Indirect Contact Chamber with Dielectric Layers for Pulsed Electric Field Treatment of Microorganisms
Abstract
1. Introduction
2. Experimental Procedures
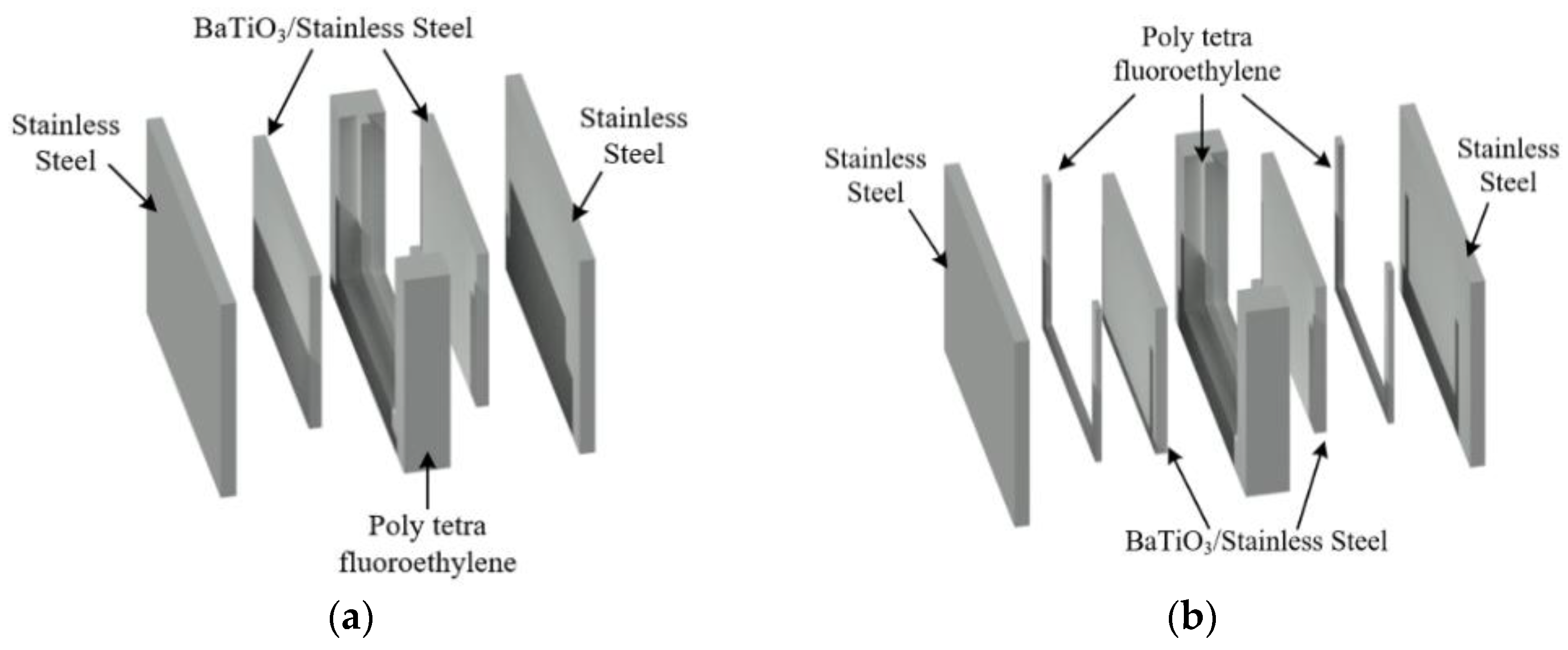
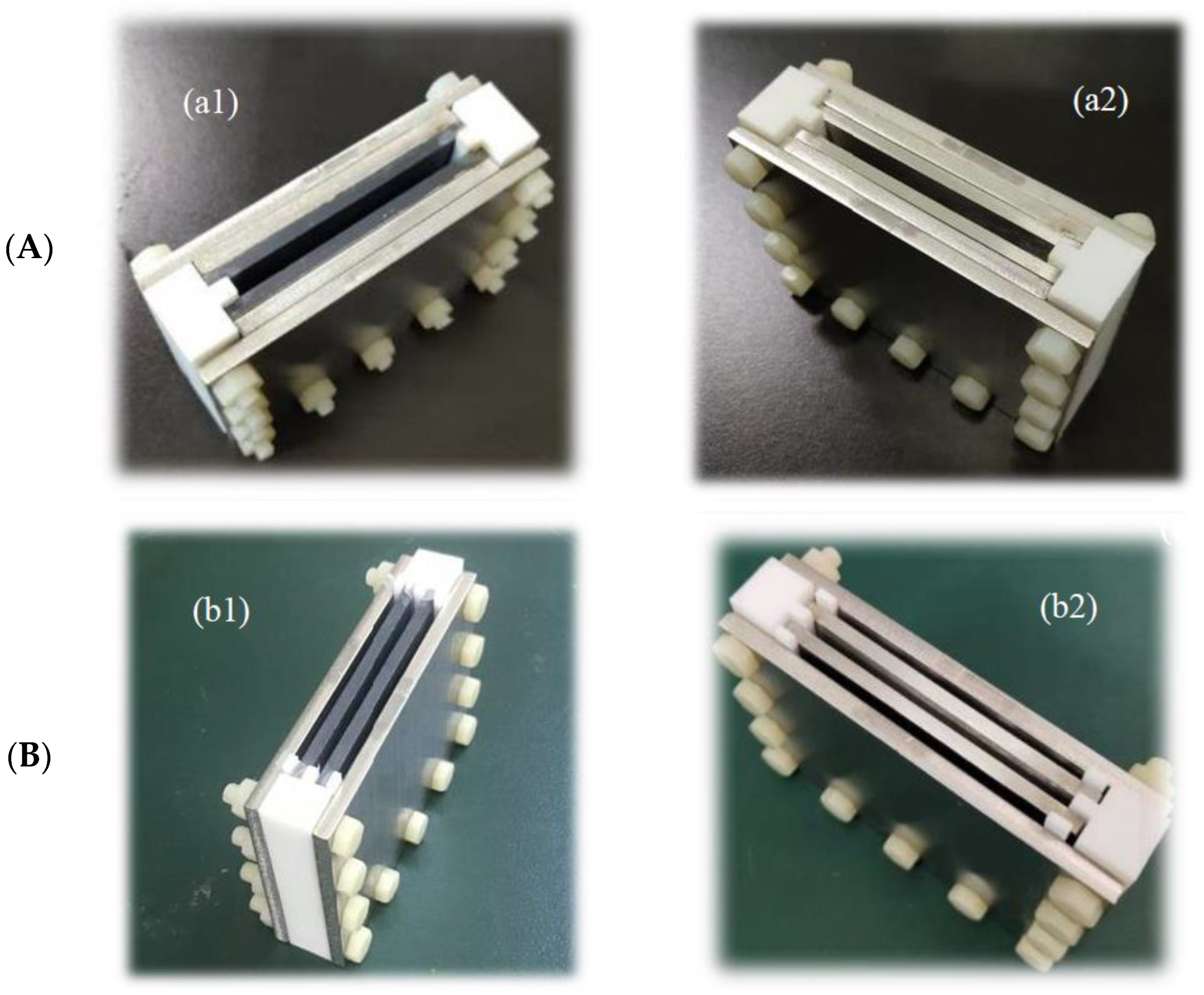
2.1. PEF Chambers with BaTiO3 Layers
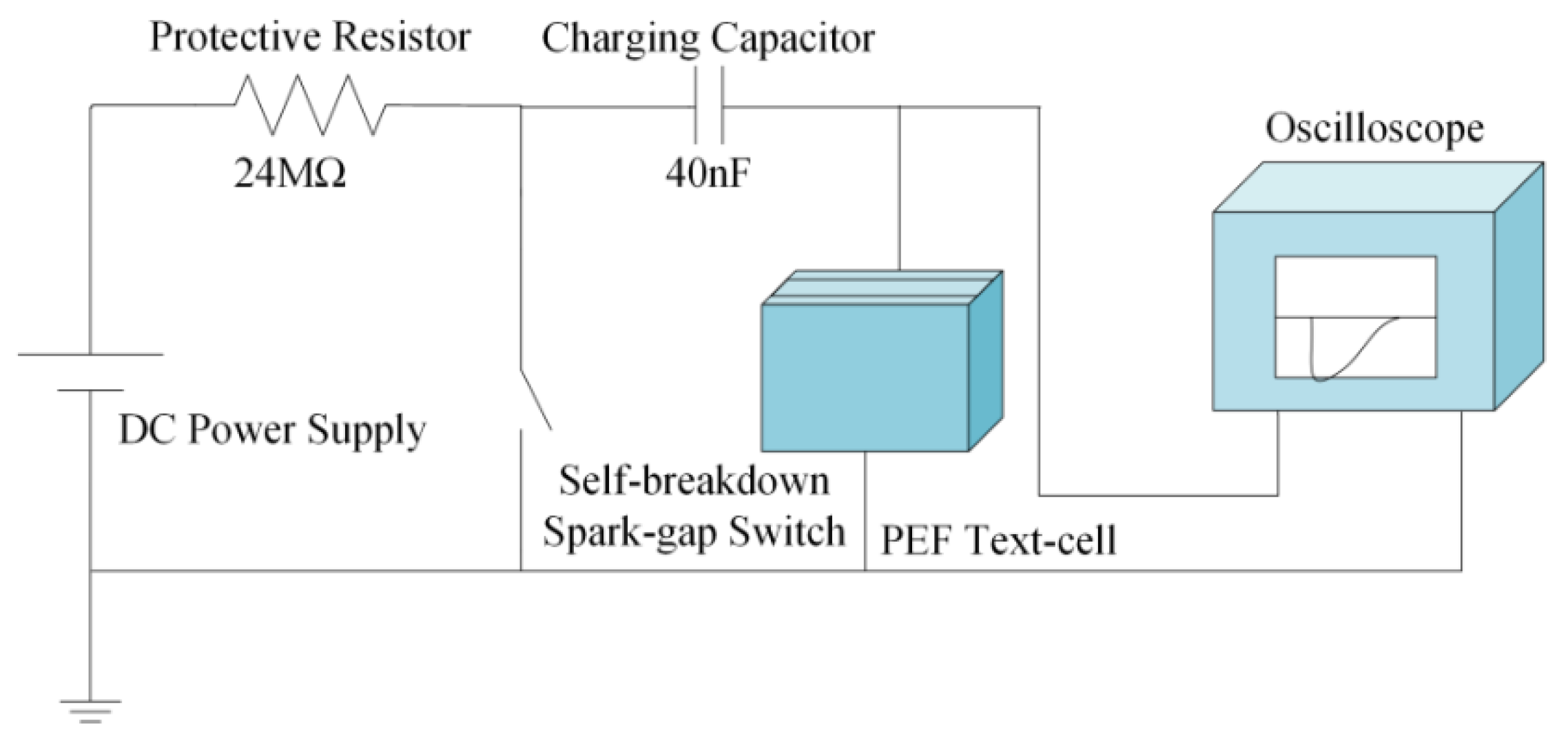
2.2. PEF System Setups
2.3. Preparation of Yeast Suspension
2.4. Assessment of PEF Inactivation
2.5. Determination of Metallic Ions and (∙OH) Concentrations
3. Results and Discussion
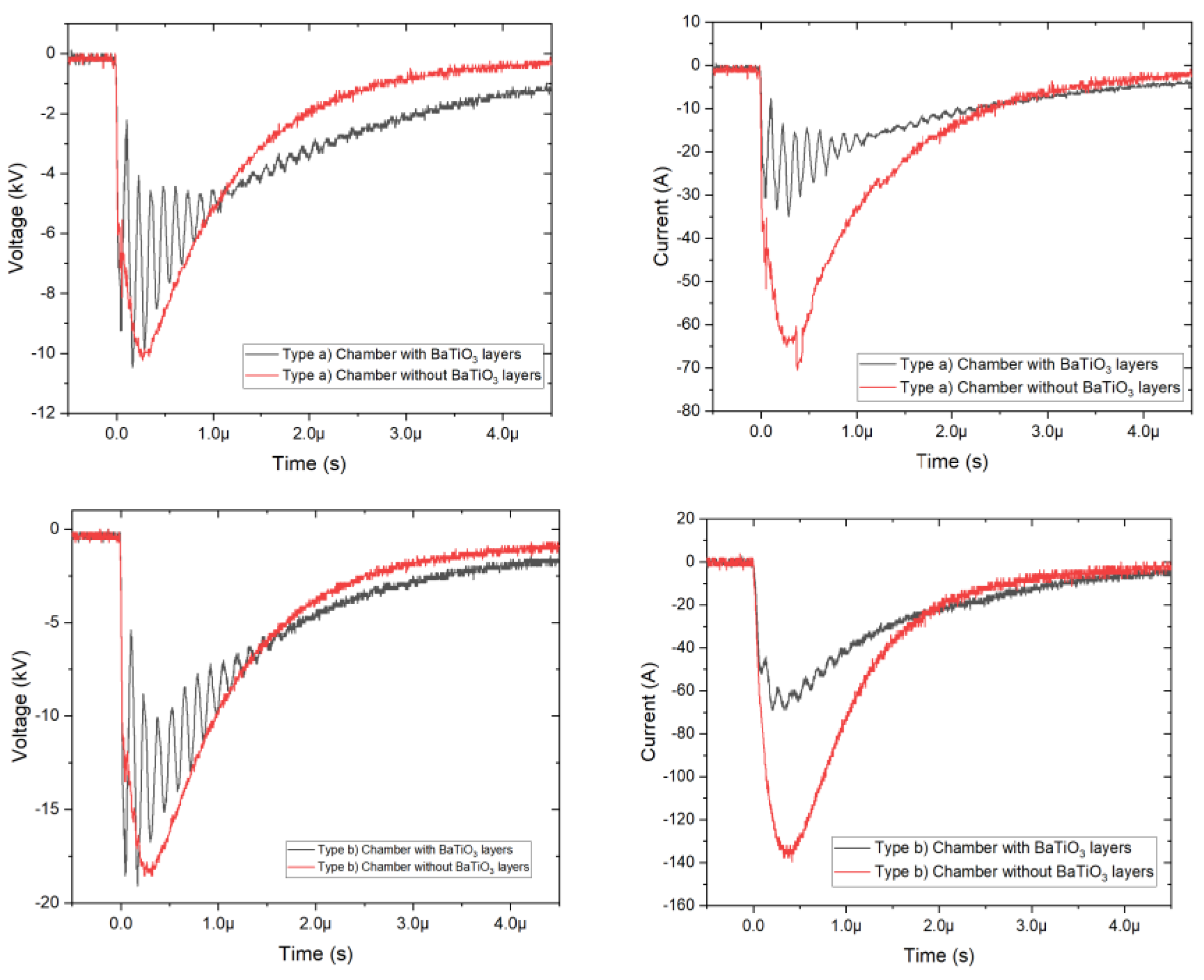
3.1. Voltage and Current Waveforms
3.2. Effects on Sterile Rate
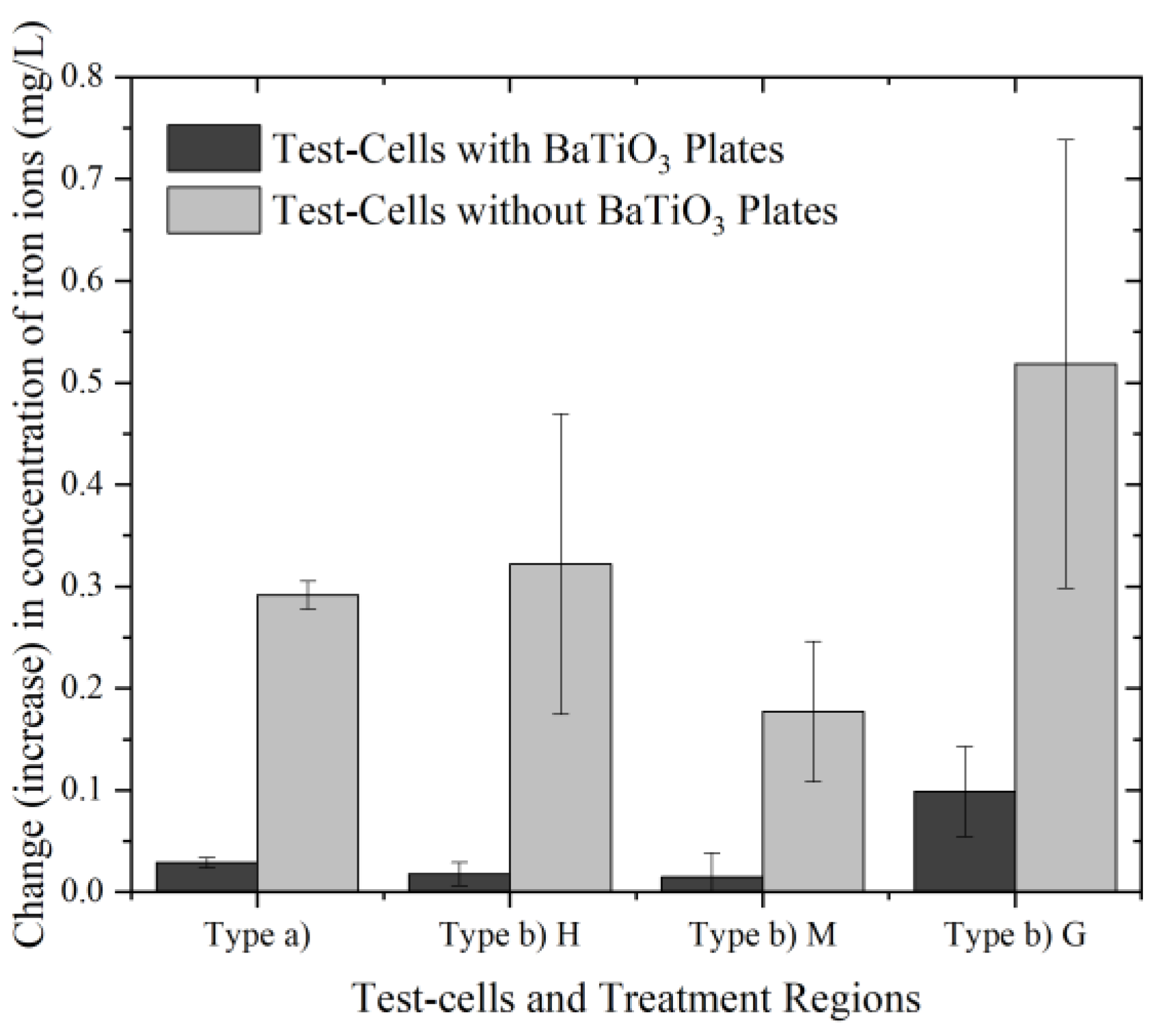
3.3. Effects on Concentrations of Metallic Ions
3.4. Effects on (∙OH) Concentrations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chem. Eng. Process 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Zimmermann, U. Electrical breakdown, electropermeabilization and electrofusion. Rev. Physiol. Biochem. Pharmacol. 1986, 105, 175–256. [Google Scholar]
- Brito, P.S.; Canacsinh, H.; Mendes, J.P.; Redondo, L.M.; Pereira, M.T. Comparison between monopolar and bipolar microsecond range pulsed electric fields in enhancement of apple juice extraction. IEEE Trans. Plasma Sci. 2012, 40, 2348–2354. [Google Scholar] [CrossRef]
