Abstract
Diabetes is coupled with hyperglycemia, a state in which elevated glucose levels trigger oxidative stress (OS) in various body functions. One of the organs most afflicted by diabetes is the kidney. Despite this, specific treatments to mitigate the harmful effects of hyperglycemia-induced OS in the kidney have not been extensively explored. This study evaluates the anti-hyperglycemic efficacy of magnesium-enhanced alkaline-reduced water (MARW) in human kidney-2 (HK-2) cells. OS, mitogen-activated protein kinase (MAPK) signaling and fibrosis markers were assessed in high glucose (HG)-induced HK-2 cells, followed by treatment with experimental water for 24 h. Surprisingly, MARW rescued the vitality of HG-induced HK-2 cells, in contrast to that seen with other experimental waters. Additionally, MARW maintained reactive oxygen species, nitric oxide, catalase, glutathione peroxidase, hepatocyte growth factor and glucose uptake in HG-induced HK-2 cells but not in tap water and mineral water. Similarly, MARW downregulated the expression of MAPK and fibrosis-linked signaling proteins such as p-p38, phospho-c-Jun N-terminal kinase, α-smooth muscle actin, matrix metalloproteinase-3 and cleaved caspase 3 in HG-induced HK-2 cells. In conclusion, MARW protects HK-2 cells from the deleterious effects of HG by stabilizing antioxidant defenses and by signaling cascades related to metabolism, apoptosis and fibrosis.
1. Introduction
Diabetes mellitus (DM) is a known metabolic disease which affects millions of people globally and is characterized by chronic hyperglycemia conditions, and it is usually linked with several microvascular and macrovascular complications [1]. Diabetic nephropathy (DN) is known as a progressive microvascular complication of the kidney in DM, whose specific mechanism still remains idiopathic. Several studies have reported that oxidative stress (OS) plays a vital function in the development of DN [2,3,4]. In DN, persistent hyperglycemia results in the formation of advanced glycation end products in the kidney, specifically in proximal tubular epithelial cells and the glomeruli region, resulting in the increased generation of reactive oxygen species (ROS) and ultimately causing OS [3]. Excessive OS due to hyperglycemia may offset the endogenous antioxidant defense system in DM conditions, followed by the oxidation of several biomolecules, such as DNA, proteins, carbohydrates and lipids [5]. Additionally, ROS act as secondary messengers that activate various signaling cascades, ultimately damaging the epithelial cells of the kidney and contributing to the progression of DN in diabetic patients. Furthermore, one of the studies has demonstrated that a signaling pathway such as the mitogen-activated protein kinase (MAPK) plays an important function in DN pathogenesis, as this pathway plays a key role in inflammation, OS and apoptosis [6]. In addition, one research study has demonstrated that renal proximal tubular epithelial cells [human kidney-2 (HK-2)] incubated in high glucose (HG) conditions leads to the activation of p38 MAPK and c-Jun N-terminal kinase (JNK) pathways, causing increased cell apoptosis due to OS [6,7]. In addition, the phosphorylated p38 MAPK signaling pathway increases the levels of inflammatory cytokines, promoting glomerulonephritis and DN development [8]. Furthermore, studies have shown that OS promotes the action of matrix metalloproteinases (MMPs) in DN. Accumulating evidence has shown that MMPs are the main factors of extracellular matrix (ECM) degradation and that they activate multiple growth factors and cytokines linked with hypertrophy of kidneys, proliferation of tubular cells and fibrosis [9,10]. In addition, studies have shown that hepatocyte growth factor (HGF) and α-smooth muscle actin (α-SMA) play a central role in ECM accumulation in diabetic kidneys [11,12]. Therefore, protecting kidney cells by suppressing OS in DM may be a possible therapeutic approach for DN treatment.
In recent years, some evidence suggests that supplementation with antioxidant-rich agents may have significant benefits in diabetic kidney disease [13,14]. The benefits of alternative products such as mineral-rich supplements and alkaline-reduced water (ARW) in OS-induced diseases is a topic of active research. It is well known that magnesium is a vital mineral that is needed as a co-factor for more than 600 enzymatic reactions in our body [15]. Additionally, studies have shown that magnesium is required for the regulation of several biochemical functions in numerous metabolic pathways in cells, including calcium ion regulation, the transporting of potassium ions across the membranes of cells, the production of adenosine triphosphate (ATP) and the stabilization of DNA and RNA structures [16]. Studies have reported that magnesium is a vital element in DM due to the fact that patients with DM have low levels of magnesium, which is considered an indicator of the disease [17,18]. Thus, supplementation with magnesium-enhanced supplements may be a beneficial treatment modality to overcome DM and DM-associated complications, such as DN. Additionally, evidence has shown that antioxidant supplementation is an effective therapeutic strategy for reducing diabetic complications by suppressing the excessive production of free radicals, such as ROS or reactive nitrogen species, or by increasing the capabilities of the antioxidant defense system [19,20]. Recently, ARW has been widely studied in numerous metabolic syndromes [21] and OS-related diseases, owing to its strong antioxidative and ROS-scavenging properties [22]. In addition, it exhibits other properties such as alkaline pH, micro-clustered molecules of water, dissolved molecular hydrogen (H2) and an extremely negative oxidation reduction potential (ORP) [23]. Previous studies conducted in vivo have shown that ARW has anti-diabetic effects by reducing blood glucose levels, triglycerides and total cholesterol in the blood [24]. Considering the aforementioned aspects regarding the function of magnesium/minerals and ARW, magnesium-enhanced ARW (MARW) is a potential low-cost and non-caloric medical treatment due to its strong antioxidant activity, which is improved by its high H2 content, high mineral content and low ORP. HK-2 cells are known cells in the proximal tubular epithelial region that are obtained from the normal human kidney, as used in the present study.
In this study, we hypothesized that, due to its properties, MARW can neutralize free radicals and reduce OS, preventing diabetes by mitigating cell damage. This reduction in OS may help cells to maintain glucose homeostasis. Thus, we want to explore the effect of MARW on HG-induced HK-2 cells.
