Numerical Study of Electrostatic Desalting: A Detailed Parametric Study
Abstract
:1. Introduction
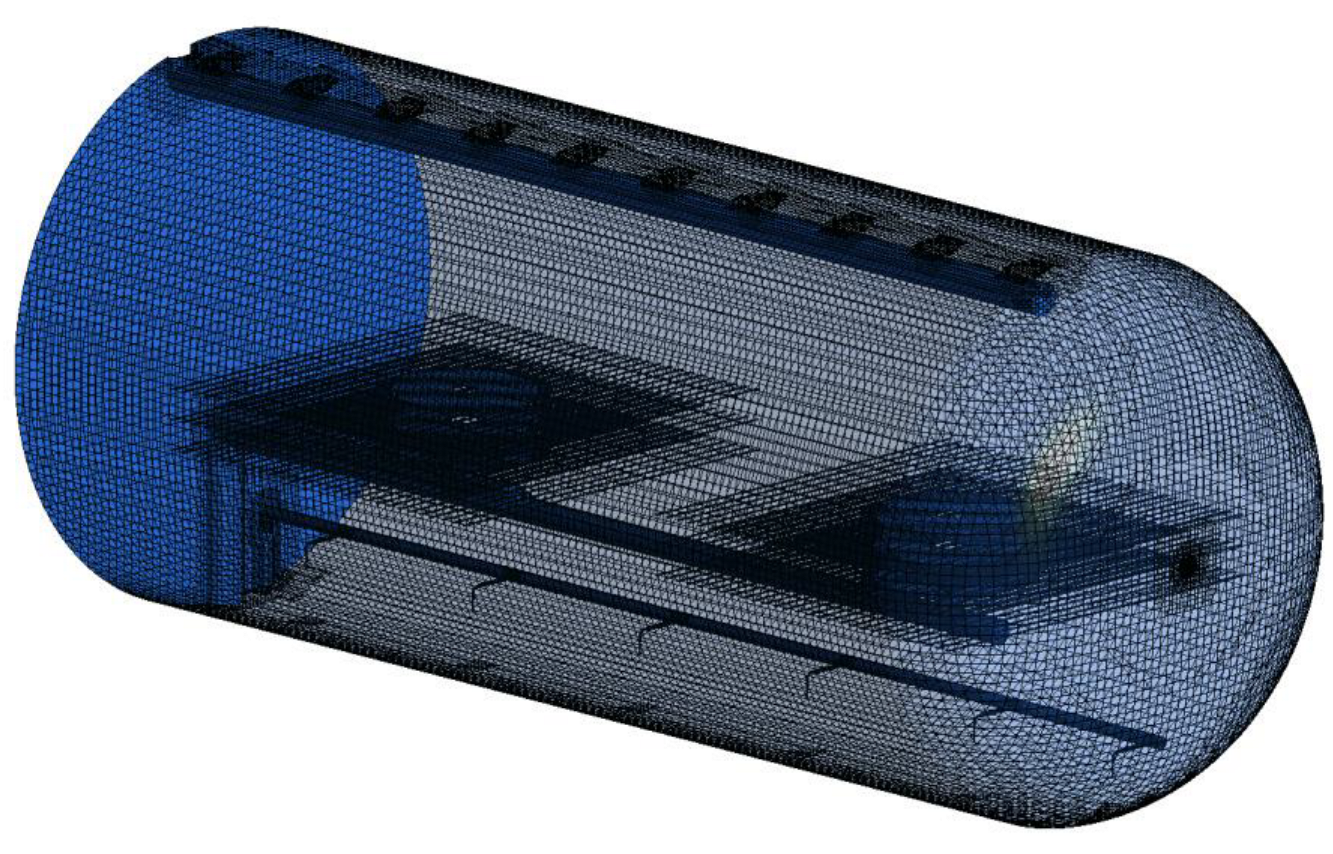
Mathematical Modeling
2. Results
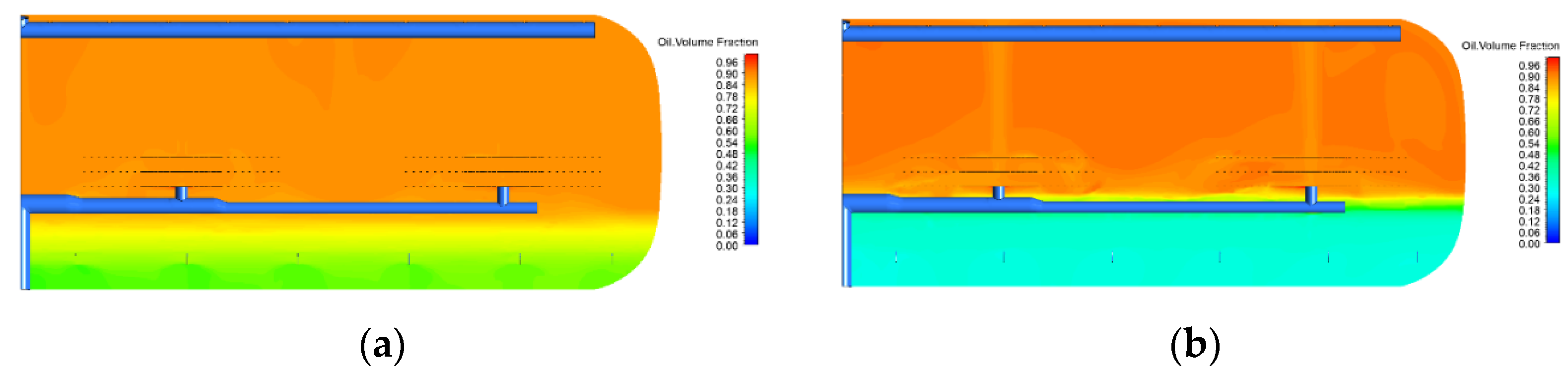
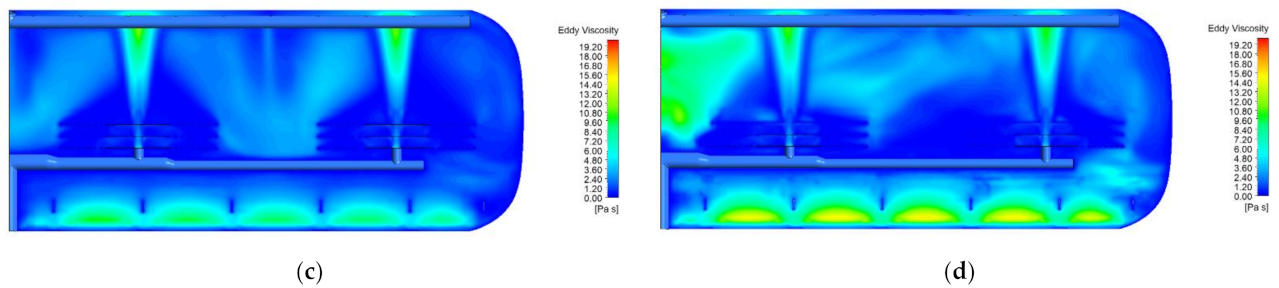
2.1. Effect of Electric Field
2.2. Effect of Temperature (Oil Viscosity)
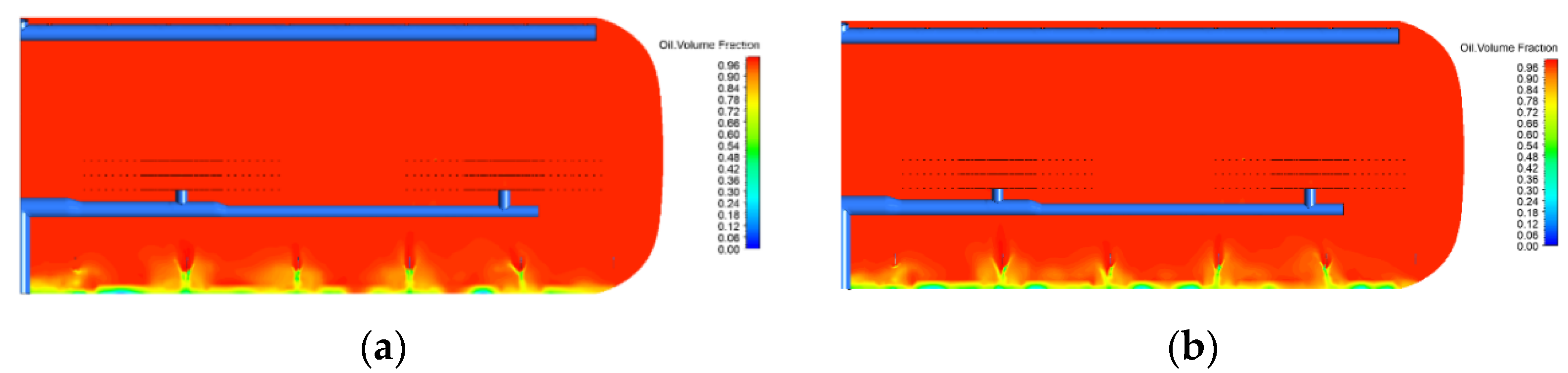
2.3. Effect of Water Content
2.4. Effect of Droplet Size
3. Discussion
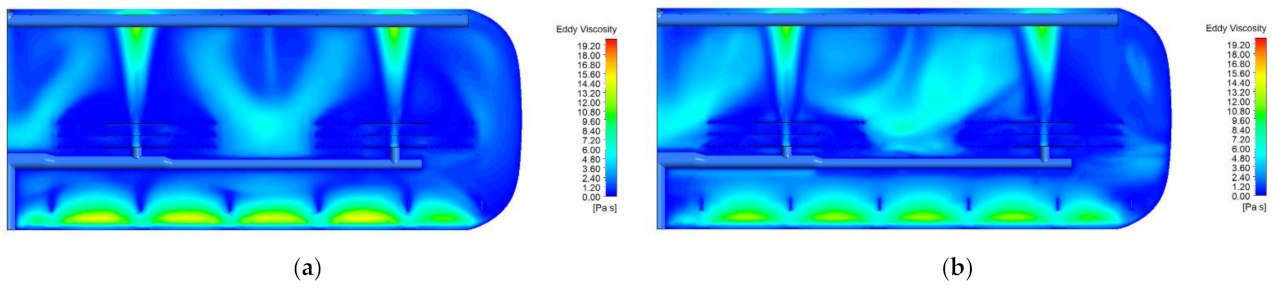
3.1. Hydrodynamic Analysis
3.2. Statistical Analysis and Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| Symbol | Meaning | Units | Symbol | Meaning |
| density | [kg m−3] | Sub index | ||
| Volume fraction | [-] | M | mixture | |
| N | Number of phases | [-] | I | i-th phase |
| Viscosity | [kg m−1 s−1] | T | Turbulence | |
| Average velocity | [m s−1] | D | Diffusion | |
| Pressure | [N m−2] | Drift of i-th phase | ||
| Stress tensor | [N m−2] | w | Water | |
| Fluctuating velocity | [m s−1] | o | Oil | |
| Gravitational constant | 9.81 [m s−2] | c | Coalescence | |
| Kinematic viscosity | [m2 s−1] | b | Breakup | |
| Pr | Prandtl number | [-] | ||
| Drag coefficient | [-] | |||
| Re | Reynolds number | [-] | ||
| Droplet diameter | [m] | |||
| Interfacial area concentration | [m2 m−3] | |||
| Droplet breakup | [m−1 s−1] | |||
| Droplet Coalescence | [m−1 s−1] | |||
