Seed Waste from Custard Apple (Annona squamosa L.): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile
Abstract
1. Introduction
2. Proximate Composition of Custard Apple Seeds
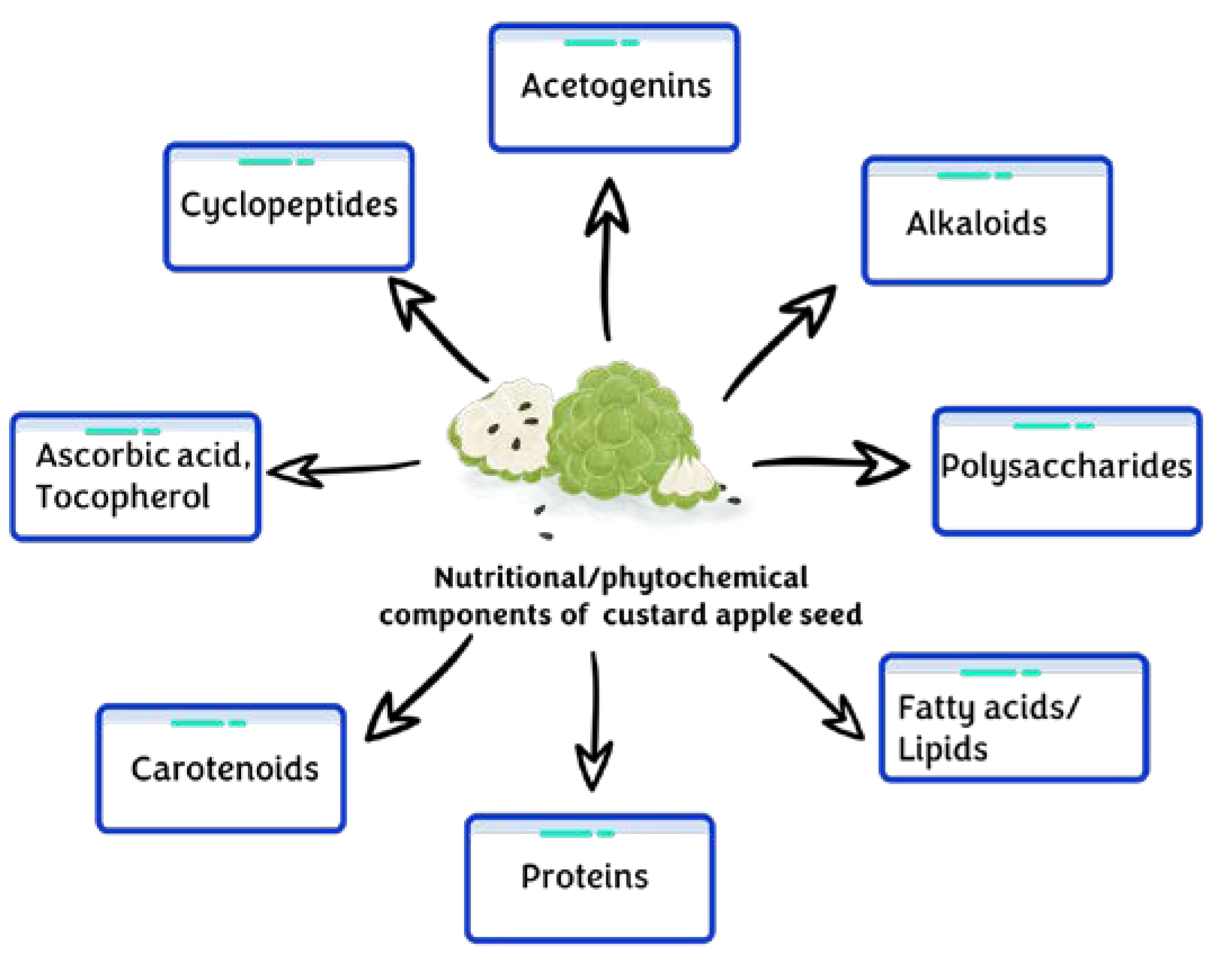
3. Phytochemical Profile of Custard Apple Seeds
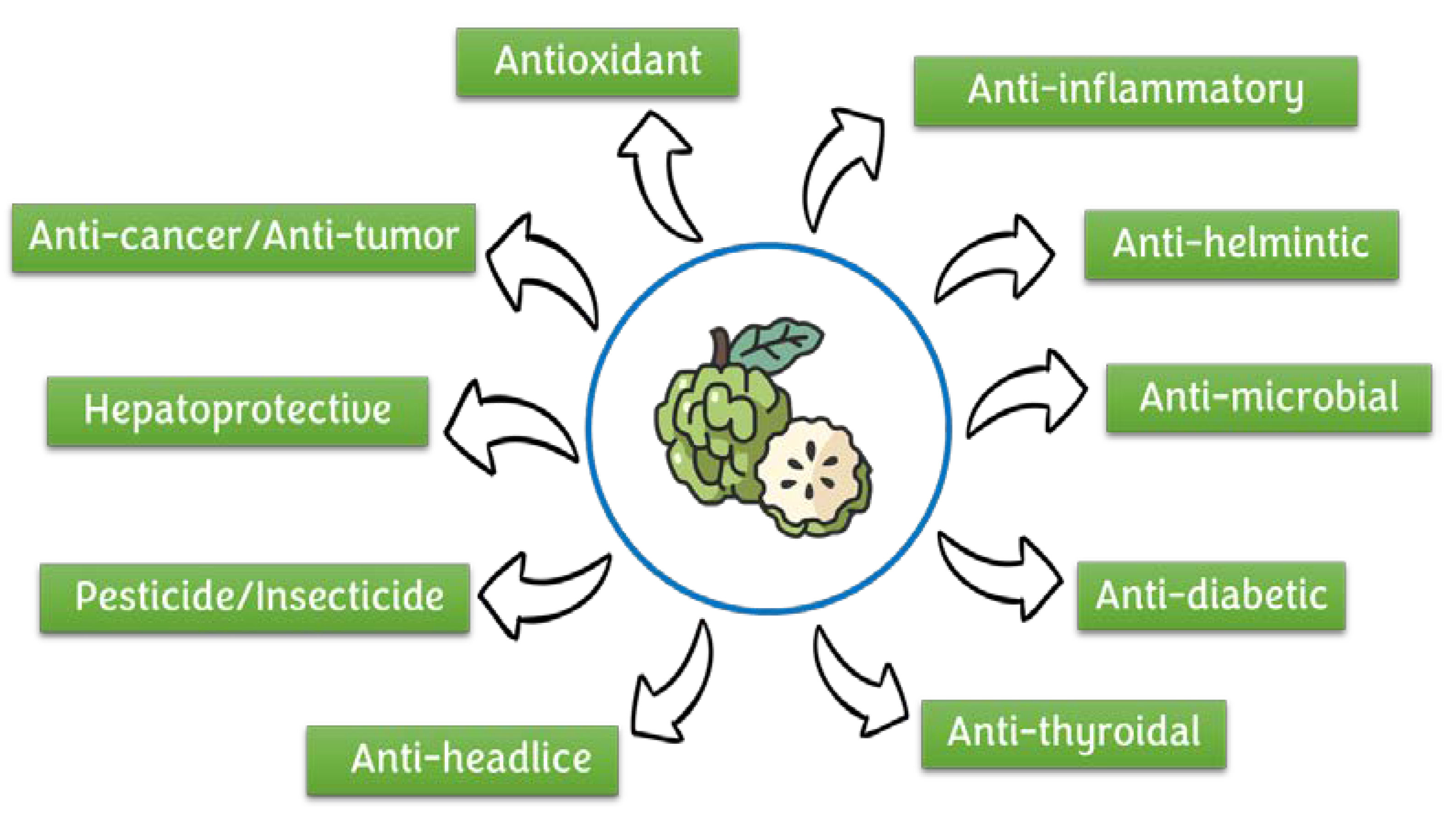
4. Pharmacological Properties
4.1. Antimicrobial Activity
4.2. Antidiabetic Activity
4.3. Anti-Inflammatory
4.4. Anticancer/Antitumor Activity
4.5. Antioxidant Activity
4.6. Hepatoprotective Activity
4.7. Other Activities
5. Toxicity of Annona squamosa Seeds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Mekhemar, M. Custard apple (Annona squamosa L.) leaves: Nutritional composition, phytochemical profile, and health-promoting biological activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Tamang, A.; Subba, S.K.; Chhetri, S. Wild edible and minor fruits of Odisha. Pharma Innov. J. 2021, 10, 609–613. [Google Scholar]
- Kohli, A.; Ahmad, T.; Singh, S. Aegle marmelos (Bael) and Annona squamosa (Sugar Apple). In Herbs Shrubs Trees Potential Medicinal Benefits, 1st ed.; Husen, A., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 339–364. [Google Scholar]
- Irwan, Z.; Kamarudin, W.F.W.; Korish, U.A.S.A.; Rusli, A.S.; Sallehuddin, S. Effectiveness of Annona squamosa and Annona muricata Seed Extracts as Ingredients in Bio-pesticides Spray. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1176, 012007. [Google Scholar] [CrossRef]
- Katole, R.M.; Sharma, M.K.; Joshi, C.K. Annona squamosa L. As a potential natural botanical pesticide and its futuristic research scope: A review. Plant Cell Biotechnol. Mol. Biol. 2021, 41–42, 75–98. [Google Scholar]
- Sundaramahalingam, M.A.; Karthikumar, S.; Kumar, R.S.; Samuel, K.J.; Shajahan, S.; Sivasubramanian, V.; Moorthy, I.G. An intensified approach for transesterification of biodiesel from Annona squamosa seed oil using ultrasound-assisted homogeneous catalysis reaction and its process optimization. Fuel 2021, 291, 120–195. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef]
- Mondal, P.; Biswas, S.; Pal, K.; Ray, D.P. Annona squamosa as a potential botanical insecticide for agricultural domains: A review. Int. J. Bioresour. Sci. 2018, 5, 81–89. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Y.; Chen, J.; Li, X.; Chen, Y. A review on Annona squamosa L.: Phytochemicals and biological activities. Am. J. Chin. Med. 2017, 45, 933–964. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, Y.; Liu, Y.; Jiao, S.; Zhang, M. Chemical Constituents with nitric oxide inhibition from the fruit peel of Annona squamosa. Chem. Nat. Compd. 2021, 57, 1153–1156. [Google Scholar] [CrossRef]
- Pardhasaradhi, B.V.V.; Reddy, M.; Ali, A.M.; Kumari, A.L.; Khar, A. Antitumour activity of Annona squamosa seed extracts is through the generation of free radicals and induction of apoptosis. Indian J. Biochem. Biophys. 2004, 41, 167–172. [Google Scholar]
- Chen, J.; Chen, Y.; Li, X. Beneficial aspects of custard apple (Annona squamosa L.) seeds. In Nuts and Seeds in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: London, UK, 2011; pp. 439–445. [Google Scholar]
- Poyer, S.; Laboureur, L.; Hebra, T.; Elie, N.; Van der Rest, G.; Salpin, J.Y.; Touboul, D. Dereplication of Acetogenins from Annona muricata by Combining Tandem Mass Spectrometry after Lithium and Copper Postcolumn Cationization and Molecular Networks. J. Am. Soc. Mass Spectrom. 2022, 33, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Fareed, M.M.; Ali, M.M. The Revelation and Therapeutic Role of Medicinal Phytochemicals in the Treatment of Cancer: A Brief. Comput. Intell. Oncol. Appl. Diagn. Progn. Ther. Cancers 2022, 1016, 335. [Google Scholar]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Rabbee, M.F.; Roy, A.; Chowdhury, R.; Banik, A.; Kubra, K.; Baek, K.H. Therapeutic promises of medicinal plants in Bangladesh and their bioactive compounds against ulcers and inflammatory diseases. Plants 2021, 10, 1348. [Google Scholar] [CrossRef]
