Chemical Constituents, Quantitative Analysis, Anti-SARS-CoV-2 and Antioxidant Activities of Herbal Formula “Ping An Fang Yu Yin”
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Reagent
2.2. Fingerprints Analysis in PAFYY
2.3. Bioactivity Assay
2.4. Molecular Modeling Docking Study
3. Results
3.1. Determination of Multiple Chemical Ingredients
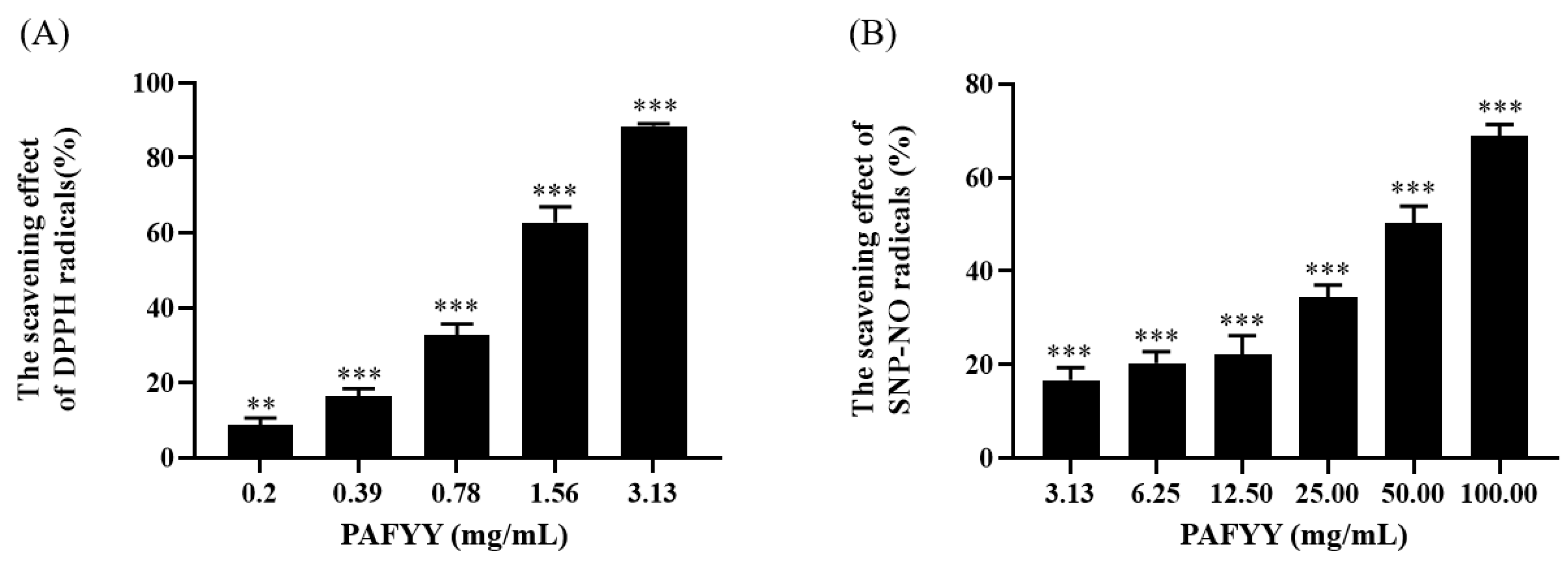
3.2. Anti-Oxidant Activity of PAFYY and Herbs Extraction
3.3. Anti-LOX Activity of PAFYY and Herbs Extraction
3.4. Effect of PAFYY and Herbs Extraction on SARS-CoV-2 Spike with ACE2 Interaction
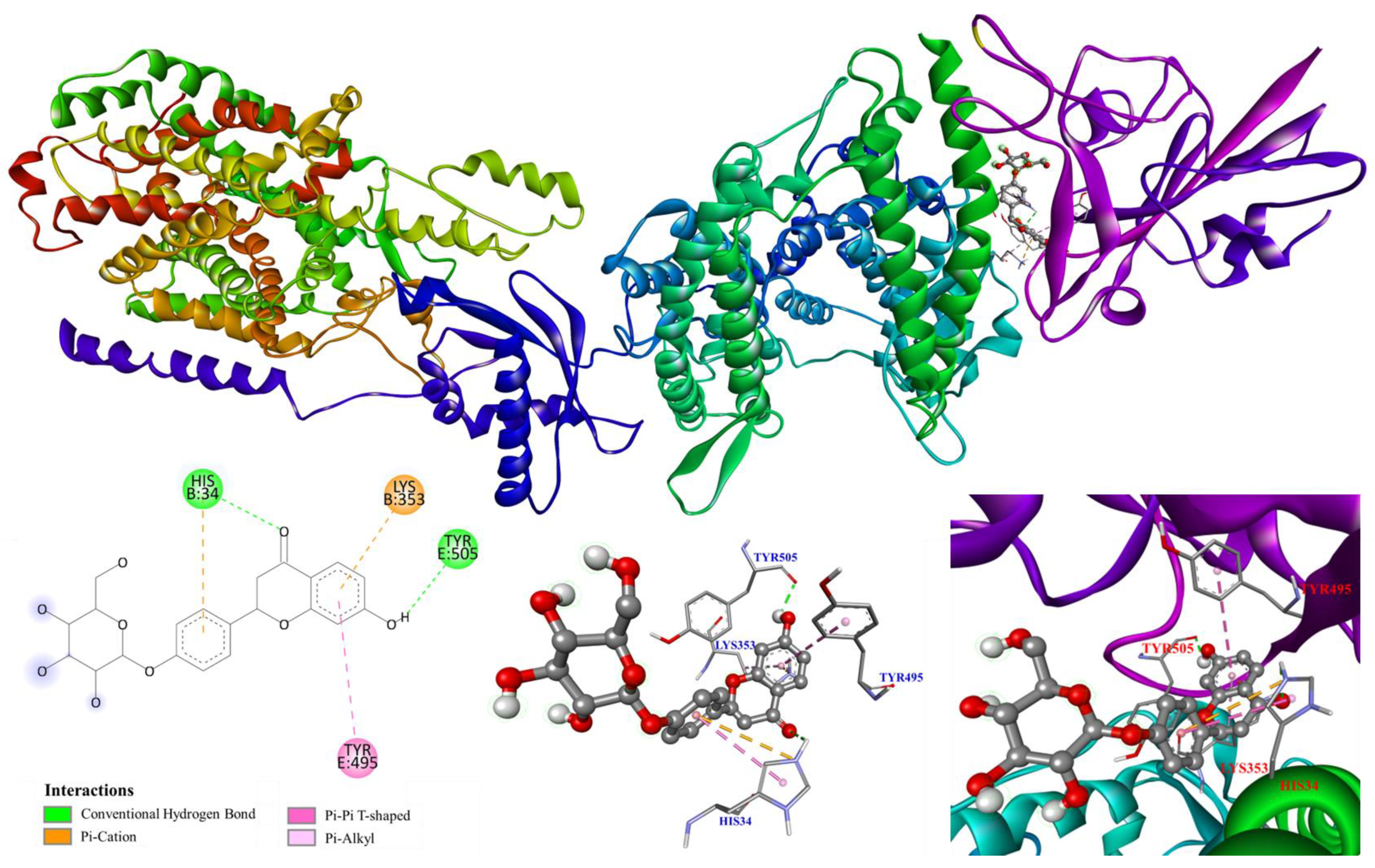
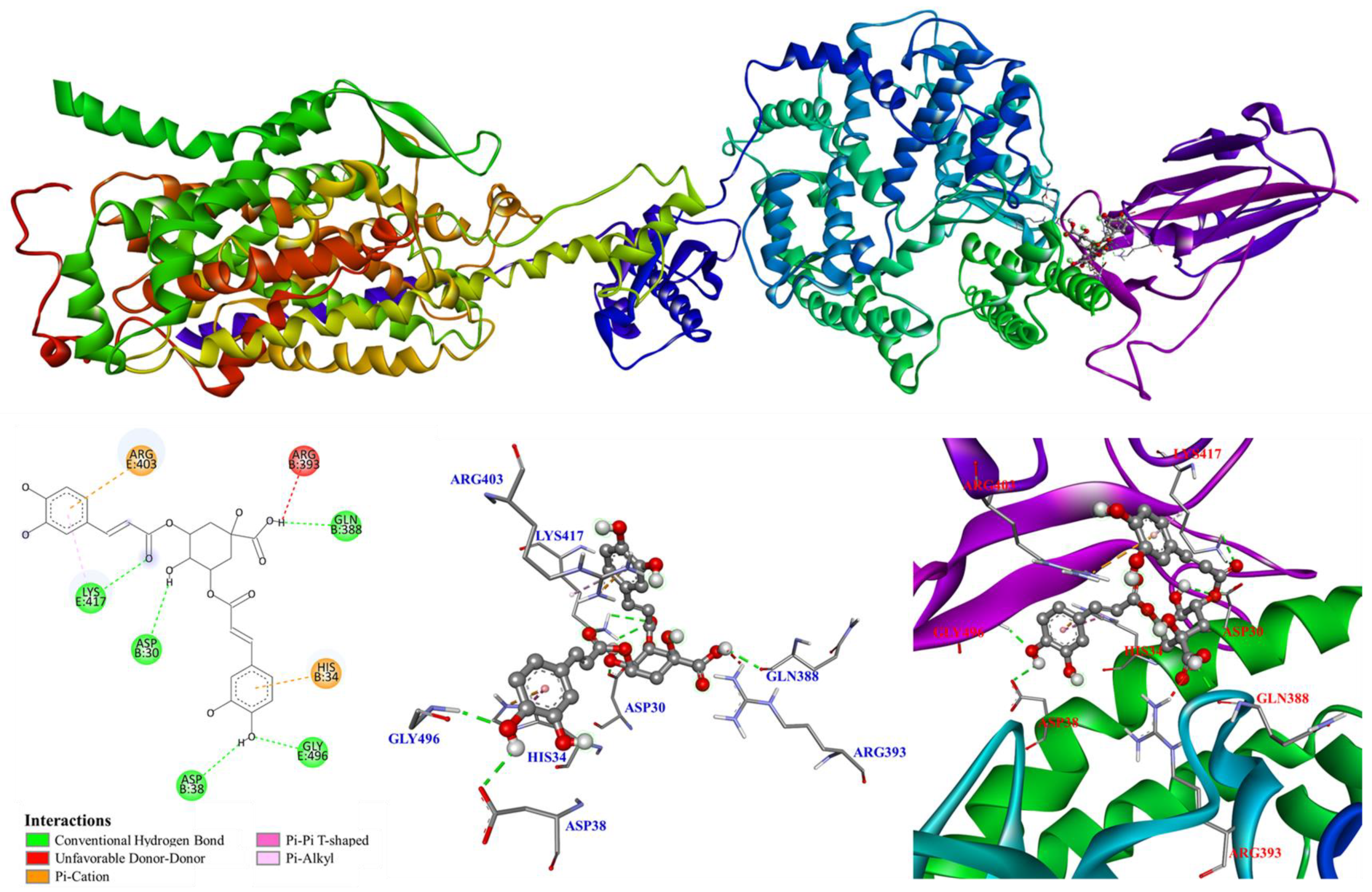
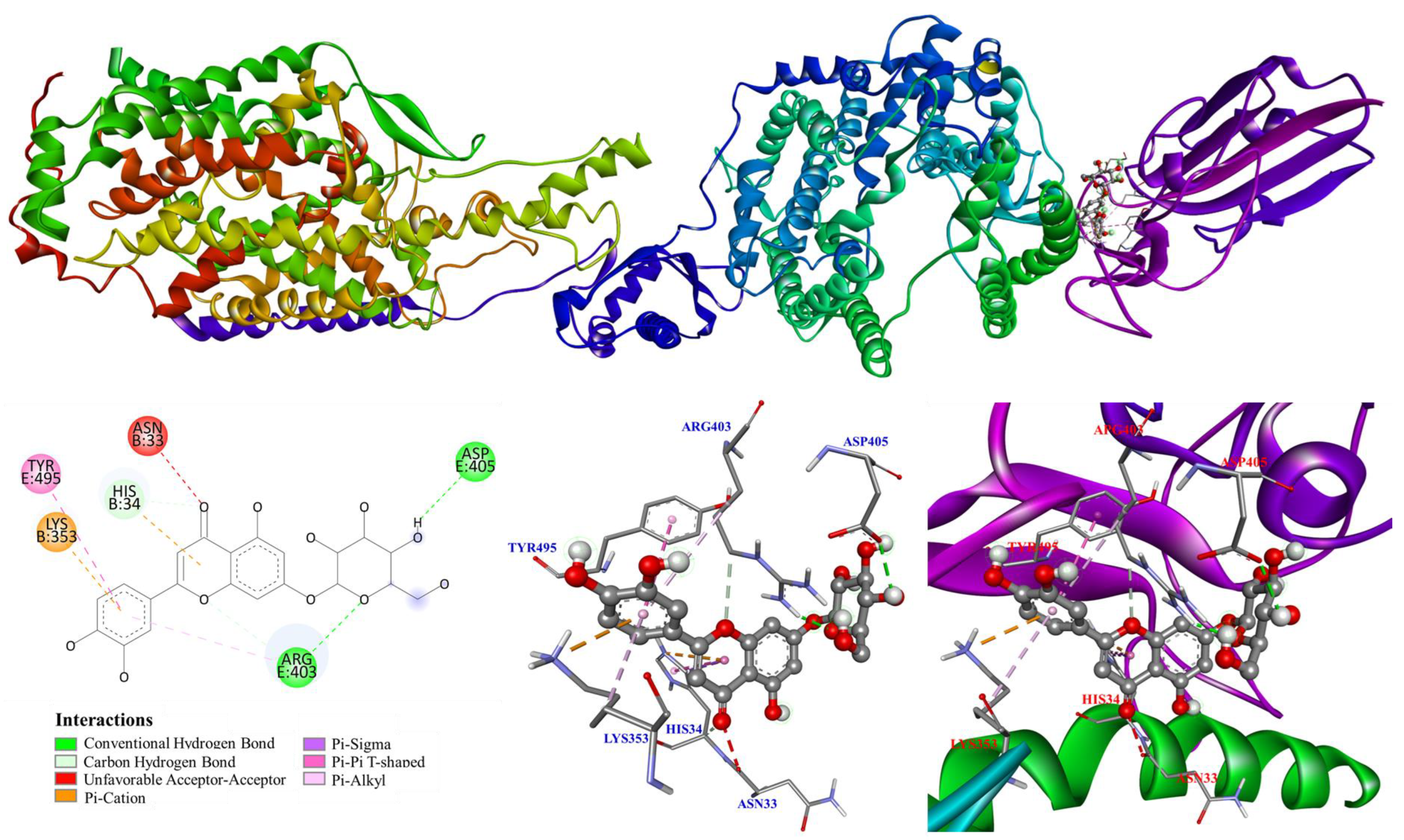
3.5. Molecular Modeling Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 September 2022).
- World Health Organization. Coronavirus Disease (COVID-19). Available online: https://www.who.int/health-topics/coronavirus#tab=tab_1 (accessed on 6 June 2022).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta. Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune response in COVID-19: What is next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Ripon, M.A.R.; Bhowmik, D.R.; Amin, M.T.; Hossain, M.S. Role of arachidonic cascade in COVID-19 infection: A review. Prostaglandins Other Lipid Mediat. 2021, 154, 106539. [Google Scholar] [CrossRef] [PubMed]