| Number of droplets per volume of the mixture | [m−3] | |||
| Frequency of collisions | [s−1] | |||
| Coalescence probability | [-] | |||
| Number of eddies per volume of the mixture | [m−3] | |||
| Frequency of collisions due to turbulence | [s−1] | |||
| Breakup efficiency | [-] | |||
| The maximum water volume fraction | [-] | |||
| Energy dissipation rate | [m2 s−3] | |||
| k | Turbulent kinetic energy | [m2 s−2] | ||
| Production of turbulence | [m2 s−3] | |||
| Strain tensor | [s−1] | |||
| Constants of the k-ε realizable turbulence model | [-] | |||
References
- Lovell, J. Climate report calls for green ‘New Deal’. Reuters 2008. [Google Scholar]
- Chohan, U.W. A Green New Deal: Discursive Review and Appraisal. Notes on the 21st Century (CBRI). Notes 21st Century CBRI 2019. [Google Scholar] [CrossRef]
- Varadaraj, R.; Savage, D.W.; Brons, C.H. Chemical Demulsifier for Desalting Heavy Crude. U.S. Patent 6,168,702, 28 January 2001. [Google Scholar]
- Alshehri, A. Modeling and Optimization of Desalting Process in Oil Industry. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2009; p. 74. [Google Scholar]
- Abdel-Aal, H.K.; Aggour, M.A.; Fahim, M.A. Petroleum and Gas Field Processing; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Al-Otaibi, M.B.; Elkamel, A.; Nassehi, A.V.; Abdul-Wahab, S.A. A Computational Intelligence Based Approach for the Analysis and Optimization of a Crude Oil Desalting and Dehydration Process. Energy Fuels 2005, 19, 2526–2534. [Google Scholar] [CrossRef]