- Imadi, S.R.; Mahmood, I.; Gul, A. Medicinal Plants Against Cancer. Plant Hum. Health 2018, 1, 139–196. [Google Scholar]
- Chen, Y.; Xu, S.S.; Chen, J.W.; Wang, Y.; Xu, H.Q.; Fan, N.B.; Li, X. Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J. Ethnopharmacol. 2012, 142, 462–466. [Google Scholar] [CrossRef]
- Bhoir, S.S.; Vishwapathi, V.; Singh, K.K. Antipsoriatic potential of Annona squamosa seed oil: An in vitro and in vivo evaluation. Phytomedicine 2018, 54, 265–277. [Google Scholar] [CrossRef]
- Mehta, S.D.; Paliwal, S. Hepatoprotective activity of hydroalcohilic extract of Annona squamosa seeds. Int. J. Pharmacol. Phytol. Res. 2017, 9, 997–1000. [Google Scholar] [CrossRef][Green Version]
- Rana, V.S. Fatty oil and fatty acid composition of Annona squamosa Linn. Seed kernels. Int. J. Fruit Sci. 2015, 15, 79–84. [Google Scholar] [CrossRef]
- Mariod, A.A.; Elkhier, S.; Ahmed, Y.M.; Matthaus, B. Annona squamosa and Catunaregam nilotica seed, the effect of extraction method on oil composition. J. Am. Chem. Soc. 2010, 87, 763–769. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abu-Serie, M.M.; El-Aziz, A.; Mohammad, N.; El-Sohaimy, S.A. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Ahvanooei, M.R.; Norouzian, M.A.; Vahmani, P. Beneficial effects of vitamins, minerals, and bioactive peptides on strengthening the immune system against COVID-19 and the role of cow’s milk in the supply of these nutrients. Biol. Trace Elem. Res. 2022, 200, 4664–4677. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Role of Metal ions in Biological System. Contemp. Adv. Sci. Technol. 2022, 2, 67. [Google Scholar]
- Dare, C.A.; Oyedapo, O.O.; Akinlalu, A.O.; Komolafe, I.J.; Fajobi, A.O.; Ogunsusi, M. Genotoxic Activities of Polysaccharides from Cotyledon and Coat of Fermented and Unfermented Annona squamosa L. Seed. Egypt. Acad. J. Biol. Sci. 2021, 12, 189–207. [Google Scholar] [CrossRef]
- Abdualrahman, M.A.Y.; Haile, M.; Cunshan, Z.; Abu, E.G.A.Y.; Ali, O.A.; Haroon, E.T.; Asif, W. Postharvest physicochemical properties of the pulp and seed oil from Annona squamosa L. (Gishta) fruit grown in Darfur region, Sudan. Arab. J. Chem. 2016, 12, 4514–4521. [Google Scholar] [CrossRef]
- Leite, D.O.D.; Camilo, C.J.; Nonato, C.D.F.A.; Carvalho, N.K.G.D.; Salazar, G.J.T.; de Morais, S.M.; Costa, J.G.M.D. Chemical Profile and Evaluation of the Antioxidant and Anti-Acetylcholinesterase Activities of Annona squamosa L. (Annonaceae) Extracts. Foods 2021, 10, 2343. [Google Scholar] [CrossRef]
- Kowalska, M.T.; Puett, D. Potential biomedical applications for tropical fruit products. Trop. Gard. Fruit World 1990, 1, 126–127. [Google Scholar]
- Hiwale, S. Custard apple (Annona squamosa L.). In Sustainable Horticulture in Semiarid Dry Lands; Springer: New Delhi, India, 2015; pp. 135–152. [Google Scholar]
- Zahid, M.; Arif, M.; Rahman, M.A.; Singh, K.; Mujahid, M. Solvent extraction and gas chromatography–mass spectrometry analysis of Annona squamosa L. seeds for determination of bioactives, fatty acid/fatty oil composition, and antioxidant activity. J. Diet. Suppl. 2018, 15, 613–623. [Google Scholar] [CrossRef]
- Vetal, D.S.; Pardeshi, A.B. Insecticidal potential of ethanol and hexane solvent seed extract of Annona squamosa against Spodoptera litura Fab. J. Pharmacogn. Phytochem. 2019, 8, 842–845. [Google Scholar]
- Al-Kazman, B.S.; Harnett, J.E.; Hanrahan, J.R. The Phytochemical Constituents and Pharmacological Activities of Annona atemoya: A Systematic Review. Pharmaceuticals 2020, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.; Patel, P.K.; Suthar, R.; Shah, K.R. Identification of Phytochemicals from seed extract of Custard Apple (Annona squamosa L.). Biosci. Biotechnol. Res. Commun. 2021, 14, 397–402. [Google Scholar] [CrossRef]