- Jaeger, H.; Meneses, N.; Knorr, D. Impact of PEF treatment inhomogeneity such as electric field distribution, flow characteristics and temperature effects on the inactivation of E. coli and milk alkaline phosphatase. Innov. Food Sci. Emerg. Technol. 2009, 10, 470–480. [Google Scholar] [CrossRef]
- Guionet, A.; Fujiwara, T.; Sato, H.; Takahashi, K.; Takaki, K.; Matsui, M.; Tanino, T.; Ohshima, T. Pulsed electric fields act on tryptophan to inactivate α-amylase. J. Electrost. 2021, 112, 103597. [Google Scholar] [CrossRef]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Electrost. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Min, S.; Evrendilek, G.A.; Zhang, H.Q. Pulsed electric fields: Processing system, microbial and enzyme inhibition, and shelf life extension of foods. IEEE Trans. Plasma Sci. 2007, 35, 59–73. [Google Scholar] [CrossRef]
- Jayaram, S.H. Sterilization of liquid foods by pulsed electric fields. IEEE Electr. Insul. Mag. 2000, 16, 17–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Barbosa-Cánovas, G.V.; Swanson, B.G. Engineering aspects of pulsed electric field pasteurization. J. Food Eng. 1995, 25, 261–281. [Google Scholar] [CrossRef]
- Narsetti, R.; Curry, R.D.; McDonald, K.F.; Clevenger, T.E.; Nichols, L.M. Microbial inactivation in water using pulsed electric fields and magnetic pulse compressor technology. IEEE Trans. Plasma Sci. 2006, 34, 1386–1393. [Google Scholar] [CrossRef]