2. Materials and Methods
2.1. Cells and Chemicals
In this study, a human proximal epithelial HK-2 cell line was obtained from the Korean Cell Line Bank (Seoul National University, Cancer Institute, Seoul, Korea). In summary, cells were cultured in a Roswell Park Memorial Institute (RPMI) 1640 medium. Furthermore, in RPMI media, 10% fetal bovine serum (HyClone Laboratories, GE Healthcare Life Sciences, South Logan, UT, USA) and 1% antibiotic-antimycotic by Gibco (Life Technologies Corporation, Grand Island, NY, USA) were added in culture plates and were incubated at 37 °C in a humidified 5% CO2 incubator. In addition, to induce OS in the cells, different concentrations of glucose (Welgene Inc., Daegu, Korea) were used in this study.
2.2. Experimental Design
OS was induced in HK-2 cells with 71.68 mM glucose treatment for 24 h, and they were divided into five different groups: normal control (NC), induction with HG only (HG only), HG induction and tap water (TW) treatment (HG + TW), HG induction and mineral water (MW) treatment (HG + MW) and HG induction and MARW treatment (HG + MARW). The HK-2 cells were separated, numbered, and seeded for 24 h until the confluence of cells in the culture medium reached roughly 80% confluence. The cells were washed and incubated with HG (71.68 mM) and were treated with 10% TW, MW (Samdasoo, Kwang Dong Pharmaceutical Co., Ltd., Seoul, Korea) and MARW (Mineral Maker Mini, AMMIAQ250, Aquamine Inc., Gyeonggi-do, Korea) after 24 h. After the 24 h treatment, the cells were washed thrice with 1X-PBS and were utilized for further experiments. To obtain the supernatant, furthermore, the cells were washed thrice with 1X-PBS before lysis, and the supernatant was collected. One part of the resulting supernatant was preserved at −80 °C for Western blotting, whereas the other part was used for enzymatic testing.
2.3. Characteristics of MARW
TW and MW were used as controls, and MARW was created using a water bottle containing the processed magnesium ore balls (diameter 3 mm) after reacting with MW for 30 min. The following characteristics of TW, MW and MARW were measured: pH (HM-31P, TOA DKK, Tokyo, Japan), ORP (RM-30P, TOA DKK, Tokyo, Japan), total dissolved solids (TDS) (BOMEX, Beijing, China), H2 content (MARK-509, Hydrogen Meter, Nizhny Novgorod, Russia) and Magnesium content (Korea Conformity Laboratories, Seoul, Korea) (Table 1).

Table 1.
Properties and magnesium content of TW, MW and MARW.
2.4. Measurement of Cell Viability
In this study, the Quanti-Max™ cell counting kit-8 (CCK-8) assay (Biomax, Seoul, Korea) was used according to the guidelines provided by the manufacturer to investigate cell viability and proliferation. In summary, HK-2 cells (2 × 103 cells/well) were seeded in 96-well plates in RPMI 1640 media and were incubated at 37 °C in a humidified 5% CO2 incubator for 24 h. After treatment with HG, TW, MW and MARW for 24 h, the cells were washed 3 times and were resuspended in RPMI 1640 media. After that, 10 µL of CCK-8 reagent was added to each well, and then the plate was incubated at 37 °C for 2 h. Furthermore, the microplate reader SpectraMax® ABS Plus (Molecular Devices, San Jose, CA, USA) was used at 380 nm to measure the optical density (OD).
2.5. In-Cell Enzyme-Linked Immunosorbent Assay (ELISA)
An in-cell ELISA protocol was used to detect cleaved caspase 3 in HK-2 proximal epithelial cells using specific antibodies in a 96-well plate. In brief, the cells were fixed in a microplate containing 8% paraformaldehyde. After incubation, the cells were permeabilized using a solution and were blocked. The cells were then exposed to specific primary antibodies and were stored overnight at 4 °C. On the next day, after washing them with washing buffer 3 times, the cells were incubated with secondary antibodies for 2 h. Finally, the proteins were developed in HRP-labelled microplates and were read with a spectrophotometer at 450 nm.
2.6. Analysis of OS
For OS measurement in HK-2 proximal epithelial cells, a cell assay kit for ROS detection (Abcam, Cambridge, MA, USA) was used according to the manufacturer’s guidelines. In summary, 10 µL samples and 100 µL of 10 µM 2′-7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA) were added to wells in 96-well plates and were incubated in a humidified 5% CO2 incubator at 37 °C for 30 min. After that, a DTX 880 multimode microplate reader (Beckman Coulter Inc., Brea, CA, USA) was used to measure ROS absorbance with 488 nm excitation/525 nm emissions.
In addition, the production of nitric oxide was evaluated by quantifying the accumulation of nitrite in a medium treated with the Griess reagent (Promega Corp., Madison, WI, USA) in HK-2 cells following manufacturer’s guidelines. To measure the nitrate levels, 50 µL samples were placed in 96-well plates and were incubated with 50 µL sulfanilamide solution at room temperature for approximately 10 min. After that, 50 µL of N-(1-naphthyl)-ethylenediamine dihydrochloride solution was added and incubated at room temperature for an additional 10 min. Furthermore, the nitrite content was measured at 520 nm using a microplate spectrophotometer reader, SpectraMax® ABS Plus (Molecular Devices, San Jose, CA, USA).
2.7. Measurement of Antioxidant Enzyme Activities
Antioxidant enzyme activities were measured in proximal epithelial HK-2 cell lysates following the protocol of the manufacturer. In summary, cells were seeded in culture plates with media (1 × 106 cells/well), and cell lysates were collected in an epitube using an assay buffer. In addition, cell lysates were centrifuged at 13,000× g rpm at 4 °C for 15 min. Then, a microplate spectrophotometer reader (SpectraMax® ABS Plus, Molecular Devices, San Jose, CA, USA) was used to measure the OD of glutathione peroxidase (GPx) (Biovision Inc., Milpitas, CA, USA) and catalase (CAT) (Biomax Co., Ltd., Seoul, Korea) at 340 nm and 560 nm, respectively.