- Mangal, M.; Khan, M.I.; Agarwal, S.M. Acetogenins as Potential Anticancer Agents. Anticancer Agent. Med. Chem. 2015, 16, 138–159. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.P.; Zanardi, O.Z.; Gonçalves, G.; Ansante, T.F.; Yamamoto, P.T.; Vendramim, J.D. Toxicity of an Annonin-Based Commercial Bioinsecticide Against Three Primary Pest Species of Stored Products. Neotrop. Entomol. 2018, 47, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-infective and anti-cancer properties of the Annona species: Their ethno medicinal uses, alkaloid diversity, and pharmacological activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef]
- Thangaraj, V.; Khan, M.R.; Rajendran, A.; Selvam, D. Phytochemical Screening of Annona squamosa (L.) Seed Extracts: A Potential Source of Ethnomedicine. Indo Am. J. Pharm. Res. 2017, 7, 8068–8072. [Google Scholar]
- Nguyen, V.; Nguyen, Q.; Muoi, N.; Mai, C. Effect of Extraction Conditions on Total Polyphenol and Flavonoid Content of Sugar Apple Seeds (Annona squamosa L.). Asian. J. Chem. 2020, 32, 1741–1745. [Google Scholar] [CrossRef]
- Kumar, Y. New compound 6, 7-dimethoxy-2-methylisoquinolinium from Indian medicinal plant Annona squamosa L. Int. J. Chem. Anal. Sci. 2013, 4, 161–168. [Google Scholar]
- Ravaomanarivo, L.H.R.; Razafindraleva, H.A.; Raharimalala, F.N.; Rasoahantaveloniaina, B.; Ravelonandro, P.H.; Mavingui, P. Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae). Asian Pac. J. Trop. Biomed. 2014, 4, 798–806. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer. 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef] [PubMed]
- Vikas, B.S.; Malar, J.P.W.; Remani, P. Antibacterial activity of Annona squamosa seed extract. Int. J. Pharm. Technol. 2013, 5, 5651–5659. [Google Scholar]
- Aher, P.S.; Shinde, Y.S.; Chavan, P.P. In vitro evaluation of antibacterial potential of Annona squamosa L. against pathogenic bacteria. Int. J. Pharm. Sci. Res. 2012, 3, 1457. [Google Scholar]
- Aamir, J.; Kumari, A.; Khan, M.N.; Medam, S.K. Evaluation of the combinational antimicrobial effect of Annona squamosa and Phoenix dactylifera seeds methanolic extract on standard microbial strains. Int. Res. J. Biol. Sci. 2013, 2, 68–73. [Google Scholar]
- Baranwal, A.; Arora, S.; Kumar, R.G.; Praharsha, J.; Javed, A.; Sanobar, N. Evaluation of the combinational antimicrobial effect of Prunus persia and Annona squamosa seeds methanolic extract on standard microbial strains. Glob. J. Biosci. Biotechnol. 2013, 2, 571–575. [Google Scholar]
- Singh, P.; Singh, K.R.; Singh, J.; Das, S.N.; Singh, R.P. Tunable electrochemistry and efficient antibacterial activity of plant-mediated copper oxide nanoparticles synthesized by Annona squamosa seed extract for agricultural utility. RSC Adv. 2021, 11, 18050–18060. [Google Scholar] [CrossRef]
- Kebir, N.E.; Zahzeh, T. Magnesium Deficiency Associated with Stress, Systemic Inflammation, and Insulin Resistance in Diabetes Mellitus: A review. Egypt. Acad. J. Biol. Sci. C Physiol. Molecul. Biol. 2022, 14, 31–46. [Google Scholar] [CrossRef]
- Alam, A.; Akbar, S.; Khan, I.A.; Gul, R.; Rehman, R.; Noreen, S. Risk factors assessment of type 2 diabetes mellitus in adult male population of Hayatabad, Peshawar: A Cross-sectional Study. Khyber J. Med. Sci. 2021, 14, 13. [Google Scholar]
- Sangala, R.; Kodati, D.R.; Burra, S.; Gopu, J.; Dubasi, A. Evaluation of antidiabetic activity of Annona squamosa Linn Seed in alloxan–induced diabetic rats. Diabetes 2011, 2, 100–106. [Google Scholar]
- Yang, A.; Wu, Y.; Yu, G.; Wang, H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: Mechanisms and development. Respir. Res. 2021, 22, 1–17. [Google Scholar] [CrossRef]
- Hossain, M.R.; Ansary, T.M.; Komine, M.; Ohtsuki, M. Diversified stimuli-induced inflammatory pathways cause skin pigmentation. Int. J. Mol. Sci. 2021, 22, 3970. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Gibbs, J.E. Adaptive immunity, chronic inflammation and the clock. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–16. [Google Scholar]