- Qin, B.-L.; Zhang, Q.; Barbosa-Cánovas, G.V.; Swanson, B.G.; Pedrow, P.D. Inactivation of microorganisms by pulsed electric fields of different voltage waveforms. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 1047–1057. [Google Scholar]
- Aronssona, K.; Lindgrena, M.; Johanssonb, B.R.; Rönner, U. Inactivation of microorganisms using pulsed electric fields: The influence of process parameters on Escherichia coli, Listeria innocua, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2001, 2, 41–54. [Google Scholar] [CrossRef]
- Lubicki, P.; Jayaram, S. High Voltage Pulse Application for the Destruction of the Gram-negative Bacterium Yersinia enterocolitica. Bioelectrochem. Bioenerg. 1997, 43, 135–141. [Google Scholar] [CrossRef]
- Qin, S.; Timoshkin, I.; Maclean, M.; MacGregor, S.; Wilson, M.; Given, M.; Wang, T.; Anderson, J. TiO2-Coated Electrodes for Pulsed Electric Field Treatment of Microorganisms. IEEE Trans. Plasma Sci. 2016, 44, 2121–2128. [Google Scholar] [CrossRef]
- Tanino, T.; Hirosawa, M.; Moteki, R.; Matsui, M.; Ohshima, T. Engineering of pulsed electric field treatment using carbon materials as electrode and application to pasteurization of sake. J. Electrost. 2020, 104, 103424. [Google Scholar] [CrossRef]
- Timoshkin, I.V.; MacGregor, S.J.; Fouracre, R.A.; Crichton, B.H.; Anderson, J.G. Transient electrical field across cellular membranes: Pulsed electric field treatment of microbial cells. J. Phys. D Appl. Phys. 2006, 39, 569–603. [Google Scholar] [CrossRef]
