Phytosterol, Tocopherol and Carotenoid Retention during Commercial Processing of Brassica napus (Canola) Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Processing Plant Samples
2.3. Sample Preparation and Laboratory Oil Extraction
2.4. Tocopherol, Sterol, and Carotenoid Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Bioactive Concentrations in Untreated Seed
3.2. Effect of Seed Preparation on Bioactive Retention
3.3. Effect of Crude Oil Extraction on Bioactive Retention
3.4. Effect of Refining on Bioactive Retention
3.4.1. Phytosterols
3.4.2. Tocopherols
3.4.3. Carotenoids
3.5. Examination of Second Press Oil from Process CB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daun, J.K.; Eskin, N.A.M.; Hickling, D. Canola: Chemistry, Production, Processing, and Utilisation; Elsevier Science: Urbana, IL, USA, 2015. [Google Scholar]
- Australian Oilseeds Federation. Crop Report: Canola September 2016; Australian Oilseeds Federation: Sydney, Australia, 2016; p. 1. [Google Scholar]
- Australian Oilseeds Federation Industry Facts and Figures. Available online: http://www.australianoilseeds.com/oilseeds_industry/industry_facts_and_figures (accessed on 1 December 2016).
- Matthäus, B.; Brühl, L. Why is it so difficult to produce high-quality virgin rapeseed oil for human consumption? Eur. J. Lipid Sci. Technol. 2008, 110, 611–617. [Google Scholar] [CrossRef]
- Alander, J.; Andersson, A.; Bagge, C.; Bringsarve, K.; Hjorth, M.; Johansson, M.; Granroth, B.; Norberg, S.; Pedersen, M.; Persson, M.; et al. Raw materials. In Handbook of Vegetable Oils and Fats, 2nd ed.; Lidefelt, J., Ed.; Alfaprint: Sundbyberg, Sweden, 2007. [Google Scholar]
- Gül, M.K.; Amar, S. Sterols and the phytosterol content in oilseed rape (Brassica napus L.). J. Mol. Cell Biol. 2006, 5, 71–79. [Google Scholar]
- Li, C.; Yao, Y.; Zhao, G.; Cheng, W.; Liu, H.; Liu, C.; Shi, Z.; Chen, Y.; Wang, S. Comparison and analysis of fatty acids, sterols, and tocopherols in eight vegetable oils. J. Agric. Food Chem. 2011, 59, 12493–12498. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, E.; Carvajal-Lérida, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A.; Hornero-Méndez, D. Carotenoids bioavailability from foods: From plant pigments to efficient biological activities. Food Res. Int. 2012, 46, 438–450. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Granado, F.; Olmedilla, B.; Blanco, I. Nutritional and clinical relevance of lutein in human health. Brit. J. Nutr. 2003, 90, 487–502. [Google Scholar] [CrossRef] [Green Version]
