Abstract
The most widely applied methods to unlock heavy oil and natural bitumen resources in the world are still based on steam injection techniques. Improving the efficiency of hydrothermal processes poses a great challenge. The co-injection of various additives is practiced to lower the steam-to-oil ratio (SOR), viscosity alteration and to improve heavy oil properties. Organic solvents, non-condensable gases, air and surfactants are the preferred chemicals to be combined with steam. This study provides an investigation of the surfactant-assisted hydrothermal upgrading of heavy oil at 200 °C. The thermal stability and salt resistivity of two non-ionic surfactants (SA–3 and Biolub Green) were investigated. Moreover, the improved performance of the surfactants was established by performing an SARA analysis, elemental analysis, FT-IR spectroscopy, and EPR analysis, and by studying the viscosity reduction degree. The experimental results showed that surfactants lead to the in-depth destructive hydrogenation of the high-molecular components of heavy oil such as resins and asphaltenes. However, the content of light fractions increased. According to the results of the elemental analysis, the surfactants assist in the hydrodesulphurization of heavy oil. Overall, the physical and chemical consequences of hydrothermal upgrading in the presence of surfactants led to the irreversible viscosity reduction of heavy oil.
1. Introduction
The United States Geological Survey (USGS) reports the amount of estimated global heavy oil and natural bitumen resources as about three trillion barrels [1]. This is twice as much as the proven conventional reserves of crude oil [2]. Nevertheless, the progress of human society has led to an essential demand for energy resources. Therefore, heavy oil and natural bitumen have been considered as a promising energy source. However, the extraction of heavy oil poses a great challenge due to its high viscosity and low API gravity. These properties derive from the lower hydrogen-to-carbon ratio and the high percentage of asphaltene compounds, sulfur and heavy metals [3,4,5]. Asphaltenes are high-molecular weight compounds with a cyclic structure and paraffin chains that are connected to O, N and S atoms [6,7]. It is worth mentioning that the molecular weight of these compounds ranges from several hundred to several thousand based on their type and structure. Moreover, the polar and nonpolar fragments of asphaltenes make them act like surfactants [8,9,10].
Thermal-enhanced oil recovery methods are widely applied to unlock heavy oil and natural bitumen reservoirs. Specifically, steam-based heavy oil production techniques are the most efficient in terms of upgrading and enhancing the recovery factor [1,11]. Steam injection has both physical and chemical consequences, which lead to an irreversible viscosity reduction [12,13,14]. The technique of heavy oil recovery using steam injection was first introduced in 1966 in Trinidad and Tobago during a small segment project in the Palo Seco region [15]. Briefly, the mechanism of oil recovery using the steam injection method is based on the constant or intermittent injection of a hot fluid into the formation. The hot fluid increases the temperature of the oil-saturated reservoir rocks, which decreases the viscosity of crude oil and improves the mobility of heavy oil. Since steam injection has a high capacity for heat transfer, it has been used frequently around the world [16,17,18,19,20]. On the other hand, the chemical interactions between oil and steam in the presence of rock minerals have been widely reported following the synopsis of work by J.B. Hyne and his colleagues [21,22,23]. The authors propose that in situ hydrothermal processes improve the quality of crude oil by hydrocracking, hydrogenolysis, acid polymerization, hydrodesulfurization, hydrodenitrogenation, destructive hydrogenation and water–gas-shift reactions. The mechanism of irreversible viscosity reduction is explained by the chemical changes in the structure and composition of heavy oil after the aforementioned chemical reactions, for which the authors coined the term “aquathermolysis”. Later, many other scholars reported the enhancement in the composition of crude oil after hydrothermal treatment in the presence of ion metals, such that the content of saturated and aromatics is increased and the content of high-molecular components—resins and asphaltenes—is reduced after hydrothermal upgrading [24,25]. Moreover, the molecular weight of asphaltenes is also altered [26]. It was reported that the significant yield of gaseous products after the addition of ion metals indicates the acceleration of chemical reactions during the steam treatment of heavy oil. However, the steam-based oil recovery techniques suffered from efficiency, serious steam channeling and sweep problems. Despite the high cost of steam generation, significant amounts of CO2 evolved, which is harmful and has an