- Dellai, A.; Maricic, I.; Kumar, V.; Arutyunyan, S.; Bouraoui, A.; Nefzi, A. Parallel synthesis and anti-inflammatory activity of cyclic peptides cyclosquamosin D and Met-cherimolacyclopeptide B and their analogs. Bioorg. Med. Chem. Lett. 2010, 20, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Hua, K.F.; Chuang, P.H.; Wu, S.H.; Wu, K.Y.; Chang, F.R.; Wu, Y.C. New cyclic peptides from the seeds of Annona squamosa L. and their anti-inflammatory activities. J. Agric. Food Chem. 2008, 56, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Velleuer, E. Cancer Biology: How Science Works; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Vijg, J. From DNA damage to mutations: All roads lead to aging. Ageing Res. Rev. 2021, 68, 101316. [Google Scholar] [CrossRef]
- El-Habibi, M.F.; Megdad, M.M.; Al-Qadi, M.H.; AlQatrawi, M.J.; Sababa, R.Z.; Abu-Naser, S.S. A Proposed Expert System for Obstetrics & Gynecology Diseases Diagnosis. Int. J. Acad. Multidiscip. Res. 2022, 6, 305–321. [Google Scholar]
- Ranganathan, P.; Sengar, M.; Chinnaswamy, G.; Agrawal, G.; Arumugham, R.; Bhatt, R. Impact of COVID-19 on cancer care in India: A cohort study. Lancet Oncol. 2021, 22, 970–976. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, Y.; Ma, C.; Wang, X.; Li, Y.; Li, X. Antitumor activity of Annona squamosa seed oil. J. Ethnopharmacol. 2016, 193, 362–367. [Google Scholar] [CrossRef]
- Liaw, C.C.; Yang, Y.L.; Chen, M.; Chang, F.R.; Chen, S.L.; Wu, S.H.; Wu, Y.C. Mono-tetrahydrofuran annonaceous acetogenins from Annona squamosa as cytotoxic agents and calcium ion chelators. J. Nat. Prod. 2008, 71, 764–771. [Google Scholar] [CrossRef]
- Ao, H.; Lu, L.; Li, M.; Han, M.; Guo, Y.; Wang, X. Enhanced Solubility and Antitumor Activity of Annona squamosa Seed Oil via Nanoparticles Stabilized with TPGS: Preparation and In Vitro and In Vivo Evaluation. Pharmaceutics 2022, 14, 1232. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Bano, A.; Gupta, A.; Rai, S.; Fatima, T.; Sharma, S.; Pathak, N. Mechanistic role of reactive oxygen species and its regulation via the antioxidant system under environmental stress. In Plant Stress Physiology. Perspectives in Agriculture; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2022; Volume 11. [Google Scholar]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic antioxidants in lipid emulsions: Partitioning effects and interfacial phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food–a review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Seshadri, S. Antioxidant activity of seed extracts of Annona squamosa and Carica papaya. Nutr. Food Sci. 2010, 40, 403–408. [Google Scholar] [CrossRef]
- Luzia, D.M.M.; Jorge, N. Soursop (Annona muricata L.) and sugar apple (Annona squamosa L.): Antioxidant activity, fatty acids profile and determination of tocopherols. Nut. Food Sci. 2012, 42, 434–441. [Google Scholar] [CrossRef]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef]
- Polimati, H.; Pragada, R.R.; Thuan, N.H.; Tatipamula, V.B. Hepatoprotective potential of bioflavonoids. Stud. Nat. Prod. Chem. 2022, 72, 259–285. [Google Scholar]
- Li, S.; Liu, Z.; Joseph, P.; Hu, B.; Yin, L.; Tse, L.A.; PURE-China Investigators. Modifiable risk factors associated with cardiovascular disease and mortality in China: A PURE substudy. Eur. Heart J. 2022, 43, 2852–2863. [Google Scholar] [CrossRef]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological aspects of alcohol metabolism in the liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef]
- Singal, A.K.; Bashar, H.; Anand, B.S.; Jampana, S.C.; Singal, V.; Kuo, Y.F. Outcomes after liver transplantation for alcoholic hepatitis are similar to alcoholic cirrhosis: Exploratory analysis from the UNOS database. Hepatology 2012, 55, 1398–1405. [Google Scholar] [CrossRef]
- WHO. Global Status Report on Alcohol and Health. World Health Organization, 2014. Available online: Chrome-extension://oemmndcbldboiebfnladdacbdafmadadm/https://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf (accessed on 3 June 2022).
- Morita, H.; Iizuka, T.; Choo, C.Y.; Chan, K.L.; Takeya, K.; Kobayashi, J.I. Vasorelaxant activity of cyclic peptide, cyclosquamosin B, from Annona squamosa. Bioorg. Med. Chem. Lett. 2006, 16, 4609–4611. [Google Scholar] [CrossRef]