- Ayola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S.; et al. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm. Res. 2021, 70, 877–889. [Google Scholar] [CrossRef]
- DE Flora, S.; Balansky, R.; LA Maestra, S. Antioxidants and COVID-19. J. Prev. Med. Hyg. 2021, 62 (Suppl. S3), 34–45. [Google Scholar]
- Tsai, C.I.; Chiou, C.T.; Lin, C.J.; Wei, W.C.; Lin, S.J.; Tseng, Y.H.; Yeh, K.M.; Lin, Y.L.; Jan, J.T.; Liang, J.J.; et al. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: A bedside-to-bench study. Biomed. Pharmacother. 2021, 133, 111037. [Google Scholar] [CrossRef]
- Ping, Y.H.; Yeh, H.; Chu, L.W.; Lin, Z.H.; Hsu, Y.C.; Lin, L.C.; Hsu, C.H.; Fu, S.L.; Lin, T.Y. The traditional Chinese medicine formula Jing Guan Fang for preventing SARS-CoV-2 infection: From clinical observation to basic research. Front. Pharmacol. 2022, 13, 744439. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhou, L.; Zhou, X.; Xie, B.; Zhang, W.; Sun, J. Prevention and treatment of COVID-19 using traditional Chinese medicine: A review. Phytomedicine 2021, 85, 153308. [Google Scholar] [CrossRef]
