Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture
2.3. Animals
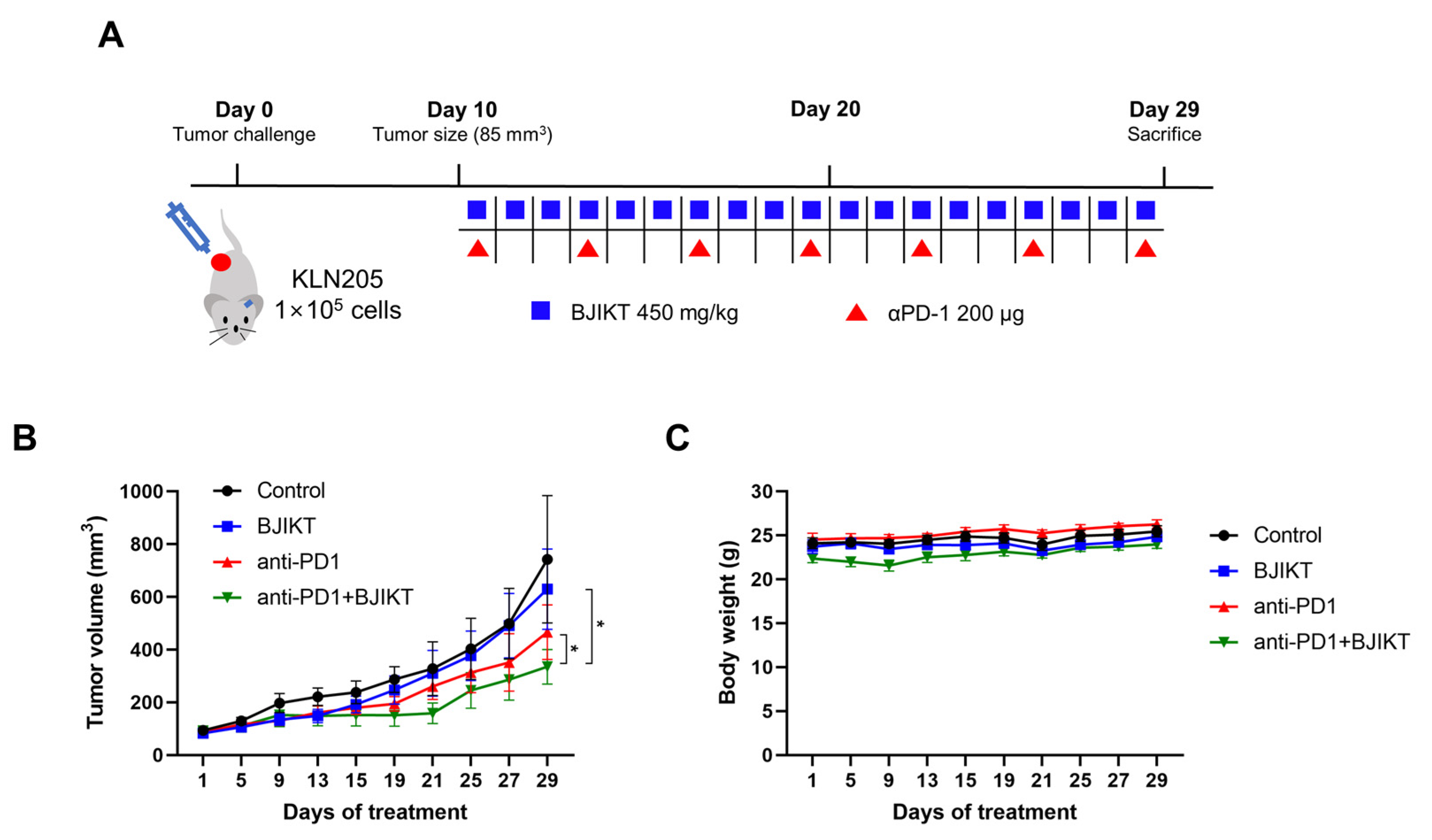
2.4. Establishment of Tumor-Bearing Mice and Treatment
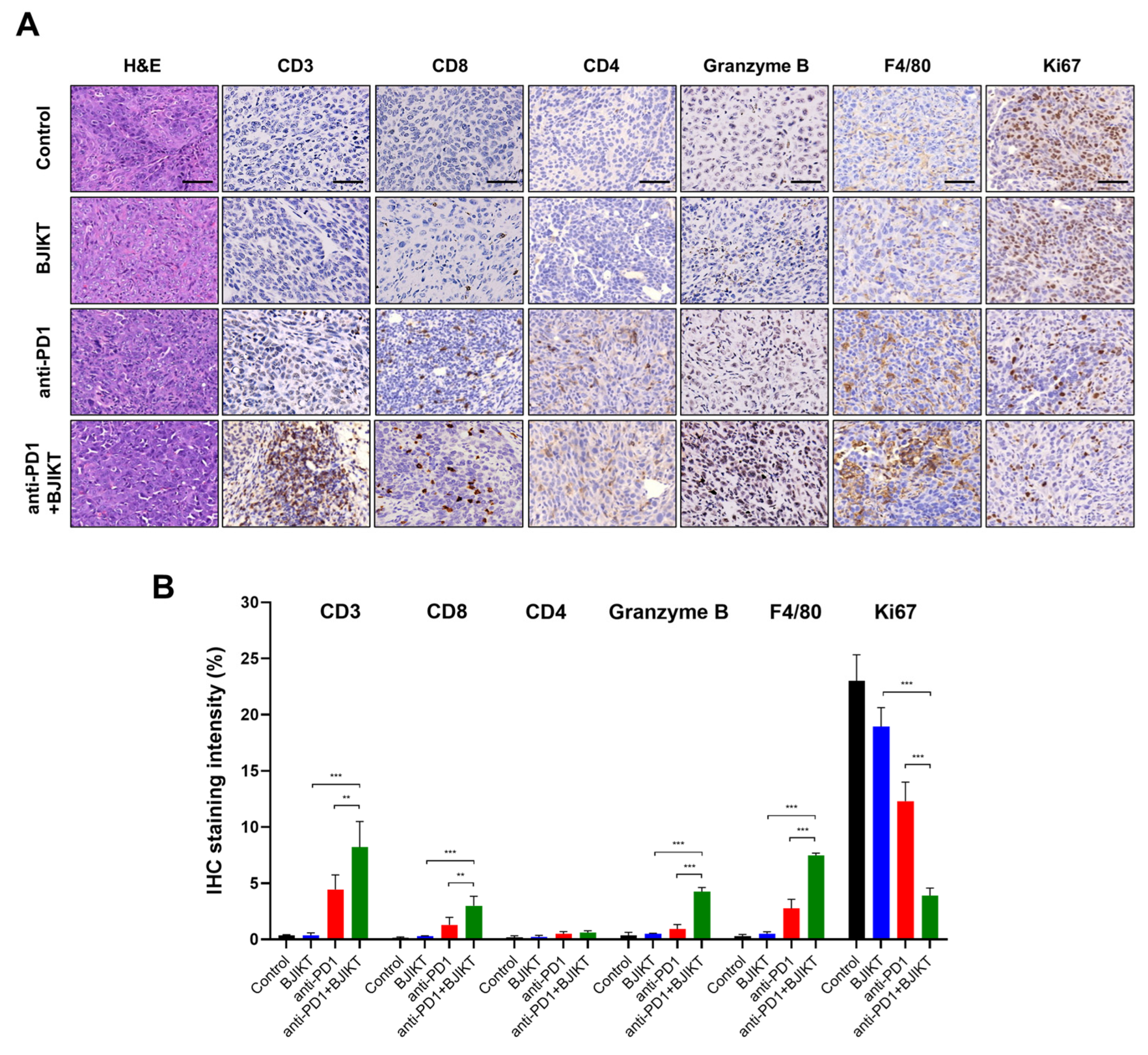
2.5. Immunohistochemistry (IHC)
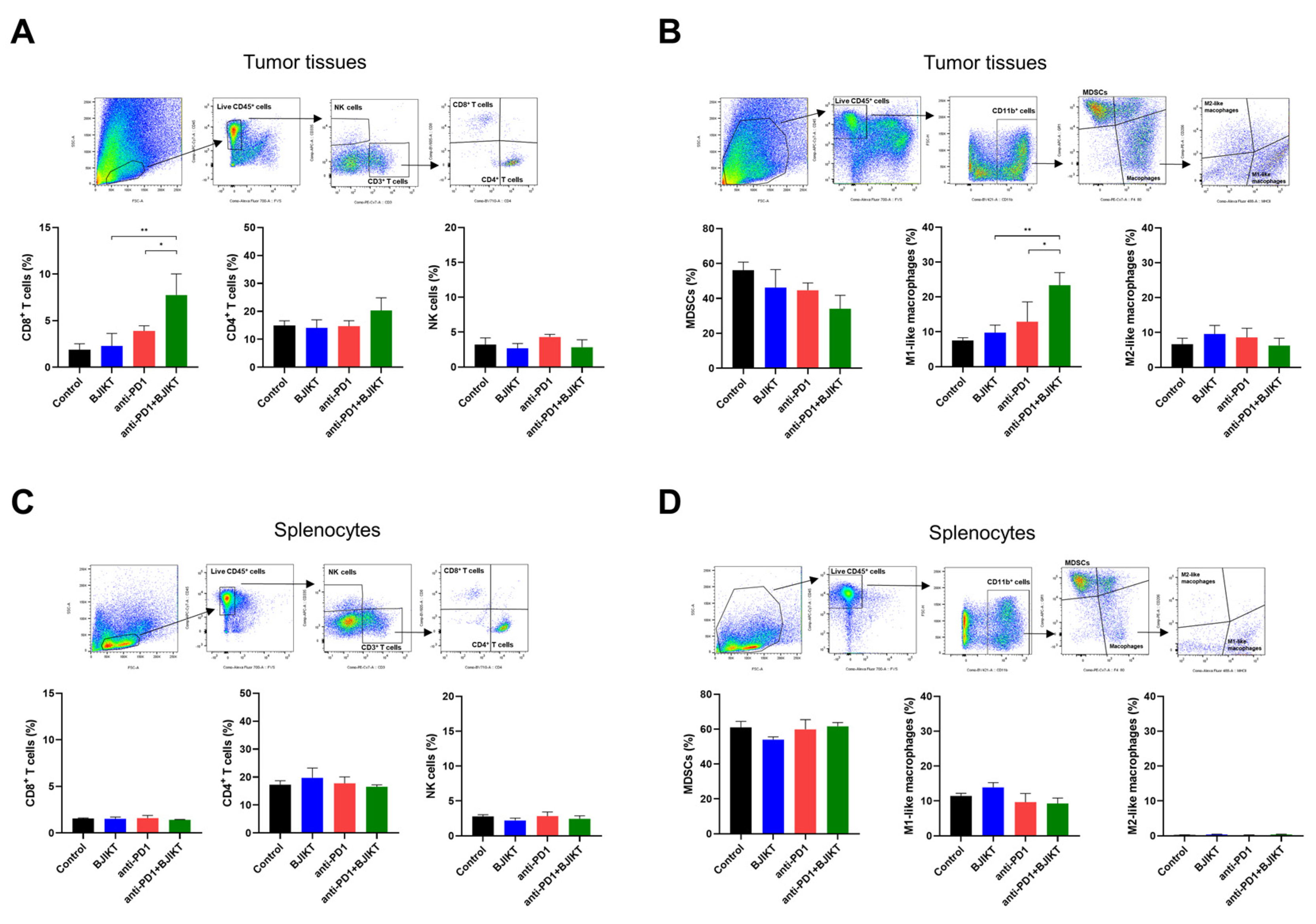
2.6. Flow Cytometry
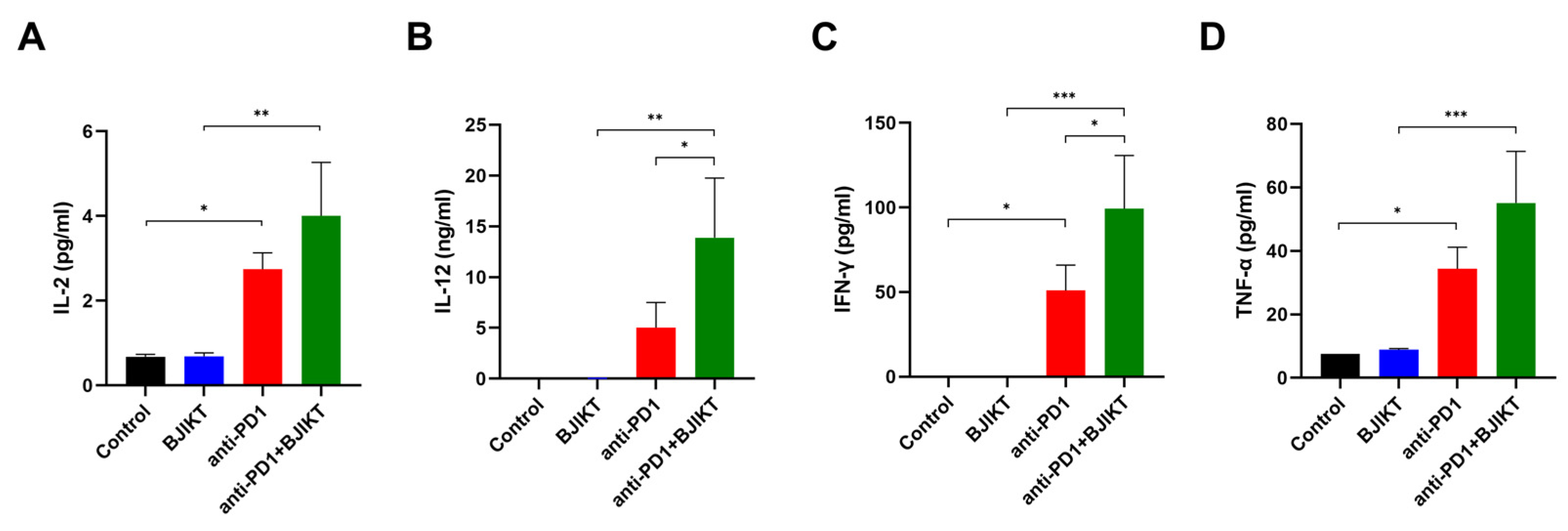
2.7. Measurement of Cytokine Production
2.8. Network Pharmacological Analysis of BJIKT
2.9. Statistical Analysis
3. Results
3.1. BJIKT Plus an Anti-PD-1 Antibody Inhibited Tumor Progression in the Lung Cancer Xenograft Model
3.2. BJIKT Plus an Anti-PD-1 Antibody Increased the Infiltration of Cytotoxic T Lymphocytes (CTLs) and Macrophages
3.3. BJIKT Plus an Anti-PD-1 Antibody Regulated Antitumor Immune Response through CTLs, MDSCs, and Macrophages
3.4. BJIKT Combined with an Anti-PD-1 Antibody Altered the Concentration of Th1 Cytokines in the Peripheral Blood
3.5. Network Pharmacology Analysis Predicted the Target Pathways of BJIKT to Treat NSCLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef]
- Liu, W.-J.; Du, Y.; Wen, R.; Yang, M.; Xu, J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol. Ther. 2020, 206, 107438. [Google Scholar] [CrossRef]
- Assi, H.I.; Kamphorst, A.O.; Moukalled, N.M.; Ramalingam, S.S. Immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer 2018, 124, 248–261. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Vasievich, E.A.; Huang, L. The suppressive tumor microenvironment: A challenge in cancer immunotherapy. Mol. Pharmacol. 2011, 8, 635–641. [Google Scholar] [CrossRef]
