Abstract
Coal mining subsidence leads to reductions in soil fertility. In order to improve soil physical and chemical properties and to promote vegetation restoration, a nitrogen-fixing bacterium named S1 was isolated from the coal mining subsidence area in the Shendong mining area, and a zeolite-immobilized nitrogen-fixing bacterium was studied to improve the soil in the subsidence area. The results show that the immobilized nitrogen-fixing bacteria can significantly improve the ammonium nitrogen and nitrate nitrogen of soil by 50 times and 0.6 times, respectively, at 20 days, and it can also improve organic matter. In pot experiments, it was found that immobilized microorganisms can improve germination rate, plant height and the dry and fresh weight of maize. The results of the above soil culture tests and pot experiments were then compared and analyzed. It was found that plants made obvious use of soil ammonium nitrogen and nitrate nitrogen, and planting the plants was conducive to increases in soil organic matter.
1. Introduction
Coal mining activities have a long-term adverse impact on soil ecological structure [1,2]. The generation of soil fissures in mining subsidence areas increases the evaporation and infiltration of soil moisture. The soil nutrients penetrate vertically with soil fissures and surface runoff, or they are eroded by surface runoff, thus decreasing the concentrations of total nitrogen, total phosphorus, organic matter and other elements in soil [3,4]. Coal mining subsidence leads to the loss of soil nutrients, thereby restricting plant growth and inhibiting ecological reconstruction. The lack of soil nitrogen is a critical problem for plant growth [5,6]. Naturally existing microorganisms in the soil have a certain remediation function for the ecological environment of the soil. Compared with other remediation technologies, microbial remediation is low-cost, without the generation of secondary pollutants. However, under natural conditions, the efficiency of microbial remediation is low. Therefore, microbial-strengthening remediation has attracted more attention due to its low cost and high efficiency [7,8,9,10].
Immobilized-microorganism technology mainly refers to the method of immobilizing dispersed and free microorganisms in a certain limited space area by physical or chemical methods. It can increase the concentration of microbial cells and maintain high biological activity [11,12] Immobilized microorganisms have the advantages of high microbial density, fast reaction speed, resistance to environmental impacts and being capable of significantly improving remediation efficiency. In addition, immobilized-microorganism technology has the characteristics of simple operation, low requirements and low cost. This technology is expected to overcome the vicious competition between exogenous microorganisms and indigenous microorganisms [13,14] Immobilized microbial technology has been widely reported in wastewater treatment, but very little research has been reported with respect to soil remediation [15].
Current research shows that fixing microorganisms on a carrier can significantly improve the remediation efficiency of microorganisms [15,16]. Microbial immobilization technology can provide a stable microenvironment for target microorganisms in soils, thereby reducing the adverse effects caused by some environmental factors and improving the survival rate, stability and remediation efficiency of target microorganisms [11,17,18]. Therefore, it is crucial to select a carrier material with rich pores and a strong adsorption capacity. Zeolite has a silicon (aluminum) oxygen tetrahedron structure, with a regular structure, uniform pores, strong thermal stability, a large specific surface area and strong adsorption capacity [19,20,21]. Thus, it is considered an ideal microbial adsorption carrier. Moreover, zeolite can also improve soil quality. Yamada et al. [22] found that Ca-type artificial zeolite can improve the ion balance of soil, improve the water-holding capacity of soil and promote the growth of tomatoes and sugar beets. Ippolito et al. [23] found that adding clinoptilolite to the soil can improve the yield of alfalfa. When the addition amount was 20%, the yield, plant height and root weight of alfalfa reached the highest. Ozbahce et al. [24] used natural zeolite for soil improvement. It was found that the addition of zeolite improved the absorption of nutrient elements such as N, K, Zn, Mn and Cu by plants. The content of nutrient elements increased with increases in zeolite doses while increasing plant yield. However, to the best of our knowledge, there are few studies on immobilizing microorganisms on zeolite to improve soil physical and chemical properties and to explore its impact on plant growth.
The objective of this study is to promote soil nutrient cycling and increase plant biomass via zeolite-immobilized microorganism technology. Therefore, in this study, nitrogen-fixing bacteria were used as functional microorganisms and were immobilized on three zeolites by physical adsorption. Immobilized bacteria, free bacteria and sterilization carriers at different rates were added to the soil in the coal mining area. The purposes were: (1) to analyze its effect on soil physical and chemical properties (pH, electrical conductivity, nitrate nitrogen and ammonium nitrogen) through soil culture tests, and (2) to evaluate the improvement effect (soil nutrients and maize growth characteristics) using pot experiments.
2. Materials and Methods
2.1. Study Area
The Shendong mining area is located in the transition zone between the Loess Plateau and Mu Us sandy land. Its ecological environment is fragile. It is in the early stage of mining construction. It is dry and rainless, with an average annual rainfall of 360 mm and annual evaporation of 2300 mm. The species of primary vegetation is single, with an average vegetation coverage rate of only 3~11%, and the area of wind erosion accounts for 70% of the total area. It is a key monitoring and control area for soil erosion in China [25]. The sampling area includes the coal mining subsidence area (109°59′0″ E, 39°25′29″ N) and the control area far from the coal mining area (109°58′56″ E, 39°26′10″ N). There are two sampling areas in total. Five sampling points were randomly selected in each sampling area, and five sub-samples were set at each sampling point for collecting surface soil (0–20 cm). The soil samples from each sampling point were mixed evenly, and the plant residues and stones in the soil samples were taken out for preservation.
2.2. Materials
The basic physical and chemical properties of the soil were determined by the methods shown in Table 1. The basic physical and chemical properties of the soil were determined by our laboratory. Alkali hydrolysable nitrogen was calculated as the sum of ammonium nitrogen and nitrate nitrogen.