- Sams, G.W.; Zaouk, M. Emulsion Resolution in Electrostatic Processes. Energy Fuels 1999, 14, 31–37. [Google Scholar] [CrossRef]
- Mahdi, K.; Gheshlaghi, R.; Zahedi, G.; Lohi, A. Characterization and modeling of a crude oil desalting plant by a statistically designed approach. J. Pet. Sci. Eng. 2008, 61, 116–123. [Google Scholar] [CrossRef]
- Herrmann, H.; Bucksch, H. Mud Logging. In Dictionary Geotechnical Engineering/Wörterbuch GeoTechnik; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bresciani, A.E.; Alves, R.M.B.; Nascimento, C.A.O. Coalescence of Water Droplets in Crude Oil Emulsions: Analytical Solution. Chem. Eng. Technol. 2010, 33, 237–243. [Google Scholar] [CrossRef]
- Pruneda, E.F.; Rivero, E.; Escobedo, B.; Javier, F.; Vázquez, G. Optimum Temperature in the Electrostatic Desalting of Maya Crude Oil. J. Mex. Chem. Soc. Vol. 2005, 49, 14–19. [Google Scholar]
- Wilkinson, D.; Waldie, B.; Nor, M.I.M.; Lee, H.Y. Baffle plate configurations to enhance separation in horizontal primary separators. Chem. Eng. J. 2000, 77, 221–226. [Google Scholar] [CrossRef]