- Kumari, S.; Advani, D.; Sharma, S.; Ambasta, R.K.; Kumar, P. Combinatorial therapy in tumor microenvironment: Where do we stand? Biochim. Biophys. Acta 2021, 1876, 188585. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.E.; Kim, J.N.; Kwon, M.J.; Lee, J.R.; Kim, S.C.; Nam, J.H.; Kim, B.J. The traditional medicine bojungikki-tang increases intestinal motility. Phcog. Mag. 2021, 17, 1–8. [Google Scholar] [CrossRef]
- Jeong, J.S.; Ryu, B.H.; Kim, J.S.; Park, J.W.; Choi, W.C.; Yoon, S.W. Bojungikki-tang for cancer-related fatigue: A pilot randomized clinical trial. Integr. Cancer Ther. 2010, 9, 331–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.-J.; Kim, K.-I.; Choi, C.-W.; Kim, J.Y.; Lee, J.-H. Long-term progression-free survival in a patient with advanced non-small-cell lung cancer treated with low-dose gefitinib and traditional herbal medicine: A case report. Medicine 2021, 100, e24292. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kita, K.; Sato, C.; Kaneda, A. Hochuekkito (Buzhongyiqitang), a herbal medicine, enhances cisplatin-induced apoptosis in HeLa cells. Mol. Med. Report. 2015, 12, 6215–6220. [Google Scholar] [CrossRef] [PubMed]
- Utsuyama, M.; Seidlar, H.; Kitagawa, M.; Hirokawa, K. Immunological restoration and anti-tumor effect by Japanese herbal medicine in aged mice. Mech. Ageing Dev. 2001, 122, 341–352. [Google Scholar] [CrossRef]
- Li, T.; Tamada, K.; Abe, K.; Tada, H.; Onoe, Y.; Tatsugami, K.; Harada, M.; Kubo, C.; Nomoto, K. The restoration of the antitumor T cell response from stress-induced suppression using a traditional Chinese herbal medicine Hochu-ekki-to (TJ-41:Bu-Zhong-Yi-Qi-Tang). Immunopharmacology 1999, 43, 11–21. [Google Scholar] [CrossRef]
