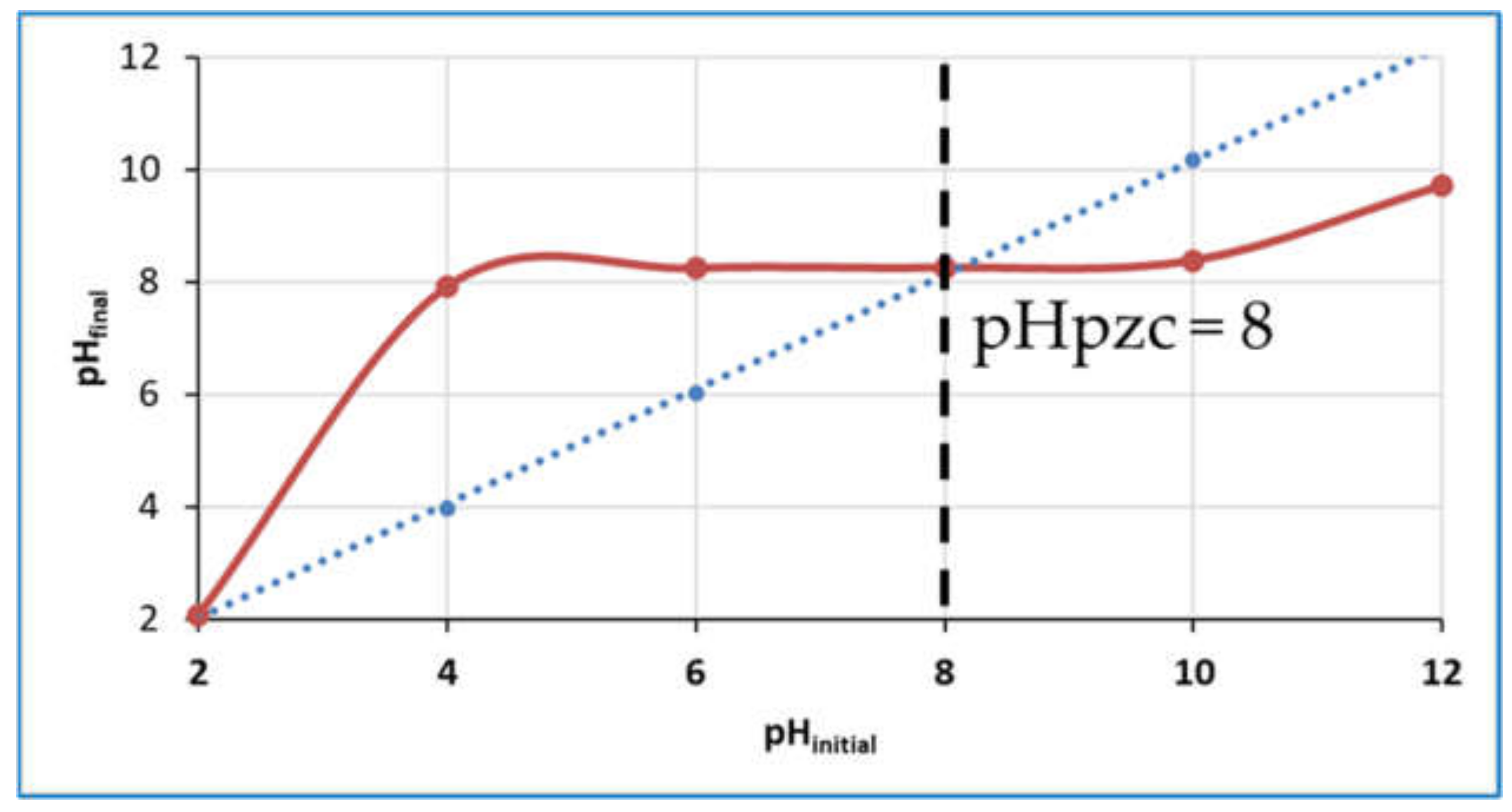
- pH Point of Zero Charge (pHpzc) Value
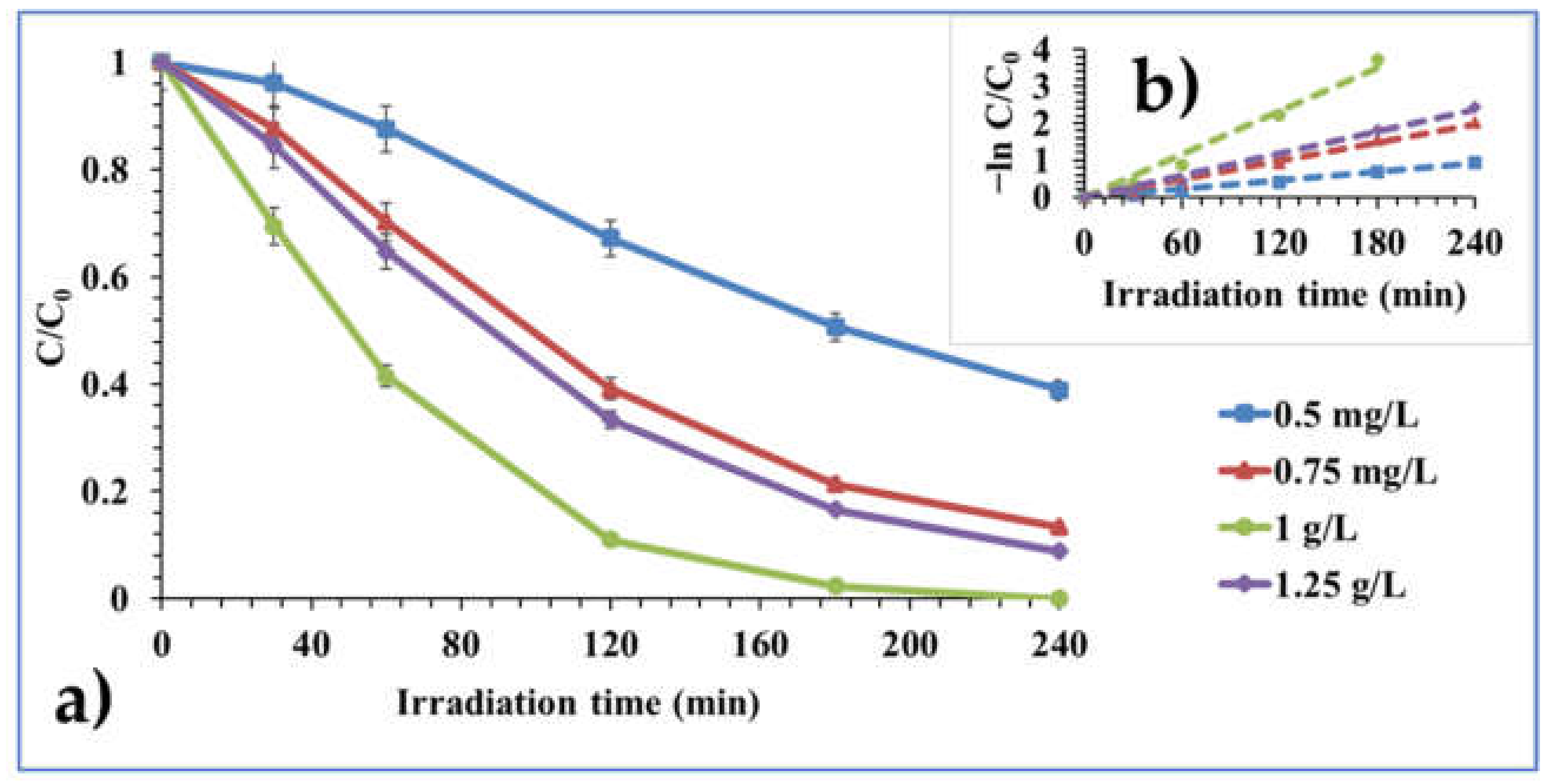
2.2.2. Effect of Catalyst Dosage
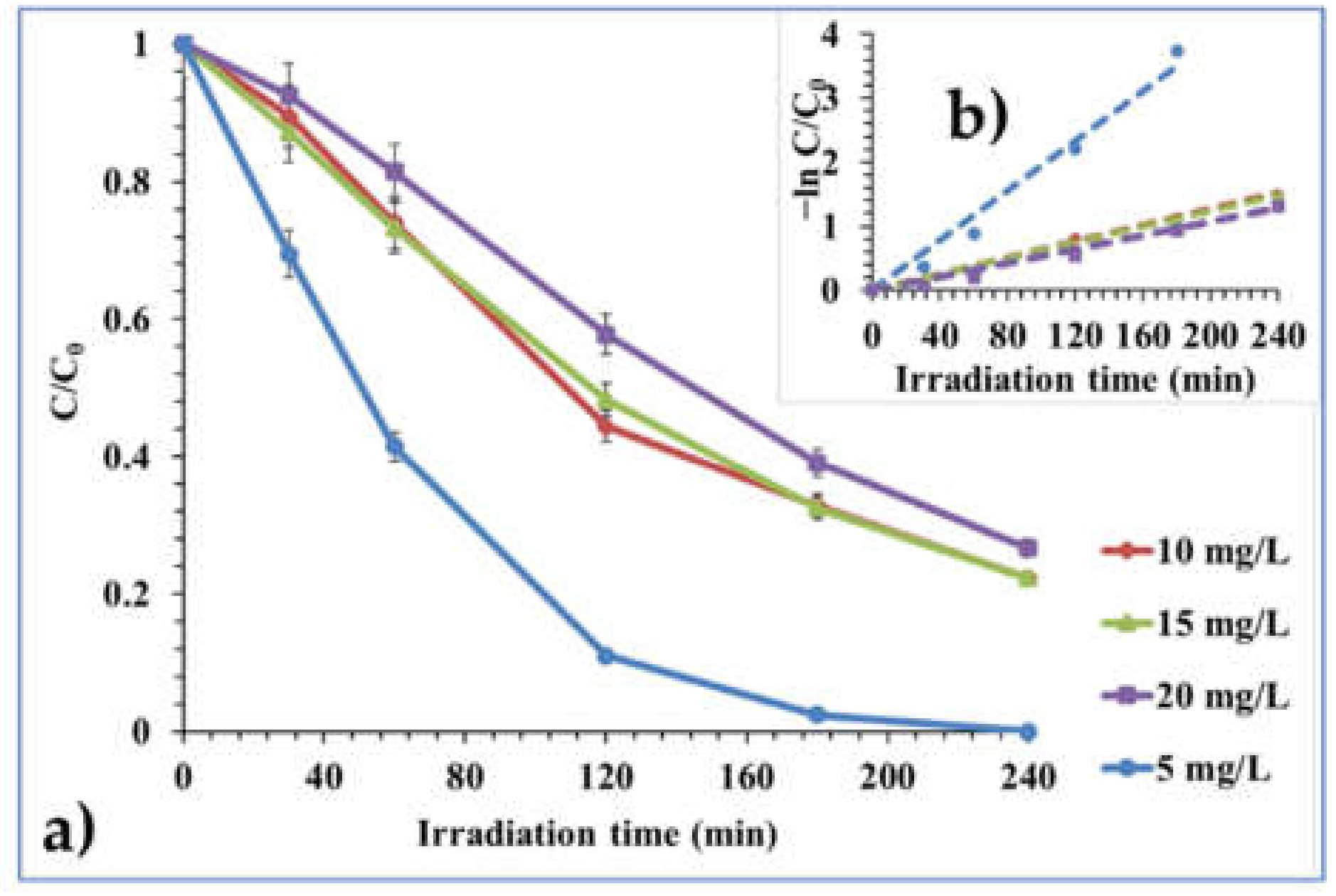
2.2.3. Effect of Initial Pollutant Concentration
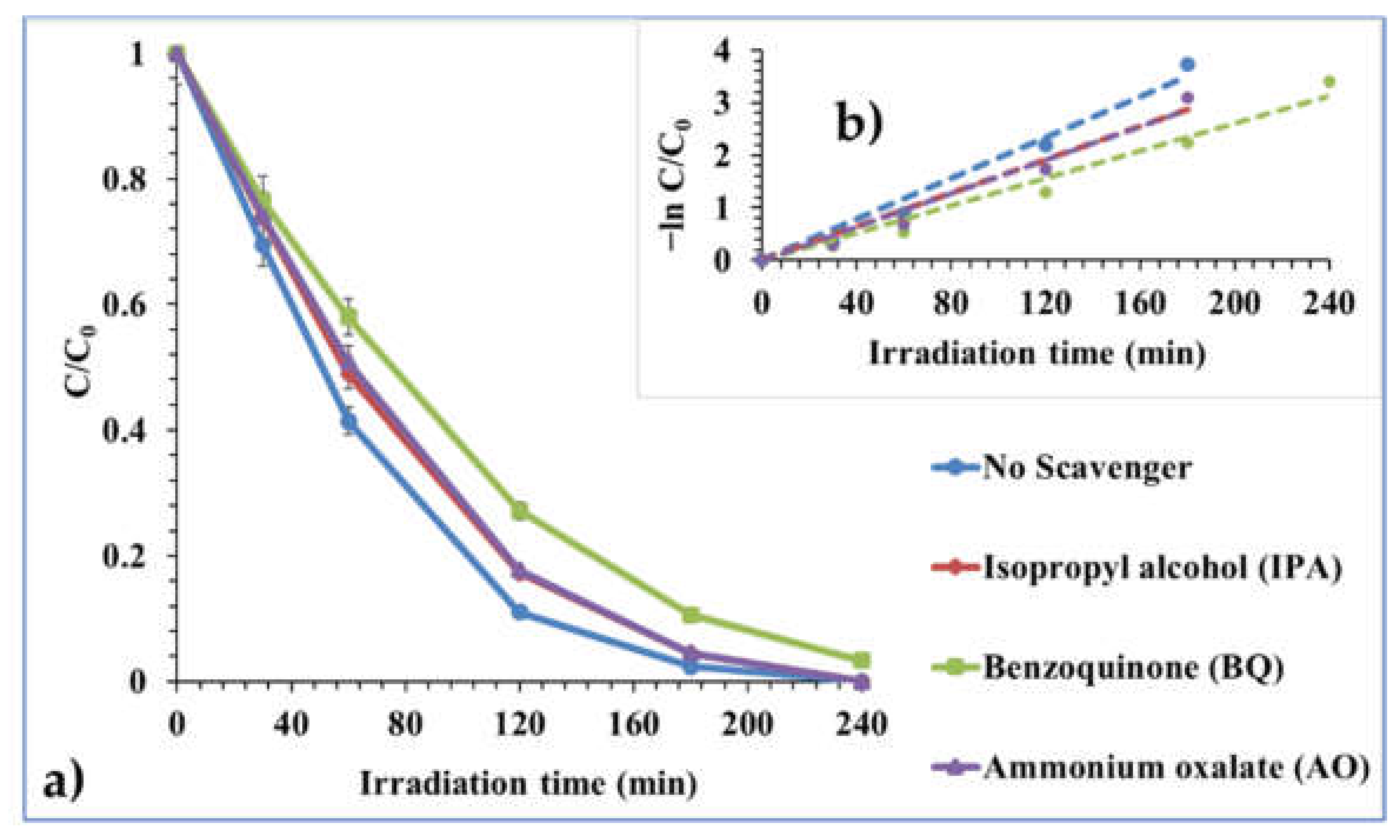
2.2.4. Effect of Chemical Scavengers
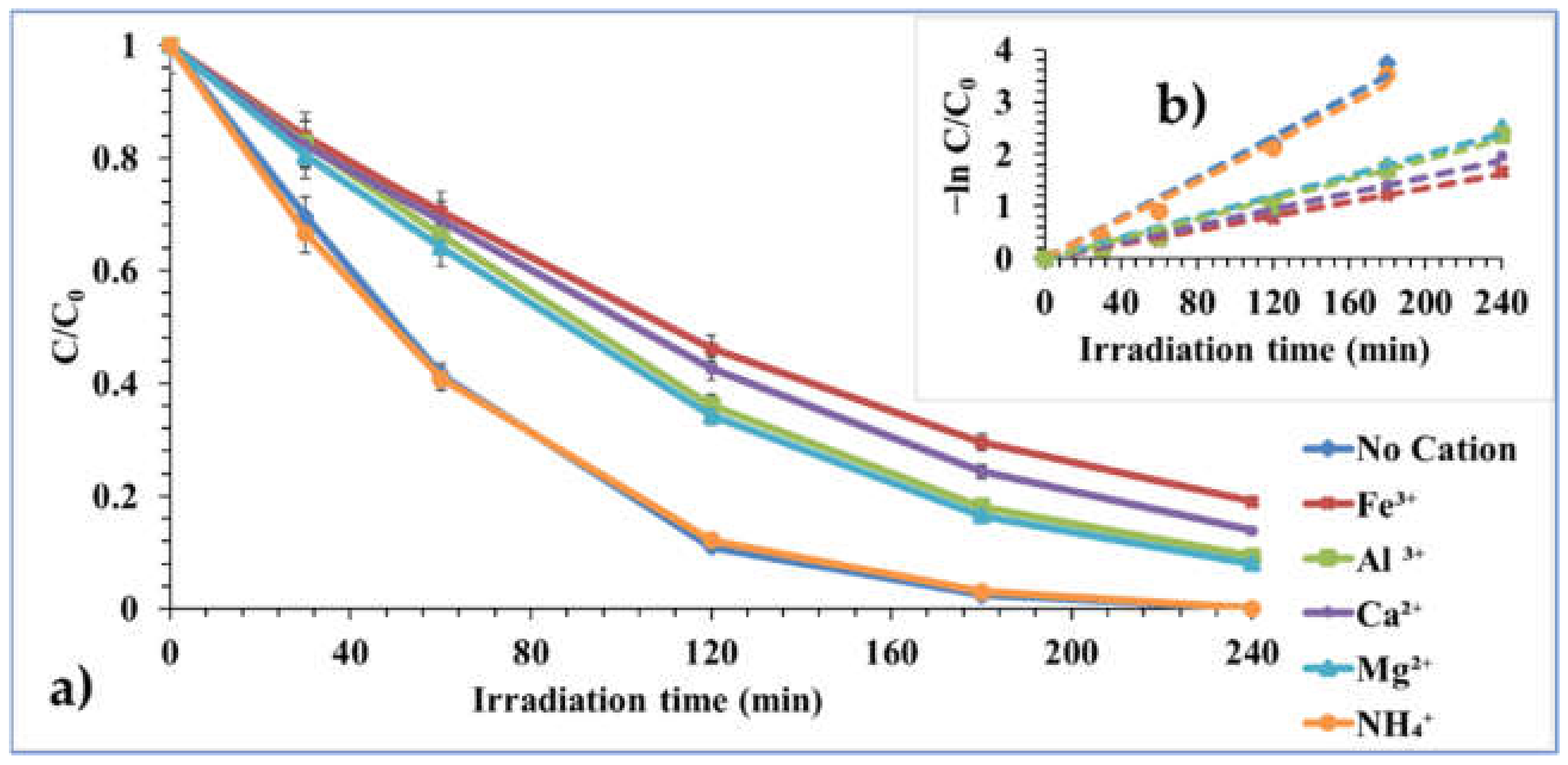
2.2.5. Effect of Contaminant Ion (Cations and Anions)
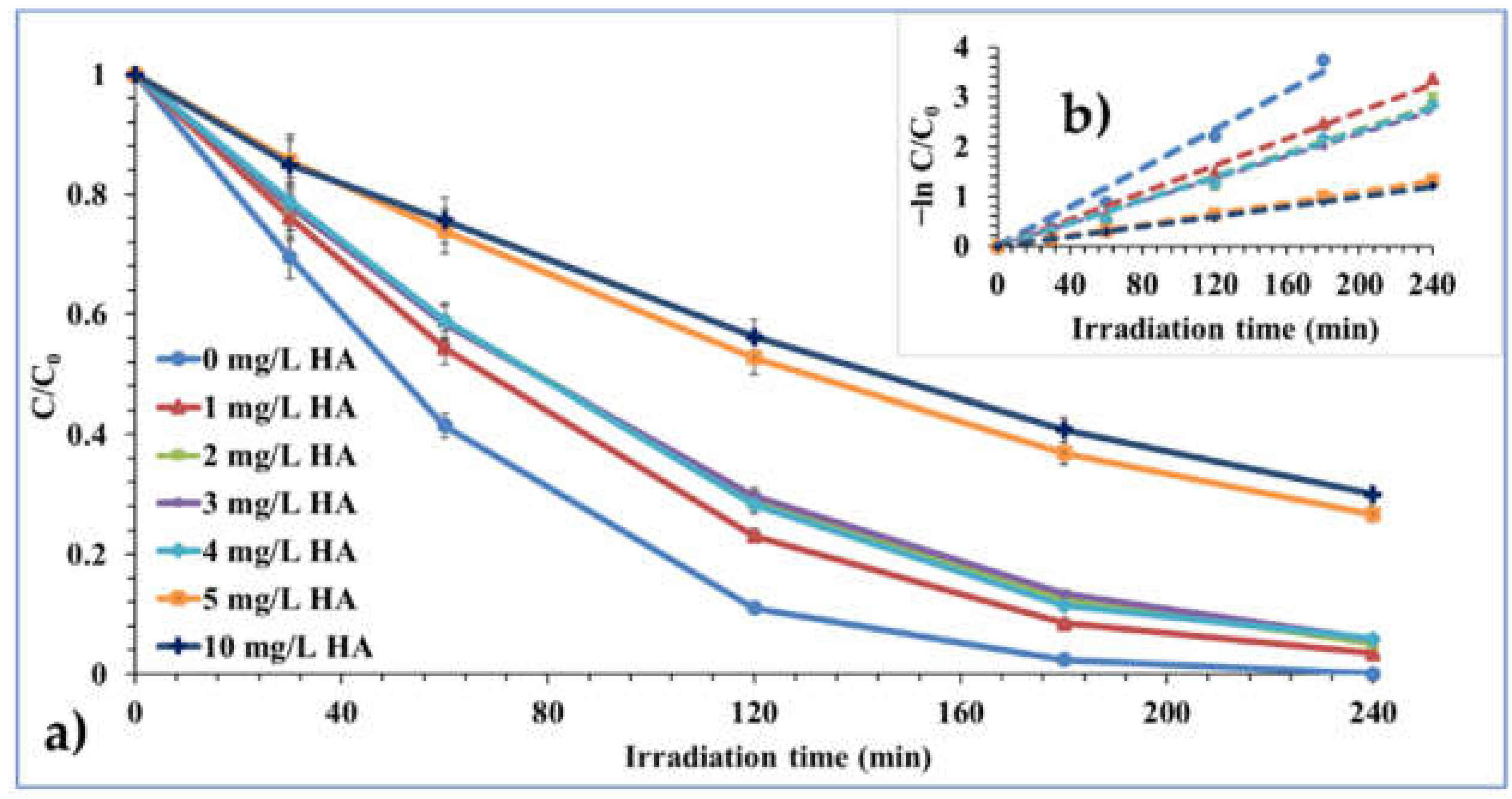
2.2.6. Effect of Hydrogen Peroxide (H2O2) and Humic Acid (HA)
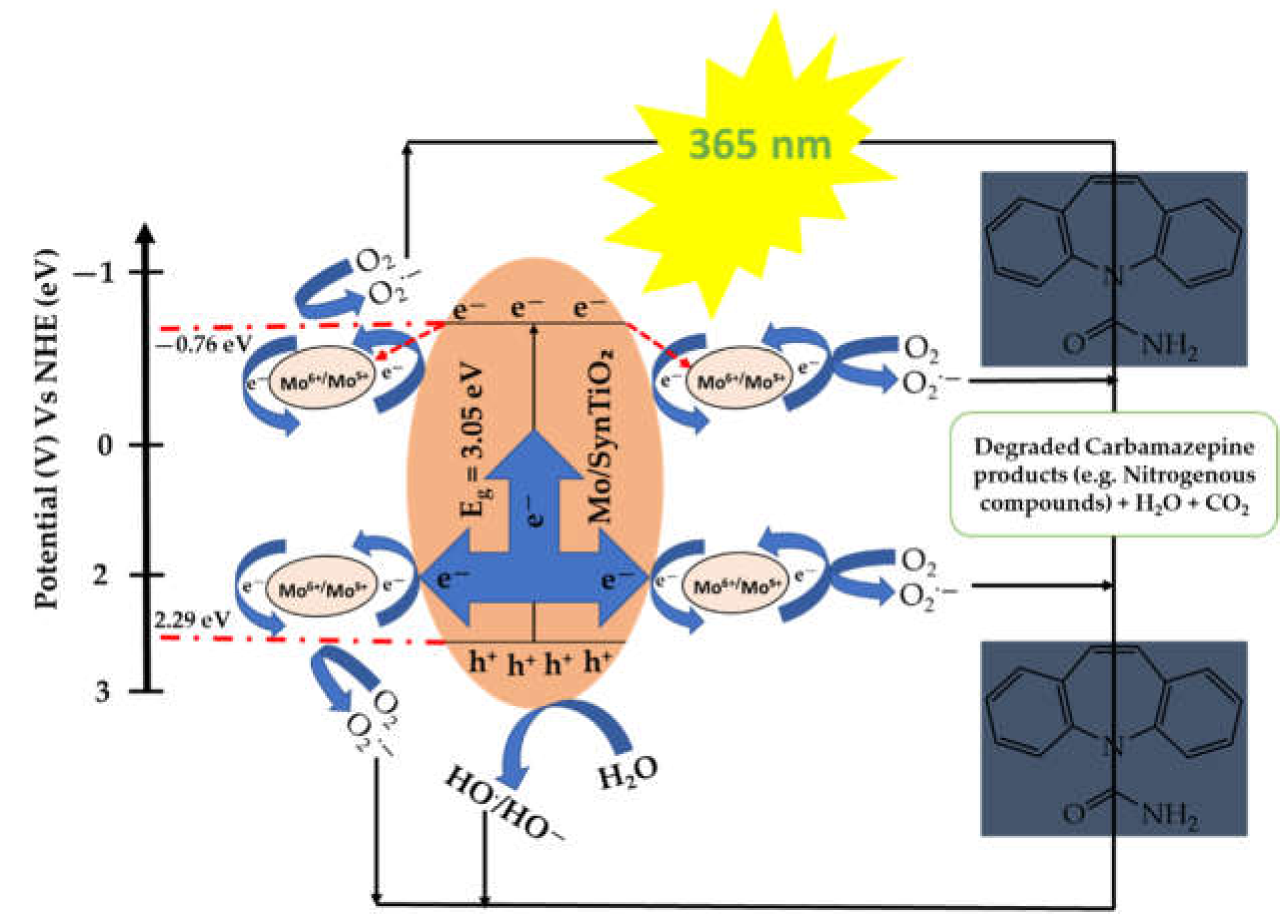
2.3. Proposed Reaction Mechanism
3. Materials and Methods
3.1. Chemical Reagents
3.2. Synthesis of Mo/SynTiO2 Material
