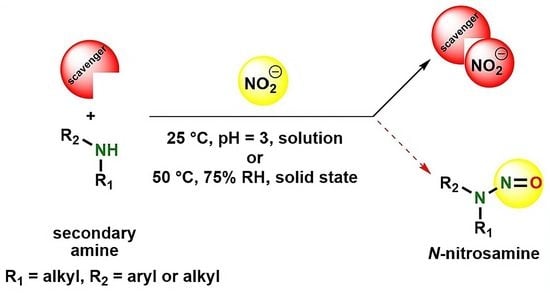
Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scavenger Screening Experiments
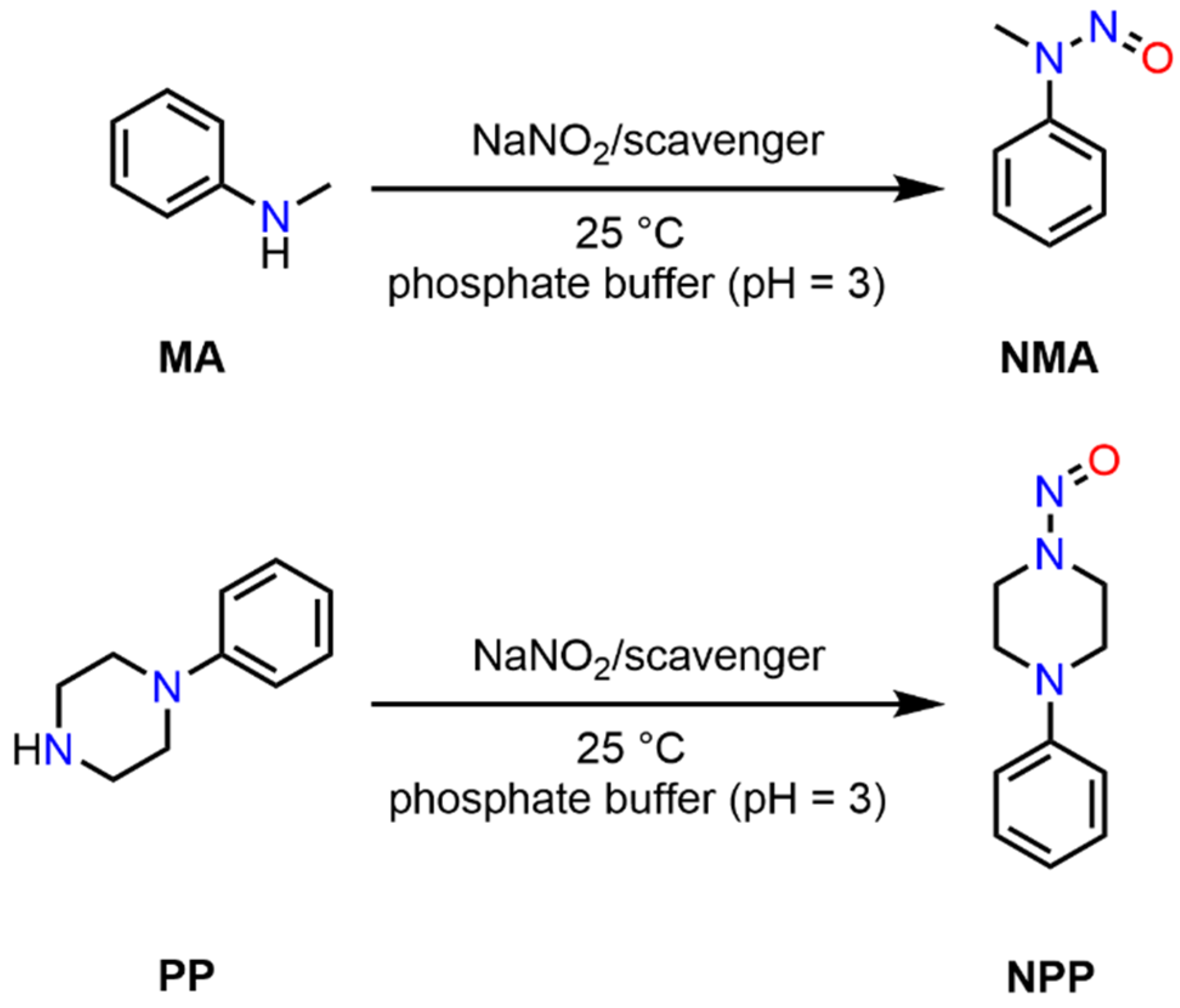
2.3. Model N-Nitrosamine Synthesis
2.4. Scavenger Evaluation on Inhibition of Model API N-Nitrosation in Solution
2.5. Scavenger Evaluation on Inhibition of Model API N-Nitrosation in Tablets
2.6. Liquid Chromatography-Mass Spectrometry Method for NMA/NPP Quantification
3. Results
3.1. Scavenger Screening Experiments
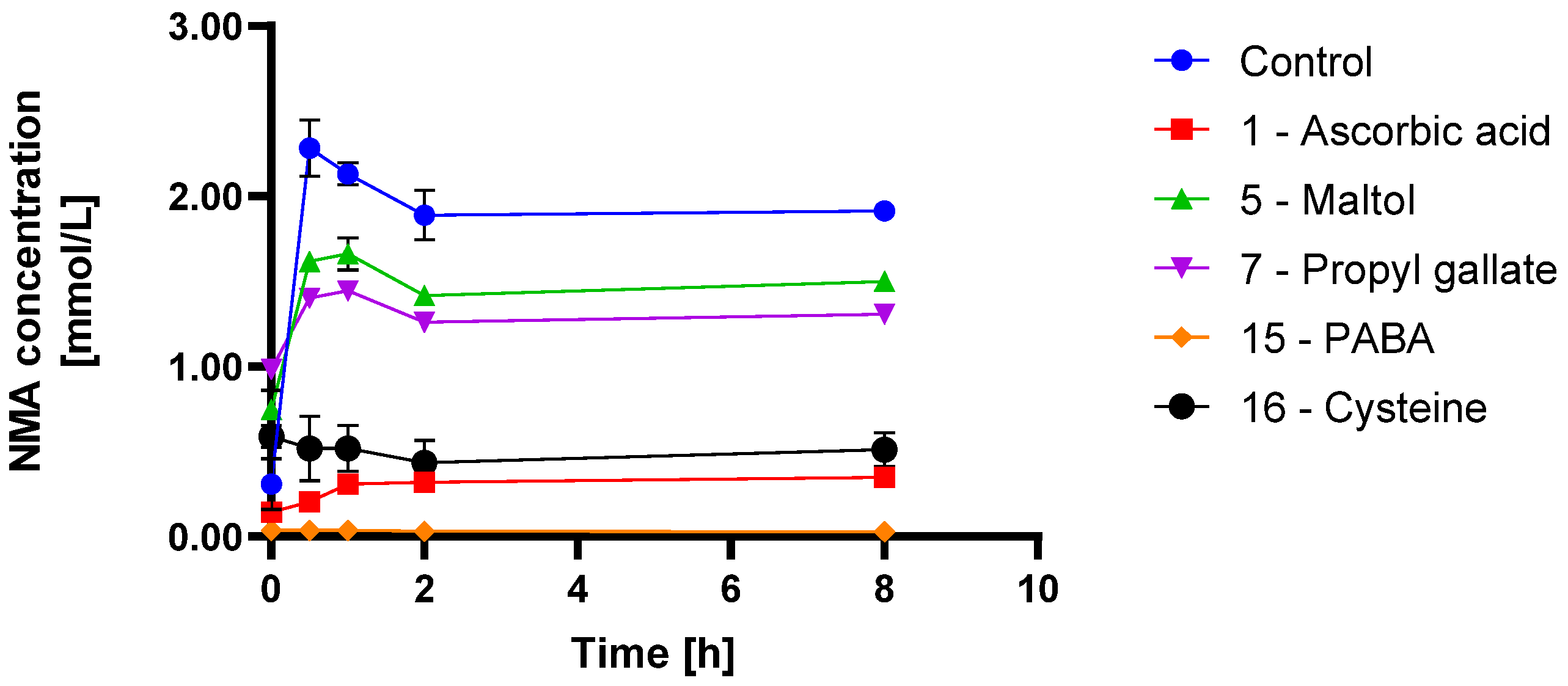
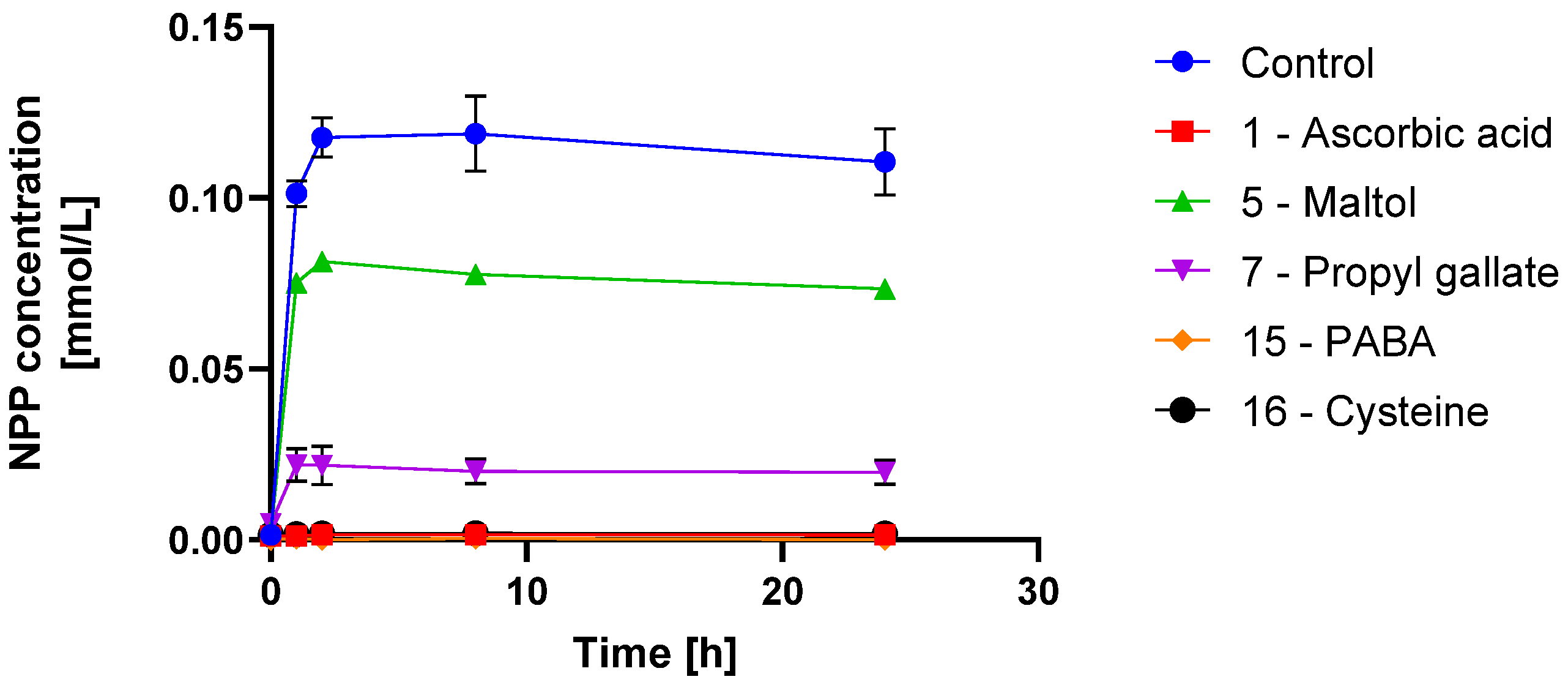
3.2. Scavenger Evaluation on Inhibition of Model API N-Nitrosation in Solution
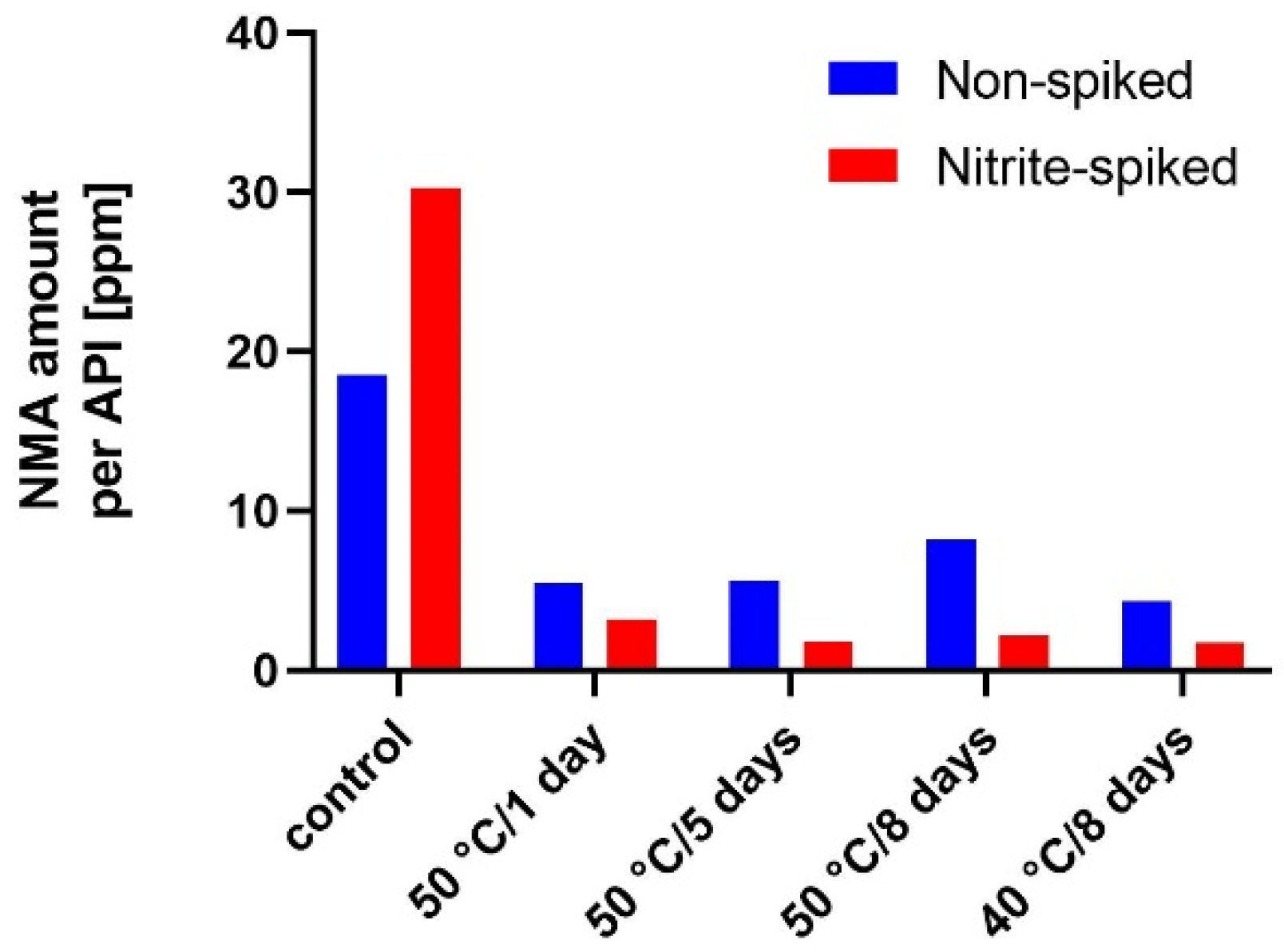
3.3. Scavenger Evaluation on Inhibition of Model API N-Nitrosation in Tablets
4. Discussion
4.1. Nitrite Scavenging
4.2. N-Nitrosamine Formation Inhibition in Solution
4.3. N-Nitrosation Inhibition Activity Comparison of Selected Scavengers in Solid State and Solution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fritzsche, M.; Blom, G.; Keitel, J.; Goettsche, A.; Seegel, M.; Leicht, S.; Guessregen, B.; Hickert, S.; Reifenberg, P.; Cimelli, A.; et al. NDMA Analytics in Metformin Products: Comparison of Methods and Pitfalls. Eur. J. Pharm. Sci. 2022, 168, 106026. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.M.; Enners, S.; Kieble, M.; Mahfoud, F.; Böhm, M.; Laufs, U.; Schulz, M. Impact of Angiotensin Receptor Blocker Product Recalls on Antihypertensive Prescribing in Germany. J. Hum. Hypertens. 