- Srivastava, S.; Lal, V.K.; Pant, K.K. Medicinal potential of Annona squamosa: At a glance. J. Pharmacol. Res. 2011, 4, 4596–4598. [Google Scholar]
- Srilakshmi, S.; Sravanthi, K.C.; Sarvani, M.; Krishnaharsha, A.; Karteek, P. Anti-helminthic activity of Annona squamosa seed extract. Int. J. Pharm. Technol. 2011, 3, 1623–1628. [Google Scholar]
- Leatemia, J.A.; Isman, M.B. Insecticidal activity of crude seed extracts of Annona spp., Lansium domesticum and Sandoricum koetjape against lepidopteran larvae. Phytoparasitica 2004, 32, 30–37. [Google Scholar] [CrossRef]
- Dang, Q.L.; Kim, W.K.; Nguyen, C.M.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Kim, J.C. Nematicidal and antifungal activities of annonaceous acetogenins from Annona squamosa against various plant pathogens. J. Agric. Food Chem. 2011, 59, 11160–11167. [Google Scholar] [CrossRef] [PubMed]
- Sneeha, V.; Safreen, S.D.A.; Kumaresan, R. Anti-cancer efficacy of ethanolic extracts from various parts of Annona Squamosa on MCF-7 cell line. J. Pharmacogn. Phyther. 2016, 8, 147–154. [Google Scholar]
- Panda, S.; Kar, A. Annona squamosa seed extract in the regulation of hyperthyroidism and lipid-peroxidation in mice: Possible involvement of quercetin. Phytomedicine 2007, 14, 799–805. [Google Scholar] [CrossRef]
- Intaranongpai, J.; Chavasiri, W.; Gritsanapan, W. Anti-head lice effect of Annona squamosa seeds. Southeast Asian J. Trop. Med. Public Health 2006, 37, 532. [Google Scholar]
- Souza, M.; Bevilaqua, C.M.; Morais, S.M.; Costa, C.T.; Silva, A.R.; Braz-Filho, R. Anthelmintic acetogenin from Annona squamosa L. Seeds. An. Acad. Bras. Ciênc. 2007, 80, 271–277. [Google Scholar] [CrossRef]
- Jose, V.; Raphel, L.; Aiswariya, K.S.; Mathew, P. Green synthesis of silver nanoparticles using Annona squamosa L. seed extract: Characterization, photocatalytic and biological activity assay. Bioprocess Biosyst Eng. 2021, 44, 1819–1829. [Google Scholar] [CrossRef]
- Nagaraja, H.; Kugar, T.; Shivanna, Y.; Agrawal, A.; Shetty, R. Ocular toxicity by seeds of Annona squamosa (custard apple). Indian J. Opthalmol. 2016, 64, 611. [Google Scholar] [CrossRef]
- Mwihia, S.K. In vitro Antibacterial and Antioxidant Activities of Methanolic and Dichloromethanolic Seed Extracts of Kenyan Annona squamosa Linn; Kenyatta University: Nairobi, Kenya, 2017. [Google Scholar]
- Bhardwaj, R.; Pareek, S.; Sagar, N.A.; Vyas, N. Bioactive compounds of Annona. In Bioactive Compounds in Underutilized Fruits Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 37–62. [Google Scholar]
- Dahiya, R.; Dahiya, S. Natural bio-effective cyclooligopeptides from plant seeds of Annona genus. Eur. J. Med. Chem. 2021, 214, 113221. [Google Scholar] [CrossRef] [PubMed]
- Quilez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Champy, P. Acetogenins from the Seeds of the Custard Apple (Annona squamosa L.) and their Health Outcomes. In Nuts and Seeds in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Elsevier: London, UK, 2011; pp. 429–437. [Google Scholar]
- Sookvanichsilp, N.; Gritsanapan, W.; Somanabandhu, A.O.; Lekcharoen, K.; Tiankrop, P. Toxicity testing of organic solvent extracts from Annona squamosa: Effects on rabbit eyes and ear skin. Phyther. Res. 1994, 8, 365–368. [Google Scholar] [CrossRef]


| Variety | Category | Compound | Yield/Concentration | Ref. |
|---|---|---|---|---|
| Annona squamosa seeds | Fatty acids (%) | Margaric acid | 0.2 | [21] |
| Linoleic acid | 22.9 | |||
| Eicosanoic acid | 0.9 | |||
| Palmitic acid | 12.1 | |||
| Heneicosanoic acid | 2.3 | |||
| Stearic acid | 13.6 | |||
| Oleic acid | 47.4 | |||
| 11-eicosanoic acid | 0.2 | |||
| Dihydrosterculic acid | 0.1 | |||
| 17-Methyloctadecanoic acid | 0.1 | |||
| Palmitoleic acid | 0.01 | |||
| Annona squamosa seeds | Amino acids (g/100 g protein) | Leucine | 0.845 | [22] |
| Isoleucine | 0.464 | |||
| Glutamic acid | 0.995 | |||
| Phenylalanine + Tyrosine | 0.671 | |||
| Aspartic acid | 0.684 | |||
| Serine | 0.299 | |||
| Alanine | 0.594 | |||