2.8. Assessment of Human HGF Level
Both the treated and untreated cells were harvested after 24 h, and the protein concentration was measured. After normalization, an ELISA assay for HGF content (#EK711042; AFG Bioscience, Northbrook, IL, USA) was performed according to the manufacturer’s instructions. In summary, 50 µL of the sample and standard were added to the 96-well ELISA kit and were incubated. Furthermore, 50 µL of HRP conjugate reagent along with chromogen reagents A and B were added during the experiment. Finally, 50 µL of stop solution was added, and the absorbance was measured at 420 nm using the SpectraMax® ABS Plus (Molecular Devices, San Jose, CA, USA).
2.9. Assessment of Glucose Uptake Assay
In this study, we performed a glucose uptake assay (Abcam, Seoul, Korea) according to the guidelines of the manufacturer. In summary, HK-2 cells were serum-starved and washed with a Krebs–Ringer Phosphate HEPES buffer. Furthermore, cells were extracted using an extraction buffer and were heated at 85 °C for 40 min. Subsequently, 10 µL of neutralization buffer was added along with the sample and an assay buffer. This step was further processed by mixing the reaction mix (A), extraction buffer, neutralization buffer and reaction mix (B). Finally, the data were obtained and calculated after measuring the plate at 412 nm (Molecular Devices, San Jose, CA, USA).
2.10. Western Blot Analysis
Total proteins in proximal epithelial HK-cells were extracted using a radio-immunoprecipitation assay (1X-RIPA) buffer using standard protocol, and then the concentrations of the sample protein were quantified by using a bicinchoninic acid (BCA) protein assay kit. After protein extraction from the sample and normalization of sample, an equal amount of protein extract from cell lysates was loaded using 12% SDS-PAGE (80 V, 30 min and then 120 V, 60 min). Then, gels were electro transferred to the nitrocellulose membranes for 2 h. After the electro transfer of the membranes, furthermore, the membranes were blocked with protein-free blocking buffer (Takara Bio Inc., Kusatsu, Japan) for 2 h at room temperature and were incubated with primary antibodies against β-actin, p-p38, p-JNK, α-SMA and MMP3 (1:1000) (Cell Signaling Technology, Danvers, MA, USA) overnight for 12–16 h. On the next day, after washing with Tris-buffered saline/Tween 20 (1X TBST), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5000) (Cell Signaling Technology, Danvers, MA, USA) for 2 h at room temperature. The membrane was developed using enhanced chemiluminescence (ECL) (ECL Pierce Biotechnology) in a UVP Biospectrum 600 Imaging System (UVP, LLC, Upland, CA, USA). Furthermore, protein activity was determined using the Image J software (Bio-rad, Hercules, CA, USA).
2.11. Cytokines Analysis
The supernatants from the proximal epithelial HK-2 cell lysate sample were analyzed using a Bio-plex Pro Human Cytokine 17-plex Assay kit (NovoBiotechnology Co. Ltd., Beijing, China) to investigate different cytokines such as [interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor (TNF)-α] according to the instructions of manufacturer. In summary, a standard with various concentrations and a sample (supernatant) were prepared. The bead solution was then diluted and plated in a 96-well plate. After washing, 50 µL of the sample and standard were added to each desired well and were incubated by covering with foil. Next, diluted primary and secondary antibodies were added to each well in a 96-well plate and were incubated for a specific time period. Subsequently, streptavidin solution was added to a 96-well plate and was incubated for a recommended time period. Finally, 125 µL of the assay buffer was plated in each well, and the plates were used for reading and data collection after proper shaking.
2.12. Data Management and Statistical Analysis
All the data obtained in this study are shown using the mean ± standard error of the mean (SEM). We used the Graph Pad Prism (version 5.0; Graph-Pad, San Diego, CA, USA) software to conduct the analysis of the data. In addition, we used a one-way analysis of variance (ANOVA) to examine and assess the mean values of the groups, followed by a multiple comparison test (Tukey’s post-hoc test). Statistical significance was defined at p < 0.05.
3. Results
3.1. MARW Alleviated Cell Viability and Cleaved Caspase 3 Activity in HG-Induced HK-2 Cells
Cell viability was evaluated at different concentrations of glucose and IC50 (71.68 mM) in HK-2 cells (Figure 1A,B). Moreover, to explore the effect of MARW on HG-induced HK-2 cells, we induced the cells with HG and treated them with TW, MW and MARW for 24 h. Our result shows that the cell viability of the HG only group was significantly decreased (p < 0.001) compared to that of the NC group. However, upon 10% MARW treatment, the viability of the HG + MARW group was significantly increased (p < 0.001) compared to the HG only group (Figure 1C). Similarly, cleaved caspase 3, which is involved in programmed cell death or apoptosis [25], was found to be increased after HG induction (p < 0.01). However, this increase level was attenuated by treatment with 10% MARW (p < 0.05) (Figure 1D).
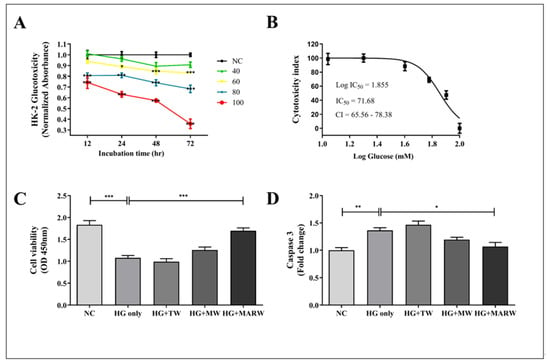
Figure 1.
Effects of MARW on cell viability and glucose resorption in HG-induced HK-2 cells: (A,B) Cell viability of HK-2 cells treated with different concentrations of D-glucose (NC, 20, 40, 60, 80, 100 mM); (C) Cell viability of HK-2 cells treated with 71.68 mM glucose, treated with 10% TW, MW and MARW after 24 h; (D) Cleaved caspase 3 was performed by the ELISA. * p < 0.05, ** p < 0.01, and *** p < 0.001 indicate significant differences when tested with Tukey’s ANOVA test. Values represent the Mean ± SEM (n = 3). Abbreviations: NC, normal control; HG, high glucose; TW, tap water; MW, mineral water; MARW, magnesium-enhanced alkaline-reduced water.