- Sol–Gel Synthesized TiO2 (SynTiO2)
- Mo doping of the Sol–Gel Synthesized TiO2 (SynTiO2)
3.3. Characterization of Mo (1.5 wt%)/SynTiO2 Material
3.4. Determination of the pH Point of Zero Charge (pHpzc) of Mo (1.5 wt%)/SynTiO2 Material
3.5. Photocatalytic Test Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Education and Cultural Organization (UNESCO). The United Nations Water Development Report 3: Water in a Changing World; UNESCO: Paris, France; Berghahn Books: New York, NY, USA, 2009. [Google Scholar]
- Fabrega, F.; Marques, M.; Ginebrade, A.; Kuzmanovic, M.; Barcelo, D.; Schuhmacher, M. Integrated Risk Index of Chemical Aquatic Pollution (IRICAP): Case Studies in Iberian Rivers. J. Hazard. Mater. 2013, 263, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Guten, U.; Wehrli, B. Global Water Pollution and Health. Annu. Rev. Environ. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.; Ternes, T.A. Pharmaceuticals and Personal Care Products in the Environment: Agents of Subtle Change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Pelog, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Pal, A.; Gin, A.Y.-C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Review of Recent Occurrences, Fates, and Effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Emerging Pollutants. Available online: http://www.norman-network.net (accessed on 21 February 2022).