- Fine, F.; Brochet, C.; Gaud, M.; Carre, P.; Simon, N.; Ramli, F.; Joffre, F. Micronutrients in vegetable oils: The impact of crushing and refining processes on vitamins and antioxidants in sunflower, rapeseed, and soybean oils. Eur. J. Lipid Sci. Technol. 2015, 118, 680–697. [Google Scholar] [CrossRef]
- Ghazani, S.; Marangoni, A. Minor components in canola oil and effects of refining on these constituents: A review. J. Am. Oil Chem. Soc. 2013, 90, 923–932. [Google Scholar] [CrossRef]
- Dunford, N.T.; Dunford, H.B. Nutritionally Enhanced Edible Oil and Oilseed Processing; AOCS Press: Urbana, IL, USA, 2004. [Google Scholar]
- Gawrysiak-Witulska, M.; Siger, A.; Nogala-Kalucka, M. Degradation of tocopherols during near-ambient rapeseed drying. J. Food Lipids 2009, 16, 524–539. [Google Scholar] [CrossRef]
- Gawrysiak-Witulska, M.; Rudzińska, M.; Siger, A.; Bartkowiak-Broda, I. A high drying temperature causes degradation of sterols and tocopherols in yellow-seeded Brassica napus oils. Eur. J. Lipid Sci. Technol. 2015, 117, 483–490. [Google Scholar] [CrossRef]
- Azadmard-Damirchi, S.; Alirezalu, K.; Achachlouei, B.F. Microwave pretreatment of seeds to extract high quality vegetable oil. World Acad. Sci. Eng. Technol. 2011, 57, 72–75. [Google Scholar]
- Rękas, A.; Wroniak, M.; Rusinek, R. Influence of roasting pretreatment on high-oleic rapeseed oil quality evaluated by analytical and sensory approaches. Int. J. Food Sci. Technol. 2015, 50, 2208–2214. [Google Scholar] [CrossRef]
- Kraljic, K.; Skevin, D.; Pospisil, M.; Obranovic, M.; Neeral, S.; Bosolt, T. Quality of rapeseed oil produced by conditioning seeds at modest temperatures. J. Am. Oil Chem. Soc. 2013, 90, 589–599. [Google Scholar] [CrossRef]
- Liu, C.S.; Yang, M.; Huang, F.H. Influence of extraction processing on rheological properties of rapeseed oils. J. Am. Oil Chem. Soc. 2012, 89, 73–78. [Google Scholar] [CrossRef]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Micronutrient content of cold-pressed, hot-pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. Eur. J. Lipid Sci. Technol. 2014, 116, 380–387. [Google Scholar] [CrossRef]