- Novac, B.M.; Banakhr, F.A.; Smith, I.R.; Pecastaing, L. Demonstration of a novel pulsed electric field technique generating neither conduction currents nor Joule effects. IEEE Trans. Plasma Sci. 2014, 42, 216–228. [Google Scholar] [CrossRef]
- Peralta, E.; Roa, G.; Hemandez-Servin, J.A. Hydroxyl radicals quantification by UV spectrophotometry. Electrochim. Acta 2014, 129, 137–141. [Google Scholar] [CrossRef]
- Roodenburg, B.; Morren, J.; Berg, H.E. Metal release in a stainless steel pulsed electric field (PEF) system: Part II. The treatment of orange juice; related to legislation and treatment chamber lifetime. Innov. Food Sci. Emerg. Technol. 2005, 6, 337–345. [Google Scholar] [CrossRef]
- Saulis, G.; Riseviciene, R.; Snitka, V. Increase of the roughness of the stainless-steel anode surface due to the exposure to high-voltage electric pulses as revealed by atomic force microscopy. Bioelectrochemistry 2007, 70, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Rodaite-Riseviciene, R.; Saule, R.; Snitka, V. Release of Iron Ions From the Stainless Steel Anode Occurring During High-Voltage Pulses and Its Consequences for Cell Electroporation Technology. IEEE Trans. Plasma Sci. 2014, 42, 249–254. [Google Scholar] [CrossRef]