2021, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Ruepp, R.; Frötschl, R.; Bream, R.; Filancia, M.; Girard, T.; Spinei, A.; Weise, M.; Whomsley, R. The EU Response to the Presence of Nitrosamine Impurities in Medicines. Front. Med. 2021, 8, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Atal, S.; Sadasivam, B. Understanding the Molecular-Pharmaceutical Basis of Sartan Recalls Focusing on Valsartan. Glob. Cardiol. Sci. Pract. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.B.; Chertow, G.M.; Bhalla, V. Hypertension Hot Potato-Anatomy of the Angiotensis Receptor Blocker Recalls. N. Engl. J. Med. 2019, 380, 1589–1591. [Google Scholar] [CrossRef]
- Gunasekaran, P.M.; Chertow, G.M.; Bhalla, V.; Byrd, J.B. Current Status of Angiotensin Receptor Blocker Recalls. Hypertension 2019, 74, 1275–1278. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA/369136/2020–Nitrosamine Impurities in Human Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf (accessed on 18 October 2022).
- Bharate, S.S. Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef]
- Schmidtsdorff, S.; Neumann, J.; Schmidt, A.H.; Parr, M.K. Risk Assessment for Nitrosated Pharmaceuticals: A Future Perspective in Drug Development. Arch. Pharm. 2022, 355, e2100435. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Control of Nitrosamine Impurities in Human Drugs. Available online: https://www.fda.gov/media/141720/download (accessed on 18 October 2022).