| Methionine + Cystine | 0.106 | |||
| Histidine | 0.139 | |||
| Arginine | 0.704 | |||
| Glycine | 0.392 | |||
| Valine | 0.642 | |||
| Threonine | 0.324 | |||
| Lysine | 0.407 | |||
| Annona squamosa seeds | Polysaccharides | Rhamnose, Fucose, Mannose, Fructose, Arabinose, Galactose, Fructosamine, Galactosamine, Xylose, Glucose, Glucosamine, and Mannuronic, Alluronic, Glucuronic and Galacturonic acid | USP = 0.67–1.27%; FSP = 2.82–3.72% | [27] |
| Annona squamosa seeds | Carbohydrates (g/100 g DW (%)) | - | 66.64 | [23] |
| Fat (g/100 g DW (%)) | - | 29.21 | ||
| Fiber (g/100 g DW (%)) | - | 32.64 | ||
| Ash (g/100 g DW (%)) | - | 1.90 | ||
| Protein (g/100 g DW (%)) | - | 2.25 | ||
| Moisture (g/100 g DW (%)) | - | 3.92 | ||
| Minerals (mg/kg) | K | 56.47–355.84 | ||
| Ca | 46.90–187.12 | |||
| P | 33.30–32.75 | |||
| Mg | 16.22–20.36 | |||
| Fe | 6.74–20.84 | |||
| Cu | 0.30–23.91 | |||
| Na | 9.29–28.27 | |||
| Zn | 0.43–22.17 | |||
| Mn | 0.25 | |||
| Annona squamosa seeds | Crude protein (%) | 18.34 | [28] | |
| Crude fiber (%) | 17.56 | |||
| Crude oil (%) | 30.41 | |||
| Total carbohydrates (%) | 21.80 | |||
| Moisture (%) | 6.65 | |||
| Ash (%) | 5.24 | |||
| Annona squamosa seeds | Moisture (%) | 6.7 | [22] | |
| Fat (%) | 26.8 | |||
| Protein (%) | 17.5 | |||
| Ash (%) | 2.2 | |||
| Fiber (%) | 16.8 | |||
| Tocopherol (mg/100 g) | 15.5–16.6 | |||
| Annona squamosa seeds | Carotenoids (μg of β-Carotene/100 mg) | 0.45 | [29] | |
| Vitamin C (mg AA/100 g) | 0.57 | |||
| Variety/Region | Activity | Extract/Solvent Used/Concentration | Study/Cell Line/Animal Model | Key Finding | Ref. |
|---|---|---|---|---|---|
| Annona squamosa seeds (Thiruvananthapuram, Kerala state) | Antimicrobial | Chloroform extract of seeds (10–60 μg/mL) | E. coli, S. typhi, K. pnemoniae, P. mirabilis, B. subtilis, S. aureus | Significant antibacterial activity with inhibition rate of 37–56, 40–60.75, 36–64, 48.5–63, 35–53.5 and 34–47% for K. pneumoniae, B. subtilis, E. coli, P. mirabilis, S. typhi and S. aureus | [45] |
| Annona squamosa seeds (Nashik, Maharashtra) | Antimicrobial | Petroleum ether, methanol and chloroform seed extract | E. coli, P. aeruginosa, S. aureus, K. pneumoniae, B. subtilis | PEE: highest growth inhibition rate was observed for S. aureus (ZOI: 12 mm) and lowest for P. aeruginosa with (ZOI 7.8 mm); ME: significant inhibition against K. pneumoniae (ZOI: 12.8 mm) and B. subtilis (ZOI: 9.2 mm); CE: inhibition against E. coli (ZOI: 14.8 mm) and B. subtilis (ZOI: 1.7 mm) | [46] |
| Annona squamosa seeds (Jayanagar, Bangalore) | Antimicrobial | Methanolic seed extract (50 mg/mL) | E. coli, S. typhi, S. aeurus, E. faecalis, P. aeruginosa, S. paratyphi, K. pneumoniae | Inhibits growth of bacterial strains with ZOI equal to 27–30 mm for E. coli, 31 mm for S. typhi, 27–32 mm for S. aeurus, 23 mm for E. faecalis, 22–24 mm for P. aeruginosa, 22–30 mm for S. paratyphi, 11–20 mm for K. pneumoniae | [47] |
| Annona squamosa seeds (Bangalore) | Antimicrobial | Methanolic seed extracts of A. squamosa and Prunus Persia (1:2) | S. aureus, E. coli, K. pneumoniae, S. typhi, Enterococcus faecalis, P. aeruginosa, S. paratyphi | ZOI ranges between 18–34 mm for all tested pathogens | [48] |
| Annona squamosa seeds (Alexandria, Egypt) | Antimicrobial | - | E. coli, C. albicans, B. subtilis, K. pneumoniae, S. senftenberg, S. aureus | ZOI ranging between 9.50, 9.53, 10.33, 12.30 6.50 and 12.50 mm against E. coli, C. albicans, K. pneumoniae, S. senftenberg, S. aureus and B. subtilis, respectively | [23] |
| Annona squamosa seeds | Antimicrobial | Aqueous seed extract (500 and 1000 mg pm- CuO NPs) | Xanthomonas oryzae | At 500 mg: ZOI = 9 mm; at 1000 mg: ZOI = 15 mm | [49] |
| Annona squamosa seeds (Nam Dinh, Vietnam) | Antifungal | Acetogenins (squamostatin-A (7), squamocin-G (5), and squamocin (8)) extracted from custard apple seeds | Phytophthora infestans | Acetogenins exhibit dose-dependent activity against the growth of zoospore and sporangium IC50 value for inhibition of sporangium germination was 1.24–2.09 μg/mL and IC50 value for zoospore germination inhibition was 1.89–3.05 μg/mL, for all acetogenins | [81] |