Table 1.
Basic physical and chemical properties of soil.
Three kinds of zeolite, 4A zeolite, NaY zeolite and USY zeolite, were selected as the carriers, which were purchased from Tianjin Nanhua catalyst Co., Ltd., Tianjin, China. The basic properties of the three kinds of zeolite were provided by the supplier (Table 2).

Table 2.
Basic physical and chemical properties of zeolite.
2.3. Immobilization of the Strain
2.3.1. Isolation and Identification of the Strain
An amount of 5 g of soil samples was fully shaken in 100 mL sterile water, was diluted and coated on Ashby solid medium and was cultured in 30 °C incubator for 5 days, and then the vigorous colonies were selected and purified 3~4 times to obtain a pure culture of nitrogen-fixing bacteria. To identify the strain, genomic DNA was extracted by a universal column genomic DNA extraction kit, and the 16S rDNA gene was amplified by PCR with universal primers 27F (AGT TTG ATC MTG GCT CAG) and 1492R (GGT TAC CTT GTT ACG ACT T) [26]. PCR product purification and sequencing was conducted at Beijing Ruiboxingke Biotechnology Co., Ltd., Beijing, China. BLAST was used to compare and analyze the homology.
2.3.2. Immobilization of the Strain
Nitrogen-fixing bacteria were inoculated into a beef extract peptone liquid medium and were cultured to OD600 = 1.0. The bacterial solution was centrifuged for 10 min at 3000 r/min. The bottom bacteria were retained, washed with sterile normal saline (0.85% NaCl) and resuspended in normal saline (bacterial solution:normal saline = 1:1) [27]. The above was the seed liquid. Moreover, 4A zeolite, NaY zeolite and USY zeolite were selected as the immobilization carriers, and the bacteria were immobilized by the physical adsorption method. The specific operation method was as follows. The seed liquid and the zeolite were mixed in a proportion of 100 mL:1 g, and the adsorption was shaken for a certain time at 30 °C and 150 r/min was filtered. The carrier, which was strain S1 immobilized on zeolite, was washed with sterile water [28].
The seed liquid and the carrier were mixed in a ratio of 100 mL:1 g and were then vibrated at 30 °C and 150 r/min, and they were sampled at an interval of 2 h. The samples were centrifuged at 1000 r/min for 10 min, and the absorbance value of the supernatant was determined. The adsorption capacity of zeolite to microorganisms was expressed by subtracting the absorbance value of each sample from the initial absorbance value of the seed liquid [27]. The adsorption curve was drawn with the time as abscissa and the adsorption capacity as ordinate, and the capacity of each carrier was compared.
2.4. Soil Culture Experiments and Pot Experiments
2.4.1. Soil Culture Experiments
To explore the effect of zeolite-immobilized bacteria on soils from the mining subsidence area, three variables, including the carrier, application ratio and additive type, were set for a comprehensive test. The levels of each variable are shown in Table 3. We set a full factorial test. Each group took 200 g of soil samples in vials and mixed them with additives evenly. All experiments were performed in triplicate. After 20 days, soil pH and conductivity, ammonium nitrogen, nitrate nitrogen, available phosphorus and organic matter were determined.

Table 3.
Test Design.
The analysis method is referred to as Soil Agrochemical Analysis [29]. Nitrate nitrogen was determined by UV spectrophotometry; ammonium nitrogen was determined by KCl extraction and indophenol blue colorimetry; available phosphorus was determined by NaHCO3 extraction and molybdenum–antimony anti-colorimetry; and organic matter was determined by the potassium dichromate volumetric method.
2.4.2. Pot Experiments
The effects of the additive type (immobilized bacteria, free bacteria and sterilization carrier), zeolite type and application proportion (high and low) on plant growth were explored through a comprehensive test. Each treatment was performed in triplicate, and a control was set up (the soil in the coal mining subsidence area was not improved). The experimental setup was the same as that of the soil culture test. Each group took 1 kg of soil into plastic flower pots, took an appropriate amount of corn seeds, sterilized them with sodium hypochlorite, immersed them in clean water for one night, sowed 3 seeds in each pot, watered them regularly, observed the growth every day and recorded the physiological indexes of plant height. All experiments were performed in triplicate.
The plant height was measured with a ruler. Fresh weight was divided into three parts: roots, stems and leaves, which were weighed and recorded with an electronic balance. The dry weight of the plant was also divided into the three parts: (1) drying at 105 °C for 1 h to kill, and at 65 °C at a constant weight; (2) placing into a dryer to cool to room temperature; and (3) weighing and recording.
2.5. Data Analysis
The correlation and variance were analyzed by SPSS Statistics v25 software (SPSS Inc., Chicago, IL, USA). All data for the experiments are reported as the mean ± standard error of the mean (SEM). Excel 2016 was used for data processing, Origin 2018 software was used for figure mapping, and SPSS Statistics v25 software was applied for the simulations of soil water infiltration parameters and statistical analyses. The least significant difference (LSD) method was utilized for significance testing (p-value < 0.05).
3. Results and Discussion
3.1. Isolation and Identification of the Strain
A strain of nitrogen-fixing bacteria, named S1, was isolated from soil samples in the mining area. The colony was light yellow, opaque, round, smooth and moist. The results of the Gram staining test show that it was Gram-negative. The consistency of the 16S rDNA gene sequence showed that between strain S1 and Sphingobium yanoikuyae was 99.8%. According to Mitra et al. [30], Sphingobium yanoikuyae was a Gram-negative bacterium. On LB medium, the colony was pale yellow, which could ferment glucose, sucrose and lactose. Starch hydrolysis was negative, and it could not grow under a salt content of 7.5~10% [29]. A previous study has shown that Sphingomonas saginata is a facultative aerobic bacterium, which can utilize galactose, salicylic acid, mannitol and other carbon sources, and it is slightly halophilic [31]. Combined with the results of the 16S rDNA identification, strain S1 should belong to Sphingobium yanoikuyae. Physiological and biochemical characteristics of strain S1 was shown in Table 4.