- Li, B.H.; Li, Z.Y.; Liu, M.M.; Tian, J.Z.; Cui, Q.H. Progress in traditional Chinese medicine against respiratory viruses: A review. Front. Pharmacol. 2021, 12, 743623. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Fan, G.; Xiao, G.; Wang, T.; Xu, D.; Gao, J.; Ge, S.; Li, Q.; Ma, Y.; Zhang, H.; et al. Traditional Chinese medicine in COVID-19. Acta. Pharm. Sin. B 2021, 11, 3337–3363. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Fang, S.; Zhang, J.; Pan, Y.; Liu, H.; Wang, Y.; Li, M.; Liu, L. Chinese herbal compounds against SARS-CoV-2: Puerarin and quercetin impair the binding of viral S-protein to ACE2 receptor. Comput. Struct. Biotechnol. J. 2020, 18, 3518–3527. [Google Scholar] [CrossRef]
- Serlahwaty, D.; Giovani, C. In silico screening of mint leaves compound (Mentha piperita L.) as a potential inhibitor of SARS-CoV-2. Pharm. Educ. 2021, 21, 81–86. [Google Scholar]
- Tang, W.F.; Tsai, H.P.; Chang, Y.H.; Chang, T.Y.; Hsieh, C.F.; Lin, C.Y.; Lin, G.H.; Chen, Y.L.; Jheng, J.R.; Liu, P.C.; et al. Perilla (Perilla frutescens) leaf extract inhibits SARS-CoV-2 via direct virus inactivation. Biomed. J. 2021, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tao, G.; Liu, J.; Cai, J.; Huang, Z.; Chen, J. Current Prevention of COVID-19: Natural products and herbal medicine. Front. Pharmacol. 2022, 11, 588508. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Liang, Y.Q.; Tang, L.J.; Ding, Y.; Wang, X.H. Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA. hy926 cells. Exp. Ther. Med. 2013, 6, 199–203. [Google Scholar] [CrossRef]
- Prieto, J.M.; Schinella, G.R. Anti-inflammatory and antioxidant Chinese herbal medicines: Links between traditional characters and the skin lipoperoxidation “Western” model. Antioxidants 2022, 11, 611. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Yu, X.; Jiang, W.; Chen, S.; Wang, R.; Wang, M.; Jiao, S.; Yang, Y.; Wang, W.; et al. Antibody-dependent enhancement of SARS-CoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Commun. Biol. 2022, 5, 262. [Google Scholar] [CrossRef]
- Lin, I.H.; Lee, M.C.; Chuang, W.C. Application of LC/MS and ICP/MS for establishing the fingerprint spectrum of the traditional Chinese medicinal preparation Gan-Lu-Yin. J. Sep. Sci. 2006, 29, 172–179. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA Discovery Studio Client 2021, v.21.1.0; Dassault Systèmes: San Diego, CA, USA, 2021.
