Current Strategies to Improve Yield of Recombinant Protein Production in Rice Suspension Cells
Abstract
:1. Introduction
2. Advantages of Plant Suspension Culture Cells
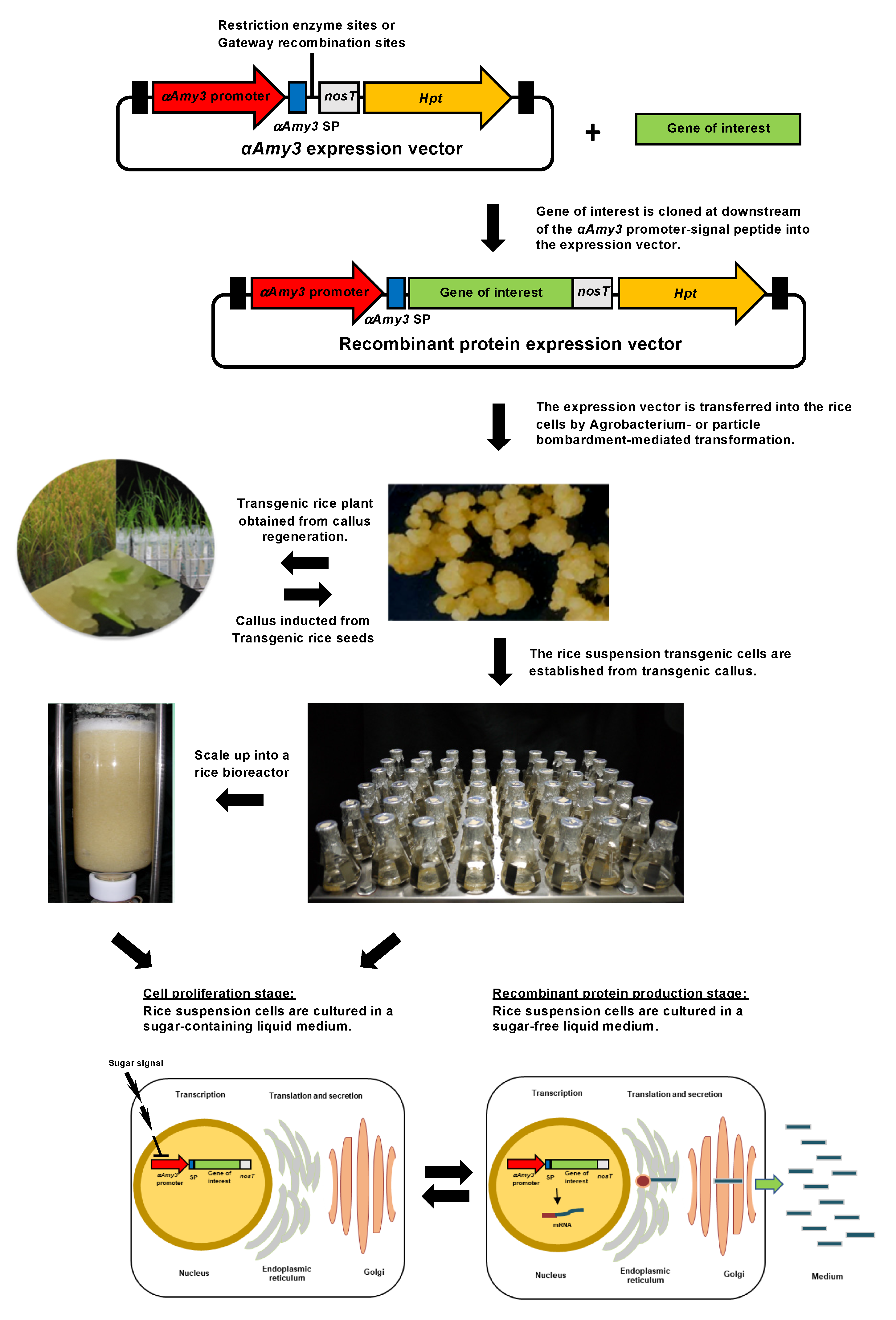
3. Rice Suspension Cell Recombinant Protein Expression System
4. Approaches Applied to Enhance the Performance of the Rice Suspension Cell Recombinant Protein Expression System
4.1. Regulation of Gene Expression in Rice
4.2. Screening of the Best Rice Signal Peptide
4.3. Regulation of Proteolytic Enzymes in Rice Cells
4.4. Optimization of the Culture Medium
4.5. Establishment of the Optimal Culture Procedure for Rice Suspension Cells
4.6. Optimization of Codon Usage
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2021, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. Main Strategies of Plant Expression System Glycoengineering for Producing Humanized Recombinant Pharmaceutical Proteins. Biochemistry. 2018, 83, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Schillberg, S.; Finnern, R. Plant molecular farming for the production of valuable proteins—Critical evaluation of achievements and future challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Singh, A.A.; Pillay, P.; Tsekoa, T.L. Engineering Approaches in Plant Molecular Farming for Global Health. Vaccines 2021, 9, 1270. [Google Scholar] [CrossRef]
- Ghag, S.B.; Adki, V.S.; Ganapathi, T.R.; Bapat, V.A. Plant Platforms for Efficient Heterologous Protein Production. Biotechnol. Bioprocess Eng. 2021, 26, 546–567. [Google Scholar] [CrossRef]
- Shaaltiel, Y.; Bartfeld, D.; Hashmueli, S.; Baum, G.; Brill-Almon, E.; Galili, G.; Dym, O.; Boldin-Adamsky, S.A.; Silman, I.; Sussman, J.L.; et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol. J. 2007, 5, 579–590. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Tan, C.C.; Ku, J.T.; Hsu, W.C.; Su, S.C.; Lu, C.A.; Huang, L.F. Improving pharmaceutical protein production in Oryza sativa. Int. J. Mol. Sci. 2013, 14, 8719–8739. [Google Scholar] [CrossRef] [Green Version]
- Singhvi, P.; Panda, A.K. Solubilization and Refolding of Inclusion Body Proteins. Methods Mol. Biol. 2022, 2406, 371–387. [Google Scholar]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies-status and outlook. Appl. Microbiol. Biotechnol. 2021, 105, 6649–6668. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Pandey, S.S.; Maji, D.; Gupta, V.; Kalra, A.; Singh, M.; Mathur, A.; Mathur, A.K. Enhanced expression of ginsenoside biosynthetic genes and in vitro ginsenoside production in elicited Panax sikkimensis (Ban) cell suspensions. Protoplasma 2018, 255, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Zebarjadi, A.; Dianatkhah, S.; Pour Mohammadi, P.; Qaderi, A. Influence of abiotic elicitors on improvement production of artemisinin in cell culture of Artemisia annua L. Cell. Mol. Biol. 2018, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-B.; Chen, J.-G.; Yin, Z.-P.; Shangguan, X.-C.; Peng, D.-Y.; Lu, T.; Lin, P. Methyl jasmonate and salicylic acid elicitation increase content and yield of chlorogenic acid and its derivatives in Gardenia jasminoides cell suspension cultures. Plant Cell Tissue Organ Cult. 2018, 134, 79–93. [Google Scholar] [CrossRef]