- Souza, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and Personal Care Products (PPCPs): A Review on Environmental Contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and Removal of Organic Micropollutants: An Overview of the Watch List of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, Sources, Receptors, and Control Measures for Three European Union Watch List Substances of Emerging Concern in Receiving Waters—A 20 Year Systematic Review. Sci. Total Environ. 2017, 574, 1140–11673. [Google Scholar] [CrossRef]
- Yan, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and Removal of Pharmaceuticals and Personal Care Products (PPCPs) in Drinking Water and Water/Sewage Treatment Plants: A Review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar]
- Anucha, C.B.; Altin, I.; Biyiklioglu, Z.; Bacaksiz, E.; Polat, I.; Stathopoulos, V.N. Synthesis, Characterization, and Photocatalytic Evaluation of Manganese (III) Phthalocyanine Sensitized ZnWO4 (ZnWO4MnPc) for Bisphenol A Degradation Under UV Irradiation. Nanomaterials 2020, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Anucha, C.B.; Altin, I.; Fabbri, D.; Degirmencioglu, I.; Calza, P.; Magnacca, G.; Stathopoulos, V.N.; Bacaksiz, E. Synthesis, and Characterization of B/NaF and Silicon Phthalocyanine- Modified TiO2 and an Evaluation of Their Photocatalytic Removal of Carbamazepine. Separations 2020, 7, 71. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Degirmencioglu, I.; Kucukomeroglu, T.; Yilmaz, S.; Stathopoulos, V.N. Immobilized TiO2/ZnO Sensitized Copper (II) Phthalocyanine Heterostructure for the Degradation of Ibuprofen under UV Irradiation. Separations 2021, 8, 24. [Google Scholar]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Kucukomeroglu, T.; Belay, M.H.; Stathopoulos, V.N. Enhanced Photocatalytic Activity of CuWO4 Doped TiO2 Photocatalyst towards Carbamazepine under UV Irradiation. Separations 2021, 8, 25. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N.; Polat, I.; Yasar, A.; Yuksel, O.F. Silver Doped Zinc Stannate (Ag-ZnSnO3) for the Photocatalytic Degradation of Caffeine under UV Irradiation. Water 2021, 13, 1290. [Google Scholar] [CrossRef]
- Jimenez-Holgado, C.; Crimatopoulos, C.; Stathopoulos, V.N.; Sakkas, V. Investigating the Utility of Fabric Phase Sorptive Extraction and HPLC-UV-Vis/DAD to Determine Antidepressant Drugs in Environmental Aqueous Samples. Separations 2020, 7, 39. [Google Scholar] [CrossRef]
- Jones, A.O.; Lester, J.N.; Voulvoulis, N. Pharmaceutical: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Heberer, T.; Mechlinski, A.; Franck, B.; Knappe, A.; Massmann, G.; Pekdeger, A.; Fritz, B. Field Studies on the Fate and Transport of Pharmaceutical of Pharmaceutical Residues in Bank Filtration. Ground Water Monit. Remediat. 2004, 24, 70–77. [Google Scholar] [CrossRef]
- Koplin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in US Streams, 1999–2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar]
- Anumol, T.; Vijayanandan, A.; Park, M.; Philip, L.; Snyder, S. Occurrence and Fate of Emerging Trace Organic Chemicals in Wastewater Plants in Chennai, India. Environ. Int. 2016, 92, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal Variation in The Occurrence of and Removal of Pharmaceuticals and Personal Care Products in Wastewater Plant in Xiamen, China. J. Hazard. Mater. 2014, 277, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, A.; Vercher, R.F.; Santos-Juanes, L.; Simon, P.J.; Lardin, C.; Martinez, M.A.; Vicente, J.; González, R.; Llosá, C.; Arques, A.; et al. Solar Photocatalysis as a Tertiary Treatment to Remove Emerging Pollutants from Wastewater Treatment Plant Effluents. Catal. Today 2011, 161, 235–240. [Google Scholar] [CrossRef]
- Prieto-Rodriguez, L.; Miralles-Cuevas, S.; Oller, I.; Aguera, A.; Puma, G.L.; Malato, S. Treatment of Emerging Contaminants in Wastewater Treatment Plant (WWTP) Effluents by Solar Photocatalysis Using Low TiO2 Concentrations. J. Hazard. Mater. 2012, 211, 131–137. [Google Scholar] [CrossRef]