3.2. MARW Suppresses the Increase in OS in HG-Induced HK-2 Cells
To assess the effect of MARW on hyperglycemic conditions in HK-2 cells, we induced the cells with HG and treated them with TW, MW or MARW for 24 h. As expected, the HG only treatment significantly increased the ROS and NO levels in HK-2 cells. We examined the effects of experimental waters against these redox imbalances. Interestingly, the MARW group showed significant changes in ROS and NO levels compared to the HG only group. Compared with the NC group, ROS and NO levels in the HG only group increased significantly (both p < 0.001). The increased levels of ROS and NO were significantly reduced after MARW treatment (both p < 0.001) (Figure 2A,B). Similarly, HG stimulation resulted in a significant decrease in GPx activity and a considerable increase in CAT activity (both p < 0.001). However, after treatment with MARW, GPx activity of the HG + MARW group was dramatically boosted (p < 0.001) compared to that of the HG only group (Figure 2C). In contrast, CAT activity was significantly reduced in the HG + MARW group (p < 0.001) compared to the HG only group (Figure 2D).
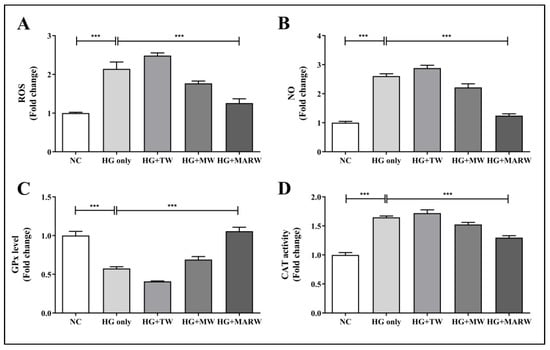
Figure 2.
Effects of MARW on ROS, NO, GPx and CAT in HG-induced HK-2 Cells. HK-2 cells were induced with glucose 71.68 mM for 24 h and then treated with TW, MW and MARW, and the (A) ROS, (B) NO, (C) GPx, and (D) CAT levels were analyzed. *** p < 0.001 indicates significant differences when tested with Tukey’s ANOVA test. Values represent the mean ± SEM (n = 3). Abbreviations: NC, normal control; HG, high glucose; TW, tap water; MW, mineral water; MARW, magnesium-enhanced alkaline-reduced water.
3.3. Effects of MARW on HGF and Glucose Uptake Level in HK-2 Cells
Next, we assessed the HGF and glucose uptake levels to analyze the effect of the MARW treatment in HG-induced HK-2 cells. We found that the HG only group in HGF decreased significantly (p < 0.01 vs. NC group); however, the HG + MARW group showed significant increments (p < 0.01) compared to the HG only group (Figure 3A). In the glucose uptake results, the HG only group showed significant increases (p < 0.05 vs. NC group); however, the HG + MARW group showed significant decreases (p < 0.05) compared to the HG only group. We did not observe any significant differences in the HG + TW and HG + MW groups (Figure 3B).
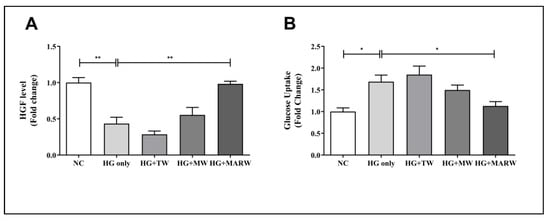
Figure 3.
Effects of HG on TW, MW and MARW in HGF and glucose uptake levels in HK-2 cells with different concentrations. HK-2 cells were induced with glucose (71.68 mM) for 24 h. After treatment, the (A) HGF and (B) glucose uptake levels were analyzed. * p < 0.05, and ** p < 0.01 represent significant differences based on Tukey’s ANOVA test. Values represent the mean ± SEM. Abbreviations: NC, normal control; HG, high glucose; TW, tap water; MW, mineral water; MARW, magnesium-enhanced alkaline-reduced water; HGF, hepatocyte growth factor.
3.4. Effects of MARW on MAPK and Fibrosis Pathways in HG-Induced HK-2 Cells
After the treatment with HG, the expressions of p-JNK (p < 0.01), p-p38 (p < 0.05), α-SMA (p < 0.05) and MMP3 (p < 0.05) were increased significantly compared to those of the NC group. However, after the MARW treatment, the expressions of p-JNK, p-p38, α-SMA and MMP3 were significantly reduced compared to the HG only groups (p < 0.05, respectively), and there was no significance in the HG + TW and HG + MW groups (Figure 4).
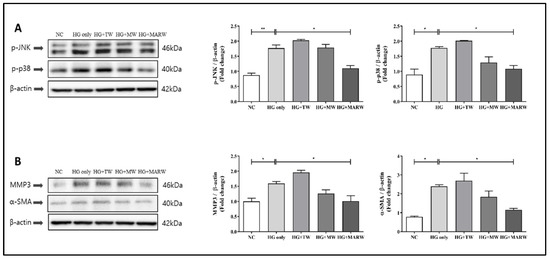
Figure 4.
Effects of MARW on MAPK and the pathway that leads to fibrosis in HG-induced HK-2 cells. HK-2 cells were induced with glucose (71.68 mM) for 24 h and were treated with 10% TW, MW and MARW. (A) MAPK (p-JNK and p-p38) pathway along with (B) fibrosis-linked (α-SMA and MMP3) proteins were analyzed. * p < 0.05, and ** p < 0.01 represent significant differences based on Tukey’s ANOVA test. Values represent the mean ± SEM. Abbreviations: NC, normal control; TW, tap water; MW, mineral water; HG, high glucose; MARW, magnesium-enhanced alkaline-reduced water; MAPK, mitogen-activated protein kinase; α-SMA, alpha-smooth muscle actin; MMP3, matrix metalloproteinase 3; p-JNK, p-jun N-terminal kinase.
3.5. Effects of MARW on Cytokine Levels in HG-Induced HK-2 Cells
Cytokine levels were measured to evaluate the effects of MARW on inflammation in HG-induced HK-2 cells. Here, the HG treatment significantly increased IL-1β (p < 0.01), IL-6 (p < 0.01) and TNF-α (p < 0.01) and significantly decreased IL-10 (p < 0.01) compared to the normal cells. However, the HG + MARW group showed significant decreases in IL-1β (p < 0.01), IL-6 (p < 0.05), and TNF-α (p < 0.001), compared to the HG group (Figure 5A,B,D). In addition, IL-10 levels were decreased in the HG only group (p < 0.01 vs. NC group) but were significantly increased in the HG + MARW group (p < 0.05 vs. HG only group) (Figure 5C).