environmental impact. Many attempts have been made to reduce the steam-to-oil ratio (SOR) in order to increase the feasibility of the steam-injecting projects and to decrease the environmental footprint. One of the alternative approaches is the co-injection of various chemical species with steam. The most widely used chemicals are hydrocarbon solvents, catalysts, hydrogen donors, gases, acids and seed oils [27]. Recently, scholars have suggested that co-injection of different surfactants into reservoirs might solve the abovementioned issues [28,29,30]. Surfactants as a surface-active substance can reduce IFT between different phases and, hence, improve the sweep efficiency. As a result, water breakthrough is diminished and the oil recovery factor can be increased. In general, surfactants have been utilized in oil recovery since the early 1970s [31,32]. They are mainly applied during secondary production methods such as waterflooding techniques. However, the co-injection of surfactants with steam is less studied, since it faces issues regarding the thermal stability, salinity resistance and adsorption processes of surfactants. Sasaki et al. [33] reported an increase in oil recovery of 16% after the co-injection of a surfactant during steam assisted gravity drainage (SAGD). Srivastava et al. [34] altered the wettability of reservoir rocks, significantly reduced the water permeability and enhanced oil permeability by implementing surfactant co-injection. In this way, they increased the recovery of heavy oil by 20%. Xuefan Gu and others studied the mechanism and influence of two non-ionic surfactants on the flow characteristics of crude oil [35]. The authors reported that the viscosity of crude oil was reduced by up to 70% with Span 80. The same authors synthesized and evaluated the performance of the chemical additive hydroxymethyl tetramide, which provided a 93% viscosity reduction degree in the case of the Yanchang Oilfield crude oil sample [36]. The results of other researchers have pointed in the same direction [29,37,38,39]. The results of the literature review lead us to conclude that the influence of surfactants on the chemical composition of heavy crude oil and hence on the viscosity reduction is a few studied. Asphaltenes are complex organic matter with a very high molecular weight [40]. They contain a significant amount of heteroatoms. Polar and nonpolar parts in the composition of asphaltenes create a micelle, making them crucial in the formation of emulsions. It is believed that the introduction of surfactants under aquathermolytic conditions can influence the polynuclear aromatic rings and nonpolar aliphatic fragments of asphaltenes in order to stabilize them and prevent precipitation. Moreover, surfactants assist in ring-opening reactions and overall aquathermolysis reactions, which lead to the intensification of heavy oil upgrading and the viscosity reduction degree.
In this study, the upgrading performance of two non-ionic surfactants SA–3 and Biolub Green (SBG) were evaluated under a heavy oil aquathemrolysis process at 200 C. The experiments were performed in a high-pressure reactor to simulate the reservoir conditions. The upgrading products were thoroughly investigated using physical and chemical analysis methods.
2. Materials and Methods
2.1. Materials
The object of this study was a heavy oil produced at the Ashal’cha field (Tatarstan, Russia), the physical and chemical properties of which are summarized in Table 1.

Table 1.
Physical and chemical properties of heavy oil sample from Ashalcha field.
The non-ionic surfactants SA–3 and Biolub Green (SBG) were purchased from Mirrico LLC. The schematic of the upgrading processes is illustrated in Figure 1.
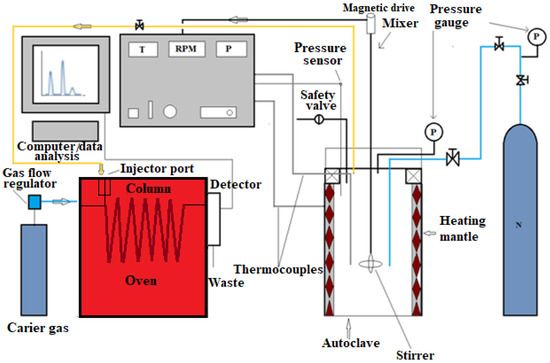
Figure 1.
Schematic of the autoclave coupled with GC.
2.2. Thermal Analysis and Salt-Resistance of Surfactants
Prior to the upgrading experiments, the surfactants were examined for thermal stability, which further determined the temperature of the upgrading experiments. A thermogravimetric analysis (TGA) of surfactants was carried out on an STA 449 F1 Jupiter Thermal Analyzer (Netzsch, Selb, Germany) in a temperature range of 20–1000 °C with heating rates of 10 °C/min and an airflow rate of 50 mL/min. Aluminum oxide was used as a solid phase, nitrogen and oxygen as a gas phase. The data processing was performed using the Proteus Analysis 5.2.1 and NETZSCH Kinetics Neo 2.1.2.2 software packages.
The salt resistance of surfactants was determined using a sodium chloride solution to imitate the reservoir formation water in a Vitag FPX-63 furnace (Daihan), which can provide a temperature of up to 1200 °C. Sodium chloride was selected at various concentrations, from 0.5% to 20%, and placed in a container with a lid, as shown in Figure 2. Then, the solutions were placed in an oven at 100 °C, 200 °C and 250 °C for an hour and kept for a week at room temperature.
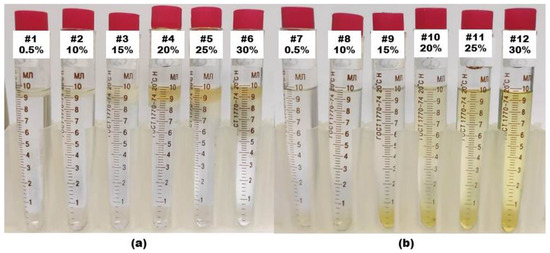
Figure 2.