- Montesano, R. Alkylation of DNA and Tissue Specificity in Nitrosamine Carcinogenesis. J. Supramol. Struct. Cell. Biochem. 1981, 17, 259–273. [Google Scholar] [CrossRef]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-Nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential. Mutat. Res. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Kroeger-Koepke, M.B.; Koepke, S.R.; McClusky, G.A.; Magee, P.N.; Michejda, C.J. α-Hydroxylation Pathway in the in Vitro Metabolism of Carcinogenic Nitrosamines: N-Nitrosodimethylamine and N-Nitroso-N-Methylaniline. Proc. Natl. Acad. Sci. USA 1981, 78, 6489–6493. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Oxidation of Toxic and Carcinogenic Chemicals by Human Cytochrome P-450 Enzymes. Chem. Res. Toxicol. 1991, 4, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hecht, S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022, 23, 4559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hecht, S.S. Metabolism and DNA Adduct Formation of Tobacco-Specific N-Nitrosamines. Int. J. Mol. Sci. 2022, 23, 5109. [Google Scholar] [CrossRef] [PubMed]
- Jakszyn, P.; González, C.A. Nitrosamine and Related Food Intake and Gastric and Oesophageal Cancer Risk: A Systematic Review of the Epidemiological Evidence. World J. Gastroenterol. 2006, 12, 4296–4303. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. List of Classifications–IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 11 November 2022).