| Annona squamosa seeds | Antidiabetic | Ethanolic and methanolic seed extract (200 mg/kg BW) | Alloxan-induced diabetic rats | Decrease in level of blood glucose after administration of ethanolic seed extract (139.8–142 mg/dL) and methanolic seed extract (139–146 mg/dL) at 7th day of treatment | [52] |
| Annona squamosa seeds | Anti-inflammatory | Cyclopeptides-met-cherimolacyclopeptide and cyclosquamosin D (A1), and B (B) | LPS-J774A.1 cell line | Reduction in IL-6 and TNF-α secretion in J774A with an IC50 value of 1.22 and 9.2 µM | [56] |
| Annona squamosa seeds (Luye, Taitung County, Taiwan) | Anti-inflammatory | Cyclosquamosin D | Lipopolysaccharide and Pam3Cys-stimulated J774A.1 macrophages | Inhibition of secretion of pro-inflammatory cytokines | [57] |
| Annona squamosa seeds (Jiangsu, China) | Antitumor | Bullatacin and 12,15-cis-squamostatin-A | A-549, Hela, HepG2 and MCF-7 (in vitro) and H22 tumor cell line in mice (in vivo) | IC50 value for MCF-7, A-549, and HepG2 and Hela, are 2.5 × 10−1, 3.2, 3.6 × 10−1 and 13.0 µg/mL, respectively, and 69.55% inhibition of H22 cell line | [18] |
| Annona squamosa seeds (Jiangsu, China) | Antitumor | Seed oil | H22 tumor cell line (mice: in vivo) | Inhibition of growth of H22 cell line with maximum inhibitory rate of 53.54% | [62] |
| Annona squamosa seeds (TaiDong County, Taiwan) | Antitumor | Squadiolins A and B and squafosacin B | MDA-MB-231, Hep G2, MCF-7 and Hep 3B, cell lines | Squadiolins A- MDA-MB-231: IC50 = 0.63 µM; squadiolins B- MDA-MB-231: IC50 = 0.28 µM; squafosacin B- HepG2: IC50 = 0.71 µM; Hep 3B: IC50 = 0.72 µM; MCF-7: IC50 = 0.96 µM | [63] |
| Annona squamosa seeds (Hyderabad, India) | Antitumor | Aqueous and organic extract from defatted seeds | AK-5 histiocytic tumor cell line in rat animal model | Significant tumor cell apoptosis, with increased caspase-3 expression, down regulation of Bcl-2 and Bclxl antiapoptotic genes | [11] |
| Annona squamosa seeds (Beijing, China) | Anticancer | Seed oil nanoparticles | 4T1-Mouse breast cancer cells | Inhibitory rate of 69.8% against 4T1 cell line | [64] |
| Annona squamosa seeds | Anticancer | Ethanolic seed extract | MCF-7 breast cancer cell line | Inhibit growth of MCF-7 (IC50 = 10 ug/mL) by inducing apoptosis | [82] |
| Annona squamosa seeds (Alexandria, Egypt) | Anticancer | - | HepG-2, MCF-7, Caco-2 and PC-3 cancer cell lines | Caco-2: IC50 = 11.55 μg/mL; HepG-2: IC50 = 7.99 μg/mL; MCF-7: IC50 = 14.34 μg/mL; PC-3: IC50 = 7.31 μg/mL | [23] |
| Annona squamosa seeds (Ahmedabad, India) | Antioxidant | Hexane, acetone, chloroform: methanol (2:1), ethanol (50%) and water seed extract | DPPH assay | Highest antioxidant activity was observed in water (777.64 g GAE/g), while lowest was observed in hexane (268.75 g GAE/g) seed extract | [69] |
| Annona squamosa seeds (Alexandria, Egypt) | Antioxidant | - | DPPH assay | IC50 value equal to 7.88 µg/mL | [23] |
| Annona squamosa seeds (Ceara, Brazil) | Antioxidant | Methanolic seed extract | Fe3+ reduction, DPPH and ABTS assay | IC50 value of 0.57, 0.36, and 0.14 mg/mL for Fe3+ reduction, DPPH and ABTS assay performed on methanolic seed extract, respectively | [29] |
| Annona squamosa seeds (Southeastern Brazil) | Antioxidant | Ethanolic seed extract | DPPH assay | EC50 value of seed extract is 63.19 µg/mL | [70] |
| Annona squamosa seeds (Lucknow, India) | Antioxidant | Ethanolic seed extract | Alcohol-induced liver damage in Sprague Dawley rats (150–210 g) (dose: 200 and 400 mg/kg po) | Significant elevation in the level of SOD, GSH and CAT and decrease in the level of TBARS | [32] |
| Annona squamosa seeds (Lucknow, India) | Hepatoprotective | Ethanolic seed extract | Alcohol-induced liver injury in Sprague Dawley rats (150–210 g) (dose: 200 and 400 mg/kg po) | Reduction in ALT, ALP, AST, LDH and SBL and cholesterol level and increase in the level of albumin (p < 0.01–p < 0.001) and total protein (p < 0.05–p < 0.001) | [32] |