Salt resistance of surfactants at high temperature. Samples numbered 1–6 refer to the surfactant (a) SA–3 at T = 230 °C and samples numbered 7–12 refer to the surfactant (b) SBG at T = 250 °C. The NaCl concentration of each sample is presented in the figure.
2.3. Surfactant-Assisted Hydrothermal Upgrading of Heavy Oil: SARA and Elemental Analysis
The hydrothermal experiments were carried out in a high pressure and high temperature (HPHT) reactor, designed by Parr Instruments, USA, with a capacity of 300 mL at 200 °C for 48 h. The system was purged with Nitrogen for 20 min to remove the air. The initial pressure was set to 10 bar in order to meet the reservoir conditions. The model system was composed of oil (69.7 wt.%), surfactant (0.3 wt.%) and water (30 wt.%).
The group composition of the initial crude oil and following hydrothermal upgrading in the absence and presence of surfactants was determined according to the SARA analysis method, which is regulated by ASTM D-2007. The elemental composition of Ashalcha oil and oil with surfactants was determined by burning them in a CHNS analyzer at a temperature of 1000 °C.
2.4. Fourier Transform Infrared Spectral (FT-IR) Analysis
A vector infrared spectrometer 22 (Bruker, Germany) was used to analyze the conversions in structural groups of core extracts after catalytic aquathermolysis. The spectra were recorded in the range of 600–1800 cm−1. In this research, the following spectrometric index was used: C1 = D1600/D720 (aromaticity), C2 = D1710/D1465 (oxidation), C3 = D1380/D1465 (branching), C4 = (D720 + D1380)/D1600 (aliphatic) and C5 = D1030/D1465 (sulfurization).
2.5. Viscosity Measurements
Heavy oil samples were measured on a rotational viscometer Alpha L from Fungilab. The device measures the torque of a rotating spindle in a liquid sample at a given rotation speed from 15 to 6,000,000 mPa.s.
2.6. Electron Paramagnetic Resonance (EPR) Analysis
The modulation frequency of 100 kHz was used for all measurements. The integrated intenESP-300 spectrometer (Bruker) was used to record EPR spectra in a continuous wave mode. The instrument provides a magnetic field of 20–1600 mT, with a maximum accuracy of 0.01 mT. The spectra of the sample were compared with the powder of DPPH and Mn2+ in MgO as a reference to estimate PC concentration. The heating process of samples was performed at a rate of 2 K/min in a stream of nitrogen blown using the quartz insert in the high-temperature resonator ER 4114 HT equipped with the VT thermostatic system. The temperature dependence of the sample’s spectra in the range of 293–780 K was obtained with an 8–15 K increment. The temperature changes of vanadyl complexes were investigated using a modulation amplitude of 0.5 mT. The saturation effects were hindered using a microwave power of 25 μW. The relative concentration (ratio [FR]/[VP]) was measured in all experiments due to mass variations inside the EPR cavity, probable changes of densities and viscosities of the studied species [41,42].
2.7. Gas Chromatography Analysis
The composition of the evolved gases during the hydrothermal experiments in the HPHT reactor was studied using gas chromatography on a Chromatec-Crystal 5000.2 instrument, which was coupled with the HPHT reactor. The obtained data were processed digitally. Gas fragmentation was carried out in a 100 m long capillary column and two absorption chambers. Chromatography was carried out in the following temperature mode: from 35 °C to 250 °C with a heating rate of 2 °C/min. Carrier gas—helium, flow rate—15 mL/min. The procedure was carried out according to the Russian Standard GOST 32507-2013, which is equivalent to the standard of the American Society for Testing and Materials (ASTM) D 5134-98.
3. Results and Discussion
3.1. Thermal Stability and Salt Resistance of the Surfactants
Both solutions of surfactants withstood 200 °C. However, at 230 °C, the SA–3 surfactant solution separated into the following three phases: water, surfactant and emulsion mixture, as shown in Figure 2a. The separation of these phases indicates that functional groups in the composition of surfactants at temperatures above 230 °C were decomposed in a saline solution. No changes in the composition of the SBG surfactant were noticed even at a temperature of 250 °C (Figure 2b). In addition, a thermogravimetric analysis (TGA) was performed to evaluate the thermal stability of the surfactants, the results of which are presented in Figure 3.