- Bai, M.D.; Bai, X.; Zhang, Z. Treatment of Red Tide in Ocean Using Non-Thermal Plasma Based Advanced Oxidation Technology. Plasma Chem. Plasma Process. 2005, 25, 539–550. [Google Scholar] [CrossRef]
- Takatsuji, Y.; Ishikawa, S.; Haruyama, T. Efficient sterilization using reactive oxygen species generated by a radical vapor reactor. Process Biochem. 2017, 54, 140–143. [Google Scholar] [CrossRef]
- Hulsheger, H.; Niemann, E.G. Lethal Effect of High Voltage Pulses on E. coli K12. Radiat. Eniron. Biophys. 1980, 18, 281–288. [Google Scholar] [CrossRef] [PubMed]




| Chamber | Regions | Mean | SD | SEM | Significant Difference (From Control Group) | Significant Difference (From Stainless Steel Group) | |
|---|---|---|---|---|---|---|---|
| At 0.05 | At 0.1 | At 0.01 | |||||
| BaTiO3 | Type a) | 0.73 | 0.407 | 0.235 | No | Yes | Yes |
| Type b) H | 1.14 | 1.255 | 0.724 | No | No | Yes | |
| Type b) M | 4.12 | 2.680 | 1.547 | No | No | Yes | |
| Type b) G | 5.40 | 1.864 | 1.076 | Yes | Yes | Yes | |
| Stainless steel | Type a) | 64.60 | 2.485 | 1.435 | Yes | Yes | N/A |
| Type b) H | 56.86 | 5.007 | 2.891 | Yes | Yes | N/A | |
| Type b) M | 62.00 | 2.238 | 1.292 | Yes | Yes | N/A | |
| Type b) G | 62.96 | 6.816 | 3.935 | Yes | Yes | N/A | |
| Chamber | Regions | Mean | SD | SEM | Significant Difference (From Control Group) | Significant Difference (From Stainless Steel Group) | ||