- Miranda-Garcia, N.; Suarez, S.; Sanchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic Degradation of Emerging Contaminants in Municipal Wastewater Treatment Plant Effluents Using Immobilized TiO2 in a Solar Pilot Plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Loaiza-Ambuludi, S.; Panizza, M.; Oturan, N.; Oturan, M.A. Removal of the Anti-inflammatory Drug Ibuprofen from Water Using Homogeneous Photocatalysis. Catal. Today 2014, 224, 29–33. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.; Tyrk, D. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis-Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Dal Santo, V.; Naldoni, A. Titanium Dioxide Photocatalysis. Catalysts 2018, 8, 591. [Google Scholar] [CrossRef] [Green Version]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; LeHunte, S. An Overview of Semiconductor Photocatalysis. J. Photochem. Photobiol. A 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.X.; Bi, Y.P.; Umezawa, N.; Oshikiri, M.; Ye, J.H. Nano-Photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A Review on the Visible Light Active Titanium Dioxide Photocatalyst for Environmental Applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid Materials for the Removal of Emerging Pollutants in Water: Classification, Synthesis, and Properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Zouraris, D.; Karnaouri, A.; Pandis, P.K.; Argirusis, C.; Topaka, E.; Karantonis, A. On the Integration of Lytic Polysaccharide MonoOxygenases (LPMOs) with Phosphoric Acid Swollen Cellulose (PASC). J. Electroanal. Chem. 2021, 897, 115540. [Google Scholar] [CrossRef]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Kanellou, E.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Ultra-Assisted Preparation Methods of Nanoparticles for Energy-Related Applications. In Nantotechnology and the Environment, 1st ed.; Sen, M., Ed.; IntechOpen: London, UK, 2020; Volume 1, p. 170. [Google Scholar]
- Aviles-Garcia, O.; Espino-Valencia, J.; Romero-Romero, R.; Rico-Cerda, J.L.; Arroyo-Albiter, M.; Solis-Casados, D.A.; Natividad-Rangel, R. Enhanced Photocatalytic Activity of Titania by Co-Doping with Mo and W. Catalysts 2018, 8, 631. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.-G.; Guo, X.-T.; Wang, B.; Li, L.-Y.; Zhao, M.-X.; Dong, L.-L.; Liu, X.-J.; Huang, Y.-T. Synthesis and Photocatalytic Activity of Mo-Doped TiO2 Nanoparticles. J. Spectrosc. 2015, 2015, 681850. [Google Scholar]
- Zhang, M.; Wu, J.; Hou, J.; Yang, J. Molybdenum and Nitrogen Co-Doped Titanium Dioxide Nanotube Arrays with Enhanced Visible Light Photocatalytic Activity. Sci. Adv. Mater. 2013, 5, 535–6541. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Li, X.; Wang, A.; Wang, Y.; Chen, Y. Photocatalytic Degradation of Methylene Blue by MoO3 Modified TiO2 under Visible Light. Chin. J. Catal. 2014, 35, 140–147. [Google Scholar]
- Ghorai, T.K. Photocatalytic Degradation of 4-Chlorophenol by CuMoO4-Doped TiO2 Nanoparticles Synthesized by Chemical Route. J. Phys. Chem. 2011, 1, 28–36. [Google Scholar]
- Surenjan, B.; Sambandam, B.; Pradeep, T.; Philip, L. Synthesis, Characterization and Performance of Visible Light Active C-TiO2 for Pharmaceutical Photodegradation. J. Environ. Chem. Eng. 2017, 5, 757–767. [Google Scholar] [CrossRef]
- Sambandam, B.; Surenjan, A.; Philip, L.; Pradeep, T. Rapid Synthesis of C-TiO2: Tuning the Shape from Spherical to Rice Grain Morphology for Visible Light Photocatalytic Application. ACS Sustain. Chem. Eng. 2015, 3, 1321–1329. [Google Scholar] [CrossRef]
- Czech, B.; Buda, W. Photocatalytic Treatment of Pharmaceutical Wastewater Using Multiwall-Carbon Nanotubes/TiO2/SiO2 Nanocomposites. Environ. Res. 2015, 137, 176–184. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.K. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Sharotri, N.; Sharma, D.; Sud, D. Experimental and Theoretical Investigations of Mn-N-co-doped TiO2 Photocatalyst for Visible Light Induced Degradation of Organic Pollutants. J. Mater. Res. Technol. 2019, 8, 3995–4009. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The Design and Development of Highly Reactive Titanium Oxide Photocatalyst Operating under Visible Light Irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Vasquez, G.C.; Peche-Herrero, M.A.; Maestra, D.; Cremades, A.; Ramirez-Castellanos, J.; Gonzales-Calbert, J.M.; Piqueras, J. Effects of Transition Metal Doping on the Growth and Properties of Rutile TiO2 Nanoparticles. J. Phys. Chem. C 2013, 117, 1941–1947. [Google Scholar] [CrossRef]
- Kale, G.; Arbuj, S.; Chothe, U.; Khore, S.; Nikam, L.; Kale, B. Highly Crystalline Ordered Cu-doped TiO2 Nanostructure by Paper Templated Method: Hydrogen Production and Dye Degradation under Natural Sunlight. J. Comp. Sci. 2020, 4, 48. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. MoS2/CdS/TiO2 Ternary Composite Incorporated into Carbon Nanofibres for the Removal of Organic Pollutants from Water. Inorg. Chem. Comm. 2019, 102, 113–119. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Ueno, T.; Saito, N.; Hunsom, M. Narrowing Band Gap Energy of Defective black TiO2 Fabricated by Solution Plasma Process and Its Photocatalytic Activity on Glycerol Transformation. J. Alloys Compd. 2018, 757, 188–199. [Google Scholar] [CrossRef]