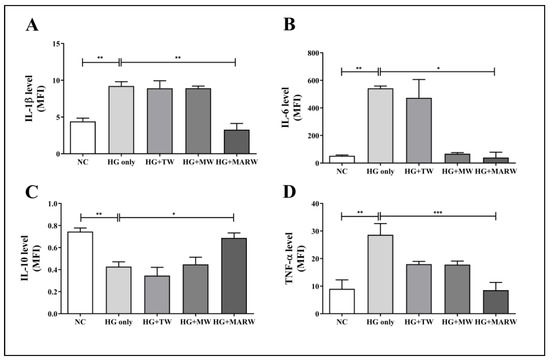
Figure 5.
Effects of MARW on various cytokines associated with fibrosis-related inflammation in HG-induced HK-2 cells. HK-2 cells were induced with glucose (71.68 mM) for 24 h and were then treated with experimental waters and cytokines, including (A) IL-1β, (B) IL-6, (C) IL-10 and (D) TNF-α, were analyzed. * p < 0.05, ** p < 0.01, and *** p < 0.001 represent significant differences based on Tukey’s ANOVA test. Values represent the mean ± SEM. Abbreviations: NC, normal control; TW, tap water; MW, mineral water; HG, high glucose; MARW, magnesium-enhanced alkaline-reduced water; IL, interleukin; TNF, tumor necrosis factor.
4. Discussion
This study establishes the cumulative action of alkaline water synthesized by magnesium beads on renal tubules against hyperglycemia. This is confirmed in several ways. Firstly, HK-2 cells, a proximal tubular epithelial cell (PTEC) line, were used as a model to investigate hyperglycemia-induced nephrotoxicity. This cell line is often used to assess cellular responses during nephrotoxicity in vitro [26,27]. Remarkably, we revealed that MARW stabilized the proliferation of the HK-2 cells grown in excessive HG conditions. HG conditions are a driving force of cellular senescence in HK-2 cells via upregulating the expression of the senescence-associated gene P21, which can be averted by the diabetes drug metformin [28]. One study has found that diabetic conditions enhance apoptosis in PTEC, as evidenced by an increase in cleaved caspase activity [29]. In this experiment, we explored whether the reduction in the viability of HK-2 cells was related to aging or cell death. Notably, our findings indicate that the decrease in viability of HG-induced HK-2 cells was caused by apoptosis, as indicated by substantial caspase 3 activation. Surprisingly, the MARW treatment prevented this higher rate of apoptosis (Figure 1). Moreover, this is the first study showing the protective effects of MARW in PTEC cells against glucotoxicity-induced apoptosis.
Next, we found that MARW enhanced the redox balance in PTEC under conditions of elevated glucose concentration by analyzing the endogenous antioxidant system. It is known that the development of diabetic complications is influenced by OS [30]. Additionally, HG upregulates a generation of ROS and NO, eventually giving rise to OS [31]. Surprisingly, we observed that MARW reduced ROS and NO levels in HK-2 cells, which increased after HG stimulation. In addition, MARW enhanced antioxidant activities against HG-induced OS, which supports our ROS and NO results. Antioxidant enzymes, such as GPx and CAT, can help cells to recover from the effects of OS [32,33,34]. These antioxidants are well known for their involvement in lowering OS effectors such as ROS and NO levels in HK-2 cell lines [32,35]. After HG-induction, MARW mediated improvements in GPx and CAT activities, implying that it has antioxidant potential (Figure 2). Thus, in this study, MARW was found to be efficient in downregulating the expression of these OS markers by enhancing the activity of strong antioxidants [32,36].
Additionally, we found that MARW ameliorated DN by sustaining HGF protein levels and glucose absorption. HGF is a neurotrophic protein that protects kidney function during diabetes by inhibiting HG-induced PTEC apoptosis [37]. Furthermore, in individuals with renal impairment, exogenous HGF therapy promotes kidney regeneration. Several studies have demonstrated that HGF has a protective function in the production, transformation and development of a variety of pathological conditions, both in vivo and in vitro [38,39,40,41]. We questioned whether kidney-derived HGF elicits pleiotropic effects on HK-2 cells to promote cell survival under elevated glucose conditions. Surprisingly, high glucose concentrations significantly diminished HGF in HK-2 cells but were recovered by MARW (Figure 3A). PTEC has been known to facilitate glucose resorption, which is mediated by HGF. We hypothesized that MARW maintained the levels of HGF in HK-2 cells to facilitate glucose trafficking. As expected, the glucose resorption in HK-2 cells remained steady albeit at elevated glucose levels (Figure 3B). However, we do not have conclusive evidence showing that stable glucose trafficking is exclusively a result of steady HGF levels in MARW-treated cells. Several studies also show that ARW can potentially affect cellular metabolism. The physiochemical characteristics of ARW enable it to easily penetrate the cell membrane, resulting in increased metabolism due to its antioxidative properties [42]. Furthermore, endogenous HGF expression in renal tubular cells has been reported in a few studies [11]. Correspondingly, this study strengthens the physiological role of HGF in PTEC, which warrants deeper investigation. However, in this study, we show that MARW can be administered in conjunction with HGF to help in restoring renal function.
Moreover, to thoroughly clarify the therapeutic mechanism of MARW against HG-induced OS, our current study focuses on stress signaling (p-JNK, p-p38) and fibrosis (α-SMA, MMP3)-related signaling pathways. p-p38 and p-JNK are downstream proteins of the MAPK pathway and play important roles in early OS and cellular responses [43,44]. Previously published studies have confirmed that, with blocking, the MAPK signaling pathway can aid in the survival of cells in a redox imbalance state [34,45,46]. However, it has been explained that ARW increases endogenous antioxidants that help to protect cells from OS [32]. Similarly, α-SMA and MMP3 proteins play vital functions in the development and progression of renal fibrosis [47,48,49]. Thus, we investigated the role of MARW in stress signaling and fibrosis-linked pathways. Interestingly, the Western blotting results reveal that MARW inhibited the HG-induced production of fibrosis-linked proteins, such as: MMP3, α-SMA, p-JNK and p-p38. This reduction in stress signaling and fibrotic protein markers may be due to increases in antioxidant activity and reductions in free radicals by MARW (Figure 4).