- Bresciani, A.E.; Mendonça, C.F.; Alves, R.M.; Nascimento, C.A. Modeling the kinetics of the coalescence of water droplets in crude oil emulsions subject to an electric field, with the cellular automata technique. Comput. Chem. Eng. 2010, 34, 1962–1968. [Google Scholar] [CrossRef]
- Aryafard, E.; Farsi, M.; Rahimpour, M.; Raeissi, S. Modeling electrostatic separation for dehydration and desalination of crude oil in an industrial two-stage desalting plant. J. Taiwan Inst. Chem. Eng. 2016, 58, 141–147. [Google Scholar] [CrossRef]
- Aryafard, E.; Farsi, M.; Rahimpour, M.R. Modeling and simulation of crude oil desalting in an industrial plant considering mixing valve and electrostatic drum. Chem. Eng. Process. Process Intensif. 2015, 95, 383–389. [Google Scholar] [CrossRef]
- Kakhki, N.A.; Farsi, M.; Rahimpour, M.R. Effect of current frequency on crude oil dehydration in an industrial electrostatic coalescer. J. Taiwan Inst. Chem. Eng. 2016, 67, 1–10. [Google Scholar] [CrossRef]
- Vafajoo, L.; Ganjian, K.; Fattahi, M. Influence of key parameters on crude oil desalting: An experimental and theoretical study. J. Pet. Sci. Eng. 2012, 90, 107–111. [Google Scholar] [CrossRef]
- Bansal, H. Ameensayal. CFD Analysis of Horizontal Electrostatic Desalter-Influence of Header Obstruction Plate Design on Crue-Water Separation. 2015. Available online: https://www.digitalxplore.org/up_proc/pdf/51-13951413061-6.pdf (accessed on 27 August 2022).