- Chun, J.; Park, S.-M.; Yi, J.-M.; Ha, I.J.; Kang, H.N.; Jeong, M.-K. Bojungikki-Tang improves response to PD-L1 immunotherapy by regulating the tumor microenvironment in MC38 tumor-bearing mice. Front. Pharmacol. 2022, 13, 901563. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Du, W.; Huang, H.; Sorrelle, N.; Brekken, R.A. Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 2018, 3, 124184. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, K.; Xiao, Y.; Feng, B.; Mikule, K.; Ma, X.; Feng, N.; Vellano, C.P.; Federico, L.; Marszalek, J.R.; et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci. Rep. 2019, 9, 1853. [Google Scholar] [CrossRef]
- Rizvi, H.; Sanchez-Vega, F.; La, K.; Chatila, W.; Jonsson, P.; Halpenny, D.; Plodkowski, A.; Long, N.; Sauter, J.L.; Rekhtman, N.; et al. Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients with Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. J. Clin. Oncol. 2018, 36, 633–641. [Google Scholar] [CrossRef]
- Sui, H.; Ma, N.; Wang, Y.; Li, H.; Liu, X.; Su, Y.; Yang, J. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J. Immunol. Res. 2018, 2018, 6984948. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive biomarkers of anti-PD-1/PD-L1 therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Deng, L.-J.; Qi, M.; Li, N.; Lei, Y.-H.; Zhang, D.-M.; Chen, J.-X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.-M.; Di, L. Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef]
- Liu, L.; Nie, S.; Xie, M. Tumor Microenvironment as a New Target for Tumor Immunotherapy of Polysaccharides. Crit. Rev. Food Sci. Nutr. 2016, 56 (Suppl. S1), S85–S94. [Google Scholar] [CrossRef]