Furthermore, we also attempted to understand the effect of MARW on HG-induced inflammatory responses. The pathogenesis of DN caused by inflammation and fibrosis is complex, and various cytokines are involved in this process [50,51,52,53]. Previous studies reported that HG administration can result in the modulation of certain pro-inflammatory cytokines, including TGF-β, IL-6, IL-1β and TNF-α, resulting in inflammation in HK-2 cells [54,55]. Similarly, alkaline-electrolyzed water was reported to be used to lower certain pro-inflammatory cytokines, which are elevated in inflammation-associated OS [56]. Moreover, IL-10 is recognized as an anti-inflammatory cytokine, which also helps reductions in pro-inflammatory factors, such as TNF-α, together with other related signals [57]. Taking into account the results of these previous studies, we performed several pro-inflammatory and anti-inflammatory cytokine analyses and observed changes in some inflammatory cytokines such as: IL-6, IL-1β, IL-10 and TNF-α. Among them, IL-1β, IL-6 and TNF-α were found to be induced by HG, which was in turn alleviated after treatment with MARW. However, we observed a reduction in IL-10 levels after HG stimulation, which was attenuated by MARW treatment. Here, among the cytokines, we observed drastic changes in IL-6 levels, which are early initiators of the inflammatory response and are highly activated in acute inflammatory reactions [58]. This result collectively implies that negative effects generated by HG on pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α and anti-inflammatory cytokines such as IL-10, can be alleviated by treatment with MARW. Thus, in this study, we found that MARW was effective in reducing HG-induced inflammation in HK-2 cells (Figure 5).
In conclusion, magnesium and ARW are known for their bioactivity against physiological malfunctions associated with hyperglycemia. However, the combined action of magnesium and ARW on renal tubular cells in response to glucose stress remains unknown. To address this, we utilized magnesium beads to fortify and alkalinize water using a non-electrolysis method, resulting in the synthesis of MARW. Next, we assessed the anti-hyperglycemic action of MARW in renal epithelial cells by examining various physiological metrics related to the OS response. Furthermore, we investigated how MARW attenuates the negative effects of OS on renal tubule glucose uptake and the onset of renal fibrosis using HK-2 cells. As a result, MARW was found to improve the survival of HK-2 cells at supramaximal glucose concentrations while maintaining normal glucose uptake. In addition, the anti-hyperglycemic action of MARW was confirmed by endogenous antioxidant machineries in which MARW stabilized GPx and CAT activities while attenuating hyperglycemia-induced ROS and NO elevation. Moreover, MARW alleviated the damaging consequences of hyperglycemia in the kidney by restoring renal-cell-derived HGF and ameliorating renal tubule fibrosis. Hence, we demonstrated the beneficial effects of MARW on stress signaling, fibrosis and inflammation.
The current study, however, has certain limitations. This research focuses on the anti-hyperglycemic properties of MARW in vitro using the proximal renal tubule, an HK-2 cell line. However, as DN is a cumulative malfunction of varieties of cells in the kidney, such as other epithelial, mesenchymal, fibroblast and immune cells, more research is needed. In addition, MARW showed pleiotropic effects on PTEC which were attributed to antioxidant, anti-apoptotic and anti-inflammatory effects, and an in-depth study on the underlying mechanisms is warranted for further elucidation. In conclusion, although several studies have shown the mediation of ARW in diabetes [21], to our knowledge, this study is the first to explore the anti-hyperglycemic effects of ARW, specifically of magnesium-enhanced ARW in renal proximal tubular epithelial cells. Our results show that MARW protects HK-2 cells from the deleterious effects of HG by stabilizing antioxidant defenses and signaling cascades related to metabolism, apoptosis and fibrosis. Furthermore, this study strengthens the therapeutic potential of MARW as an adjunctive treatment for patients with renal problems.
Author Contributions
Conceptualization, K.-J.L. and C.-S.K.; writing—original draft preparation, S.S.; writing—review and editing, S.S., C.-S.K., J.B., A.F. and J.M.A.; methodology, S.S., J.B., A.F. and J.M.A.; investigation, S.S., J.B., A.F., J.M.A., T.T.T., M.H.R., K.V. and A.-N.S.; data curation, C.-S.K., A.F. and J.B.; supervision, K.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Data Availability Statement
The data presented in this study are available in the article (table and figures).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| OS | Oxidative stress |
| MAPK | Mitogen-activated protein kinase |
| MARW | Magnesium-enhanced alkaline-reduced water |
| HK-2 | Human kidney-2 |
| JNK | c-Jun N-terminal kinase |
| MMP | Matrix metalloproteinase |
| ECM | Extracellular matrix |
| HG | High glucose |
| TW | Tap water |
| MW | Mineral water |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| SMA | Smooth muscle actin |
| HGF | Hepatocyte growth factor |
| ARW | Alkaline-reduced water |
| ATP | Adenosine triphosphate |
| DM | Diabetes mellitus |
| DN | Diabetic nephropathy |
| RPMI 1640 | Roswell Park Memorial Institute-1640 |
| DCFH-DA | 2′-7′-Dichloro-dihydro-fluorescein diacetate |
| ORP | Oxidation reduction potential |
| FBS | Fetal bovine serum |
| TDS | Total dissolved solids |
| CCK-8 | Cell counting kit-8 |
| ELISA | Enzyme-linked immune sorbent assay |
| SEM | Standard error mean |
| ANOVA | Analysis of variance |
| NC | Normal control |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| PTEC | Proximal tubular epithelial cell |