- Shariff, M.M.; Oshinowo, L.M. Debottlenecking Water-Oil Separation with Increasing Water Flow Rates in Mature Oil Fields. In Proceedings of the 5th Water Arabia 2017 Conference and Exhibition, Al-Khobar, Saudi, 17 October 2017; pp. 17–19. [Google Scholar]
- Alhajri, N.A.; White, R.J.; Oshinowo, L.M. High Efficiency Static Mixer Technology for Crude Desalting. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018; SPE-192388-MS. OnePetro: Richardson, TX, USA, 2018. [Google Scholar] [CrossRef]
- Favero, J.L.; Silva, L.F.L.; Lage, P.L. Modeling and simulation of mixing in water-in-oil emulsion flow through a valve-like element using a population balance model. Comput. Chem. Eng. 2015, 75, 155–170. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.; Chen, G.; Zhu, D. Numerical Simulation of the Oil Droplet Size Distribution Considering Coalescence and Breakup in Aero-Engine Bearing Chamber. Appl. Sci. 2020, 10, 5648. [Google Scholar] [CrossRef]
- Sofos, F. A Water/Ion Separation Device: Theoretical and Numerical Investigation. Appl. Sci. 2021, 11, 8548. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Pan, Z. Experimental Study on the Performance of a Novel Compact Electrostatic Coalescer with Helical Electrodes. Energies 2021, 14, 1733. [Google Scholar] [CrossRef]
- Sellman, E.; Sams, G.W.; Mandewalkar, S.P.K. Improved Dehydration and Desalting of Mature Crude Oil Fields. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 10 March 2013. [Google Scholar] [CrossRef]
- Ramirez-Argaez, M.; Abreú-López, D.; Gracia-Fadrique, J.; Dutta, A. Numerical Study of Electrostatic Desalting Process Based on Droplet Collision Time. Processes 2021, 9, 1226. [Google Scholar] [CrossRef]
- Dutta, A.; Ramírez-Argáez, M.A. Modeling of an Industrial Electrostatic Desalting Unit Using Computational Fluid Dynamics (CFD). Scıentıfıc Conference “Scıence, Technology and Development of Innovatıve Technologıes”. In Proceedings of the 30th Annıversary of Independence of Turkmenıstan, Ashgabat, Turkmenistan, 12–13 June 2021. [Google Scholar]
- Pericleous, K.A.; Drake, S.N. An Algebraic Slip Model of Phoenics for Multi-phase Applications. In Numerical Simulation of Fluid Flow and Heat/Mass Transfer Processes; Springer: Berlin/Heidelberg, Germany, 1986; pp. 375–385. [Google Scholar] [CrossRef]
- Kar, S.; Majumder, S.; Constales, D.; Pal, T.; Dutta, A. A comparative study of multi objective optimization algorithms for a cellular automata model. Rev. Mex. Ing. Quim. 2019, 19, 299–311. [Google Scholar] [CrossRef] [Green Version]




| Researchers | Nature | Variables | Conclusion |
|---|---|---|---|
| Abdul-Wahab et al. [5] | Laboratory scale model (bottle test) | Temperature, mixing time, residence time, chemical dosage, water content | Most important variables obtained are temperature, water content, and residence time |