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Hsiao, P.C.; Kuo, T.C.; Chiang, S.T.; Chen, S.L.; Chiou, S.J.; Ling, X.H.; Liang, M.T.; Cheng, W.Y.; Houng, J.Y. Antioxidant and anti-inflammatory activities of Lonicera japonica Thunb. var. sempervillosa Hayata flower bud extracts prepared by water, ethanol, and supercritical fluid extraction techniques. Ind. Crops Prod. 2016, 89, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Peng, P.; Cao, X.; Wu, K.; Chen, J.; Wang, K.; Tang, N.; Huang, A.L. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol. Immunol. 2022, 19, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Chirumbolo, S.; Peana, M.; Noor, S.; Menzel, A.; Dadar, M.; Bjørklund, G. The role of diet and supplementation of natural products in COVID-19 prevention. Biol. Trace Elem. Res. 2022, 200, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Al-Doori, A.; Ahmed, D.; Kadhom, M.; Yousif, E. Herbal medicine as an alternative method to treat and prevent COVID-19. Baghdad. J. Biochem. Appl. Biol. Sci. 2021, 2, 1–20. [Google Scholar] [CrossRef]
- Rathinavel, T.; Meganathan, B.; Kumarasamy, S.; Ammashi, S.; Thangaswamy, S.; Ragunathan, Y.; Palanisamy, S. Potential COVID-19 drug from natural phenolic compounds through in silico virtual screening approach. Biointerface Res. Appl. Chem. 2021, 11, 10161–10173. [Google Scholar]
- Kang, X.; Jin, D.; Jiang, L.; Zhang, Y.; Zhang, Y.; An, X.; Duan, L.; Yang, C.; Zhou, R.; Duan, Y.; et al. Efficacy and mechanisms of traditional Chinese medicine for COVID-19: A systematic review. Chin. Med. 2022, 17, 30. [Google Scholar] [CrossRef]