References
- Cho, S.J.; Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Pincha Devage Sameera Madushan, F.; Zhen, A.X.; Hyun, Y.J.; Ahn, M.J.; Kang, H.K.; Hyun, J.W. 7,8-dihydroxyflavone protects high glucose-damaged neuronal cells against oxidative stress. Biomol. Ther. 2019, 27, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.; Dekker, D.; Berden, J.; Scharstuhl, A.; Van der Vlag, J. The role of reactive oxygen species in apoptosis of the diabetic kidney. Apoptosis 2009, 14, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Coughlan, M.T.; Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008, 57, 1446–1454. [Google Scholar] [CrossRef]
- Ha, H.; Hwang, I.-A.; Park, J.H.; Lee, H.B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008, 82, S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, Y.; Zhang, T.; Xu, B.; Gao, Q.; Guan, T. Pentosan polysulfate ameliorates apoptosis and inflammation by suppressing activation of the p38 mapk pathway in high glucose-treated hk-2 cells. Int. J. Mol. Med. 2018, 41, 908–914. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, Y.; Wang, Z.; Fang, Q.; Sun, Y.; Tong, C.; Peng, K.; Wang, Y.; Miao, L.; Cai, L. Inhibition of mapk-mediated ace expression by compound c66 prevents stz-induced diabetic nephropathy. J. Cell. Mol. Med. 2014, 18, 231–241. [Google Scholar] [CrossRef]
- Hong, Z.; Hong, Z.; Wu, D.; Nie, H. Specific mapk inhibitors prevent hyperglycemia-induced renal diseases in type 1 diabetic mouse model. Mol. Cell. Biochem. 2016, 419, 1–9. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Clay Bunn, R.; Fowlkes, J.L. Matrix metalloproteinases: Their potential role in the pathogenesis of diabetic nephropathy. Endocrine 2009, 35, 1–10. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Bunn, R.C.; Moreau, C.S.; Cockrell, G.E.; Simpson, P.M.; Coleman, H.N.; Frindik, J.P.; Kemp, S.F.; Fowlkes, J.L. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care 2007, 30, 2321–2326. [Google Scholar] [CrossRef]
- Li, M.; Jiang, T.; Zhang, W.; Xie, W.; Guo, T.; Tang, X.; Zhang, J. Human umbilical cord msc-derived hepatocyte growth factor enhances autophagy in aopp-treated hk-2 cells. Exp. Ther. Med. 2020, 20, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Ziyadeh, F.N.; Sharma, K. Overview: Combating diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Sigrist, S. The protective effect of antioxidants consumption on diabetes and vascular complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Cernaro, V.; Gembillo, G.; Baggetta, R.; Buemi, M.; D’Arrigo, G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178699. [Google Scholar] [CrossRef] [PubMed]
- Schwalfenberg, G.K.; Genuis, S.J. The importance of magnesium in clinical healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Ramadass, S.; Basu, S.; Srinivasan, A. Serum magnesium levels as an indicator of status of diabetes mellitus type 2. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 42–45. [Google Scholar] [CrossRef]
- Pham, P.-C.; Pham, P.-M.; Pham, P.-A.; Pham, S.; Pham, H.; Miller, J.; Yanagawa, N.; Pham, P. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin. Nephrol. 2005, 63, 429–436. [Google Scholar] [CrossRef]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, S267. [Google Scholar] [CrossRef]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef]
- Delos Reyes, F.S.L.G.; Mamaril, A.C.C.; Matias, T.J.P.; Tronco, M.K.V.; Samson, G.R.; Javier, N.D.; Fadriquela, A.; Antonio, J.M.; Sajo, M.E. The search for the elixir of life: On the therapeutic potential of alkaline reduced water in metabolic syndromes. Processes 2021, 9, 1876. [Google Scholar] [CrossRef]
- Bajgai, J.; Kim, C.-S.; Rahman, M.; Jeong, E.-S.; Jang, H.-Y.; Kim, K.-E.; Choi, J.; Cho, I.-Y.; Lee, K.-J.; Lee, M. Effects of alkaline-reduced water on gastrointestinal diseases. Processes 2022, 10, 87. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on oletf rats. Biosci. Biotechnol. Biochem. 2006, 70, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Ryan, M.J.; Johnson, G.; Kirk, J.; Fuerstenberg, S.M.; Zager, R.A.; Torok-Storb, B. Hk-2: An immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994, 45, 48–57. [Google Scholar] [CrossRef]
- Mossoba, M.E.; Mapa, M.S.; Sprando, J.; Araujo, M.; Sprando, R.L. Evaluation of transporter expression in HK-2 cells after exposure to free and ester-bound 3-MCPD. Toxicol. Rep. 2021, 8, 436–442. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, X.; Xue, Y.; Yang, S.; Shi, M.; Wang, L. Metformin reduces the senescence of renal tubular epithelial cells in diabetic nephropathy via the mbnl1/mir-130a-3p/stat3 pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8708236. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Dong, J.-J.; Cai, T.; Shen, X.; Zhou, X.-J.; Liao, L. High glucose induces apoptosis via upregulation of bim expression in proximal tubule epithelial cells. Oncotarget 2017, 8, 24119. [Google Scholar] [CrossRef][Green Version]
- Kowluru, R.A.; Chan, P.-S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, K.M.; Jeong, J.U.; Shin, J.M.; Kang, J.H.; Bang, K.; Kim, J.-H. Nrf2-heme oxygenase-1 attenuates high-glucose-induced epithelial-to-mesenchymal transition of renal tubule cells by inhibiting ros-mediated pi3k/akt/gsk-3β signaling. J. Diabetes Res. 2019, 2019, 2510105. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.T.; Fadriquela, A.; Bajgai, J.; Sharma, S.; Rahman, M.; Goh, S.-H.; Khang, S.-S.; Khang, W.-R.; Kim, C.-S.; Lee, K.-J. Anti-oxidative effect of weak alkaline reduced water in raw 264.7 murine macrophage cells. Processes 2021, 9, 2062. [Google Scholar] [CrossRef]
- Trinh, T.T.; Fadriquela, A.; Lee, K.-J.; Bajgai, J.; Sharma, S.; Rahman, M.; Kim, C.-S.; Youn, S.-H.; Jeon, H.-T. Development of alkaline reduced water using high-temperature-roasted mineral salt and its antioxidative effect in raw 264.7 murine macrophage cell line. Processes 2021, 9, 1928. [Google Scholar] [CrossRef]