| Ali Khairan Alshehri [4] | Numerical model | Oil temperature, water content, voltage, initial water content, oil flow rate, demulsifier flow rate | Minimization of wash water and final salt content. |
| Otaibi et al. [6] | Numerical model | Demulsifier concentration, temperature, wash water %, salt content, rate of mixing water addition | Maximizing the efficiency of water and salt removal |
| Bresciani et al. [10] | Analytical model | Water content, temperature, voltage, droplet size (upper and lower droplets) | Model predicts the displacement of two droplets and the time for collision under an electric field |
| Bresciani et al. [13] | Same model as [10] but extended with cellular automata | Water content, temperature, voltage, droplet size distribution | Predicted coalescence and validated with industrial data. |
| Fetter-Pruneda et al. [11] | Experimental model (industrial scale) | Water content, temperature, crude density | The optimum temperature for Mayan crude oil and recommended practices |
| Wilkinson et al. [12] | Numerical model | Design of baffle separator in a gravity separator of water from oil | Best design of baffle enhancing the water separation from oil |
| Aryafard et al. [14,15] | Numerical model, population balance model | Pressure drop, electric field, especially wash water content. | Prediction of coalescence and break up. Analysis of one and two stages of desalting processes. Improvement of separation efficiency from 96.5 to 98.5% when wash water is changed from 3 to 6% and validated in an industrial unit. |
| Kakhki et al. [16] | Numerical model, population balance | Pressure drop, electric field, but especially wash water content (similar to [6]) | Similar results than in [13]. Increasing the rate of collision between water droplets promotes coalescence. |
| Mahdi et al. [8] | Experimental model (laboratory scale) | Demulsifying agent concentration, temperature, wash water dilution ratio, settling time, and mixing time with wash water | Optimum values of demulsifying agent concentration 15 ppm, temperature 77 °C, 10% wash water dilution ratio, settling time 3 min, and mixing time 9 min. |
| Vafajoo et al. [17] | Experimental model (laboratory scale), fuzzy logic | Temperature, injected chemicals, and the pH of the crude oil associated water | Temperature between 115 to 120 °C, best demulsifiers were C and F at levels of 50 to 100 ppm, separating 88% for water and 99% for salt. The pH has to be between 9 and 12 |
| Bansal and Ameensayal [18] | Numerical model | Design parameters | Improve fluid flow features and increase performance |
| Shariff and Oshinowo [19] | Numerical model | Fluid flow analysis | Vortices formed at the inlet reduce the separation efficiency. |
| Ilkhaani, S. [5] | Thermodynamic model | Adding a second stage of desalting to one-stage desalting to meet levels of water and salt | Improve the heat integration of the desalting process, and optimization of desalting temperature |