- Poudel, M.B.; Shin, M.; Kim, H.J. Interface Engineering of MIL-88 Derived MnFe-LDH and MnFe2O3 on Three-Dimensional Carbon Nanofibres for The Efficient Adsorption of Cr (VI), Pb(II), and AS(III) Ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel Insight into The Adsorption of Cr (VI) and Pb(II) Ions by MOF Derived Co-Al Layered Double Hydroxide @ Hematite Nanorods on 3D Porous Carbon Nanofibre Network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Li, B.; Zhao, Z.; Gao, F.; Wang, X.; Qiu, J. Mesoporous Microspheres Composed of Carbon-Coated TiO2 Nanocrystals with Exposed {001} Facets for Improved Visible Light Photocatalytic Activity. Appl. Catal. B Environ. 2014, 147, 958–964. [Google Scholar] [CrossRef]
- Husain, S.T.; Siddiqa, A.; Siddiq, M.; Ali, S. Iron-Doped Titanium Dioxide Nanotubes: A Study of Electrical, Optical and Magnetic Properties. J. Nanopart. Res. 2013, 13, 6517–6525. [Google Scholar] [CrossRef]
- Zener, B.; Matoh, L.; Carraro, G.; Miljevc, B.; Korsec, R.C. Sulfur-Nitrogen-and Platinum-Doped Titania Thin Films with High Catalytic Effiency under Visible-Light Illumination. Beilstein J. Nanotechnol. 2018, 9, 1629–1640. [Google Scholar] [CrossRef] [Green Version]
- Simsek, E.B.; Kilic, B.; Asgin, M.; Akan, A. Graphene Oxide Based Heterojunction TiO2-ZnO Catalysts with Outstanding Photocatalytic Performance for Bisphenol-A, Ibuprofen, and Flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Singh, M.; Duklan, N.; Singh, P.; Sharma, J. Synthesis and Photocatalytic Activity of Anatase TiO2 Nanoparticles for Degradation of Methyl Orange. AIP Conf. Proc. 2018, 1953, 030075. [Google Scholar] [CrossRef]
- Kumaravel, V.; Rhatigan, S.; Mathew, S.; Michel, M.C.; Bartlett, J.; Nolan, M.; Hinder, S.J.; Gasco, A.; Ruiz-Palomar, C.; Hermosilla, D.; et al. Mo Doped TiO2: Imapact on Oxygen Vacancies, Anatase Phase Stability, and Photocatalytic Activity. J. Phys. Mater. 2020, 3, 025008. [Google Scholar] [CrossRef]
- Luo, S.-Y.; Yan, B.-X.; Shen, J. Enhancement of Photoelectric and Photocatalytic Activities: Mo Doped TiO2 Thin Films Deposited by Sputtering. Thin Solid Films 2012, 522, 361–365. [Google Scholar] [CrossRef]
- Li, S.; Jena, P. Origin of the Anatase to Rutile Conversion of Metal-Doped TiO2. Phys. Rev. B 2009, 79, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-Light Induced Photodegradation of Gas Phase Acetonitrile Using Aerosol-Made Transition Metal (V, Cr, Fe, Mn, Mo, Ni, Cu, Y, Ce, and Zr) Doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Chaudri, S.M.; Gawal, P.M.; Sane, P.K.; Sontakke, S.M.; Nemade, P.R. Solar Light-Assisted Photocatalytic Degradation of Methylene Blue with Mo/TiO2: A Comparison with Cr- and Ni-Doped TiO2. Res. Chem. Intermed. 2018, 44, 3115–3134. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties of the CO Oxidation Performance of Nanostructured CeO2/TiO2. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Lykaki, M.; Stefa, S.; Carabiniero, S.A.C.; Pandis, K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effects of Ceria Morphology on CO Oxidation. Catalyst 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Tavallaei, H.; Jafarpour, M.; Feizpour, F.; Rezaeifard, A.; Farrokhi, A.A. Cooperative Effect in a Novel Bimetallic Mo-V Nanocomplex Catalyzed Selective Aerobic C-H Oxidation. ACS Omega 2019, 4, 3601–3610. [Google Scholar] [CrossRef]
- Meng, L.; Zang, X.; Tang, Y.; Su, K.; Kong, J. Hierarchically Porous-Silicon Carbon Nitrogen Hybrid Materials towards Highly Efficient and Selective Adsorption of Organic Dyes. Sci. Rep. 2015, 5, 7910. [Google Scholar] [CrossRef] [Green Version]
- Salmas, C.E.; Stathopoulos, V.N.; Pomonis, P.J.; Androutsopoulos, G.P. Pore Structure-Chemical Composition Interactions of New High Surface Area Manganese Based Mesoporous Materials. Materials Preparation, Characterization, and Catalytic Activity. Langmuir 2002, 18, 423–432. [Google Scholar] [CrossRef]
- Corberan, V.C.; Rives, V.; Stathopoulos, V.N. Recent Applications of Nanometal Oxide Catalyst in Oxidation Reactions. In Advanced Nanomaterials for Catalysis and Energy, Synthesis, Characterizations and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 227–293. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- McFarland, E.W. Catalysis by Doped Oxides. Chem. Rev. 2013, 113, 4391–4427. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, V.N.; Belessi, V.C.; Costa, C.N.; Neophytides, S.; Falaras, P.; Efstathiou, A.M.; Pomonis, P.J. Catalytic Activity of High Surface Area Mesoporous Mn-Based Mixed Oxides for the Deep Oxidation of Methane and Lean-NOx reduction. Stud. Surf. Sci. Catal. 2000, 130, 1529–1534. [Google Scholar]