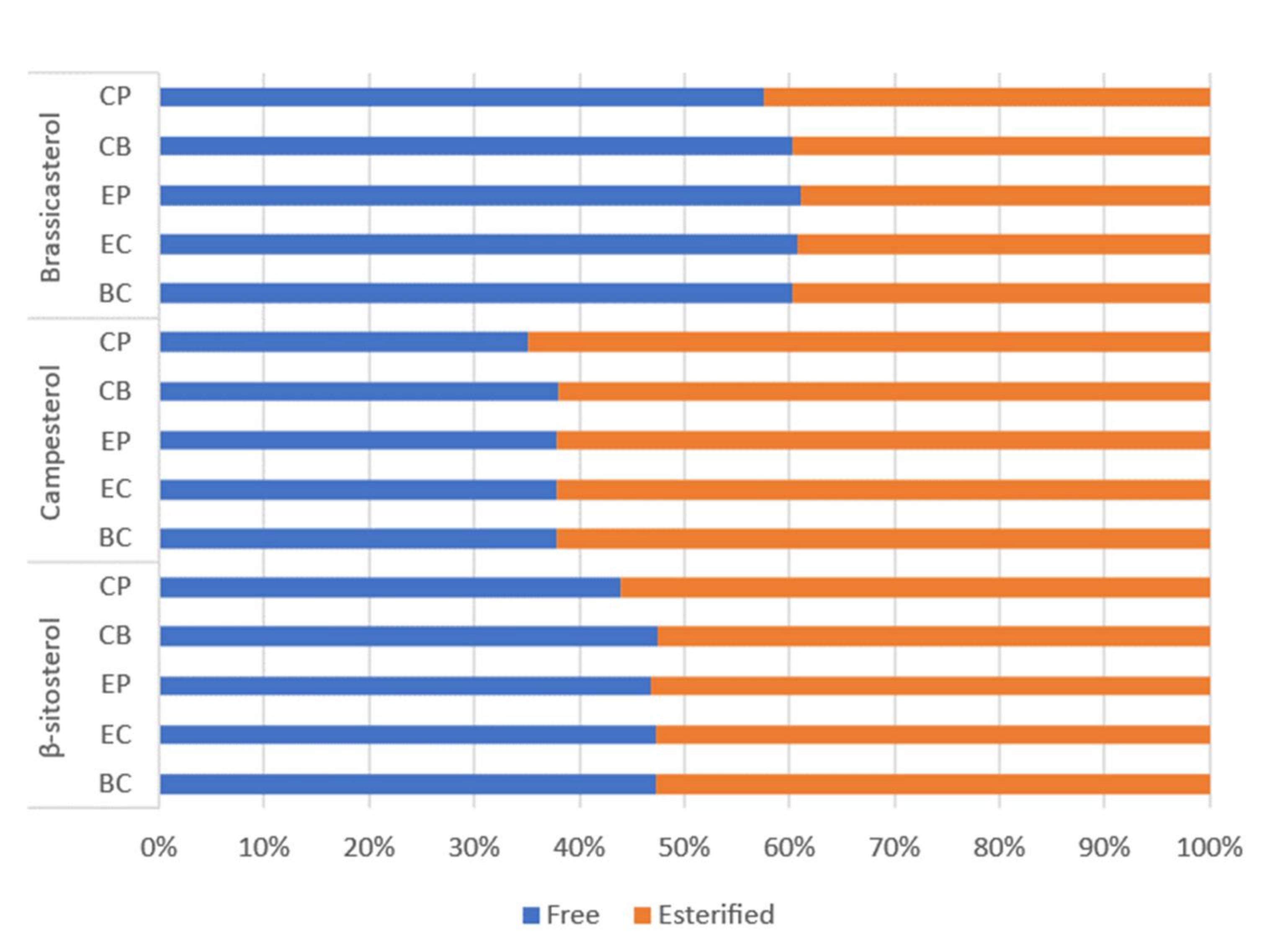
- Verleyen, T.; Sosinska, U.; Ioannidou, S.; Verhe, R.; Dewettinck, K.; Huyghebaert, A.; De Greyt, W. Influence of the vegetable oil refining process on free and esterified sterols. J. Am. Oil Chem. Soc. 2002, 79, 947–953. [Google Scholar] [CrossRef]
- Ghazani, S.M.; García-Llatas, G.; Marangoni, A.G. Minor constituents in canola oil processed by traditional and minimal refining methods. J. Am. Oil Chem. Soc. 2013, 90, 743–756. [Google Scholar] [CrossRef]
- Ferrari, R.A.; Schulte, E.; Esteves, W.; Brühl, L.; Mukherjee, K.D. Minor constituents of vegetable oils during industrial processing. J. Am. Oil Chem. Soc. 1996, 73, 587–592. [Google Scholar] [CrossRef]
- Flakelar, C.L.; Luckett, D.J.; Howitt, J.A.; Doran, G.; Prenzler, P.D. Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J. Food Compost. Anal. 2015, 42, 179–186. [Google Scholar] [CrossRef]
- Flakelar, C.L.; Prenzler, P.D.; Luckett, D.J.; Howitt, J.A.; Doran, G. A rapid method for the simultaneous quantification of the major tocopherols, carotenoids, free and esterified sterols in canola (Brassica napus) oil using normal phase liquid chromatography. Food Chem. 2017, 214, 147–155. [Google Scholar] [CrossRef]
- Flakelar, C.L.; Howitt, J.A.; Prenzler, P.D.; Doran, G.; Coombes, N.; Luckett, D.J. A multiphase experiment for the analysis of bioactive compounds in canola oil: Sources of error from field and laboratory. Chemom. Intell. Lab. 2017, 162, 55–64. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 9 July 2017).
- Rstudio: Integrated Development Environment for R; Version 1; R Studio, PBC: Boston, MA, USA, 2012; Available online: https://www.rstudio.com/ (accessed on 9 July 2017).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; Version 1.2-7; National Engineering University: Lima, Peru, 2016. [Google Scholar]