| Alhajri et al. [20] | Numerical model and plant trials | Device proposed is a static mixer at the inlet. | Turbulence is key to enhance separation of water and salt. |
| Favero et al. [21] | Numerical model | Population balance (CFD + DPM) to predict droplet size distribution in a duct that mimics pass over a globe valve | Good agreement between the experimental and predicted droplet sizes. |
| Wang et al. [22] | Numerical model | CFD + DPM in an aeroengine bearing chamber (not desalter) | Coalescence and breakup of oil droplet increases with the initial diameter of oil droplet. |
| Sofos [23] | Simulations with molecular dynamics | Novel electrostatic device consisting of separation cells | The proposed application could be exploited for the design of a desalination device. |
| Shi et al. [24] | Plant trials | Novel desalter with helical electrodes. Effect of electric field strength, frequency, water content, and fluid velocity on the performance of coalescence. | Increasing the electric field strength could contribute to the growth of small water droplets and coalescence. The study may be used for optimization |
| Assumption | Consequence |
|---|---|
| Isothermal system | There are considered thermal gradients in the desalter |
| Constant physical properties | Both water and oil are Newtonian and incompressible fluids |
| Steady state | The time derivatives are zero |
| Non-slip and impermeable walls | All components of the velocity vector are zero at the boundary and internal static walls |
| Oil is the continuous phase | Water is the disperse phase in W/O emulsions. Therefore, the reverse emulsion O/W is not considered to appear in the unit. |
| Mixture algorithm | A single set of equations: continuity, momentum, and one turbulence model to simulate the multiphase system. |
| k–epsilon realizable turbulence model | To represent the turbulence in the continuous phase. The disperse phase has no turbulence. |
| Interfacial area concentration | To account for the events of breakup and coalescence of droplets |
| Collision frequency | Coalescence depends directly on the frequency of the collisions between droplets, as was stated by Ramirez-Argaez et al. [26] |
| Name | Equation |
|---|---|
| Mixture density | |
| Mixture viscosity | |
| Mixture velocity | |
| Continuity | |
| Momentum | where the three tensors are the average viscous stress τm, the turbulence stress τTm and the diffusion stress τDm due to the phase slip: where vd,i : In the case of water droplets, according to the algebraic slip formulation by [28] where the buoyant, turbulent dispersion and drag forces acting on the water droplets are balanced: |
| Drag coefficient (Shiller–Nauman) | |
| Water phase continuity | As there are only two phases and the , |