Table 4.
Physiological and biochemical characteristics of strain S1.
3.2. Immobilization of the Strain
Strain S1 was immobilized by three types of zeolites via physical adsorption. As shown in Figure 1, among the three carriers, 4A zeolite had the best adsorption effect on S1, and NaY has the worst adsorption effect. In addition, 4A zeolite reached equilibrium at 2 h, whereas NaY zeolite and USY zeolite reached equilibrium at 8 h. Considering that too much time affects the activity of S1, the optimal adsorption time was 8 h (Figure 1).
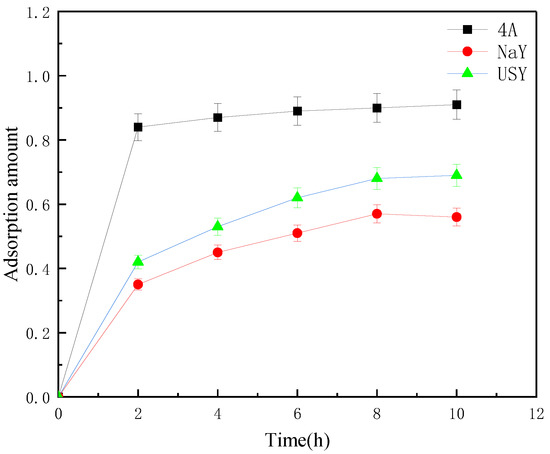
Figure 1.
Curve of adsorption amount of three carriers to strain S1 with time.
The physical adsorption method mainly used the carrier material with a rough surface to make the microorganism easy to attach to the surface. Generally, as the specific surface area of the carrier increases, the adsorption effect becomes better [32]. Compared with the basic properties of the three, 4A zeolite had the best adsorption effect on S1, although its specific surface area was smaller than the other two. There are two reasons for this: firstly, the microbial surface was negatively charged, resulting in an electrostatic interaction with the positively charged carrier [31]; secondly, it was related to the hydrophilicity of the carrier. The increase in the Si/Al ratio in zeolite resulted in stronger hydrophobicity [33], whereas the carrier with low Si/Al ratio had the best adsorption effect on S1. The fruit was better.
3.3. Effect of Immobilized Microorganism on Soil Improvement
3.3.1. Effects of pH and Electroconductibility
Figure 2 shows that zeolite and its immobilized microorganisms have no obvious law regarding the change in soil pH. However, it was obvious that the addition of USY zeolite-immobilized bacteria significantly reduced the soil pH, which was 7.65 and 7.33, respectively, making the soil pH closer to neutral, whereas the pH of other groups was improved to varying degrees. Additionally, it was found that the overall pH of the USY zeolite group was less than that of the other two groups, indicating that the application of USY zeolite improved the pH of alkaline soil to a certain extent. The application ratio had no significant relationship with soil pH, but most of them showed that the pH at a high application ratio was greater than that at a low application ratio. In addition, although the effect of loading microorganisms on soil pH was not significant, it could be seen that the pH of the immobilized bacteria group was lower than that of the blank carrier group, indicating that the addition of nitrogen-fixing bacteria S1 could also improve the pH of alkaline soil to a certain extent. By measuring the pH of the three zeolites at an early stage, we found that, except for USY zeolite, the other two zeolites were alkaline, resulting in an increase in soil pH.
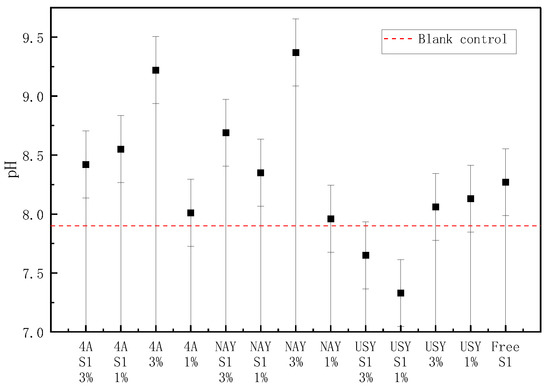
Figure 2.
The effect of zeolite and bacteria on soil pH.
Figure 3 shows that the most significant factor for the improvement of conductivity was the application ratio. The soil conductivity at a high application ratio was significantly higher than that at a low application ratio. The highest group was USY zeolite at 3%, which was 713 μs/cm (six times higher than that of the blank group). At this point, the conductivity reached a very high level. The soil under this level can be considered saline alkali soil, and the conductivity content of the control group and free bacteria group was the lowest, at 100 μs/cm. Comparing the test results between the blank carrier and the immobilized microorganism, it was found that the conductivity of the blank carrier group was higher than that of the immobilized bacteria group, and the conductivity of all test groups was significantly higher than that of the blank group. Hence, the addition of zeolite and nitrogen-fixing bacteria S1 improved the soil conductivity, whereas the type of zeolite has no significant effect on the soil conductivity. Since the soil conductivity reflected the salt content, it was concluded that the addition of zeolite and the nitrogen-fixing bacterium S1 greatly increases the salt content in the soil. This action increased the content of ionic nitrogen in the soil, thus improving the soil conductivity. Zeolite had a strong adsorption capacity and ion exchange capacity, which further improved the soil conductivity [34].