- Nawrocki, J.; Andrzejewski, P. Nitrosamines and Water. J. Hazard. Mat. 2011, 189, 1–18. [Google Scholar] [CrossRef]
- Ashworth, I.W.; Dirat, O.; Teasdale, A.; Whiting, M. Potential for the Formation of N-Nitrosamines during the Manufacture of Active Pharmaceutical Ingredients: An Assessment of the Risk Posed by Trace Nitrite in Water. Org. Process Res. Dev. 2020, 24, 1629–1646. [Google Scholar] [CrossRef]
- Langford, V.S.; Gray, J.D.C.; Maclagan, R.G.A.R.; Milligan, D.B.; McEwan, M.J. Real-Time Measurements of Nitrosamines in Air. Int. J. Mass Spectrom. 2015, 377, 490–495. [Google Scholar] [CrossRef]
- Choi, N.R.; Ahn, Y.G.; Lee, J.Y.; Kim, E.; Kim, S.; Park, S.M.; Song, I.H.; Kim, Y.P. Winter-Time Particulate Nitrosamines and Nitramines in the Atmosphere at Seoul, South Korea. Atmos. Environ. 2020, 237, 117582. [Google Scholar] [CrossRef]
- Pancholy, S.K. Formation of Carcinogenic Nitrosamines in Soils. Soil Biol. Biochem. 1978, 10, 27–32. [Google Scholar] [CrossRef]
- Yoneyama, T. Detection of N-Nitrosodimethylamine in Soils Amended with Sludges. Soil Sci. Plant Nutr. 1981, 27, 249–253. [Google Scholar] [CrossRef][Green Version]
- Khan, S.U.; Young, J.C. N-Nitrosamine Formation in Soil from the Herbicide Glyphosate. J. Agric. Food Chem. 1977, 25, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Biochemistry, Biology, and Carcinogenicity of Tobacco-Specific N-Nitrosamines. Chem. Res. Toxicol. 1998, 11, 559–603. [Google Scholar] [CrossRef] [PubMed]
- Altkofer, W.; Braune, S.; Ellendt, K.; Kettl-Grömminger, M.; Steiner, G. Migration of Nitrosamines from Rubber Products—Are Balloons and Condoms Harmful to the Human Health? Mol. Nutr. Food Res. 2005, 49, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Challis, B.C.; Trew, D.F.; Guthrie, W.G.; Roper, D.V. Reduction of Nitrosamines in Cosmetic Products. Int. J. Cosmet. Sci. 1995, 17, 119–131. [Google Scholar] [CrossRef]
- Tuesuwan, B.; Vongsutilers, V. Nitrosamine Contamination in Pharmaceuticals: Threat, Impact, and Control. J. Pharm. Sci. 2021, 110, 3118–3128. [Google Scholar] [CrossRef] [PubMed]
- Parr, M.K.; Joseph, J.F. NDMA Impurity in Valsartan and Other Pharmaceutical Products: Analytical Methods for the Determination of N-Nitrosamines. J. Pharm. Biomed. Anal. 2019, 164, 536–549. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; McManus, J.A.; Murphy, N.S.; Ott, M.A.; Burns, M.J. Pathways for N-Nitroso Compound Formation: Secondary Amines and Beyond. Org. Process Res. Dev. 2020, 24, 1558–1585. [Google Scholar] [CrossRef]
- Itoh, T.; Matsuya, Y.; Maeta, H.; Miyazaki, M.; Nagata, K.; Ohsawa, A. Effect of Oxygen on the Reaction of Secondary Amines with Nitric Oxide. Chem. Pharm. Bull. 1999, 47, 133–135. [Google Scholar] [CrossRef]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive Impurities in Excipients: Profiling, Identification and Mitigation of Drug-Excipient Incompatibility. AAPS Pharm. Sci. Tech. 2011, 12, 1248–1263. [Google Scholar] [CrossRef]
- Schlingemann, J.; Boucley, C.; Hickert, S.; Bourasseau, L.; Walker, M.; Celdran, C.; Chemarin, T.; Pegues, C.; Fritzsche, M.; Keitel, J.; et al. Avoiding N-Nitrosodimethylamine Formation in Metformin Pharmaceuticals by Limiting Dimethylamine and Nitrite. Int. J. Pharm. 2022, 620, 121740. [Google Scholar] [CrossRef] [PubMed]
- Mirvish, S.S.; Wallcave, L.; Eagen, M.; Shubik, P. Ascorbate-Nitrite Reaction: Possible Means of Blocking the Formation of Carcinogenic N-Nitroso Compounds. Science 1972, 177, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, S.R. Preventive Action of Vitamin C on Nitrosamine Formation. Int. J. Vitam. Nutr. Res. 1989, 30, 109–113. [Google Scholar]
- Rundlöf, T.; Olsson, E.; Wiernik, A.; Back, S.; Aune, M.; Johansson, L.; Wahlberg, I. Potential Nitrite Scavengers as Inhibitors of the Formation of N-Nitrosamines in Solution and Tobacco Matrix Systems. J. Agric. Food Chem. 2000, 48, 4381–4388. [Google Scholar] [CrossRef]
- Lim, D.S.; Lim, S.K.; Kim, M.K.; Kwon, Y.C.; Roh, T.H.; Choi, S.M.; Yoon, S.; Kim, H.S.; Lee, B.M. Formation and Inhibition of N-Nitrosodiethanolamine in Cosmetics under pH, Temperature, and Fluorescent, Ultraviolet, and Visual Light. J. Toxicol. Environ. Health Part A 2018, 81, 241–253. [Google Scholar] [CrossRef]