- Sijmons, P.C.; Dekker, B.M.; Schrammeijer, B.; Verwoerd, T.C.; van den Elzen, P.J.; Hoekema, A. Production of correctly processed human serum albumin in transgenic plants. Biotechnology 1990, 8, 217–221. [Google Scholar] [CrossRef]
- Schillberg, S.; Raven, N.; Fischer, R.; Twyman, R.M.; Schiermeyer, A. Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr. Pharm. Des. 2013, 19, 5531–5542. [Google Scholar] [CrossRef]
- Fischer, R.; Emans, N. Molecular farming of pharmaceutical proteins. Transgenic Res. 2000, 9, 279–299, Discussion 277. [Google Scholar] [CrossRef]
- Kusnadi, A.R.; Nikolov, Z.L.; Howard, J.A. Production of recombinant proteins in transgenic plants: Practical considerations. Biotechnol. Bioeng. 1997, 56, 473–484. [Google Scholar] [CrossRef]
- Mir-Artigues, P.; Twyman, R.M.; Alvarez, D.; Cerda Bennasser, P.; Balcells, M.; Christou, P.; Capell, T. A simplified techno-economic model for the molecular pharming of antibodies. Biotechnol. Bioeng. 2019, 116, 2526–2539. [Google Scholar] [CrossRef]
- Staub, J.M.; Garcia, B.; Graves, J.; Hajdukiewicz, P.T.; Hunter, P.; Nehra, N.; Paradkar, V.; Schlittler, M.; Carroll, J.A.; Spatola, L.; et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 2000, 18, 333–338. [Google Scholar] [CrossRef]
- Michoux, F.; Ahmad, N.; McCarthy, J.; Nixon, P.J. Contained and high-level production of recombinant protein in plant chloroplasts using a temporary immersion bioreactor. Plant Biotechnol. J. 2011, 9, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.D.; Kim, N.S.; Giap do, V.; Jang, S.H.; Oh, S.M.; Jang, S.H.; Kim, T.G.; Jang, Y.S.; Yang, M.S. Production of functional human vascular endothelial growth factor(165) in transgenic rice cell suspension cultures. Enzyme Microb. Technol. 2014, 63, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.H.; Seo, J.E.; Kim, J.; Lee, J.H.; Jang, Y.S.; Yang, M.S. Expression and secretion of the heterodimeric protein interleukin-12 in plant cell suspension culture. Biotechnol. Bioeng. 2003, 81, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Sunil Kumar, G.B.; Ganapathi, T.R.; Revathi, C.J.; Prasad, K.S.; Bapat, V.A. Expression of hepatitis B surface antigen in tobacco cell suspension cultures. Protein Expr. Purif. 2003, 32, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lienard, D.; Sourrouille, C.; Gomord, V.; Faye, L. Pharming and transgenic plants. Biotechnol. Annu. Rev. 2007, 13, 115–147. [Google Scholar] [PubMed]
- Tuse, D.; Tu, T.; McDonald, K.A. Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. Biomed. Res. Int. 2014, 2014, 256135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, N.; Rasche, S.; Kuehn, C.; Anderlei, T.; Klockner, W.; Schuster, F.; Henquet, M.; Bosch, D.; Buchs, J.; Fischer, R.; et al. Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol. Bioeng. 2015, 112, 308–321. [Google Scholar] [CrossRef]