|---|---|---|---|---|---|---|---|---|
| At 0.05 | At 0.1 | At 0.05 | At 0.1 | |||||
| BaTiO3 | Type a) | 0.029 | 0.005 | 0.003 | Yes | Yes | Yes | Yes |
| Type b) H | 0.018 | 0.011 | 0.007 | No | No | No | Yes | |
| Type b) M | 0.015 | 0.024 | 0.014 | No | No | No | Yes | |
| Type b) G | 0.100 | 0.044 | 0.026 | No | Yes | No | Yes | |
| Stainless steel | Type a) | 0.292 | 0.014 | 0.008 | Yes | Yes | N/A | N/A |
| Type b) H | 0.322 | 0.148 | 0.085 | No | Yes | N/A | N/A | |
| Type b) M | 0.177 | 0.069 | 0.040 | Yes | Yes | N/A | N/A | |
| Type b) G | 0.519 | 0.220 | 0.127 | No | Yes | N/A | N/A | |
| Chamber | Regions | Mean | SD | SEM | Significant Difference (From Control Group) | Significant Difference (From Type a) Group) | ||
|---|---|---|---|---|---|---|---|---|
| At 0.05 | At 0.1 | At 0.01 | At 0.05 | |||||
| BaTiO3 | Type a) | 0.005 | 0.003 | 0.002 | No | No | N/A | N/A |
| Type b) H | 1.217 | 0.434 | 0.251 | Yes | Yes | No | Yes | |
| Type b) M | 1.101 | 0.248 | 0.143 | Yes | Yes | No | Yes | |
| Type b) G | 0.955 | 0.176 | 0.102 | Yes | Yes | No | Yes | |
| Chamber | Regions | Mean | SD | SEM | Significant Difference (From Control Group) | Significant Difference (From Stainless Steel Group) | ||
|---|---|---|---|---|---|---|---|---|
| At 0.05 | At 0.1 | At 0.05 | At 0.1 | |||||