- Nanda, K.K.; Tignor, S.; Clancy, J.; Marota, M.J.; Allain, L.R.; D’Addio, S.M. Inhibition of N-Nitrosamine Formation in Drug Products: A Model Study. J. Pharm. Sci. 2021, 110, 3773–3775. [Google Scholar] [CrossRef]
- Combet, E.; Paterson, S.; Iijima, K.; Winter, J.; Mullen, W.; Crozier, A.; Preston, T.; McColl, K.E.L. Fat Transforms Ascorbic Acid from Inhibiting to Promoting Acid-Catalysed N-Nitrosation. Gut 2007, 56, 1678–1684. [Google Scholar] [CrossRef]
- Combet, E.; el Mesmari, A.; Preston, T.; Crozier, A.; McColl, K.E.L. Dietary Phenolic Acids and Ascorbic Acid: Influence on Acid-Catalyzed Nitrosative Chemistry in the Presence and Absence of Lipids. Free Radical Biol. Med. 2010, 48, 763–771. [Google Scholar] [CrossRef]
- Sen, N.P.; Donaldson, B.; Seaman, S.; Iyengar, J.R.; Miles, W.F. Inhibition of Nitrosamine Formation in Fried Bacon by Propyl Gallate and L-Ascorbyl Palmitate. J. Agric. Food Chem. 1976, 24, 397–401. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Granby, K.; Duedahl-Olesen, L. Formation and Mitigation of N-Nitrosamines in Nitrite Preserved Cooked Sausages. Food Chem. 2015, 174, 516–526. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, S.H.; Choi, J.H. Nitrite Scavenging Effect by Flavonoids and Its Structure-Effect Relationship. Arch. Pharm. Res. 1989, 12, 26–33. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Li, X.; He, Q. The Nitrite-Scavenging Properties of Catechol, Resorcinol, and Hydroquinone: A Comparative Study on Their Nitration and Nitrosation Reactions. J. Food Sci. 2016, 81, C2692–C2696. [Google Scholar] [CrossRef] [PubMed]
- Astill, B.D.; Mulligan, L.T. Phenolic Antioxidans and the Inhibition of Hepatotoxicity from N-Dimethylnitrosamine Formed in Situ in the Rat Stomach. Food Cosmet. Toxicol. 1977, 15, 167–171. [Google Scholar] [CrossRef]
- Hughes, M.N. Kinetic Study of the Reaction between Nitrous Acid and Sulphamic Acid. J. Chem. Soc. A 1967, 902–905. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Meyer, T.A.; O’Neill, M.E.; Williams, D.L.H. Comparison of the Reactivity of Nine Nitrous Acid Scavengers. J. Chem. Soc. Perkin Trans. 2 1984, 1984, 927–932. [Google Scholar] [CrossRef]
- Stedman, G. Mechanism of the Azide-Nitrite Reaction. Part IV. J. Chem. Soc. 1960, 1702–1709. [Google Scholar] [CrossRef]
- Hughes, M.N.; Stedman, G. Kinetics and Mechanism of the Reaction between Nitrous Acid and Hydroxylamine. Part I. J. Chem. Soc. 1963, 2824–2830. [Google Scholar] [CrossRef]
- Bryant, T.; Williams, D.L.H. Nitrosation of Ammonia. J. Chem. Soc. Perkin Trans. 2 1988, 1988, 97–99. [Google Scholar] [CrossRef]
- Kako, Y.; Toyoda, Y.; Hatanaka, Y.; Suwa, Y.; Nukaya, H.; Nagao, M. Inhibition of mutagenesis by p-aminobenzoic acid as a nitrite scavenger. Mutat. Res. 1992, 282, 119–125. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Iwaniw, M.A.; Fogler, H.S. Kinetics and Mechanism of the Reaction between Ammonium and Nitrite Ions: Experimental and Theoretical Studies. Chem. Eng. Sci. 2003, 58, 4351–4362. [Google Scholar] [CrossRef]
- Kato, T.; Kikugawa, K. Proteins and Amino Acids as Scavengers of Nitrite: Inhibitory Effect on the Formation of Nitrosodimethylamine and Diazoquinone. Food Chem. Toxicol. 1992, 30, 617–626. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Takeda, K. Benzoic Acid and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2000; Volume 5, pp. 329–342. [Google Scholar]
- Chaudhary, P.; Gupta, S.; Muniyappan, N.; Sabiah, S.; Kandasamy, J. An Efficient Synthesis of N-Nitrosamines under Solvent, Metal and Acid Free Conditions Using Tert -Butyl Nitrite. Green Chem. 2016, 18, 2323. [Google Scholar] [CrossRef]
- Bugarin, A.; Jones, K.D.; Connell, B.T. Efficient, Direct α-Methylenation of Carbonyls Mediated by Diisopropylammonium Trifluoroacetate. Chem. Commun. 2010, 46, 1715–1717. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Baydar, T. Evaluation of the Protective Effect of Ascorbic Acid on Nitrite- and Nitrosamine-Induced Cytotoxicity and Genotoxicity in Human Hepatoma Line. Toxicol. Mech. Methods 2010, 20, 45–52. [Google Scholar] [CrossRef]
- European Federation of Pharmaceutical Industries and Associations. N-Nitrosamine Impurities in Biological Medicinal Products. Available online: https://efpia.eu/media/580595/n-nitrosamine-impurities-in-biological-medicinal-products.pdf (accessed on 18 October 2022).