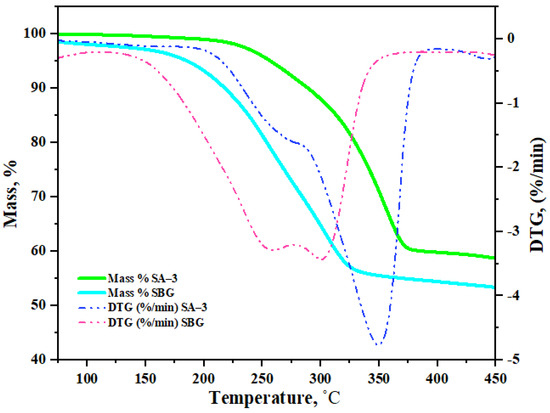
Figure 3.
Thermal analysis of surfactants.
According to the TGA curves, both samples can withstand 200 °C. Therefore, further hydrothermal treatment experiments were carried out at 200 °C.
3.2. Chemical Composition and Elemental Analysis
The results of the group composition analysis of oil samples before and after hydrothermal treatment are illustrated in Figure 4. The results of the SARA analysis show that the amount of saturated hydrocarbons in the oil sample with SA–3 has increased by 20%; however, for SBG it is almost unchanged. However, the aromatics have been improved by 19% for solutions containing SBG and by 10% in the case of SA–3 surfactant. Moreover, the amount of compounds with high molecular weight (resins) in samples containing SA–3 and SBG decreased by 27% and 17%, respectively. It should be noted that the asphaltene compounds of the samples containing SA–3 and SBG were reduced by 12% and 9%, respectively. Thus, the proposed hypothesis regarding the role of surfactants in weakening the bond interactions between asphaltene molecules is proven.
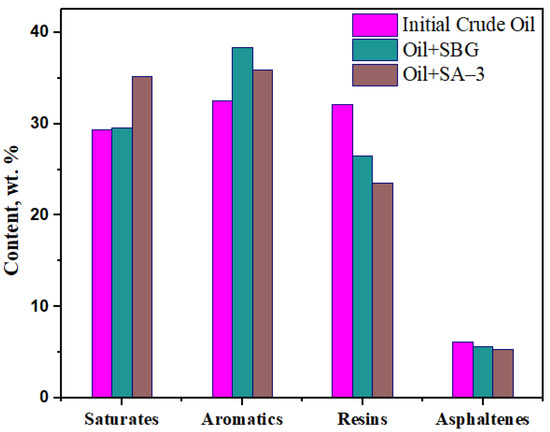
Figure 4.
The results of the SARA analysis before and after surfactant-assisted hydrothermal upgrading.
Furthermore, the results of the elemental analysis in Table 2 showed that surfactants have a considerable effect on the destruction of C-S bonds as the elemental sulfur concentration in the composition of oil was reduced. Moreover, the hydrogen-to-carbon ratio increased significantly in the presence of the SA–3 surfactant in contrast to the initial crude oil.

Table 2.
Results of elemental analysis.
3.3. IR Spectroscopy
Infrared spectra are presented in Figure 5 and the estimated spectral coefficients are summarized in Table 3. This technique is employed to study the changes in functional groups of surfactants.
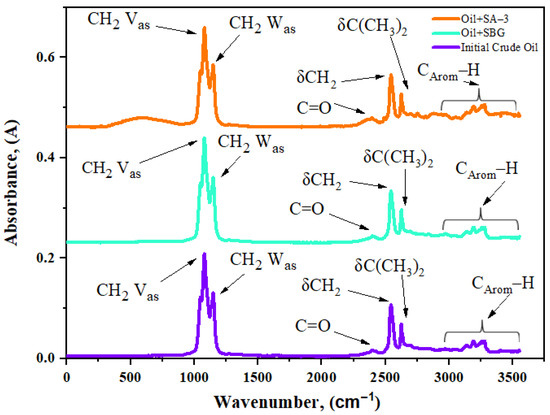
Figure 5.
IR spectra of Ashalcha oil and samples of surfactants after aquathermolysis.

Table 3.
Results of IR spectral analysis of oil samples.
The structural changes after the co-addition of surfactants were evaluated by calculating and comparing the spectral coefficients. The aromaticity (C1) was reduced from 0.86 to 0.82 following the addition of the SBG surfactant. However, the SA–3 surfactant caused a decrease in aromaticity by almost half (from 0.86 to 0.48), while aliphaticity (C4) increased from 2.52 to 6.05. The results indicate the depolymerization of polynucleic aromatic rings in the asphaltene structures. Moreover, the nonpolar aliphatic side chains of asphaltenes increased. The FT-IR coefficient estimation results are in accordance with the results of the SARA (Figure 4) and elemental analysis (Table 3). The C3 coefficient stands for branching and indicates the intensity of hydrocracking reactions. The destructive hydrogenation of heavy fragments of crude oil in the presence of the SA–3 surfactant led to the detachment of alkyl substitutes, and an increase in the length of alkyl chains. As a result, there was a decrease in the branching coefficient (C3) of the crude oil sample after surfactant-assisted steam upgrading. A significant decrease in the C5 coefficient in the case of the SA–3 surfactant indicates that C-S bonds were destroyed under hydrothermal impact, thus contributing to the in situ hydrodesulfurization of crude oil.