- Hwang, J.; Zhang, W.; Dhananjay, Y.; An, E.-K.; Kwak, M.; You, S.; Lee, P.C.; Jin, J.-O. Astragalus membranaceus polysaccharides potentiate the growth-inhibitory activity of immune checkpoint inhibitors against pulmonary metastatic melanoma in mice. Int. J. Biol. Macromol. 2021, 182, 1292–1300. [Google Scholar] [CrossRef]
- Huang, J.; Liu, D.; Wang, Y.; Liu, L.; Li, J.; Yuan, J.; Jiang, Z.; Jiang, Z.; Hsiao, W.W.; Liu, H.; et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 2022, 71, 734–745. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, R.; Wu, L.; Tang, S.; Wei, B.; Guo, L.; He, L.; Feng, Y. Atractylodes macrocephala polysaccharides regulate the innate immunity of colorectal cancer cells by modulating the TLR4 signaling pathway. Onco Targets Ther. 2019, 12, 7111–7121. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Lee, S.W.; Park, H.J.; Lee, S.H.; Im, W.K.; Kim, Y.D.; Kim, K.H.; Park, S.J.; Hong, S.; Jeon, S.H. Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complement. Altern. Med. 2018, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Ayeka, P.A.; Bian, Y.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Shi, H.; Li, K.; Ni, Y.; Liang, X.; Zhao, X. Myeloid-Derived Suppressor Cells: Implications in the Resistance of Malignant Tumors to T Cell-Based Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 707198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Sun, Q.; Zhang, W.; Liu, C.; Ma, W.; Sun, C. Natural compounds: A new perspective on targeting polarization and infiltration of tumor-associated macrophages in lung cancer. Biomed. Pharmacother. 2022, 151, 113096. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cai, Y.; Wang, Z.; He, W.; Cao, S.; Xu, R.; Chen, H. Estrogen receptors promote NSCLC progression by modulating the membrane receptor signaling network: A systems biology perspective. J. Transl. Med. 2019, 17, 308. [Google Scholar] [CrossRef]