- Mergens, W.J.; Newmark, H.L. Antioxidants as Blocking Agents against Nitrosamine Formation. In Autoxidation in Food and Biological Systems; Simic, M.G., Karel, M., Eds.; Springer: New York, NY, USA, 1980; pp. 387–403. [Google Scholar]
- Digenis, G.A.; Issidorides, C.H. Some Biochemical Aspects of N-Nitroso Compounds. Bioorg. Chem. 1979, 8, 97–137. [Google Scholar] [CrossRef]
- Delmas, D.; Lan, A.; Colin, D.; Jannin, B.; Lançon, A.; Latruffe, N. Resveratrol as a Chemopreventive Agent: A Promising Molecule for Fighting Cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Mahal, H.S.; Mukherjee, T. Scavenging of Reactive Oxygen Radicals by Resveratrol: Antioxidant Effect. Res. Chem. Intermed. 2006, 32, 59–71. [Google Scholar] [CrossRef]
- Botterweck, A.A.M.; Verhagen, H.; Goldbohm, R.A.; Kleinjans, J.; van den Brandt, P.A. Intake of Butylated Hydroxyanisole and Butylated Hydroxytoluene and Stomach Cancer Risk: Results from Analyses in the Netherlands Cohort Study. Food Chem. Toxicol. 2000, 38, 599–605. [Google Scholar] [CrossRef]
- Braida, W.; Ong, S.K. Decomposition of Nitrite under Various PH and Aeration Conditions. Water Air Soil Pollut. 2000, 118, 13–26. [Google Scholar] [CrossRef]
- Boetzel, R.; Schlingemann, J.; Hickert, S.; Korn, C.; Kocks, G.; Luck, B.; Blom, G.; Harrison, M.; François, M.; Allain, L.; et al. A Nitrite Excipient Database: A Useful Tool to Support N-Nitrosamine Risk Assessments for Drug Products. J. Pharm. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Byrn, S.R. Mechanisms of Solid-state Reactions of Drugs. J. Pharm. Sci. 1976, 65, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Singh, R.J.; Singh, N.P. Organic Solid State Reactivity. Tetrahedron 1994, 50, 6441–6493. [Google Scholar] [CrossRef]
- Ropp, R.C. Chapter 4—Mechanisms and Reactions in the Solid State. In Solid State Chemistry; Ropp, R.C., Ed.; Elsevier Science: New York, NY, USA, 2003; pp. 129–189. ISBN 9780444514363. [Google Scholar]
- Wang, Z. Fischer-Hepp Rearrangement. In Comprehensive Organic Name Reactions and Reagents; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2010; pp. 1091–1094. ISBN 9780471704508. [Google Scholar]
- Gowenlock, B.G.; Pfab, J.; Young, V.M. Photolysis of Some N-Nitroso- and N-Nitro-Anilines in Solution. J. Chem. Soc. Perkin Trans. 2 1997, 1997, 915–919. [Google Scholar] [CrossRef]