- You, I.-S.; Sharma, S.; Fadriquela, A.; Bajgai, J.; Thi, T.T.; Rahman, M.; Sung, J.; Kwon, H.-U.; Lee, S.-Y.; Kim, C.-S.J.M. Antioxidant properties of hydrogen gas attenuates oxidative stress in airway epithelial cells. Molecules 2021, 26, 6375. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Li, J.; Yu, Y.; Wei, Q.; Deng, W.; Yu, L. L-carnitine attenuates oxidant injury in hk-2 cells via ros-mitochondria pathway. Regul. Pept. 2010, 161, 58–66. [Google Scholar] [CrossRef]
- Bajgai, J.; Lee, K.-J. The drinking effect of hydrogen rich alkaline water produced by alkastone on reactive oxygen species. Korean J. Waters 2017, 6, 74. [Google Scholar]
- Matsumoto, K.; Nakamura, T. Renotropic role and therapeutic potential of hgf in the kidney. Nephrol. Dial. Transpl. 2002, 17, 59–61. [Google Scholar] [CrossRef][Green Version]
- Narayan, K.V.; Gregg, E.W.; Fagot-Campagna, A.; Engelgau, M.M.; Vinicor, F. Diabetes—A common, growing, serious, costly, and potentially preventable public health problem. Diabetes Res. Clin. Pract. 2000, 50, S77–S84. [Google Scholar] [CrossRef]
- Piero, M.; Nzaro, G.; Njagi, J. Diabetes mellitus—A devastating metabolic disorder. Asian J. Biomed. Pharm. Sci. 2015, 5, 1. [Google Scholar]
- Rout, D.; Dash, U.C.; Kanhar, S.; Swain, S.K.; Sahoo, A.K. Homalium zeylanicum attenuates streptozotocin-induced hyperglycemia and cellular stress in experimental rats via attenuation of oxidative stress imparts inflammation. J. Ethnopharmacol. 2022, 283, 114649. [Google Scholar] [CrossRef]
- Nebbioso, M.; Lambiase, A.; Armentano, M.; Tucciarone, G.; Sacchetti, M.; Greco, A.; Alisi, L. Diabetic retinopathy, oxidative stress, and sirtuins: An in depth look in enzymatic patterns and new therapeutic horizons. Surv. Ophthalmol. 2022, 67, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Quesada, I.; Ropero, A.B. The pancreatic β-cell as a target of estrogens and xenoestrogens: Implications for blood glucose homeostasis and diabetes. Endocrinology 2009, 304, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-activated protein kinases and reactive oxygen species: How can ros activate mapk pathways? J. Sign. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Behl, T.; Rana, T.; Alotaibi, G.H.; Shamsuzzaman, M.; Naqvi, M.; Sehgal, A.; Singh, S.; Sharma, N.; Almoshari, Y.; Abdellatif, A.A. Polyphenols inhibiting mapk signalling pathway mediated oxidative stress and inflammation in depression. Biomed. Pharmacother. 2022, 146, 112545. [Google Scholar] [CrossRef]
- Antonio, J.M.; Fadriquela, A.; Jeong, Y.J.; Kim, C.-S.; Kim, S.-K. Alkaline reduced water attenuates oxidative stress-induced mitochondrial dysfunction and innate immune response triggered by intestinal epithelial dysfunction. Processes 2021, 9, 1828. [Google Scholar] [CrossRef]
- Park, W.H. The effect of mapk inhibitors and ros modulators on cell growth and death of h2o2-treated hela cells. Mol. Med. Rep. 2013, 8, 557–564. [Google Scholar] [CrossRef]
- Guo, Z.; Tuo, H.; Tang, N.; Liu, F.-Y.; Ma, S.-Q.; An, P.; Yang, D.; Wang, M.-Y.; Fan, D.; Yang, Z. Neuraminidase 1 deficiency attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory via ampk-sirt3 pathway in diabetic cardiomyopathy mice. Int. J. Biol. Sci. 2022, 18, 826–840. [Google Scholar] [CrossRef]
- Wang, W.-C.; Liu, S.-F.; Chang, W.-T.; Shiue, Y.-L.; Hsieh, P.-F.; Hung, T.-J.; Hung, C.-Y.; Hung, Y.-J.; Chen, M.-F.; Yang, Y.-L. The effects of diosgenin in the regulation of renal proximal tubular fibrosis. Exp. Cell Res. 2014, 323, 255–262. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y.; Zhang, Y.; Harris, D.C.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 2, 84–89. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, J.; He, Z.; Yang, M.; Li, L.; Jiang, H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin a4 by targeting transforming growth factor β (tgf-β)/smad pathway and pro-inflammatory cytokines. Med. Sci. Monit. 2019, 25, 3069–3076. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, S.; Ji, Y.; Liang, Y. Pterostilbene ameliorates nephropathy injury in streptozotocin-induced diabetic rats. Pharmacology 2019, 104, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liao, G.; Zhang, J.; Chen, Y.; Li, L.; Li, L.; Liu, F.; Chen, B.; Guo, G. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int. J. Mol. Med. 2018, 41, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, U.; Regmi, S.C.; Awasthi, B.P.; Chaudhary, P.; Kim, Y.E.; Lee, I.H.; Nam, T.G.; Kim, J.A.; Jeong, B.S. Synthesis and activity of N-(5-hydroxy-3, 4, 6-trimethylpyridin-2-yl) acetamide analogues as anticolitis agents via dual inhibition of TNF-α-and IL-6-induced cell adhesions. Bioorg. Med. Chem. Lett. 2021, 43, 128059. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhong, L.; Li, H.; Xu, Y.; Li, X.; Zheng, D. Psoralen alleviates high glucose-induced hk-2 cell injury by inhibition of smad 2 signaling via upregulation of microrna 874. Toxicology 2020, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef]
- Rias, Y.A.; Kurniawan, A.L.; Chang, C.W.; Gordon, C.J.; Tsai, H.T. Synergistic effects of regular walking and alkaline electrolyzed water on decreasing inflammation and oxidative stress, and increasing quality of life in individuals with type 2 diabetes: A community based randomized controlled trial. Antioxidants 2020, 9, 946. [Google Scholar] [CrossRef]
- Grosick, R.; Alvarado-Vazquez, P.A.; Messersmith, A.R.; Romero-Sandoval, E.A. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J. Pain Res. 2018, 11, 1769–1778. [Google Scholar] [CrossRef]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. 2), S3. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).