- Moon, K.B.; Park, J.S.; Park, Y.I.; Song, I.J.; Lee, H.J.; Cho, H.S.; Jeon, J.H.; Kim, H.S. Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants 2019, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Nochi, T.; Takagi, H.; Yuki, Y.; Yang, L.; Masumura, T.; Mejima, M.; Nakanishi, U.; Matsumura, A.; Uozumi, A.; Hiroi, T.; et al. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl. Acad. Sci. USA 2007, 104, 10986–10991. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.J.; Kwon, J.Y.; Choi, H.Y.; Kang, S.H.; Jung, H.S.; Kim, D.I. Production and Purification of Recombinant Glucocerebrosidase in Transgenic Rice Cell Suspension Cultures. Appl. Biochem. Biotechnol. 2017, 181, 1401–1415. [Google Scholar] [CrossRef]
- Chen, T.L.; Lin, Y.L.; Lee, Y.L.; Yang, N.S.; Chan, M.T. Expression of bioactive human interferon-gamma in transgenic rice cell suspension cultures. Transgenic Res. 2004, 13, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Lee, T.S.; Kim, J.; Jung, J.H.; Choi, C.W.; Kim, T.G.; Kwon, T.H.; Jang, Y.S.; Yang, M.S. Tumor targeting of humanized fragment antibody secreted from transgenic rice cell suspension culture. Plant Mol. Biol. 2008, 68, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Kim, N.S.; Jung, J.W.; Kim, H.B.; Han, S.C.; Yang, M.S. Spontaneous pepsin C-catalyzed activation of human pepsinogen C in transgenic rice cell suspension culture: Production and characterization of human pepsin C. Enzyme Microb. Technol. 2018, 108, 66–73. [Google Scholar] [CrossRef]
- Yu, S.M.; Kuo, Y.H.; Sheu, G.; Sheu, Y.J.; Liu, L.F. Metabolic derepression of alpha-amylase gene expression in suspension-cultured cells of rice. J. Biol. Chem. 1991, 266, 21131–21137. [Google Scholar] [CrossRef]
- Huang, N.; Chandler, J.; Thomas, B.R.; Koizumi, N.; Rodriguez, R.L. Metabolic regulation of alpha-amylase gene expression in transgenic cell cultures of rice (Oryza sativa L.). Plant Mol. Biol. 1993, 23, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Tzou, W.S.; Lo, W.S.; Kuo, Y.H.; Lee, H.T.; Wu, R. Regulation of alpha-amylase-encoding gene expression in germinating seeds and cultured cells of rice. Gene 1992, 122, 247–253. [Google Scholar] [PubMed]
- Ho, S.; Chao, Y.; Tong, W.; Yu, S. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol. 2001, 125, 877–890. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef] [Green Version]
- McDonald, K.A.; Hong, L.M.; Trombly, D.M.; Xie, Q.; Jackman, A.P. Production of human alpha-1-antitrypsin from transgenic rice cell culture in a membrane bioreactor. Biotechnol. Prog. 2005, 21, 728–734. [Google Scholar] [CrossRef]
- Huang, N.; Sutliff, T.D.; Litts, J.C.; Rodriguez, R.L. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol. Biol. 1990, 14, 655–668. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The Rice Alpha-Amylase, Conserved Regulator of Seed Maturation and Germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.M.; Lee, Y.C.; Fang, S.C.; Chan, M.T.; Hwa, S.F.; Liu, L.F. Sugars act as signal molecules and osmotica to regulate the expression of alpha-amylase genes and metabolic activities in germinating cereal grains. Plant Mol. Biol. 1996, 30, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.A.; Lim, E.K.; Yu, S.M. Sugar response sequence in the promoter of a rice alpha-amylase gene serves as a transcriptional enhancer. J. Biol. Chem. 1998, 273, 10120–10131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.S.; Kim, T.G.; Jang, Y.S.; Shin, Y.J.; Kwon, T.H.; Yang, M.S. Amylase gene silencing by RNA interference improves recombinant hGM-CSF production in rice suspension culture. Plant Mol. Biol. 2008, 68, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.A.; Ho, T.H.; Ho, S.L.; Yu, S.M. Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of alpha-amylase gene expression. Plant Cell 2002, 14, 1963–1980. [Google Scholar] [CrossRef] [Green Version]
- Sinaga, D.S.; Ho, S.L.; Lu, C.A.; Yu, S.M.; Huang, L.F. Knockdown expression of a MYB-related transcription factor gene, OsMYBS2, enhances production of recombinant proteins in rice suspension cells. Plant Methods 2021, 17, 99. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Lu, C.A.; Huang, L.F. Applications of CRISPR/Cas9 in a rice protein expression system via an intron-targeted insertion approach. Plant Sci. 2022, 315, 111132. [Google Scholar] [CrossRef]
- Sojikul, P.; Buehner, N.; Mason, H.S. A plant signal peptide-hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2209–2214. [Google Scholar] [CrossRef] [Green Version]
- Firek, S.; Draper, J.; Owen, M.R.; Gandecha, A.; Cockburn, B.; Whitelam, G.C. Secretion of a functional single-chain Fv protein in transgenic tobacco plants and cell suspension cultures. Plant Mol. Biol. 1993, 23, 861–870. [Google Scholar] [CrossRef]
- Colgan, R.; Atkinson, C.J.; Paul, M.; Hassan, S.; Drake, P.M.; Sexton, A.L.; Santa-Cruz, S.; James, D.; Hamp, K.; Gutteridge, C.; et al. Optimisation of contained Nicotiana tabacum cultivation for the production of recombinant protein pharmaceuticals. Transgenic Res. 2010, 19, 241–256. [Google Scholar] [CrossRef]
- James, E.A.; Wang, C.; Wang, Z.; Reeves, R.; Shin, J.H.; Magnuson, N.S.; Lee, J.M. Production and characterization of biologically active human GM-CSF secreted by genetically modified plant cells. Protein Expr. Purif. 2000, 19, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hakkinen, S.T.; Raven, N.; Henquet, M.; Laukkanen, M.L.; Anderlei, T.; Pitkanen, J.P.; Twyman, R.M.; Bosch, D.; Oksman-Caldentey, K.M.; Schillberg, S.; et al. Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol. Bioeng. 2014, 111, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.K.; Huang, L.F.; Ho, S.L.; Liao, C.Y.; Liu, H.Y.; Lai, Y.H.; Yu, S.M.; Lu, C.A. Production of mouse granulocyte-macrophage colony-stimulating factor by gateway technology and transgenic rice cell culture. Biotechnol. Bioeng. 2012, 109, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2017, 69, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.F.; Tan, C.C.; Yeh, J.F.; Liu, H.Y.; Liu, Y.K.; Ho, S.L.; Lu, C.A. Efficient Secretion of Recombinant Proteins from Rice Suspension-Cultured Cells Modulated by the Choice of Signal Peptide. PLoS ONE 2015, 10, e0140812. [Google Scholar] [CrossRef]
- Huang, L.F.; Sinaga, D.S.; Tan, C.C.; Hsieh, S.M.; Huang, C.H. Expression of Recombinant Human Octamer-Binding Transcription Factor 4 in Rice Suspension Cells. Int. J. Mol. Sci. 2021, 22, 1409. [Google Scholar] [CrossRef]