- Senapati, S.; Banerjee, P.; Bhagavatula, S.; Kushwaha, P.P.; Kumar, S. Contributions of human ACE2 and TMPRSS2 in determining host-pathogen interaction of COVID-19. J. Genet. 2021, 100, 12. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gong, K.; Qin, Y.; Yuan, Z.; Xia, S.; Zhang, S.; Yang, J.; Yang, P.; Li, L.; Xie, M. In silico analysis of the potential mechanism of a preventive Chinese medicine formula on coronavirus disease 2019. J. Ethnopharmacol. 2021, 275, 114098. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Chaple, D.; Arora, S.; Yende, S.; Mehta, C.; Nayak, U. Prospecting for Cressa cretica to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 40, 5643–5652. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Abdalla, M.; Sharaf, M.; Al-Resayes, S.; Imededdine, K.; Mahboob, A.; Yagi, S.; Azam, M.; Yousfi, M. In-silico investigation of phenolic compounds from leaves of Phillyrea angustifolia L. as a potential inhibitor against the SARS-CoV-2 main protease (Mpro PDB ID:5R83) using a virtual screening method. J. Saudi Chem. Soc. 2022, 26, 101473. [Google Scholar]
- Mbikay, M.; Chrétien, M. Isoquercetin as an anti-COVID-19 medication: A potential to realize. Front. Pharmacol. 2022, 13, 830205. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2022, 11, 570122. [Google Scholar] [CrossRef]
- Wu, J. Tackle the free radicals damage in COVID-19. Nitric Oxide. 2020, 102, 39–41. [Google Scholar] [CrossRef]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy Metal Contaminations in Herbal Medicines: Determination, Comprehensive Risk Assessments, and Solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef]
- Balekundri, A.; Mannur, V. Quality control of the traditional herbs and herbal products: A review. Futur. J. Pharm. Sci. 2020, 6, 67. [Google Scholar] [CrossRef]
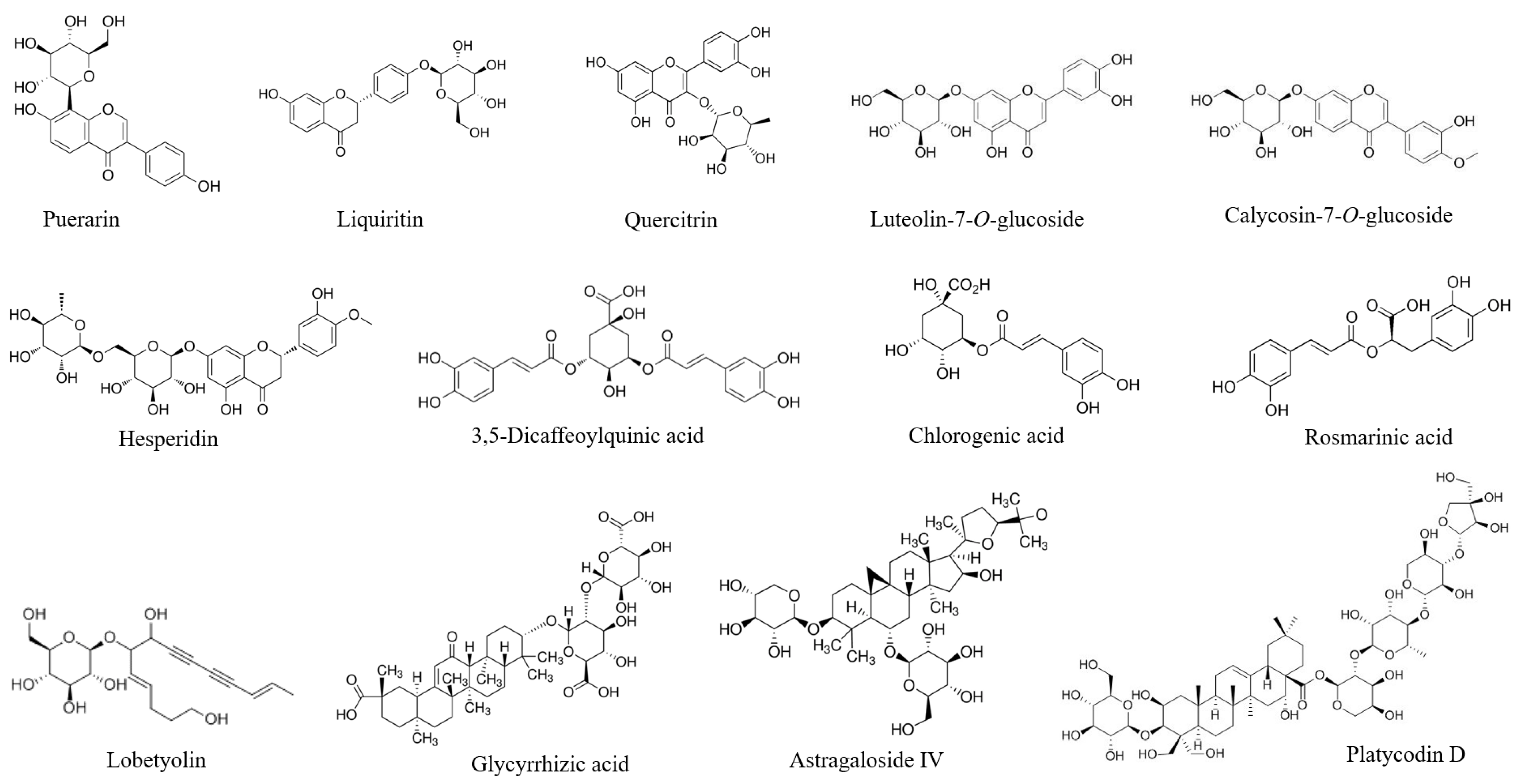
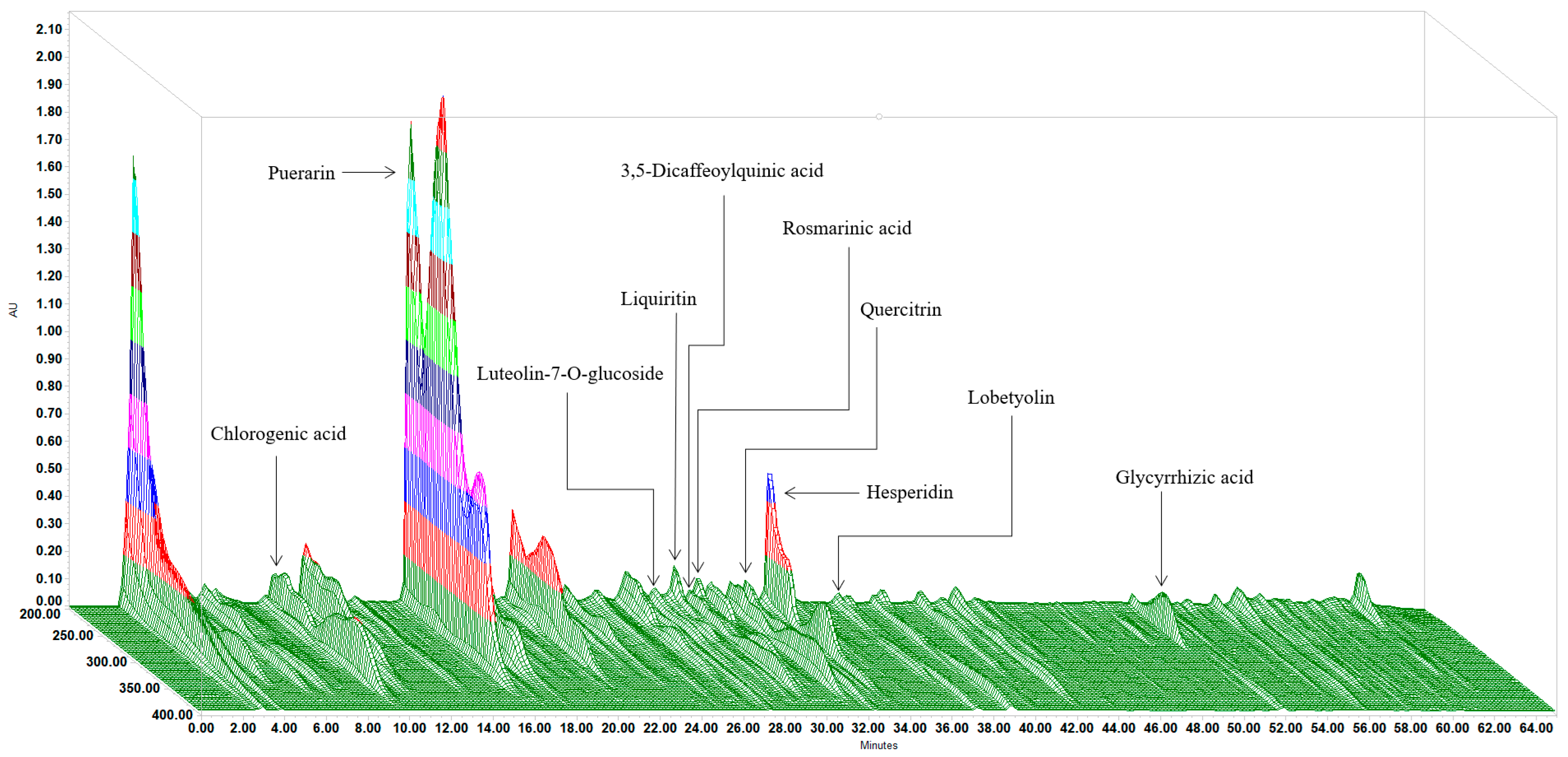
| Herbal Material in PAFYY | Reference Substance | Retention Time (min) | Content (mg/g) |
|---|---|---|---|
| Astragalus | Astragaloside IV | 32.00 | 0.12 |
| Astragalus | Calycosin-7-O-glucoside | 25.49 | BQL |