- Pandis, P.K.; Perros, D.E.; Stathopoulos, V.N. Doped Apatite-type Lanthanum Silicates in CO Oxidation Reaction. Catal. Comm. 2018, 114, 98–103. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Stathopoulos, V.N.; Efstathiou, A.M. The Effect of CeO2 Preparation Method on the Carbon Pathways in Dry Reforming of Methane on Ni/CeO2 Studied by Transient Techniques. Catalysts 2019, 9, 621. [Google Scholar] [CrossRef] [Green Version]
- Goula, M.A.; Charisiou, N.D.; Pandis, P.K.; Stathopoulos, V.N. A Ni/Apatite-type Lanthanum Silicate Supported Catalyst in Glycerol Steam Reforming Reaction. RSC Adv. 2016, 6, 78954–78958. [Google Scholar] [CrossRef]
- Bespalko, Y.; Kuznetsova, T.; Kriger, T.; Chesalov, Y.; Lapina, O.; Ischenko, A.; Larina, T.; Sadykov, V.; Stathopoulos, V. La2Zr2O7/LaAlO3 Composite Prepared by Mixing Precipitated Precursors: Evolution of its Structure under Sintering. Mater. Chem. Phys. 2020, 251, 123093. [Google Scholar] [CrossRef]
- Trens, P.; Sthatopoulos, V.N.; Hudson, M.J.; Pomonis, P. Synthesis and Characterization of Packed Mesoporous Tungstenosilicates: Application to the Catalytic Dehydrogenation of 2-Propanol. Appl. Catal. A 2004, 263, 103–108. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface Modification of TiO2 Photocatalyst for Environmental Applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Esposito, S.; Ditaranto, N.; Dell’Aglli, G.; Nasi, R.; Rivolo, P.; Bonelli, B. Effective Inclusion of Sizeable Amounts of Mo within TiO2 Nanoparticles Can Be Obtained by Reverse Micelle Sol-Gel Synthesis. ACS Omega 2021, 6, 5379–5388. [Google Scholar] [CrossRef]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and Solar Photodegradation of Ibuprofen and Carbamazepine Catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Sin, J.-C.; Lam, S.-M.; Zheng, H.; Lin, H.; Li, H.; Tham, K.-O.; Mohamed, A.R.; Lim, J.-W.; Qin, Z. Magnetic NiFe2O4 Nanoparticles Decorated on N-Doped BiOBr Nanosheets for Expeditious Visible Light Photocatalytic Phenol Degradation and Hexavalent Chromium Reduction Via a Z-Scheme Heterojunction Mechanism. Appl. Surf. Sci. 2021, 559, 149966. [Google Scholar] [CrossRef]
- Lam, S.-M.; Jaffari, Z.H.; Sin, J.-C.; Zheng, H.; Lin, H.; Li, H.; Mohamed, A.R.; Ng, D.-Q. Surface Decorated Coral-Like Magnetic BiFeO3 with Au Nanoparticles for Effective Sunlight Photodegradation of 2,4-D and E-coli Inactivation. J. Mol. Liq. 2021, 326, 115372. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Zheng, H.; Lin, H.; Li, H.; Qin, Z.; Lim, J.W.; Mohamed, A.R. Z-Scheme MoO3 Anchored-Hexagonal Rod Like ZnO/Zn Photoanode for Effective Wastewater Treatment, Copper Reduction Accompanied with Electricity Production in Sunlight-Powered Photocatalytic Cell. Sep. Purif. Technol. 2021, 265, 118495. [Google Scholar] [CrossRef]
- Kudlek, E.; Dudziak, M.; Bohdziewicz, J. Influence of Inorganic Ions and Organic Substances on the Degradation of Pharmaceutical Compound in Water Matrix. Water 2016, 8, 532. [Google Scholar] [CrossRef] [Green Version]
- Haroun, L.; Salaun, M.; Menard, A.; Lagault, C.-Y.; Bellenger, J.-P. Photodegradation of Carbamazepine and Three Derivatives Using TiO2 and ZnO: Effect of pH, Ionic Strength, and Natural Organic Matter. Sci. Total Environ. 2014, 475, 16–22. [Google Scholar] [CrossRef]
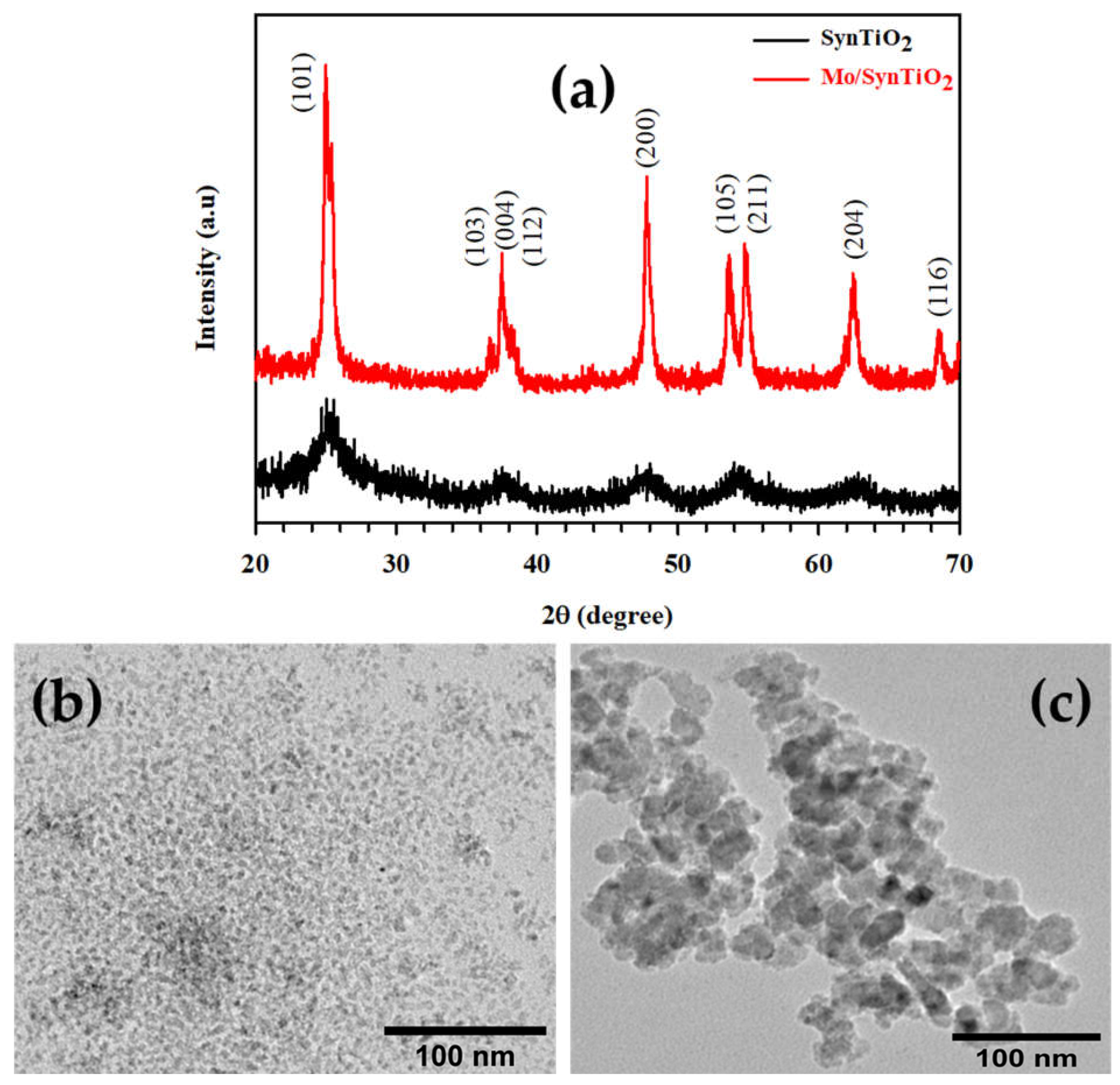
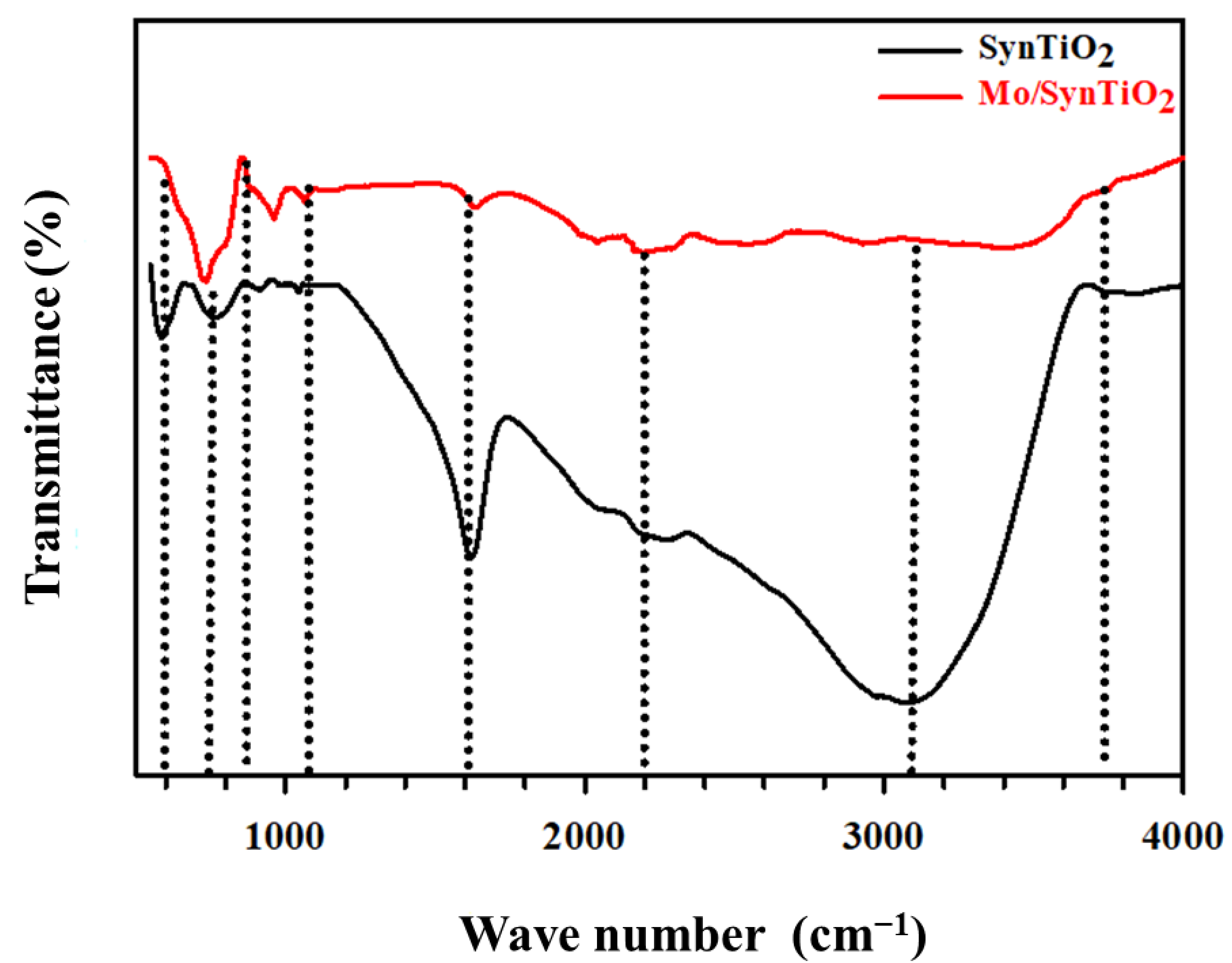
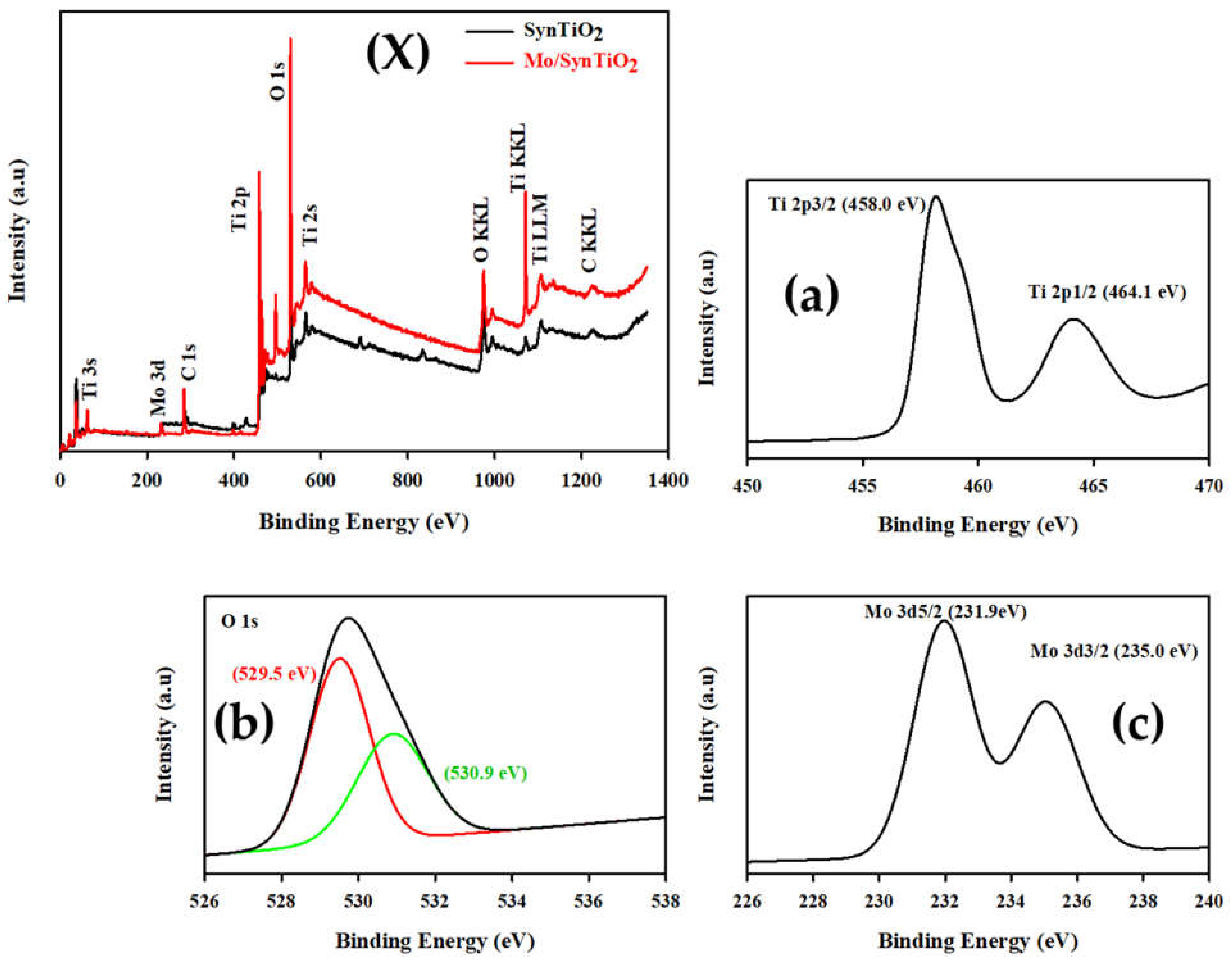

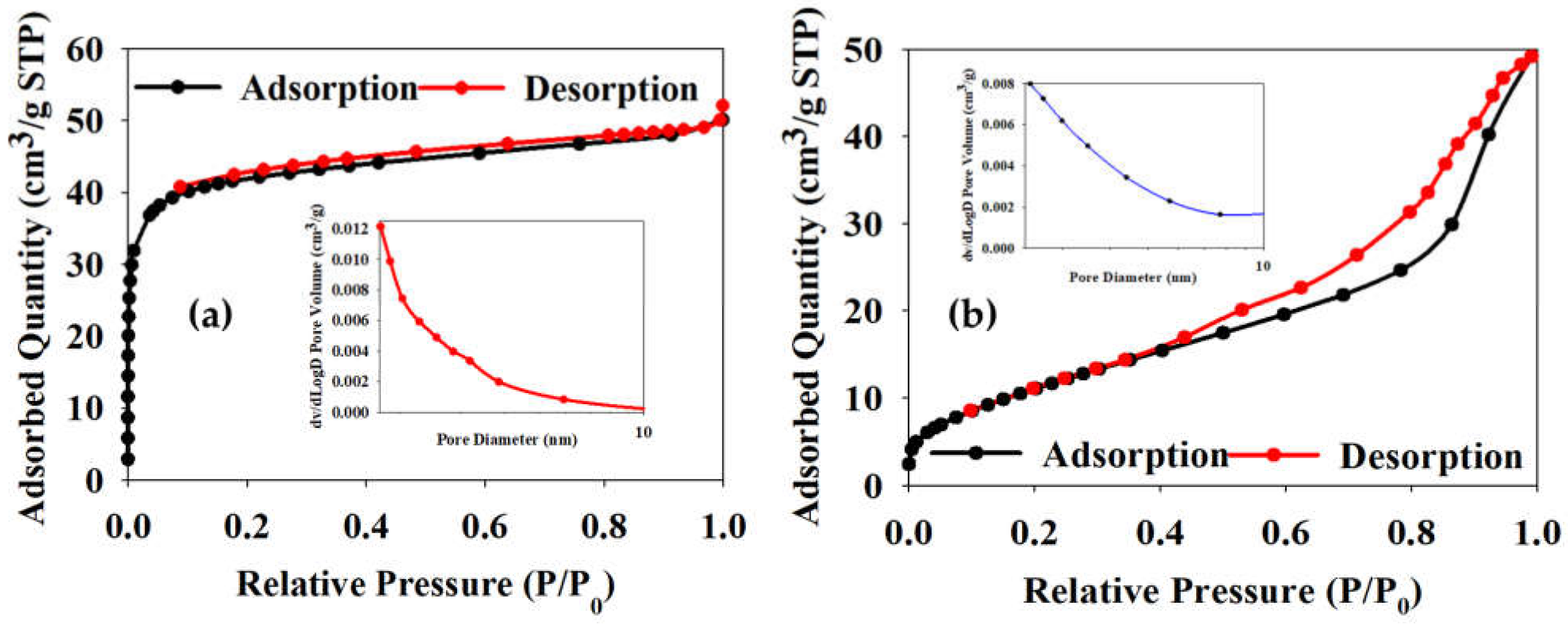




| Estimated/Measured Characterization Data | ||||||
|---|---|---|---|---|---|---|
| Materials | * dXRD (nm) | # dTEM (nm) | SBET (m2/g) | Vp (cm3/g) | Dp (nm) | Eg (eV) |
| SynTiO2 | 14.24 | 16.02 | 133.87 | 0.08 | 2.32 | 3.30 |
| Mo/SynTiO2 | 16.10 | 24.55 | 43.30 | 0.07 | 6.23 | 3.05 |
| Chemical Property/Name | Carbamazepine |
|---|---|
| Chemical structure |  |
| Molecular formula | C15H12N2O |
| CAS No. | 298-46-4 |
| Molecular weight, g/mol | 236.27 |
| Solubility in ethanol, g/L | 50 |
| pKa | 13.9 |
| Catalyst Material and Dopant Amount | Pollutant and Initial Conc. | Catalyst Amount | Radiation Source | Efficiency (%) | Irradiation. Time (min) | Ref |
|---|---|---|---|---|---|---|
| (1 wt% W-1 wt% Mo)-TiO2 | 15.56 × 105 mol/L 4CP | 2 × 10−4 kg/L | UV | 97% Degradation, 74% Mineralization | 100 | [41] |
| Mo (2%)-TiO2 | 20 mg/L MB | 0.2 g | Solar Light | Almost 100% Degradation | 300 | [42] |