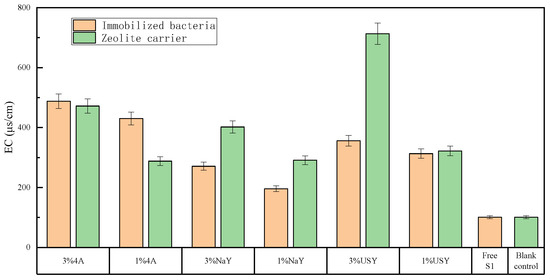
Figure 3.
The effect of zeolite and bacteria on soil EC.
As shown in Table 5, the carrier, application ratio and additive type had no significant effect on EC, available phosphorus and urease. The carrier and application ratio had no significant effect on NO3− and NH4+. The carrier had a significant effect on organic matter. The additive type had a very significant effect on NO3−, NH4+ and organic matter.

Table 5.
Variance analysis.
3.3.2. Effects of Ammonium Nitrogen and Nitrate Nitrogen
As shown in Figure 4, the addition of zeolite did not significantly increase the content of nitrate nitrogen in soil, but it significantly increased the content of nitrate nitrogen when it was used as a carrier to immobilize microorganisms. The nitrate nitrogen content of the immobilized microbial group was higher than that of the blank carrier group. The two optimal groups were 4A and USY zeolite-immobilized microorganisms with a high proportion. The nitrate nitrogen contents were 46.04 mg/kg and 46.24 mg/kg, respectively. When there were loaded microorganisms, the soil nitrate nitrogen under a high application ratio was higher than that under a low application ratio. When there were no loaded microorganisms, the law was opposite to the former. The effects of different zeolites on soil nitrate nitrogen content also had a certain correlation. The specific performance was that the USY zeolite group was higher than the other two groups, whereas the NaY zeolite group had the lowest nitrate nitrogen content. The content of nitrate nitrogen in the free bacteria group was roughly between that of the immobilized bacteria group and the blank carrier group, and it was slightly higher than that in the control group.
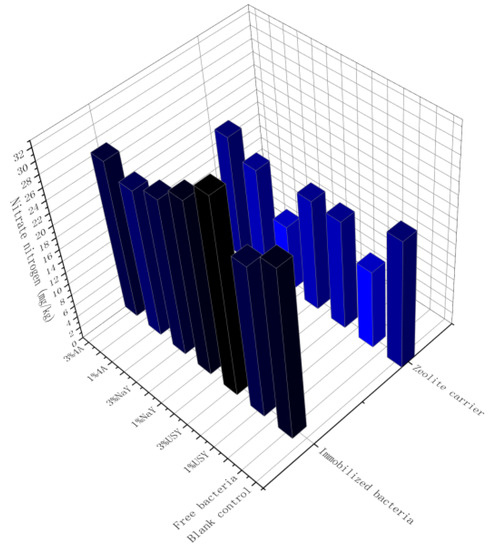
Figure 4.
The effect of zeolite and bacteria on soil nitrate nitrogen.
Based on the obtained results, it was found that the main factors affecting the content of soil nitrate nitrogen are related to immobilized microorganisms. We found that nitrogen-fixing bacteria S1 significantly affect the content of nitrogen in soil, and their nitrogen-fixation effect is enhanced through immobilization. Combined with the effect of the test of immobilization, we found that, as the adsorption effects of the carrier on microorganisms became stronger, the microbial increase in nitrogen in the soil became more significant. This may be due to nitrogen-fixing bacteria that capture nitrogen in the air and convert it into a nitrogen form that can be used by plants in the soil.
As shown in Figure 5, the change in soil ammonium nitrogen content was similar to that of nitrate nitrogen. However, some significant differences were observed among different groups. The content of high-content groups was more than 100 mg/kg, whereas the content of some groups was less than 10 mg/kg, which are values that are far from each other. The application ratio had an obvious influence on the content of ammonium nitrogen. Under a high application ratio, the content of ammonium nitrogen was significantly higher than that under a low application ratio, and this law is not associated with whether to load microorganisms, indicating that the addition of zeolite also had a certain effect on the content of soil ammonium nitrogen. The type of zeolite had no significant effect on ammonium nitrogen, but it was found that USY zeolite was better than the other two groups. Whether the microorganism was loaded or not was the most significant condition. The ammonium nitrogen content of the loaded microorganism group could be over 10 times higher than that of the unloaded microorganism group. Among them, the ammonium nitrogen content of 4A and the USY zeolite-immobilized microorganism group under the ratio of 3% was the highest (127.86 mg/kg and 131.40 mg/kg, respectively), which was about 50 times and 52 times higher than that of the blank group and about 15 times and 10 times higher than that of the blank carrier under the same ratio. It was found that, by adding zeolite and nitrogen-fixing bacteria S1, the contents of nitrate nitrogen and ammonium nitrogen in soil increased from a very barren level to a high level. It was concluded that the effect of nitrogen-fixing bacteria S1 on soil ammonium nitrogen was very significant. Since nitrogen-fixing bacteria can convert nitrogen in the air into ammonium nitrogen and exist in the soil, through immobilization, the survival rate and nitrogen-fixing ability of the microorganisms were increased, resulting in an increase in soil ammonium nitrogen content. Soil nitrate nitrogen was derived from the transformation of ammonium nitrogen. Some ammonium nitrogen was transformed into nitrate nitrogen through nitrification due to the action of other microorganisms in the soil. Therefore, there was a significant positive correlation between ammonium nitrogen (the correlation coefficient was 0.689) and nitrate nitrogen content, and some differences may come from the strength of nitrification in different groups of soil. For example, the ammonium nitrogen content of the 4A zeolite-immobilized microbiome is low at 1%, most likely due to strong nitrification, and more of that part was converted into nitrate nitrogen, thereby increasing the content of nitrate nitrogen.