| Houttuynia | Quercitrin | 32.64 | 0.50 |
| Houttuynia, Honeysuckle flower | Chlorogenic acid | 9.96 | 2.98 |
| Honeysuckle flower | 3,5-Dicaffeoylquinic acid | 29.77 | 0.55 |
| Honeysuckle flower | Luteolin-7-O-glucoside | 28.45 | 0.10 |
| Citrus Peel | Hesperidin | 33.70 | 2.49 |
| Codonopsis | Lobetyolin, | 36.04 | 0.16 |
| Licorice | Glycyrrhizic acid | 51.02 | 2.00 |
| Licorice | Liquiritin | 26.85 | 1.12 |
| Mentha, Perilla leaf | Rosmarinic acid | 30.13 | 0.27 |
| Platycodon | Platycodin D | 23.5 | BQL |
| Pueraria | Puerarin | 16.39 | 10.90 |
| SC50 (mg/mL) a | ||
|---|---|---|
| Herbs | DPPH Scavenging Assay | SNP-NO Scavenging Assay |
| Houttuynia | 1.72 ± 0.18 *** | 70.80 ± 2.19 |
| Pueraria | 1.07 ± 0.08 *** | 25.99 ± 2.54 *** |
| Astragalus | 24.44 ± 1.34 | >100 |
| Honeysuckle flower | 0.58 ± 0.04 *** | 25.01 ± 4.07 *** |
| Agastache | 3.74 ± 0.62 | 62.26 ± 15.96 |
| Mentha | 0.25 ± 0.00 *** | 19.62 ± 1.65 *** |
| Codonopsis | 11.52 ± 1.85 | >100 |
| Licorice | 2.09 ± 0.17 *** | >100 |
| Platycodon | 6.89 ± 1.03 | >100 |
| Perilla leaf | 0.53 ± 0.05 ** | 12.11 ± 0.46 *** |
| Citrus peel b | 3.96 ± 0.11 | 75.60 ± 3.10 |
| PAFYY | 1.24 ± 0.09 *** | 48.93 ± 4.96 ** |
| Inhibition Rate (%) | |||
|---|---|---|---|
| SOD Activity (%) | LOX Inhibition Rate (%) | Interaction of SARS-CoV-2 Spike with ACE2 Activity (%) | |
| PAFYY | 68.71 ± 1.28 *** | 75.96 ± 7.64 *** | 48.04 ± 3.18 *** |
| Herbs | SOD Activity (Unit/mL) a | LOX IC50 (mg/mL) b | SARS-CoV-2 Spike with ACE2 Interaction Activity IC50 (mg/mL) b |
|---|---|---|---|
| Houttuynia | 14.89 ± 1.56 *** | 2.31 ± 0.14 ** | 22.08 ± 3.70 *** |
| Pueraria d | 3.82 ± 0.16 | >100 | 91.69 ± 7.44 |
| Astragalus | 12.90 ± 1.93 ** | 40.75 ± 21.15 | >100 |
| Citrus peel | 8.43 ± 0.18 ** | >100 | >100 |
| Agastache | 12.91 ± 1.76 ** | >100 | >100 |
| Mentha | 4.21 ± 0.26 | 3.70 ± 0.52 ** | 12.33 ± 2.13 *** |