- De Muynck, B.; Navarre, C.; Nizet, Y.; Stadlmann, J.; Boutry, M. Different subcellular localization and glycosylation for a functional antibody expressed in Nicotiana tabacum plants and suspension cells. Transgenic Res. 2009, 18, 467–482. [Google Scholar] [CrossRef]
- Wang, H.J.; Wan, A.R.; Hsu, C.M.; Lee, K.W.; Yu, S.M.; Jauh, G.Y. Transcriptomic adaptations in rice suspension cells under sucrose starvation. Plant Mol. Bio.l 2007, 63, 441–463. [Google Scholar] [CrossRef]
- Kim, N.S.; Kim, T.G.; Kim, O.H.; Ko, E.M.; Jang, Y.S.; Jung, E.S.; Kwon, T.H.; Yang, M.S. Improvement of recombinant hGM-CSF production by suppression of cysteine proteinase gene expression using RNA interference in a transgenic rice culture. Plant Mol. Biol. 2008, 68, 263–275. [Google Scholar] [CrossRef]
- Dunse, K.M.; Stevens, J.A.; Lay, F.T.; Gaspar, Y.M.; Heath, R.L.; Anderson, M.A. Coexpression of potato type I and II proteinase inhibitors gives cotton plants protection against insect damage in the field. Proc. Natl. Acad. Sci. USA 2010, 107, 15011–15015. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.G.; Lee, H.J.; Jang, Y.S.; Shin, Y.J.; Kwon, T.H.; Yang, M.S. Co-expression of proteinase inhibitor enhances recombinant human granulocyte-macrophage colony stimulating factor production in transgenic rice cell suspension culture. Protein Expr. Purif. 2008, 61, 117–121. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Finck, B.N. Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem. J. 2015, 466, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terashima, M.; Ejiri, Y.; Hashikawa, N.; Yoshida, H. Utilization of an alternative carbon source for efficient production of human alpha(1)-antitrypsin by genetically engineered rice cell culture. Biotechnol. Prog. 2001, 17, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Yu, H.Y.; Chung, N.D.; Kwon, T.H.; Yang, M.S. High-level production of recombinant trypsin in transgenic rice cell culture through utilization of an alternative carbon source and recycling system. Enzyme Microb. Technol. 2014, 63, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Terashima, M.; Ejiri, Y.; Hashikawa, N.; Yoshida, H. Effects of sugar concentration on recombinant human alpha(1)-antitrypsin production by genetically engineered rice cell. Biochem. Eng. J. 2000, 6, 201–205. [Google Scholar] [CrossRef]
- Park, C.-I.; Lee, S.-J.; Kang, S.-H.; Jung, H.-S.; Kim, D.-I.; Lim, S.-M. Fed-batch cultivation of transgenic rice cells for the production of hCTLA4Ig using concentrated amino acids. Process Biochem. 2010, 45, 67–74. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, J.H.F.; Quinhones, C.G.S.; Schertl, P.; Brito, D.S.; Eubel, H.; Hildebrandt, T.; Nunes-Nesi, A.; Braun, H.P.; Araujo, W.L. Differential impact of amino acids on OXPHOS system activity following carbohydrate starvation in Arabidopsis cell suspensions. Physiol. Plant 2017, 161, 451–467. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Liu, Y.K.; Lu, C.W.; Chang, J.Y.; Lu, C.F.; Tan, C.C.; Huang, L.F. Optimization of the culture medium for recombinant protein production under the control of the αAmy3 promoter in a rice suspension-cultured cell expression system. Plant Cell Tissue Organ Cult. 2018, 132, 383–391. [Google Scholar] [CrossRef]