3.4. Dynamic Viscosity and EPR
Viscosity is one of the most important parameters during the extraction and transportation of heavy oil. The results of the viscosity measurements show that the viscosity of heavy oil is almost constant in the presence of both surfactants without hydrothermal treatment (Figure 6). However, the viscosity of heavy oil sample was significantly reduced after hydrothermal upgrading in the presence of surfactant, as displayed in Figure 7. This excludes the solubility and emulsification impacts of surfactants on the viscosity reduction degree during hydrothermal upgrading.
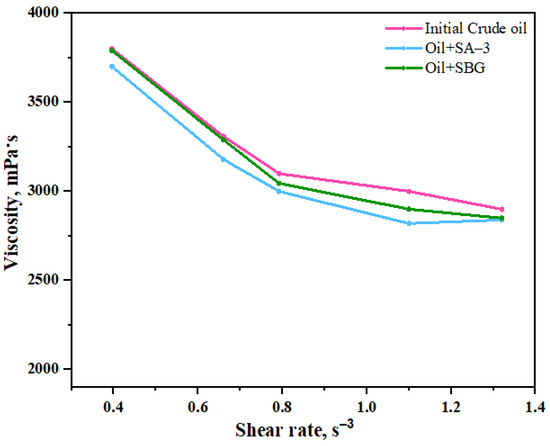
Figure 6.
Dynamic viscosity of model systems before hydrothermal treatment measured at 20 °C.
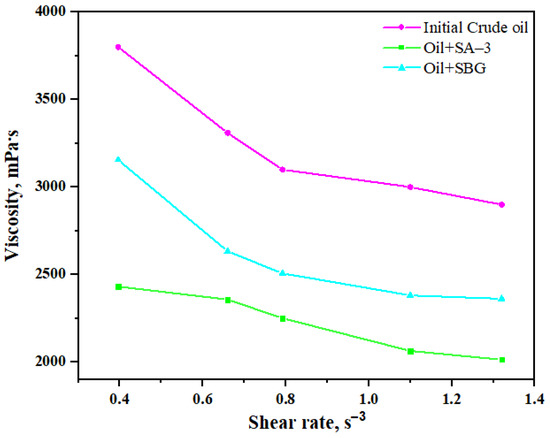
Figure 7.
Dynamic viscosity of model systems after hydrothermal treatment measured at 20 °C.
The dynamic viscosity of Ashalcha oil at 20 °C was about 3000 mPa∙s with a shear rate of 1.3 s−1. The viscosity at the same shear rate decreased by 22% and 30% following co-addition of the SBG and SA–3 surfactants, respectively, to the reaction medium. The apparent influence of surfactants on the viscosity reduction degree is explained by the structural and molecular changes mostly in the heavy components of heavy oil such as resins and asphaltenes. It is well known that even a small decrease in the content of asphaltenes can significantly reduce the viscosity of oil. Thus, it is proposed that the decrease in viscosity may occur due to the weakening of the π = π bonds, which are mostly concentrated in resins and asphaltenes.
The results of the EPR analysis (Figure 8) show an increase in the amount of free radicals when surfactants are added. The initial crude oil was assumed as a unit of intensity, and the intensity of other samples was compared relative to the initial crude oil. The EPR results also support the destruction of the double bond π = π in asphaltene packs. The EPR spectra of oil samples before and after surfactant-assisted hydrothermal upgrading are illustrated in Figure 8.
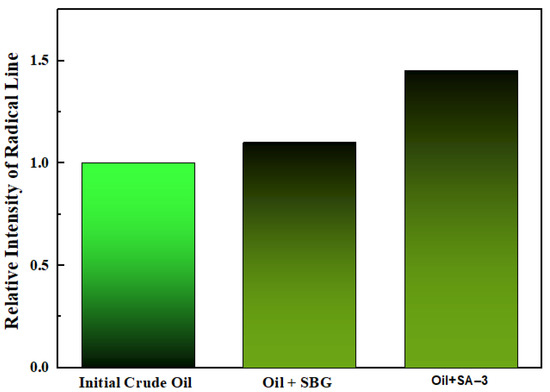
Figure 8.
EPR analysis of oil and surfactant samples after aquathermolysis.
According to the spectra of oil samples (Figure 9), the intensity of free radicals in the crude oil significantly increased after an aquathermolysis process in the presence of the SA–3 surfactant. However, the SBG surfactant leads to the lowered formation of free radicals. The obtained results support the idea about the influence of surfactants on the double bond loosening, leading to the formation of free radicals. Thus, the co-injection of the appropriate concentration of surfactant with steam is a promising method to enhance heavy oil quality and recovery.