- Richards, A.; Wijesundera, C.; Salisbury, P. Genotype and growing environment effects on the tocopherols and fatty acids of Brassica napus and B. juncea. J. Am. Oil Chem. Soc. 2008, 85, 159–168. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compost. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Verleyen, T.; Forcades, M.; Verhe, R.; Dewettinck, K.; Huyghebaert, A.; Greyt, W. Analysis of free and esterified sterols in vegetable oils. J. Am. Oil Chem. Soc. 2002, 79, 117–122. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Toivo, J.I.; Swank, M.A.; Simpkins, A.H. Free and esterified sterol composition of edible oils and fats. J. Food Compost. Anal. 2002, 15, 123–142. [Google Scholar] [CrossRef]
- Prior, E.; Vadke, V.; Sosulski, F. Effect of heat treatments on canola press oils. I. Non- triglyceride components. J. Am. Oil Chem. Soc. 1991, 68, 401–406. [Google Scholar] [CrossRef]
- Rossi, M.; Gianazza, M.; Alamprese, C.; Stanga, F. The effect of bleaching and physical refining on color and minor components of palm oil. J. Am. Oil Chem. Soc. 2001, 78, 1051–1055. [Google Scholar] [CrossRef]
- De Greyt, W.F.; Kellens, M.J.; Huyghebaert, A.D. Effect of physical refining on selected minor components in vegetable oils. Lipid/Fett 1999, 101, 428–432. [Google Scholar] [CrossRef]

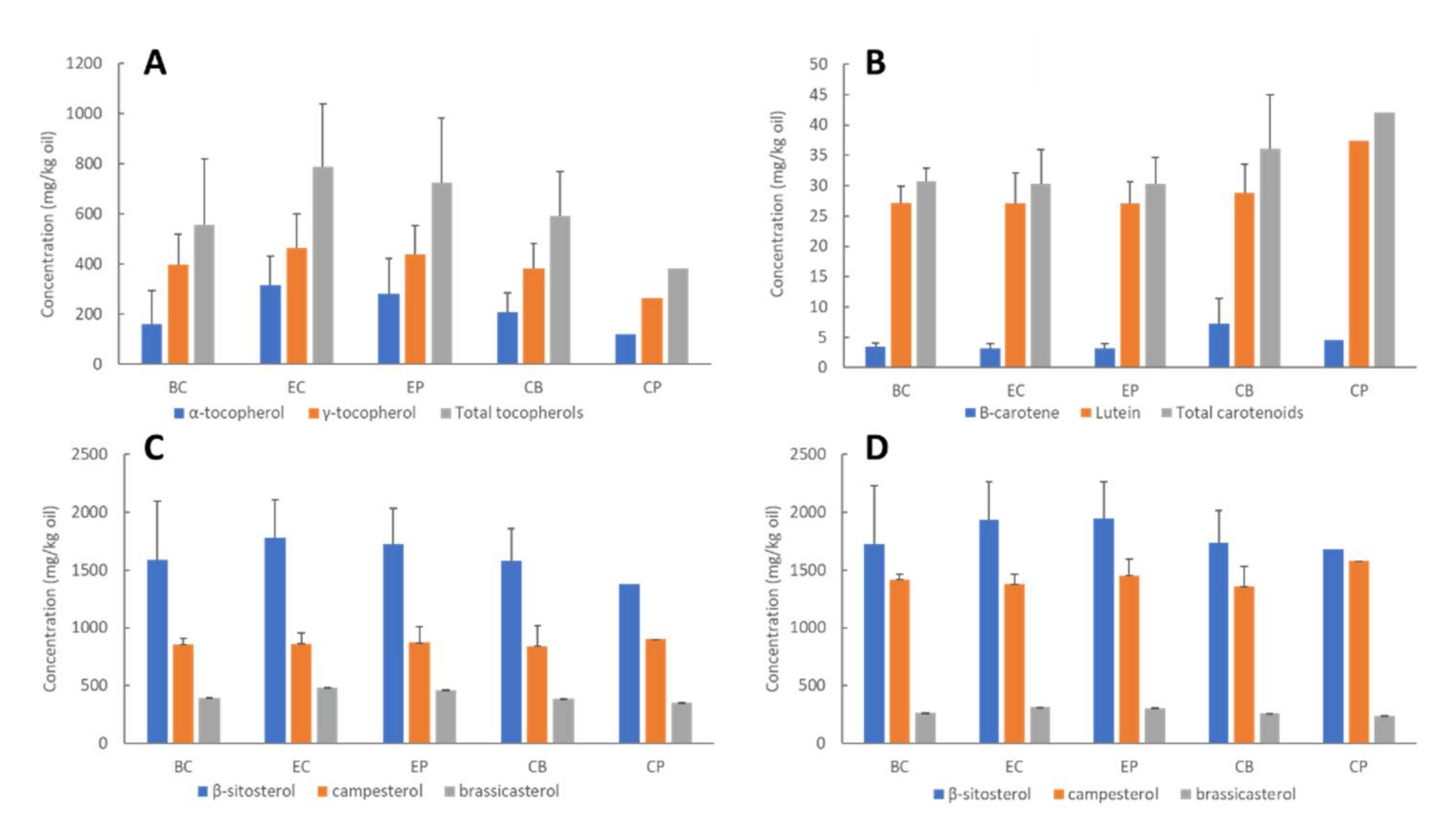
| Process Sample | mg/kg Oil ± Standard Deviation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Carotene | Lutein | Total Carotenoids | α-Tocopherol | γ-Tocopherol | Total Tocopherols | β-Sitosterol | Campesterol | Brassicasterol | Total Phytosterol | |
| Seed | 3.48 b ± 0.49 | 27.2 b ± 3.21 | 30.7 b ± 2.94 | 161 a ± 133 | 397 a ± 123 | 558 a ± 261 | 3310 a ± 918 | 2270 a ± 221 | 655 b ± 72.4 | 6240 b ± 769 |
| Preconditioned seed | 3.70 b ± 0.65 | 28.2 b ± 3.67 | 31.9 b ± 3.24 | 210 a ± 104 | 401 a ± 140 | 611 a ± 248 | 3130 a ± 981 | 2190 a ± 136 | 632 b ± 111 | 5950 b ± 956 |
| Flakes | 3.60 b ± 0.40 | 32.2 b ± 3.78 | 35.8 b ± 3.58 | 198 a ± 127 | 409 a ± 146 | 606 a ± 278 | 3310 a ± 758 | 2350 a ± 376 | 644 b ± 56.0 | 6310 b ± 438 |
| Expeller press cake | 5.80 a ± 0.51 | 34.4 b ± 4.12 | 40.2 a ± 3.62 | 291 a ± 156 | 441 a ± 145 | 737 a ± 308 | 4410 a ± 801 | 2860 a ± 497 | 842 a ± 82.1 | 8110 a ± 386 |
| Process Sample | mg/kg Oil ± Standard Deviation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Carotene | Lutein | Total Carotenoids | α-Tocopherol | γ-Tocopherol | Total Tocopherols | β-Sitosterol | Campesterol | Brassicasterol | Total Phytosterols | |