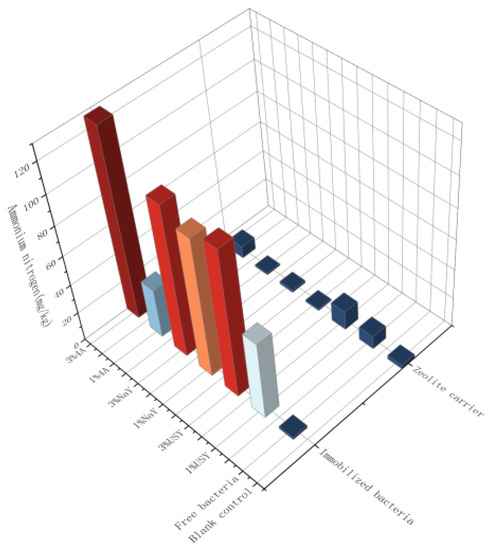
Figure 5.
The effect of zeolite and bacteria on soil ammonium nitrogen.
3.3.3. Effects of Organic Matter
Zeolite and its immobilized microorganisms improved soil organic matter to a certain extent. The most obvious two groups were 4A zeolite-immobilized microorganisms under the application ratios of 3% and 1%. The organic matter contents were 14.53 g/kg and 12.74 g/kg, respectively. Comparing the results between the immobilized bacteria group and the blank carrier group, it was found that the content of organic matter in the group added with nitrogen-fixing bacteria S1 was slightly higher than that in the group with only added zeolite, but the effect was not significant. Comparing the results among the three zeolites, the organic matter of the 4A zeolite group was slightly higher than that of the other two groups. Interestingly, the type of the carrier and the addition of microorganisms have a high impact on the content of organic matter. However, the organic matter content of more than half of the experimental groups was still at a very low level, indicating that the improvement of organic matter content needs to be further studied (Figure 6). Relevant research has shown that, although the content of soil organic matter decreases at a rate of 2% per year [35], the long-term application of chemical fertilizers can also lead to continuous reductions in soil organic matter [36], and the application of microbial fertilizers effectively improves soil organic matter [37].
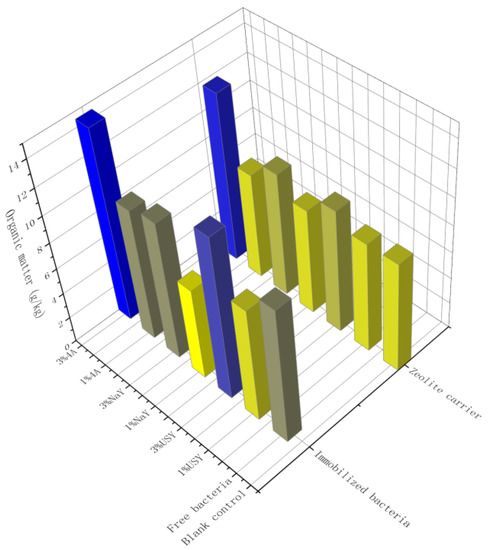
Figure 6.
The effect of zeolite and bacteria on soil organic matter.
3.3.4. Effects of Available Phosphorus
As presented in Figure 7, the content of available phosphorus in soil by zeolite was not increased significantly, but there were some differences among the groups. The content of the available phosphorus in each group was in the range of 2.5 mg/kg~7.5 mg/kg, belonging to a very low level. The group with the highest available phosphorus content was 4A zeolite-immobilized bacteria at a ratio of 1%, and the content was 7.26 mg/kg, whereas the lowest group was 4A zeolite-immobilized bacteria at a ratio of 3%, and the content was 2.74 mg/kg. Except for the 4A zeolite-immobilized microbiome, the application ratios in other groups had no significant effect on the available phosphorus. There were some differences in the content of the available phosphorus under different types of zeolites. The content of the available phosphorus in the USY zeolite group was higher than that in the other two groups. Compared with the control group, the content of the available phosphorus was significantly improved. The overall level of the NaY zeolite group was lower, and the content was below that in the control group. It was concluded that only USY zeolite and 4A zeolite-immobilized microorganisms with low application ratios had remarkable effects on the improvement of soil available phosphorus. The possible reason is that the addition of zeolite enhanced the water retention capacity of the soil and the activity of other microorganisms; promoted the mineralization of phosphorus by other microorganisms in soil; and led to the improvement of available phosphorus. The results show that the phosphorus dissolution in soil was related to pH and organic matter. Therefore, the low level of available phosphorus in this test may inhibit the availability of soil phosphorus due to a high pH and low organic matter.
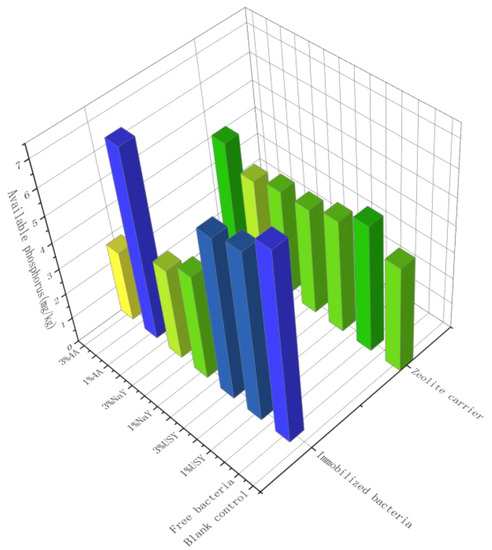
Figure 7.
The effect of zeolite and bacteria on soil available phosphorus.