- Gong, K.; Zhou, H.; Liu, H.; Xie, T.; Luo, Y.; Guo, H.; Chen, J.; Tan, Z.; Yang, Y.; Xie, L. Identification and Integrate Analysis of Key Biomarkers for Diagnosis and Prognosis of Non-Small Cell Lung Cancer Based on Bioinformatics Analysis. Technol. Cancer Res. Treat. 2021, 20, 15330338211060202. [Google Scholar] [CrossRef]
- Lin, X.M.; Luo, W.; Wang, H.; Li, R.Z.; Huang, Y.S.; Chen, L.K.; Wu, X.P. The Role of Prostaglandin-Endoperoxide Synthase-2 in Chemoresistance of Non-Small Cell Lung Cancer. Front. Pharmacol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Lee, S.-E.; Hong, J.-E.; Lee, S.-H.; Shin, J.-Y.; Ro, S.-S. Study on Apoptosis Effect and Mechanism by Bojungikki-tang on Human Cancer Cell Line H460. J. Int. Kor. Med. 2004, 25, 274–288. [Google Scholar]
- Yu, N.; Xiong, Y.; Wang, C. Bu-Zhong-Yi-Qi Decoction, the Water Extract of Chinese Traditional Herbal Medicine, Enhances Cisplatin Cytotoxicity in A549/DDP Cells through Induction of Apoptosis and Autophagy. BioMed Res. Int. 2017, 2017, 3692797. [Google Scholar] [CrossRef]
- Kao, S.T.; Yeh, C.C.; Hsieh, C.C.; Yang, M.D.; Lee, M.R.; Liu, H.S.; Lin, J.G. The Chinese medicine Bu-Zhong-Yi-Qi-Tang inhibited proliferation of hepatoma cell lines by inducing apoptosis via G0/G1 arrest. Life Sci. 2001, 69, 1485–1496. [Google Scholar] [CrossRef]
- Dong, Y.; Duan, L.; Chen, H.W.; Liu, Y.M.; Zhang, Y.; Wang, J. Network Pharmacology-Based Prediction and Verification of the Targets and Mechanism for Panax Notoginseng Saponins against Coronary Heart Disease. Evid. Based Complement. Alternat. Med. 2019, 2019, 6503752. [Google Scholar] [CrossRef] [PubMed]