| Crude press oil | 3.90 b ± 0.50 | 44.6 a ± 6.12 | 48.5 a ± 6.60 | 246 a ± 156 | 466 a ± 149 | 712 a ± 309 | 3750 a ± 617 | 2610 a ± 414 | 721 b ± 65.2 | 7080 b ± 268 |
| Crude blended oil | 4.93 a ± 0.46 | 46.1 a ± 11.1 | 51.0 a ± 11.5 | 268 a ± 173 | 481 a ± 178 | 749 a ± 356 | 4130 a ± 726 | 2730 a ± 375 | 784 a ± 69.0 | 7640 a ± 420 |
| Oil Sample | mg/kg Oil ± Standard Deviation | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Sitosterol | Campesterol | Brassicasterol | Total Sterols | |||||||||||||||||
| BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | |
| Crude | 4130 | 4240 | 4310 | 3550 | 3300 | 2730 | 2600 | 2600 | 2510 | 2770 | 784 | 846 | 850 | 680 | 680 | 7640 | 7690 | 7760 | 6740 | 6750 |
| ±725 | ±405 | ±1030 | ±242 | N/A | ±375 | ±587 | ±322 | ±725 | N/A | ±68.6 | ±18.6 | ±115 | ±59.1 | N/A | ±419 | ±163 | ±819 | ±424 | N/A | |
| Degummed/ Neutralised | 3940 (−4.6) | 4350 (+2.6) | 4400 (+2.1) | – | 3274 (−0.8) | 2610 (−4.4) | 2630 (+1.2) | 2620 (+0.8) | – | 2644 (−4.5) | 739 (−5.7) | 829 (−2.0) | 850 (0.0) | – | 718 (+5.6) | 7280 (−4.7) | 7810 (+1.6) | 7870 (+1.4) | – | 6640 (−1.6) |
| ±812 | ±751 | ±896 | N/A | ±269 | ±410 | ±383 | N/A | ±90.2 | ±86.3 | ±95.0 | N/A | ±634 | ±427 | ±608 | N/A | |||||
| Bleached | 3930 (−0.3) | 4260 (−2.1) | 4170 (−5.2) | 3400 (−4.2) | 2980 (−9.0) | 2630 (+0.8) | 2560 (−2.7) | 2630 (+0.4) | 2370 (−5.4) | 2450 (−7.3) | 721 (−2.4) | 815 (−1.7) | 850 (0.0) | 690 (+1.0) | 626 (−12.8) | 7280 (0.0) | 7640 (−2.2) | 7910 (+0.5) | 6460 (+8.6) | 6050 (−8.9) |
| ±788 | ±773 | ±1090 | ±672 | N/A | ±338 | ±336 | ±244 | ±343 | N/A | ±76.9 | ±74.0 | ±120 | ±116 | N/A | ±527 | ±511 | ±1020 | ±445 | N/A | |
| Deodorised | 3870 (−1.5) | 4200 (−1.4) | 4210 (+1.0) | – | 2860 (−4.0) | 2610 (−0.8) | 2560 (0.0) | 2470 (−6.1) | – | 2380 (−2.9) | 715 (−0.8) | 789 (−3.2) | 790 (−7.1) | – | 590 (−5.8) | 7200 (−1.1) | 7550 (−1.2) | 7440 (−6.0) | – | 5830 (−3.6) |
| ±788 | ±1050 | ±1020 | N/A | ±313 | ±195 | ±194 | N/A | ±85.1 | ±144 | ±142 | N/A | ±561 | ±1000 | ±1040 | N/A | |||||
| Total loss/gain (%) | −6.3 | −0.9 | −2.3 | −4.2 | −13.3 | −4.4 | −1.5 | −5.0 | −5.4 | −14.1 | −8.8 | −6.7 | −7.1 | +1.0 | −13.2 | −5.8 | −1.8 | −4.1 | −9.6 | −13.6 |
| Oil Sample | mg/kg Oil ± Standard Deviation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Tocopherol | γ-Tocopherol | Total Tocopherols | |||||||||||||
| BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | |
| Crude | 268 | 365 | 353 | 245 | 130 | 481 | 467 | 531 | 417 | 282 | 749 | 840 | 893 | 663 | 412 |
| ±173 | ±88.0 | ±180 | ±93.0 | N/A | ±178 | ±117 | ±187 | ±119 | N/A | ±356 | ±205 | ±367 | ±211 | N/A | |
| Degummed/ Neutralised | 252 (−6.0) | 386 (+5.8) | 367 (+4.0) | – | 80 (−38.5) | 474 (−1.5) | 521 (+11.6) | 534 (+0.6) | – | 276 (−2.1) | 726 (−3.1) | 919 (+9.4) | 910 (+1.9) | – | 356 (−13.6) |
| ±193 | ±156 | ±152 | N/A | ±170 | ±185 | ±198 | N/A | ±368 | ±341 | ±351 | N/A | ||||
| Bleached | 261 (+ 3.6) | 367 (−4.9) | 406 (+10.6) | 197 (−19.6) | 123 (+53.8) | 496 (+4.6) | 530 (+1.7) | 533 (−0.2) | 406 (−2.6) | 293 (+6.2) | 757 (+4.3) | 909 (−1.1) | 950 (+4.4) | 604 (−8.9) | 416 (+16.9) |
| ±184 | ±138 | ±210 | ±98.3 | N/A | ±164 | ±191 | ±183 | ±134 | N/A | ±358 | ±328 | ±393 | ±233 | N/A | |
| Deodorised | 246 (−5.7) | 311 (−15.3) | 357 (−12.1) | – | 85 (−30.9) | 490 (−1.2) | 521 (−1.7) | 492 (−7.7) | – | 263 (−10.2) | 736 (−2.8) | 840 (−7.6) | 853 (−10.2) | – | 348 (−16.4) |
| ±182 | ±143 | ±187 | N/A | ±158 | ±189 | ±172 | N/A | ±345 | ±332 | ±360 | N/A | ||||
| Total loss/gain (%) | −8.1 | −14.8 | +1.1 | −19.6 | −34.6 | +1.9 | +11.6 | −7.3 | −2.6 | −6.7 | −1.8 | 0.0 | −4.5 | −8.9 | −15.5 |
| Oil Sample | mg/kg Oil ± Standard Deviation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Carotene | Lutein | Total Carotenoids | |||||||||||||
| BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | BC | EC | EP | CB | CP | |
| Crude | 4.93 | 5.25 | 5.03 | 8.36 | 4.62 | 46.1 | 48.9 | 50.7 | 40.0 | 33.4 | 51.0 | 54.1 | 55.7 | 48.3 | 38.0 |
| ±0.55 | ±2.69 | ±0.66 | ±4.05 | N/A | ±13.2 | ±24.6 | ±15.9 | 13.3 | N/A | 13.8 | 27.3 | 16.5 | 9.23 | N/A | |
| Degummed/ Neutralised | 5.11 (+3.7) | 6.2 (+18.1) | 5.14 (+2.2) | – | 4.41 (−4.5) | 29.6 (−35.8) | 34.0 (−30.5) | 39.8 (−21.5) | – | 36.0 (+7.8) | 34.7 (−32.0) | 40.2 (−25.7) | 44.9 (−19.4) | – | 40.4 (+6.3) |
| ±0.53 | ±1.74 | ±0.57 | N/A | ±4.37 | ±5.71 | ±13.4 | N/A | ±4.91 | ±7.46 | ±13.9 | N/A | ||||
| Bleached | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL | <DL |
| Deodorised | <DL | <DL | <DL | – | <DL | <DL | <DL | <DL | – | <DL | <DL | <DL | <DL | – | <DL |