3.3.5. Effects of Urease Activity
The change level of soil urease activity was low, and the range of urease activity in each group was 0.78~0.88, with little difference. However, as can be seen in Figure 8, the urease activity of most treatment groups was higher than that of the control group, and the immobilized bacteria group was slightly higher than that of the blank carrier group, indicating that the addition of zeolite and its immobilized microorganisms had a certain impact on soil urease activity. However, the impact was small (Figure 8). Although the three factors had no significant effect on urease activity, it can be seen that the most influential factor was whether S1 was added.
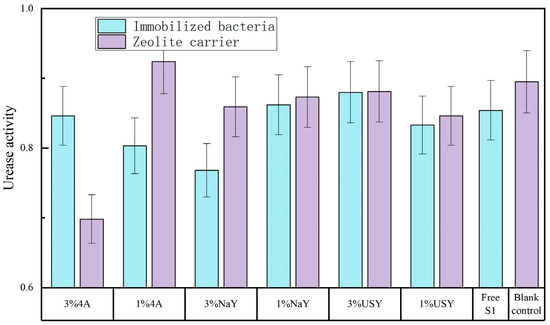
Figure 8.
The effect of zeolite and bacteria on soil urease activity.
3.4. Effect on Plant Growth
3.4.1. Effects of Germination Percentage
The final germination percentage of corn seeds in each group was between 88% and 100%. Zeolite and its immobilized microorganisms promoted the germination rate of corn to a certain extent. The group with the best effect was that with the 1% NaY zeolite-immobilized microorganisms. The corn in this treatment group began to germinate after 2 days of sowing, and all germinated after 4 days. The other groups began to germinate at 3 days. Overall, the germination of each group, from high to low, was in the following order: 1% immobilized bacteria, 1% blank carrier, 3% immobilized bacteria, free bacteria and 3% blank carrier. The effect of zeolite types on germination rate was not obvious. Thus, the main factors affecting seed germination were microorganisms and the application proportion. When 3% zeolite was applied, it showed a certain inhibitory effect. It began to germinate on the fifth day, and the overall germination rate was low, as only 88% germinated (Figure 9).
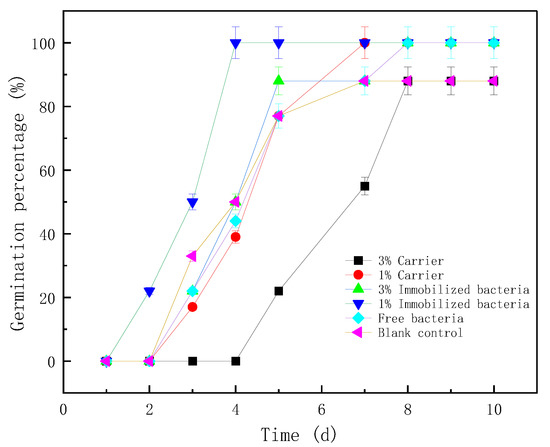
Figure 9.
The effect of zeolite and bacteria on corn germination percentage.
3.4.2. Effects of Height
Zeolite-immobilized microorganisms promoted the growth of maize slightly. Zeolite had no obvious effect on plant height when it was not loaded with microorganisms. After loading microorganisms, plant height increased to a certain extent. The difference in plant height among different groups was mainly reflected after 25 days. Among the three zeolites, 4A zeolite had the most obvious promoting effect after the immobilizing microorganisms, which increased by 23.28% compared with the blank group at 35 days. The application proportion not only affected the germination rate, but it also had a certain impact on plant height. Due to the high application proportion of zeolite, it was found that it can easily lead to soil lumping and hardening and can affect the extension of plant roots. Therefore, it led to slower growth in the initial stage, and the final height was lower than that of the 1% application group. When loaded with microorganisms, the inhibitory effect of zeolite with a high application ratio for plant growth was not so obvious (Figure 10).
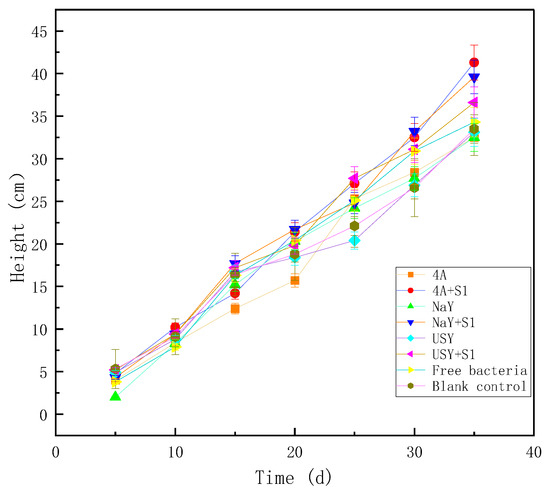
Figure 10.
The effect of zeolite and bacteria on plant height.
3.4.3. Effect of Weight
The addition of zeolite and its immobilized microorganisms significantly improved the fresh weight of plants. Among them, 4A zeolite was the carrier, and the effect of the immobilized microorganisms was the best at an application ratio of 1%. The total dry weight was 4.4897 g, which was an increase of 136.21% compared with the blank group. The fresh weight of leaves was 2.2631 g, which was an increase of 243.97% compared with the blank group. The 3% USY zeolite showed a certain inhibitory effect. The total fresh weight was 1.6018 g, which was 15.72% lower than that of the blank group. Among the three parts of corn fresh weight, the most significant change was the leaf fresh weight, followed by the roots, and the stems changed the least (Figure 11).
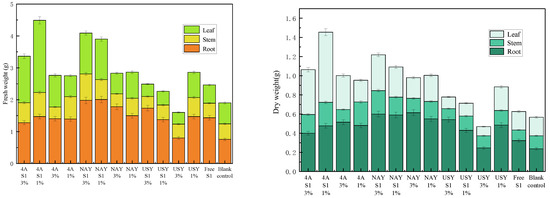
Figure 11.