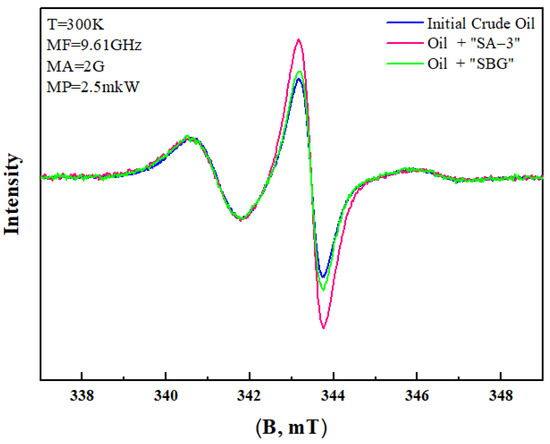
Figure 9.
Intensity spectra of free radicals.
3.5. The Composition of Evolved Gases after Surfactant-Assisted Hydrothermal Treatment
Uncondensed gases such as carbon dioxide, nitrogen, hydrogen sulfur and low-molecular n-alkane gasses play a crucial role in increasing the thermal efficiency of steam recovery methods. It is reported that uncondensed gases are the products of aquathermolysis reactions, thus indicating the complexity of heavy oil upgrading [43,44]. The composition of the evolved gases after surfactant-assisted hydrothermal upgrading are summarized in Table 4.

Table 4.
The composition of gases after surfactant-assisted hydrothermal treatment.
The results show that the SA–3 surfactant leads to the formation of hydrocarbon gases, the total amount of which are more than 20% higher than the gases evolved in the case of the SBG surfactant. Specifically, the content of ethane and propane compounds is twice as high as for the SBG sample. The formation of low-molecular n-alkanes can be achieved as a result of the hydrocracking of C-C bonds, which requires a high amount of energy during conventional steaming without chemical additives, or the hydrogenation of carbon dioxide [45]. The surfactants probably shift the cracking temperature ranges to lower values by weakening the interaction forces between molecules of asphaltenes and resins. This statement is also supported by the results of the SARA-analysis, where the content of resins and asphaltenes is the lowest in the presence of the SA–3 surfactant. The resins and asphaltenes are considered to be the most polar components of oil-dispersed systems [46]. However, the side chains of aromatic rings are generally unpolar molecules, and thus can be under the influence of surfactants.
4. Conclusions
This study presents the results of laboratory studies on the surfactant-assisted hydrothermal upgrading of heavy oil. The advantages of using surfactants are the content reduction of high-molecular weight components of oil, such as asphaltenes and resins. Moreover, they can increase the efficiency of the hydrothermal processes via partial upgrading in the reservoir formation. The application of surfactants during steam injection techniques is believed to reduce the SOR. Furthermore, the results of the elemental analysis and FT-IR spectroscopy analysis showed a significant decrease in the concentration of sulfur after the addition of surfactant. The results of the EPR analysis clearly showed an improvement in free radicals, which confirms a decrease in the viscosity of heavy oil. Thus, the co-injection of thermally stable and salt-resistant surfactants with steam can play a significant role in improving the quality and production of heavy oil. The obtained laboratory results can be considered as a first step towards the modification of steam-based enhanced oil recovery techniques.