- Dix, L.R.; Oh, S.M.N.Y.F.; Williams, D.L.H. Denitrosation of Nitrosamines-a Quantitative Study. Reactions of N-Methyl-N-Nitrosoaniline, N-Nitrosoproline, Dimethyl Nitrosamine and N-Nitrososarcosine. J. Chem. Soc. Perkin Trans. 2 1991, 1991, 1099–1104. [Google Scholar] [CrossRef]
- Al-Kaabi, S.S.; Hallett, G.; Meyer, T.A.; Williams, D.L.H. Unusual Rate-Limiting Proton Transfer in the Acid-Catalysed Reactions of N-Nitroso Compounds. J. Chem. Soc. Perkin Trans. 2 1984, 1984, 1803–1807. [Google Scholar] [CrossRef]
- Johal, S.S.; Williams, D.L.H.; Buncel, E. Kinetics and Mechanism of the Denitrosation of Nitrosamines in Ethanol. J. Chem. Soc. Perkin Trans. 2 1980, 1980, 165–169. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Cisneros, R.; Derosier, F. Maltol: To the End of Nitrosamines in Nail Polish? Available online: https://www.premiumbeautynews.com/en/maltol-to-the-end-of-nitrosamines,19250 (accessed on 18 October 2022).
- Jutkus, R.A.L.; Li, N.; Taylor, L.S.; Mauer, L.J. Effect of Temperature and Initial Moisture Content on the Chemical Stability and Color Change of Various Forms of Vitamin C. Int. J. Food Prop. 2015, 18, 862–879. [Google Scholar] [CrossRef]
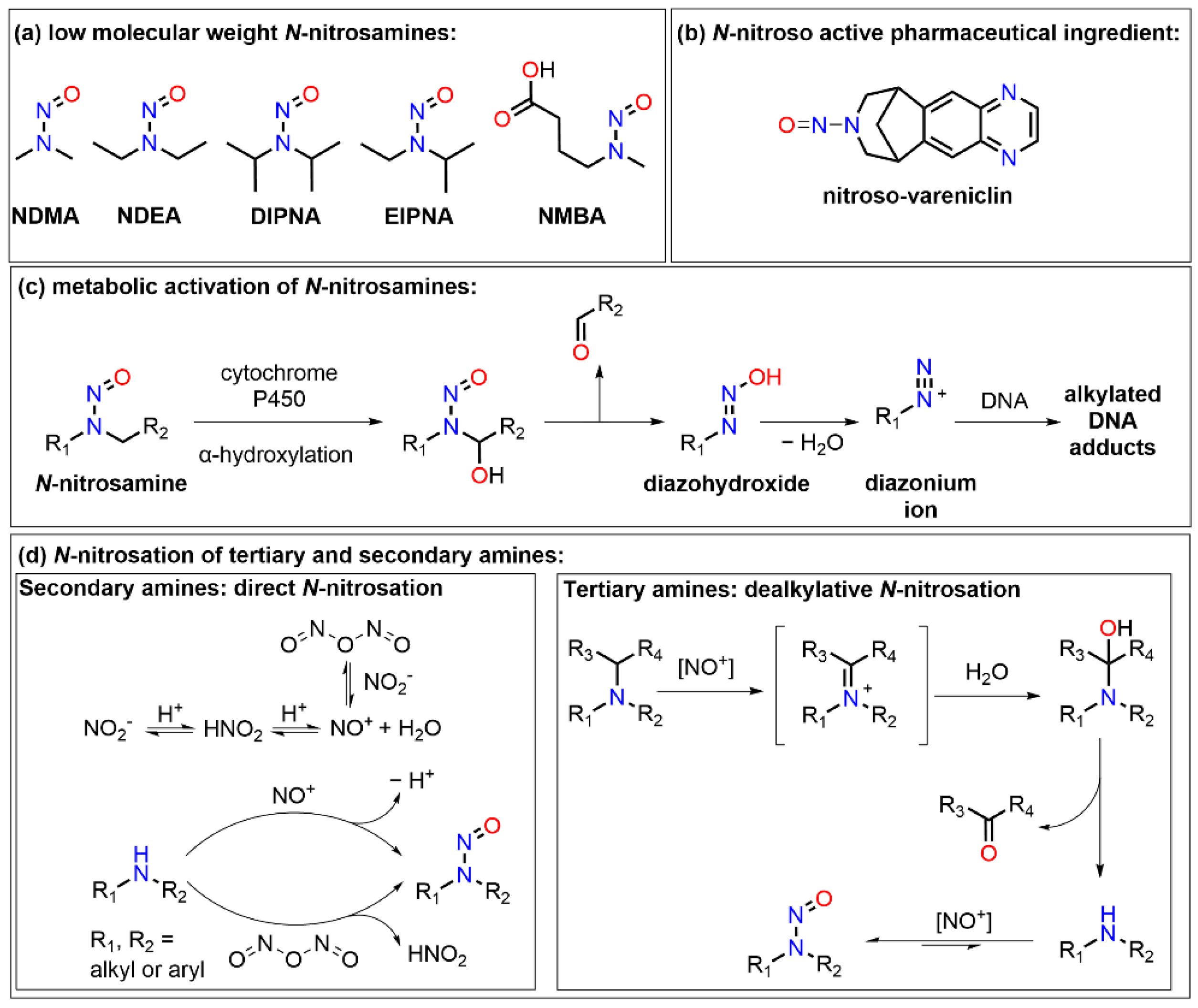
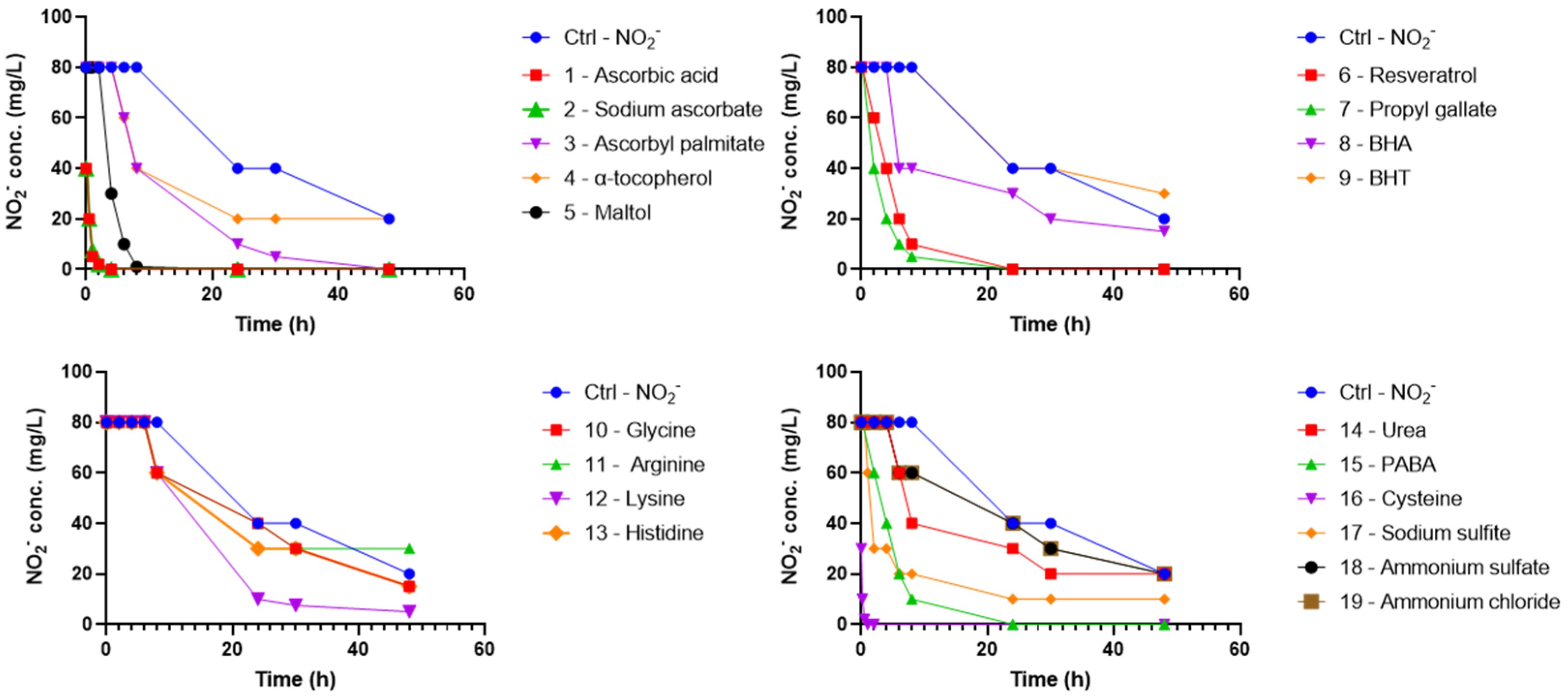
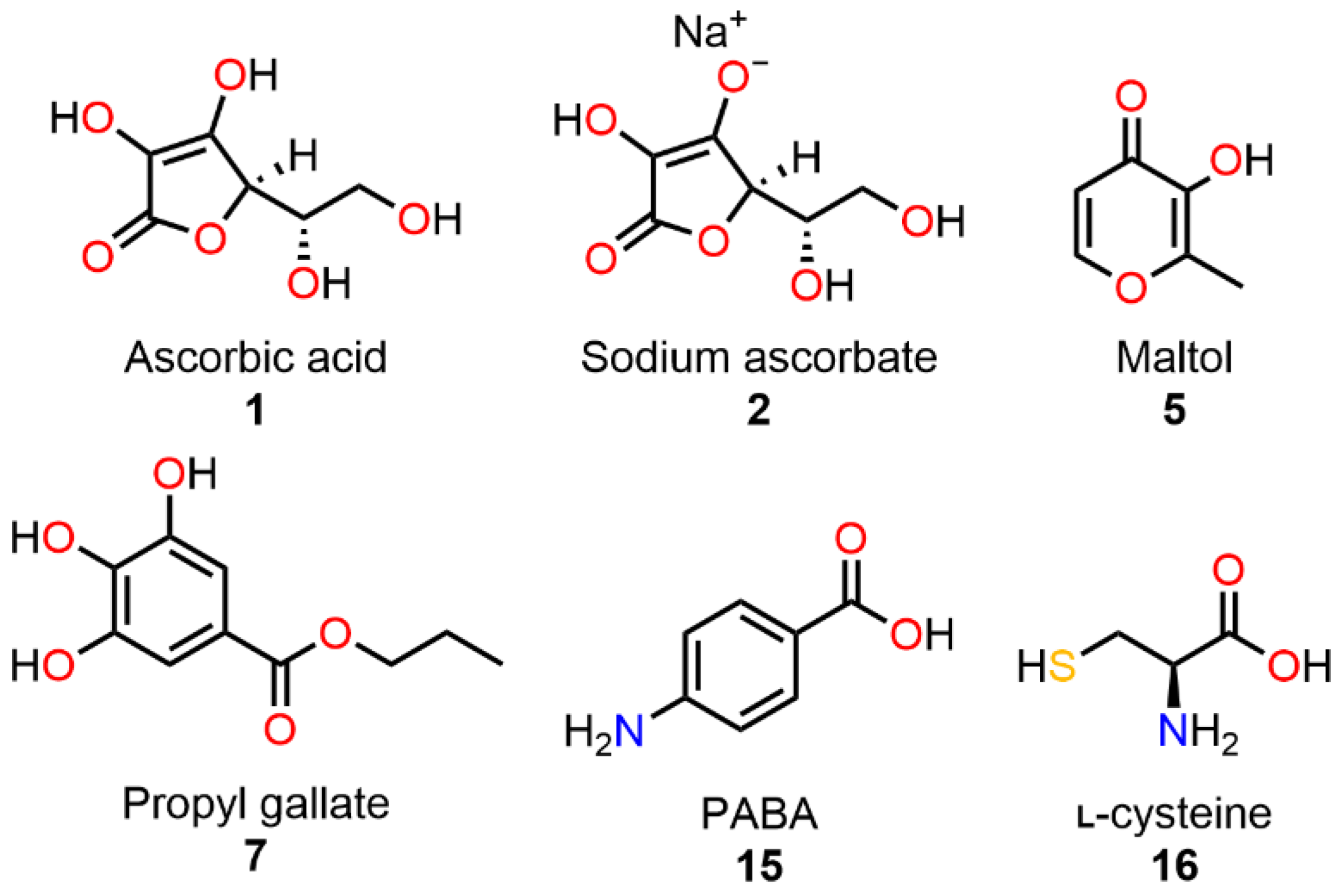
| Ingredient | Control Tablets [w/w%] | NO2− Spiked Control Tablets [w/w%] | Tablets with Scavenger [w/w%] | NO2− Spiked Tablets with Scavenger 1 [w/w%] |
|---|---|---|---|---|
| API (MA × HCl/PP × HCl) | 8.3 | 8.3 | 8.2 | 8.2 |
| Scavenger | 0 | 0 | 0.82 | 0.82 |
| MS | 0.83 | 0.83 | 0.82 | 0.82 |
| MCC | 83 | 0 | 82 | 0 |
| NO2− spiked MCC | 0 | 83 | 0 | 82 |
| PVP | 8.3 | 8.3 | 8.2 | 8.2 |
| Scavenger Number | Scavenger Name | Scavenger Number | Scavenger Name |
|---|---|---|---|
| 1 | Ascorbic acid [37,39,59] | 11 | Arginine [60] |
| 2 | Sodium ascorbate [39] | 12 | Lysine [39,60] |
| 3 | Ascorbyl palmitate [42,44,61] | 13 | Histidine [39] |
| 4 | α-tocopherol [37] | 14 | Urea [48,62] |
| 5 | Maltol [44] | 15 | PABA [52] |
| 6 | Resveratrol [63,64] | 16 | l-Cysteine [37] |
| 7 | Propyl gallate [42,61] | 17 | Sodium sulfite [61,62] |
| 8 | BHA [65] | 18 | Ammonium sulfate [53,62] |