The effect of zeolite and bacteria on plant fresh weight and dry weight.
The addition of zeolite and its immobilized microorganisms improved the dry weight of plants, and the law was similar to that of the fresh weight. Among them, with 4A zeolite as the carrier, when the immobilized microorganism was added at a proportion of 1%, the effect was the best, and its promoting effect was mainly reflected in the promotion of plant leaf growth. The effect was the worst when 3% USY zeolite was added. From the point of view of NaY zeolite, the effect of increasing the dry weight of plant bacteria was better than that of NaY zeolite. NaY zeolite and its immobilized microorganisms had a better effect on increasing root dry weight than other groups. The dry weight of plant stems had no obvious law.
The promotion effect of zeolite and its immobilized microorganisms on plant fresh weight was mainly reflected in the leaves. The promotion effect of loaded microorganisms on plant fresh weight was significantly higher than that of unloaded microorganisms, and the effect of the application ratio on fresh weight was not obvious. The promotion effect of a high application ratio of 4A zeolite fixed microorganisms and USY was lower than that with a low application ratio, whereas the difference between high and low application ratios of other groups was small.
3.5. Mechanism Analysis of Plant Growth Promotion
In the pot experiments, the physical and chemical properties of soil were analyzed, mainly including ammonium nitrogen, nitrate nitrogen, organic matter and urease activity, and they were compared with the above indexes in the soil culture experiment. The results are shown in Table 6.

Table 6.
Changes in soil physical and chemical properties between the soil culture experiment and pot experiment.
As shown in Table 7, NO3− and NH4+ had a significant effect on height and fresh weight. NO3− and NH4+ had a very significant effect on dry weight. Organic matter and urease activity had no significant effect on height, fresh weight or dry weight.

Table 7.
Correlation analysis between soil physical and chemical properties and plant growth indexes.
4. Conclusions
In this study, we found that using zeolite molecular sieves to immobilize microorganisms to improve poor soil in mining areas promoted nutrient cycling and plant growth. The soil culture test found that zeolite and its immobilized microorganisms had a significant impact on soil ammonium nitrogen, nitrate nitrogen and electrical conductivity. The best group increased ammonium nitrogen by 50 times, nitrate nitrogen by 61.49% and organic matter from a very low to a medium level in 7 days. In the pot experiment, it was found that zeolite and its immobilized microorganisms promoted the germination and plant height of Maize under a low application ratio, and the molecular sieve under a high application ratio inhibited the growth of plants. Among them, the immobilized microbiome had the best effect on plant growth. This provides theoretical and technical support for zeolite-molecular-sieve immobilized microorganisms to improve barren soil in mining areas.
Author Contributions
Conceptualization, L.B. and Y.Y.; methodology, J.J. and Y.Z.; validation, Z.S., Y.Z. and H.Z.; investigation, Z.S. and Y.Z.; resources, L.B. and Y.Y.; data curation, Z.S.; writing—original draft preparation, Z.S.; writing—review and editing, L.B., Y.Z. and J.J.; visualization, Z.S., Y.Z. and H.Z.; supervision, L.B. and Y.Y.; project administration, L.B. and Y.Y.; funding acquisition, L.B. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Open Fund of the State Key Laboratory of Water Resource Protection and Utilization in Coal Mining (GJNY-18-73.19), the National Natural Science Foundation of China (52004012) and the Key Technologies of Ecological Restoration and Stability Improvement in the Western Mining Area (GJNY2030XDXM-19-03.1).
Acknowledgments
All support that was given is covered by the author contributions or funding sections.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Hu, L.; Bai, L.; Kang, J.; Jia, J. Contamination level and potential health risk assessment of hexavalent chromium in soils from a coal chemical industrial area in Northwest China. Hum. Ecol. Risk Assessment Int. J. 2019, 26, 1300–1312. [Google Scholar] [CrossRef]
- Jing, Z.; Wang, J.; Zhu, Y.; Feng, Y. Effects of land subsidence resulted from coal mining on soil nutrient distributions in a loess area of China. J. Clean. Prod. 2017, 177, 350–361. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wei, Y.; Meng, H.; Cao, Y.; Lead, J.; Hong, J. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Sci. Total Environ. 2020, 739, 139882. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Hu, L.; Yu, J.; Luo, H.; Wang, H.; Xu, P.; Zhang, Y. Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation. Chem. Eng. J. 2020, 382, 123001. [Google Scholar] [CrossRef]
- Neina, D. The Role of Soil pH in Plant Nutrition and Soil Remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.; Das, N.; Pandey, B. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250, 272–291. [Google Scholar] [CrossRef]
- Jia, J.; Zong, S.; Hu, L.; Shi, S.; Zhai, X.; Wang, B.; Li, G.; Zhang, D. The dynamic change of microbial communities in crude oil-contaminated soils from oil fields in China. Soil Sediment Contam. Int. J. 2017, 26, 171–183. [Google Scholar] [CrossRef]
- Hu, L.; Wang, H.; Xu, P.; Zhang, Y. Biomineralization of hypersaline produced water using microbially induced calcite precipitation. Water Res. 2021, 190, 116753. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Xu, Y.; Wang, H.-Y.; Zhao, J.; Gao, D.-M.; Li, F.-M.; Xing, B. Biodegradation of crude oil in contaminated soils by free and immobilized microorganisms. Pedosphere 2012, 22, 717–725. [Google Scholar] [CrossRef]