| Oil Sample | mg/kg Oil ± Standard Deviation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Carotene | Lutein | Total Carotenoids | α-Tocopherol | γ-Tocopherol | Total Tocopherols | β-Sitosterol | Campesterol | Brassicasterol | Total Phytosterols | |
| First press crude oil | 8.40 b ± 4.05 | 39.9 c ± 13.3 | 48.3 c ± 9.23 | 244 a ± 93.0 | 417 a ± 119 | 662 a ± 211 | 3550 b ± 242 | 2510 a ± 725 | 683 a ± 59.1 | 6740 b ± 424 |
| Second press crude oil | 14.5 a ± 7.94 | 84.5 a ± 2.96 | 99.0 a ± 10.9 | 311 a ± 119 | 447 a ± 151 | 758 a ± 270 | 4760 a ± 571 | 3050 a ± 765 | 843 a ± 123 | 8660 a ± 71.3 |
| Storage tank—first press | 8.70 b ± 4.94 | 38.8 c ± 0.61 | 47.5 c ± 4.33 | 230 a ± 107 | 402 a ± 135 | 632 a ± 242 | 3420 b ± 727 | 2400 a ± 396 | 683 a ± 139 | 6500 b ± 469 |
| Storage tank—second press | 15.2 a ± 6.67 | 87.9 a ± 4.46 | 103 a ± 11.1 | 316 a ± 120 | 453 a ± 123 | 770 a ± 243 | 5010 a ± 733 | 3190 a ± 765 | 897 a ± 126 | 9100 a ± 93.5 |
| Premium bleached oil from first press | <DL | <DL | <DL | 197 a ± 120 | 406 a ± 134 | 603 a ± 233 | 3400 b ± 672 | 2370 a ± 343 | 688 a ± 117 | 6460 b ± 445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flakelar, C.L.; Adjonu, R.; Doran, G.; Howitt, J.A.; Luckett, D.J.; Prenzler, P.D. Phytosterol, Tocopherol and Carotenoid Retention during Commercial Processing of Brassica napus (Canola) Oil. Processes 2022, 10, 580. https://doi.org/10.3390/pr10030580
Flakelar CL, Adjonu R, Doran G, Howitt JA, Luckett DJ, Prenzler PD. Phytosterol, Tocopherol and Carotenoid Retention during Commercial Processing of Brassica napus (Canola) Oil. Processes. 2022; 10(3):580. https://doi.org/10.3390/pr10030580
Chicago/Turabian StyleFlakelar, Clare L., Randy Adjonu, Gregory Doran, Julia A. Howitt, David J. Luckett, and Paul D. Prenzler. 2022. "Phytosterol, Tocopherol and Carotenoid Retention during Commercial Processing of Brassica napus (Canola) Oil" Processes 10, no. 3: 580. https://doi.org/10.3390/pr10030580
APA StyleFlakelar, C. L., Adjonu, R., Doran, G., Howitt, J. A., Luckett, D. J., & Prenzler, P. D. (2022). Phytosterol, Tocopherol and Carotenoid Retention during Commercial Processing of Brassica napus (Canola) Oil. Processes, 10(3), 580. https://doi.org/10.3390/pr10030580