| 9 | BHT [65] | 19 | Ammonium chloride [53] |
| 10 | Glycine [39,60] | Ctrl | NO2− 1 |
| Scavenger Number | Initial NMA [ppm] no NO2− Spiking | Final NMA [ppm] no NO2− Spiking 3 | Initial NMA [ppm] with NO2− Spiking | Final NMA [ppm] with NO2− Spiking 3 |
|---|---|---|---|---|
| Ctrl 2 | 16.82 | n.d. 4 | 33.78 | n.d. 4 |
| 1 | 14.84 (−11.8%) | n.d. 4 | 46.75 (+38.4%) | n.d. 4 |
| 2 | 14.47 (−13.9%) | n.d. 4 | 28.66 (−15.2%) | n.d. 4 |
| 5 | 20.74 (+23.4%) | n.d. 4 | 54.12 (+60.2%) | n.d. 4 |
| 7 | 21.23 (+26.3%) | n.d. 4 | 97.31 (+188.1%) | n.d. 4 |
| 15 | 21.69 (29.0%) | n.d. 4 | 28.60 (−15.3%) | n.d. 4 |
| 16 | 25.95 (+54.3%) | n.d. 4 | 36.76 (+8.8%) | n.d. 4 |
| Scavenger Number | Initial NPP [ppm] No NO2− Spiking | Final NPP [ppm] No NO2− Spiking 3 | Initial NPP [ppm] with NO2− Spiking | Final NPP [ppm] with NO2− Spiking 3 |
|---|---|---|---|---|
| Ctrl 2 | 1.98 | 2.37 | n.d. 4 | 2.67 |
| 1 | 1.89 (−4.2%) | n.d.4 (−100.0%) | n.d. 4 | 1.29 (−51.8%) |
| 2 | n.d. 4 (−100.0%) | 7.84 (+128.4%) | n.d. 4 | 11.18 (+316.2%) |
| 5 | n.d. 4 (−100.0%) | 2.53 (+8.0%) | n.d. 4 | 2.96 (+11.0%) |
| 7 | n.d. 4 (−100.0%) | 2.50 (+7.6%) | n.d. 4 | 2.74 (+1.8%) |
| 15 | n.d. 4 (−100.0%) | 1.98 (−15.3%) | 1.88 (N.A.) 5 | 1.07 (−60.2%) |
| 16 | 2.02 (+2.3%) | 1.06 (−55.4%) | n.d. 4 | 2.42 (−10.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homšak, M.; Trampuž, M.; Naveršnik, K.; Kitanovski, Z.; Žnidarič, M.; Kiefer, M.; Časar, Z. Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives. Processes 2022, 10, 2428. https://doi.org/10.3390/pr10112428
Homšak M, Trampuž M, Naveršnik K, Kitanovski Z, Žnidarič M, Kiefer M, Časar Z. Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives. Processes. 2022; 10(11):2428. https://doi.org/10.3390/pr10112428
Chicago/Turabian StyleHomšak, Miha, Marko Trampuž, Klemen Naveršnik, Zoran Kitanovski, Mateja Žnidarič, Markus Kiefer, and Zdenko Časar. 2022. "Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives" Processes 10, no. 11: 2428. https://doi.org/10.3390/pr10112428
APA StyleHomšak, M., Trampuž, M., Naveršnik, K., Kitanovski, Z., Žnidarič, M., Kiefer, M., & Časar, Z. (2022). Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives. Processes, 10(11), 2428. https://doi.org/10.3390/pr10112428