| Mo-N (1%)-TiO2 | 10 mg/L MB | 4 cm2 TNA thin film of Mo-N-TiO2 | VL | Almost 100% Degradation | 180 | [43] |
| 0.25 (MoO3/P25) | 15 mg/L MB | 0.01 g | VL | 38% Removal | 150 | [44] |
| CuMoO4 (5 mol %)-TiO2 | 50 ppm 4CP | 1 g/L | UV-Vis | 96.9% decolouration | 180 | [45] |
| Mo (1.5 wt%)/TiO2 | 5 mg/L CBZ | 1 g/L | UV | Almost 100% Degradation | 240 | This Study |
| Test | Degradation Rate Constant k (min−1) | R2 | Half-Life (t1/2) (min) | pHinital | pHfinal |
|---|---|---|---|---|---|
| Preliminary Test for Photocatalyst Material # | |||||
| SynTiO2 (control) | 0.0004 | 0.9133 | 1732.50 | 3.33 | 3.03 |
| 2 wt% Mo/SynTiO2 | 0.0195 | 0.9898 | 35.54 | 5.84 | 5.20 |
| Tested Parametric Effects Over Photocatalyst Material Performance of Mo (1.5 wt%)/SynTiO2 | |||||
| Effect of pH # | |||||
| pHnatural 5.84 | 0.0195 | 0.9898 | 35.54 | 5.84 | 5.16 |
| pH 3 | 0.0013 | 0.984 | 533.08 | 3.32 | 3.71 |
| pH 9 | 0.0101 | 0.9611 | 68.61 | 9.17 | 7.47 |
| pH 11 | 0.0068 | 0.9915 | 101.91 | 10.89 | 10.46 |
| Effect of Catalyst Dosage # | |||||
| 0.5 g/L | 0.0039 | 0.9923 | 177.69 | 5.89 | 5.11 |
| 0.75 g/L | 0.0082 | 0.9968 | 84.51 | 6.40 | 5.12 |
| 1 g/L | 0.0195 | 0.9898 | 35.54 | 6.37 | 5.18 |
| 1.25 g/L | 0.0099 | 0.9972 | 70.00 | 6.50 | 5.36 |
| Effect of Initial Pollutant Concentration # | |||||
| 5 mg/L | 0.0195 | 0.9898 | 35.54 | 5.83 | 5.36 |
| 10 mg/L | 0.0064 | 0.9976 | 108.28 | 6.42 | 5.16 |
| 15 mg/L | 0.0062 | 0.9991 | 111.77 | 6.52 | 4.97 |
| 20 mg/L | 0.0054 | 0.9937 | 128.33 | 6.35 | 5.11 |
| Effect of Chemical Scavengers # | |||||
| No scavenger | 0.0195 | 0.9898 | 35.54 | 5.85 | 5.19 |
| 1 mM Isopropyl alcohol (IPA) | 0.016 | 0.9877 | 43.31 | 6.35 | 5.99 |
| 1 mM Benzoquinone (BQ) | 0.013 | 0.9878 | 53.31 | 6.40 | 6.01 |
| 1 mM Ammonium oxalate (AO) | 0.0159 | 0.985 | 43.58 | 6.42 | 6.52 |
| Effect of Contaminant Ion: cations # | |||||
| No Cation | 0.0195 | 0.9898 | 35.54 | 5.84 | 5.28 |
| 1 mM Fe3+ | 0.0068 | 0.9987 | 101.91 | 6.43 | 4.95 |
| 1 mM Mg2+ | 0.01 | 0.9944 | 69.30 | 6.41 | 7.79 |
| 1 mM Ca2+ | 0.0079 | 0.9961 | 87.72 | 6.52 | 5.50 |
| 1 mM Al3+ | 0.0094 | 0.9941 | 73.72 | 6.46 | 7.88 |
| 1 mM NH4+ | 0.0185 | 0.9935 | 37.46 | 5.82 | 6.52 |
| Effect of Contaminant Ion: anions # | |||||
| No Anion | 0.0195 | 0.9898 | 35.54 | 5.87 | 5.24 |
| 1 mM HCO3− | 0.0145 | 0.9881 | 47.79 | 6.33 | 5.89 |
| 1 mM CO32− | 0.0154 | 0.9974 | 45.00 | 6.42 | 7.94 |
| 1 mM SO42− | 0.0163 | 0.9769 | 42.52 | 6.45 | 6.23 |
| 1 mM HPO42− | 0.013 | 0.9896 | 53.31 | 6.51 | 8.11 |
| 1 mM Cl− | 0.0158 | 0.9877 | 43.86 | 5.89 | 6.54 |
| Effect of Hydrogen Peroxide (H2O2) # | |||||
| 0 mM H2O2 | 0.0195 | 0.9898 | 35.54 | 5.89 | 5.13 |
| 1 mM H2O2 | 0.0091 | 0.9945 | 76.15 | 6.34 | 5.77 |
| 2 mM H2O2 | 0.0087 | 0.9941 | 79.66 | 6.37 | 7.75 |
| 3 mM H2O2 | 0.0086 | 0.9898 | 80.58 | 6.40 | 5.33 |
| 4 mM H2O2 | 0.0067 | 0.9897 | 103.43 | 6.52 | 7.69 |
| 5 mM H2O2 | 0.0054 | 0.9984 | 128.33 | 6.32 | 6.4 |
| 10 mM H2O2 | 0.0047 | 0.9963 | 147.45 | 6.33 | 7.75 |
| Effect of Humic Acid (HA) # | |||||
| 0 mg/L HA | 0.0195 | 0.9898 | 35.54 | 5.85 | 5.12 |
| 1 mg/L HA | 0.0135 | 0.9951 | 51.33 | 6.33 | 6.74 |
| 2 mg/L HA | 0.0117 | 0.9932 | 59.23 | 6.42 | 8.24 |
| 3 mg/L HA | 0.0116 | 0.9955 | 59.74 | 6.47 | 6.22 |
| 4 mg/L HA | 0.0113 | 0.9954 | 61.33 | 6.52 | 6.22 |
| 5 mg/L HA | 0.0055 | 0.9996 | 126.00 | 6.51 | 7.65 |
| 10 mg/L HA | 0.005 | 0.9996 | 138.60 | 6.45 | 5.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anucha, C.B.; Bacaksiz, E.; Stathopoulos, V.N.; Pandis, P.K.; Argirusis, C.; Andreouli, C.-D.; Tatoudi, Z.; Altin, I. Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation. Processes 2022, 10, 1113. https://doi.org/10.3390/pr10061113
Anucha CB, Bacaksiz E, Stathopoulos VN, Pandis PK, Argirusis C, Andreouli C-D, Tatoudi Z, Altin I. Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation. Processes. 2022; 10(6):1113. https://doi.org/10.3390/pr10061113
Chicago/Turabian StyleAnucha, Chukwuka Bethel, Emin Bacaksiz, Vassilis N. Stathopoulos, Pavlos K. Pandis, Christos Argirusis, Constantina-Dia Andreouli, Zoi Tatoudi, and Ilknur Altin. 2022. "Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation" Processes 10, no. 6: 1113. https://doi.org/10.3390/pr10061113
APA StyleAnucha, C. B., Bacaksiz, E., Stathopoulos, V. N., Pandis, P. K., Argirusis, C., Andreouli, C.-D., Tatoudi, Z., & Altin, I. (2022). Molybdenum Modified Sol–Gel Synthesized TiO2 for the Photocatalytic Degradation of Carbamazepine under UV Irradiation. Processes, 10(6), 1113. https://doi.org/10.3390/pr10061113