- Liu, Y.K.; Li, Y.T.; Lu, C.F.; Huang, L.F. Enhancement of recombinant human serum albumin in transgenic rice cell culture system by cultivation strategy. New Biotechnol. 2015, 32, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Macharoen, K.; Du, M.; Jung, S.; McDonald, K.A.; Nandi, S. Production of recombinant butyrylcholinesterase from transgenic rice cell suspension cultures in a pilot-scale bioreactor. Biotechnol. Bioeng. 2021, 118, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.F.; Liu, Y.K.; Lu, C.A.; Hsieh, S.L.; Yu, S.M. Production of human serum albumin by sugar starvation induced promoter and rice cell culture. Transgenic Res. 2005, 14, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Gemeda, H.B.; McNulty, M.J.; McDonald, K.A.; Nandi, S.; Knipe, J.M. Immobilization of transgenic plant cells towards bioprinting for production of a recombinant biodefense agent. Biotechnol. J. 2021, 16, e2100133. [Google Scholar] [CrossRef]
- Wang, X.; Karki, U.; Abeygunaratne, H.; UnnoldCofre, C.; Xu, J. Plant cell-secreted stem cell factor stimulates expansion and differentiation of hematopoietic stem cells. Process. Biochem. 2021, 100, 39–48. [Google Scholar] [CrossRef]
- Zhou, Z.; Dang, Y.; Zhou, M.; Li, L.; Yu, C.H.; Fu, J.; Chen, S.; Liu, Y. Codon usage is an important determinant of gene expression levels largely through its effects on transcription. Proc. Natl. Acad. Sci. USA 2016, 113, E6117–E6125. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Dang, Y.; Counter, C.; Liu, Y. Codon usage regulates human KRAS expression at both transcriptional and translational levels. J. Biol. Chem. 2018, 293, 17929–17940. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Medina, S.G.; Kushawah, G.; DeVore, M.L.; Castellano, L.A.; Hand, J.M.; Wright, M.; Bazzini, A.A. Translation affects mRNA stability in a codon-dependent manner in human cells. Elife 2019, 8, e45396. [Google Scholar] [CrossRef]
- Yang, Q.; Lyu, X.; Zhao, F.; Liu, Y. Effects of codon usage on gene expression are promoter context dependent. Nucleic Acids Res. 2021, 49, 818–831. [Google Scholar] [CrossRef]
- Kim, T.G.; Baek, M.Y.; Lee, E.K.; Kwon, T.H.; Yang, M.S. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008, 27, 885–891. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.-H.; Huang, L.-F. Current Strategies to Improve Yield of Recombinant Protein Production in Rice Suspension Cells. Processes 2022, 10, 1120. https://doi.org/10.3390/pr10061120
Chi Y-H, Huang L-F. Current Strategies to Improve Yield of Recombinant Protein Production in Rice Suspension Cells. Processes. 2022; 10(6):1120. https://doi.org/10.3390/pr10061120
Chicago/Turabian StyleChi, Yu-Hsiang, and Li-Fen Huang. 2022. "Current Strategies to Improve Yield of Recombinant Protein Production in Rice Suspension Cells" Processes 10, no. 6: 1120. https://doi.org/10.3390/pr10061120
APA StyleChi, Y.-H., & Huang, L.-F. (2022). Current Strategies to Improve Yield of Recombinant Protein Production in Rice Suspension Cells. Processes, 10(6), 1120. https://doi.org/10.3390/pr10061120