| BaTiO3 | Type b) H | 0.010 | 0.022 | 0.012 | Yes | Yes | No | No |
| Type b) M | 0.067 | 0.030 | 0.017 | No | Yes | No | No | |
| Type b) G | 0.108 | 0.020 | 0.011 | Yes | Yes | No | Yes | |
| Stainless steel | Type b) H | 0.106 | 0.023 | 0.013 | Yes | Yes | N/A | N/A |
| Type b) M | 0.125 | 0.038 | 0.022 | Yes | Yes | N/A | N/A | |
| Type b) G | 0.184 | 0.041 | 0.024 | Yes | Yes | N/A | N/A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, S.; Zhang, Z.; Han, J.; Huang, S.; Meng, J. Indirect Contact Chamber with Dielectric Layers for Pulsed Electric Field Treatment of Microorganisms. Processes 2022, 10, 2432. https://doi.org/10.3390/pr10112432
Qin S, Zhang Z, Han J, Huang S, Meng J. Indirect Contact Chamber with Dielectric Layers for Pulsed Electric Field Treatment of Microorganisms. Processes. 2022; 10(11):2432. https://doi.org/10.3390/pr10112432
Chicago/Turabian StyleQin, Si, Zixin Zhang, Jiangang Han, Shihai Huang, and Jianzong Meng. 2022. "Indirect Contact Chamber with Dielectric Layers for Pulsed Electric Field Treatment of Microorganisms" Processes 10, no. 11: 2432. https://doi.org/10.3390/pr10112432
APA StyleQin, S., Zhang, Z., Han, J., Huang, S., & Meng, J. (2022). Indirect Contact Chamber with Dielectric Layers for Pulsed Electric Field Treatment of Microorganisms. Processes, 10(11), 2432. https://doi.org/10.3390/pr10112432