- Poczobutt, J.M.; De, S.; Yadav, V.K.; Nguyen, T.T.; Li, H.; Sippel, T.R.; Weiser-Evans, M.C.; Nemenoff, R.A. Expression Profiling of Macrophages Reveals Multiple Populations with Distinct Biological Roles in an Immunocompetent Orthotopic Model of Lung Cancer. J. Immunol. 2016, 196, 2847–2859. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, A.J.; Kimball, A.K.; Johnson, A.M.; Arnold, B.W.; Bullock, B.L.; Kaspar, R.E.; Kleczko, E.K.; Kwak, J.W.; Wu, M.H.; Heasley, L.E.; et al. Cancer cell-intrinsic expression of MHC II in lung cancer cell lines is actively restricted by MEK/ERK signaling and epigenetic mechanisms. J. Immunother. Cancer 2020, 8, e000441. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, J.; Kang, H.N.; Yi, J.-M.; Hong, S.H.; Park, S.-M.; Jeong, M.-K. Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma. Processes 2022, 10, 1683. https://doi.org/10.3390/pr10091683
Chun J, Kang HN, Yi J-M, Hong SH, Park S-M, Jeong M-K. Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma. Processes. 2022; 10(9):1683. https://doi.org/10.3390/pr10091683
Chicago/Turabian StyleChun, Jaemoo, Han Na Kang, Jin-Mu Yi, Se Hyang Hong, Sang-Min Park, and Mi-Kyung Jeong. 2022. "Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma" Processes 10, no. 9: 1683. https://doi.org/10.3390/pr10091683
APA StyleChun, J., Kang, H. N., Yi, J.-M., Hong, S. H., Park, S.-M., & Jeong, M.-K. (2022). Bojungikki-Tang Enhances the Effect of PD-1 Blockade in a Syngeneic Murine Model of Lung Carcinoma. Processes, 10(9), 1683. https://doi.org/10.3390/pr10091683