- Wang, T.; Su, D.; Wang, X.; He, Z. Adsorption-Degradation of Polycyclic Aromatic Hydrocarbons in Soil by Immobilized Mixed Bacteria and Its Effect on Microbial Communities. J. Agric. Food Chem. 2020, 68, 14907–14916. [Google Scholar] [CrossRef]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J.; Shen, Z.; Lian, C.; Chen, Y. Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: A review. Ecotoxicol. Environ. Saf. 2020, 207, 111261. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Ivshina, I.B.; Lozinsky, V.I.; Kuyukina, M.S.; Philp, J.C. Bioremediation of diesel-contaminated soil by microorganisms immobilised in polyvinyl alcohol. Int. Biodeterior. Biodegradation 2004, 54, 167–174. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2018, 17, 241–257. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Alhewairini, S.S.; Peng, C.; Naz, I. Application of microbial immobilization technology for remediation of Cr(VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Tsuji, K.; Asakawa, M.; Anzai, Y.; Sumino, T.; Harada, K.-I. Degradation of microcystins using immobilized microorganism isolated in an eutrophic lake. Chemosphere 2006, 65, 117–124. [Google Scholar] [CrossRef]
- Shin, D.-C.; Kim, J.-S.; Park, C.-H. Study on physical and chemical characteristics of microorganism immobilized media for advanced wastewater treatment. J. Water Process Eng. 2019, 29, 100784. [Google Scholar] [CrossRef]
- Fernández, N.; Montalvo, S.; Fernández-Polanco, F.; Guerrero, L.; Cortés, I.; Borja, R.; Sánchez, E.; Travieso, L. Real evidence about zeolite as microorganisms immobilizer in anaerobic fluidized bed reactors. Process Biochem. 2007, 42, 721–728. [Google Scholar] [CrossRef]
- Montalvo, S.; Guerrero, L.; Borja, R.; Sánchez, E.; Milán, Z.; Cortés, I.; de la la Rubia, M.A. Application of natural zeolites in anaerobic digestion processes: A review. Appl. Clay Sci. 2012, 58, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of Produced Water in the Permian Basin for Hydraulic Fracturing: Comparison of Different Coagulation Processes and Innovative Filter Media. Water 2020, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Fujiyama, H.; Endo, T.; Rikimaru, M.U.; Sasaki, Y.; Yamamoto, S.; Honna, T.; Yamamoto, T. Effect of K-type and Ca-type artificial zeolites applied to high sodic soil on the growth of plants different in salt tolerance. Soil Sci. Plant Nutr. 2007, 53, 471–479. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite Soil Application Method Affects Inorganic Nitrogen, Moisture, and Corn Growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Ozbahce, A.; Tari, A.F.; Gönülal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2014, 61, 615–626. [Google Scholar] [CrossRef]
- Song, H.; Xu, J.; Fang, J.; Cao, Z.; Yang, L.; Li, T. Potential for mine water disposal in coal seam goaf: Investigation of storage coefficients in the Shendong mining area. J. Clean. Prod. 2019, 244, 118646. [Google Scholar] [CrossRef]
- Iruene, I.T.; Wafula, E.N.; Kuja, J.; Mathara, J.M. Phenotypic and genotypic characterization of lactic acid bacteria isolated from spontaneously fermented vegetable amaranth. Afr. J. Food Sci. 2021, 15, 254–261. [Google Scholar]
- You, M.; Fang, S.; MacDonald, J.; Xu, J.; Yuan, Z.-C. Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol. Res. 2020, 233, 126395. [Google Scholar] [CrossRef]
- Reeve, P.J.; Fallowfield, H. Natural and surfactant modified zeolites: A review of their applications for water remediation with a focus on surfactant desorption and toxicity towards microorganisms. J. Environ. Manag. 2018, 205, 253–261. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Xiong, X.; Zhang, B.; Wang, L. Determination of the best conditions for modified biochar immobilized petroleum hydrocarbon degradation microorganism by orthogonal test. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Sanya, China, 20–22 November 2017; IOP Publishing: Bristol, UK, 2017; p. 012191. [Google Scholar] [CrossRef]
- Mautusi, M.; Nguyen, K.M.-A.-K.; Wayland, B.T.; Gilpin, J.S.; Hamby, S.R.; Berry, T.L.; Harper, D.E. Isolation and characterization of a novel Sphingobium yanoikuyae strain variant that uses biohazardous saturated hydrocarbons and aromatic compounds as sole carbon sources. F1000Research 2020, 9, 767. [Google Scholar]
- Kourkoutas, I.; Bekatorou, A.; Banat, I.; Marchant, R.; Koutinas, A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Peng, Y.; Tong, L.; Feng, L.; Ma, K. Characterization of the diethyl phthalate-degrading bacterium Sphingobium yanoikuyae SHJ. Data Brief 2018, 20, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ren, X.; He, J.; Zhang, Q.; Qiu, C.; Fan, B. Application of natural mixed bacteria immobilized carriers to different kinds of organic wastewater treatment and microbial community comparison. J. Hazard. Mater. 2019, 377, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Schulman, E.; Wu, W.; Liu, D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials 2020, 13, 1822. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Sheng, Z.; Braun, R.J. Salinity Management Using an Anionic Polymer in a Pecan Field with Calcareous-Sodic Soil. J. Environ. Qual. 2011, 40, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Kapkiyai, J.J.; Karanja, N.K.; Qureshi, J.N.; Smithson, P.C.; Woomer, P.L. Soil organic matter and nutrient dynamics in a Kenyan nitisol under long-term fertilizer and organic input management. Soil Biol. Biochem. 1999, 31, 1773–1782. [Google Scholar] [CrossRef]
- Riley, H.; Pommeresche, R.; Eltun, R.; Hansen, S.; Korsaeth, A. Soil structure, organic matter and earthworm activity in a comparison of cropping systems with contrasting tillage, rotations, fertilizer levels and manure use. Agric. Ecosyst. Environ. 2008, 124, 275–284. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).