Author Contributions
Conceptualization, F.A. and A.V.; methodology, A.D.; investigation, T.K. and O.M.; writing original draft, A.T.; review and editing, F.A., T.K. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No. 075-15-2022-299 within the framework of the development program for a world-class Research Center “Efficient development of the global liquid hydrocarbon reserves”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vakhin, A.V.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Mukhamatdinova, R.E.; Tajik, A.; Slavkina, O.V.; Malaniy, S.Y.; Gafurov, M.R.; Nasybullin, A.R.; Morozov, O.G. Changes in Heavy Oil Saturates and Aromatics in the Presence of Microwave Radiation and Iron-Based Nanoparticles. Catalysts 2022, 12, 514. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-heavy oil aquathermolysis using nickel-based catalyst: Some aspects of in-situ transformation of catalyst precursor. Catalysts 2021, 11, 189. [Google Scholar] [CrossRef]
- Nemati Zadeh Haghighi, A.; Dabiri, A.; Azdarpour, A.; Karaei, M.A. Oxidation behavior and kinetics of Iranian crude oil samples using thermal analysis (TA). Energy Sources Part A Recover. Util. Environ. Eff. 2019, 1–13. [Google Scholar] [CrossRef]
- Suwaid, M.A.; Varfolomeev, M.A.; Al-Muntaser, A.A.; Yuan, C.; Starshinova, V.L.; Zinnatullin, A.; Vagizov, F.G.; Rakhmatullin, I.Z.; Emelianov, D.A.; Chemodanov, A.E. In-situ catalytic upgrading of heavy oil using oil-soluble transition metal-based catalysts. Fuel 2020, 281, 118753. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Nikookar, M.; Omidkhah, M.R.; Pazuki, G.R.; Mohammadi, A.H. An insight into molecular weight distributions of asphaltene and asphalt using Gel Permeation Chromatography. J. Mol. Liq. 2022, 362, 119736. [Google Scholar] [CrossRef]
- Khaleel, A.T.; Sisco, C.J.; Tavakkoli, M.; Vargas, F.M. An Investigation of the Effect of Asphaltene Polydispersity on Asphaltene Precipitation and Deposition Tendencies. Energy Fuels 2022, 36, 8799–8808. [Google Scholar] [CrossRef]
- Rahimi, P.M.; Gentzis, T. The chemistry of bitumen and heavy oil processing. In Practical Advances in Petroleum Processing; Springer: New York, NY, USA, 2006; pp. 597–634. [Google Scholar]
- Groenzin, H.; Mullins, O.C. Asphaltene molecular size and structure. J. Phys. Chem. A 1999, 103, 11237–11245. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Experimental investigation of the asphaltene deposition in porous media: Accounting for the microwave and ultrasonic effects. J. Pet. Sci. Eng. 2018, 163, 453–462. [Google Scholar] [CrossRef]
- Speight, J.G. Introduction to Enhanced Recovery Methods for Heavy Oil and Tar Sands; Gulf Professional Publishing: Houston, TX, USA, 2016; ISBN 0128018755. [Google Scholar]
- Ali, S.M. Current status of steam injection as a heavy oil recovery method. J. Can. Pet. Technol. 1974, 13, 54–68. [Google Scholar] [CrossRef]
- Alkindi, A.; Al-Azri, N.; Said, D.; AlShuaili, K.; Te Riele, P. Persistence in EOR-design of a field trial in a carbonate reservoir using solvent-based water-flood process. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, OnePetro, Muscat, Oman, 21–23 March 2016. [Google Scholar]
- Taylor, S.E. Interfacial chemistry in steam-based thermal recovery of oil sands bitumen with emphasis on steam-assisted gravity drainage and the role of chemical additives. Colloids Interfaces 2018, 2, 16. [Google Scholar] [CrossRef]
- Meyer, R.F.; Attanasi, E.D.; Freeman, P.A. Heavy oil and natural bitumen resources in geological basins of the world: Map showing klemme basin classification of sedimentary provinces reporting heavy oil or natural bitumen. US Geol. Surv. Open File Rep. 2007, 2007, 1084. [Google Scholar]
- Farouq Ali, S.M. Practical Heavy Oil Recovery; SM Farouq Ali: Calgary, AB, Canada, 1999. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Marin, R.J., Sr. Hydrocarbon Solvent Injection Study for Heavy Oil Recovery in the Colombian Oil Sands. Search Discov. 2015, 41528. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Wang, Q.; Pang, Z.; Wang, C. Non-Newtonian flow characterization of heavy crude oil in porous media. J. Pet. Explor. Prod. Technol. 2013, 3, 43–53. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Hyne, J.B.; Lesage, K.L. The high-temperature reaction of thiophene and tetrahydrothiophene with aqueous solutions of aluminium and first-row transition-metal cations. Fuel 1987, 66, 1353–1357. [Google Scholar] [CrossRef]
- Hyne, J.B.; Clark, P.D.; Clarke, R.A.; Koo, J.; Greidanus, J.W. Aquathermolysis of heavy oils. Rev. Tec. INTEVEP 1982, 2, 87–94. [Google Scholar]
- Clark, P.D.; Clarke, R.A.; Hyne, J.B.; Lesage, K.L. Studies on the effect of metal species on oil sands undergoing steam treatments. AOSTRA J. Res. 1990, 6, 53–64. [Google Scholar]