| Annona squamosa seeds (Bangalore, India) | Hepatoprotective | Hydroalcoholic seed extract | CCl4-induced hepatotoxicity in rats | Reduction in the level of SGOT (51.22–87.37 U/L), SGPT (38.21–96.22 U/L), ALP (98.28–159.25 U/L) and total bilirubin (0.71–1.47 mg/dL) | [20] |
| Annona squamosa seeds (Mumbai, India) | Antipsoriatic/antiproliferative | Seed oil | HaCaT cell line in Oxazolone-induced psoriasis in female Balb/C | Inhibition of growth of HaCaT cells | [19] |
| Annona squamosa seeds (Madhya Pradesh, India) | Antithyroidal | Methanolic seed extract (dose: 200 mg/kg) | L-T4 (0.5 mg/kg/day) caused hyperthyroid in rats | After treating T4-induced hyperthyroid mice with seed extract (200 mg/kg) for 10 days, the effects of L-T4 were reversed, demonstrating the potential of custard apple seed in controlling hyperthyroidism | [83] |
| Annona squamosa seeds | Vasorelaxant | Cyclosquamosin B | Rat animal model | Inhibitory effect on Ca2+ channel, at concentration of 10−5 M | [77] |
| Annona squamosa seeds | Antiheadlice | Petroleum ether seed extract | In vitro | Petroleum ether extract along with coconut oil (1:1), kills 90% of lice | [78] |
| Annona squamosa seeds (Pak Chong, Thailand) | Antiheadlice | Hexane seed extract | In vitro against headlice | Seed extract contains oleic acid and a triglyceride with one oleate ester that kills lice in 11–49 min | [84] |
| Annona squamosa seeds | Antihelminthic | - | - | Seed extract exhibit antihelminthic activity against Pheritima posthuman and Haemonchus contortus | [79] |
| Annona squamosa seeds (Fortaleza, Brazil) | Antihelminthic | Ethyl acetate seed extract | Haemonchus contortus | C37 trihydroxy adjacent bis- tetrahydrofuran acetogenin repressed egg hatching of H. contortus at 25 mg mL−1 | [85] |
| Annona squamosa seeds | Antilarval | Crude ethanolic seed extract | - | Inhibit larval growth (20-fold) in Spodptera litura | [80] |
| Annona squamosa seeds (Thrissur, Kerala, India) | Larvicidal activity | Silver nanoparticles (AgNPs) of aqueous seed extract | III and IV instars of Anopheles stephensi | At 60 μg/mL, 100% mortality at III instar is observed. LC50 = 22.44 μg/mL; LC90 = 40.65 μg/mL at III instar stage. At IV instar LC50 = 27.83 μg/mL; LC90 = 48.92 μg/mL | [86] |
| Annona squamosa seeds (Thrissur, Kerala, India) | Antibacterial | Silver nanoparticles (AgNPs) of aqueous seed extract | Staphylococcus aureus, Klebsiella pnuemoniae | Antibacterial activity of AgNPs was found to be efficient compared with plant extract and commercial antibiotic tetracycline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, N.; Prakash, S.; Kumar, M.; Radha; Zhang, B.; Sheri, V.; Rais, N.; Chandran, D.; Dey, A.; Sarkar, T.; et al. Seed Waste from Custard Apple (Annona squamosa L.): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile. Processes 2022, 10, 2119. https://doi.org/10.3390/pr10102119
Kumari N, Prakash S, Kumar M, Radha, Zhang B, Sheri V, Rais N, Chandran D, Dey A, Sarkar T, et al. Seed Waste from Custard Apple (Annona squamosa L.): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile. Processes. 2022; 10(10):2119. https://doi.org/10.3390/pr10102119
Chicago/Turabian StyleKumari, Neeraj, Suraj Prakash, Manoj Kumar, Radha, Baohong Zhang, Vijay Sheri, Nadeem Rais, Deepak Chandran, Abhijit Dey, Tanmay Sarkar, and et al. 2022. "Seed Waste from Custard Apple (Annona squamosa L.): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile" Processes 10, no. 10: 2119. https://doi.org/10.3390/pr10102119
APA StyleKumari, N., Prakash, S., Kumar, M., Radha, Zhang, B., Sheri, V., Rais, N., Chandran, D., Dey, A., Sarkar, T., Dhumal, S., Kumar, S., Mahato, D. K., Vishvanathan, M., Mohankumar, P., Pateiro, M., & Lorenzo, J. M. (2022). Seed Waste from Custard Apple (Annona squamosa L.): A Comprehensive Insight on Bioactive Compounds, Health Promoting Activity and Safety Profile. Processes, 10(10), 2119. https://doi.org/10.3390/pr10102119