| Codonopsis | 5.68 ± 0.25 | >100 | 11.29 ± 2.09 *** |
| Licorice | 1.72 ± 0.17 | >100 | 55.53 ± 4.00 |
| Platycodon | 17.29 ± 2.70 ** | 31.16 ± 2.37 | 19.85 ± 2.72 *** |
| Perilla leaf | 3.20 ± 0.04 | >100 | 13.02 ± 1.50 *** |
| Honeysuckle flower c | 6.79 ± 0.23 | 13.83 ± 3.60 | 55.21 ± 31.97 |
| Compounds | Affinity (kcal/mol) |
|---|---|
| Chlorogenic acid | −7.8 |
| 3,5-Dicaffeoylquinic acid | −8.1 |
| Glycyrrhizic acid | −3.5 |
| Hesperidin | −7.7 |
| Liquiritin | −8.2 |
| Lobetyolin | −6.7 |
| Luteolin-7-O-glucoside | −8.1 |
| Puerarin | −7.8 |
| Quercitrin | −7.1 |
| Rosmarinic acid | −8.0 |
| Quercetin a | −7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-C.; Lee, M.-C.; Hsieh, Y.-H.; Wang, K.-T.; Chen, C.-Y.; Chuang, W.-C.; Chen, J.-J. Chemical Constituents, Quantitative Analysis, Anti-SARS-CoV-2 and Antioxidant Activities of Herbal Formula “Ping An Fang Yu Yin”. Processes 2022, 10, 2213. https://doi.org/10.3390/pr10112213
Tsai Y-C, Lee M-C, Hsieh Y-H, Wang K-T, Chen C-Y, Chuang W-C, Chen J-J. Chemical Constituents, Quantitative Analysis, Anti-SARS-CoV-2 and Antioxidant Activities of Herbal Formula “Ping An Fang Yu Yin”. Processes. 2022; 10(11):2213. https://doi.org/10.3390/pr10112213
Chicago/Turabian StyleTsai, Yun-Chen, Ming-Chung Lee, Yu-Hui Hsieh, Kun-Teng Wang, Chao-Yu Chen, Wu-Chang Chuang, and Jih-Jung Chen. 2022. "Chemical Constituents, Quantitative Analysis, Anti-SARS-CoV-2 and Antioxidant Activities of Herbal Formula “Ping An Fang Yu Yin”" Processes 10, no. 11: 2213. https://doi.org/10.3390/pr10112213
APA StyleTsai, Y.-C., Lee, M.-C., Hsieh, Y.-H., Wang, K.-T., Chen, C.-Y., Chuang, W.-C., & Chen, J.-J. (2022). Chemical Constituents, Quantitative Analysis, Anti-SARS-CoV-2 and Antioxidant Activities of Herbal Formula “Ping An Fang Yu Yin”. Processes, 10(11), 2213. https://doi.org/10.3390/pr10112213