- Rahham, Y.; Rane, K.; Goual, L. Characterization of the Interfacial Material in Asphaltenes Responsible for Oil/Water Emulsion Stability. Energy Fuels 2020, 34, 13871–13882. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nasyrova, Z.R.; Gareev, B.I.; Vakhin, A.V. Catalytic hydrothermal conversion of heavy oil in the porous media. Energy Fuels 2021, 35, 1297–1307. [Google Scholar] [CrossRef]
- Ma, H.; Yang, Y.; Chen, Z. Numerical simulation of bitumen recovery via supercritical water injection with in-situ upgrading. Fuel 2022, 313, 122708. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Molecular dynamics simulation of oil detachment from hydrophobic quartz surfaces during steam-surfactant Co-injection. Energy 2022, 254, 124434. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Yue, Q.; Gao, Y.; Shen, D. Conformance control of CSS and steam drive process with a carbamide surfactant. J. Can. Pet. Technol. 2009, 48, 16–18. [Google Scholar] [CrossRef]
- Adkins, S.; Arachchilage, G.P.; Solairaj, S.; Lu, J.; Weerasooriya, U.; Pope, G. Development of thermally and chemically stable large-hydrophobe alkoxy carboxylate surfactants. In Proceedings of the SPE Improved Oil Recovery Symposium, OnePetro, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Cayias, J.L.; Schechter, R.S.; Wade, W.H. The utilization of petroleum sulfonates for producing low interfacial tensions between hydrocarbons and water. J. Colloid Interface Sci. 1977, 59, 31–38. [Google Scholar] [CrossRef]
- Hongyan, W.; Xulong, C.; Jichao, Z.; Aimei, Z. Development and application of dilute surfactant–polymer flooding system for Shengli oilfield. J. Pet. Sci. Eng. 2009, 65, 45–50. [Google Scholar] [CrossRef]
- Sasaki, K.; Akibayashi, S.; Kosukegawa, H.; Kato, M.; Ono, K. Experimental study on initial stage of SAGD process using 2-dimensional scaled model for heavy oil recovery. In Proceedings of the International Conference on Horizontal Well Technology, OnePetro, Calgary, AB, Canada, 18 November 1996. [Google Scholar]
- Srivastava, P.; Debord, J.; Sadetsky, V.; Stefan, B.; Orr, B. Laboratory evaluation of a chemical additive to increase production in steam assisted gravity drainage (SAGD). In Proceedings of the SPE Improved Oil Recovery Symposium, OnePetro, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Gu, X.; Gao, L.; Li, Y.; Chen, S.; Zhang, J.; Du, W.; Qu, C.; Chen, G. Performance and mechanism of span surfactants as clean flow improvers for crude oil. Pet. Chem. 2020, 60, 140–145. [Google Scholar]
- Gu, X.; Wang, P.; Guo, Z.; Du, W.; Dong, S. Synthesis and evaluation of hydroxymethyl tetramides as flow improvers for crude oil. J. Chem. Soc. Pak. 2020, 42, 488–494. [Google Scholar]
- Lau, H.C.; Borchardt, J.K. Improved steam-foam formulations: Concepts and laboratory results. SPE Reserv. Eng. 1991, 6, 470–476. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Liu, Q.; Lu, K.; Wang, L. Research on the effect of non-condensable gas and viscosity reducer for better SAGD performance. In Proceedings of the SPE Heavy Oil Conference, OnePetro, Calgary, AB, Canada, 10–12 June 2014. [Google Scholar]
- Yusupova, T.N.; Ganeeva, Y.M.; Romanov, G.V.; Barskaya, E.E.; Morozov, V.I.; Okhotnikova, E.S.; Vakhin, A.V. Change in the structural-group composition of bitumen asphaltenes upon thermal bitumen recovery. Pet. Chem. 2017, 57, 198–202. [Google Scholar] [CrossRef]
- Mullins, O.C. The asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Volodin, M.A.; Rodionov, A.A.; Sorokina, A.T.; Dolomatov, M.Y.; Petrov, A.V.; Vakhin, A.V.; Mamin, G.V.; Orlinskii, S.B. EPR study of spectra transformations of the intrinsic vanadyl-porphyrin complexes in heavy crude oils with temperature to probe the asphaltenes’ aggregation. J. Pet. Sci. Eng. 2018, 166, 363–368. [Google Scholar] [CrossRef]
- Gafurov, M.R.; Ponomarev, A.A.; Mamin, G.V.; Rodionov, A.A.; Murzakhanov, F.F.; Arash, T.; Orlinskii, S.B. Application of pulsed and high-frequency electron paramagnetic resonance techniques to study petroleum disperse systems. Georesursy 2020, 22, 2–14. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Lapuk, S.E.; Buzyurov, A.V.; Ignashev, N.E.; Shmeleva, E.I.; Mukhamatdinov, I.I.; Vakhin, A.V. Thermal Behavior of Heavy Oil Catalytic Pyrolysis and Aquathermolysis. Catalysts 2022, 12, 449. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Khelkhal, M.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Vakhin, A.V. Iron oxide nanoparticles impact on improving reservoir rock minerals catalytic effect on heavy oil aquathermolysis. Fuel 2022, 327, 124956. [Google Scholar] [CrossRef]
- de la Cruz Parejas, R.; Moura, F.J.; de Avillez, R.R.; de Souza Mendes, P.R. Effects of Al2O3-NiO, TiO2 and (Mg, Ni) O particles on the viscosity of heavy oil during aquathermolysis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126863. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).