| Interfacial area concentration | Source due to coalescence SRC: Source due to breakup, STI: |
| Number of droplets per unit volume of the mixture | |
| Frequency of collisions [26] | with a coalescence probability =1. |
| Number of eddies per unit volume [26] | |
| Frequency of collision due to turbulence | |
| Breakup efficiency | |
| Water droplet diameter | |
| Turbulent kinetic energy | |
| Energy dissipation rate | is the production of turbulence, and S is the strain tensor. |
| Boundary | Condition |
|---|---|
| Non-slip conditions at the internal and external walls | Zero velocity of all components, no turbulence (standard wall functions) |
| Inlets | Inlet velocity of the emulsion with a volume fraction of water |
| Outlets | Pressure outlet (gauge pressure equal zero) |
| Level/Variable | E (kV/cm) | X | µ (kg/ms) | D (µm) |
|---|---|---|---|---|
| (+) | 3 | 0.12 | 0.071 | 20 |
| (−) | 0.1 | 0.03 | 0.017 | 1 |
| Case Number | E | X | µ | D |
|---|---|---|---|---|
| 1 | − | − | − | − |
| 2 | − | − | − | + |
| 3 | − | − | + | − |
| 4 | − | − | + | + |
| 5 | − | + | − | − |
| 6 | − | + | − | + |
| 7 | − | + | + | − |
| 8 | − | + | + | + |
| 9 | + | − | − | − |
| 10 | + | − | − | + |
| 11 | + | − | + | − |
| 12 | + | − | + | + |
| 13 | + | + | − | − |
| 14 | + | + | − | + |
| 15 | + | + | + | − |
| 16 | + | + | + | + |
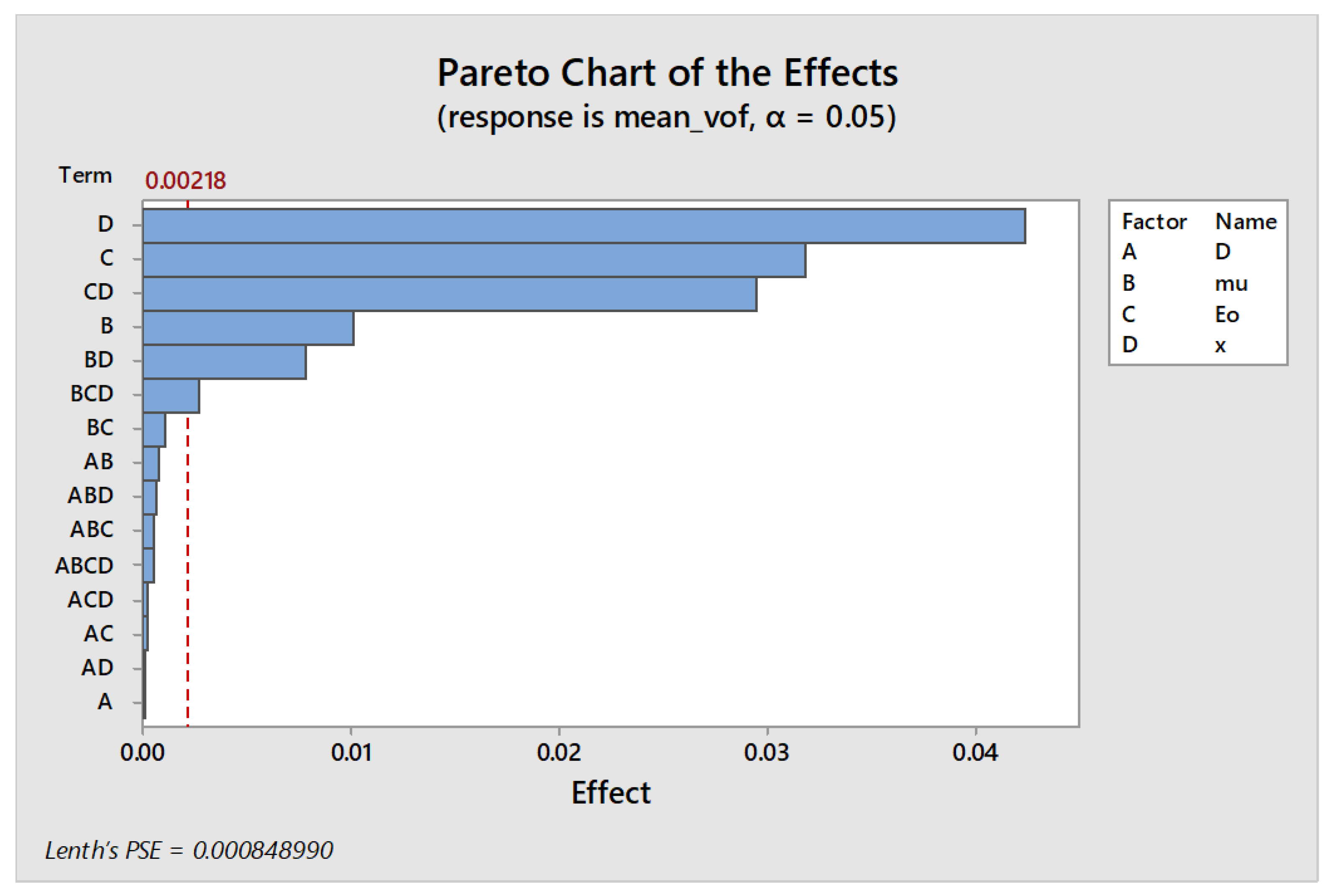
| Term | Effect | Coefficient | Std Dev. | p-Value |
|---|---|---|---|---|
| µ | 0.010134 | 0.005067 | 0.000656 | 0.001 |
| E | −0.03191 | −0.01596 | 0.000656 | 0.000 |
| X | 0.04239 | 0.02119 | 0.000656 | 0.000 |
| µ × x | 0.007805 | 0.003903 | 0.000656 | 0.002 |
| E × x | −0.02950 | −0.01475 | 0.000656 | 0.000 |
| Optimum Point | D | μ | E | x | Mean Volume Fraction of Water |
|---|---|---|---|---|---|
| 1 | 3.06095597 | 0.01707254 | 2.99339092 | 0.03003833 | 0.02526776 |
| 2 | 3.52471021 | 0.01701073 | 2.99998767 | 0.03001266 | 0.02524942 |
| 3 | 3.55716842 | 0.01703218 | 2.98611306 | 0.03003497 | 0.02527249 |
| 4 | 6.1115551 | 0.01701526 | 2.99889999 | 0.03004764 | 0.02524273 |
| 5 | 7.33734703 | 0.01701039 | 2.9964797 | 0.03003354 | 0.02523891 |
| 6 | 7.7335968 | 0.01704495 | 2.99988084 | 0.03008657 | 0.0252396 |
| 7 | 7.83882932 | 0.01704015 | 2.94999207 | 0.03003251 | 0.0253042 |
| 8 | 8.06589326 | 0.01707272 | 2.91442089 | 0.03008012 | 0.02536068 |
| 9 | 8.52232293 | 0.0170021 | 2.99859394 | 0.03000871 | 0.02522771 |
| 10 | 10.0636939 | 0.01710667 | 2.99625853 | 0.03005619 | 0.0252359 |
| 11 | 10.2583472 | 0.01702999 | 2.96275447 | 0.03005567 | 0.02527663 |
| 12 | 10.3801102 | 0.01704703 | 2.99860591 | 0.03009518 | 0.02523013 |
| 13 | 11.7521461 | 0.01704481 | 2.99105772 | 0.03001022 | 0.02522692 |
| 14 | 12.2213506 | 0.01702969 | 2.98366032 | 0.03006916 | 0.02523927 |
| 15 | 12.4154352 | 0.01703886 | 2.99939996 | 0.03004532 | 0.02521476 |
| 16 | 12.9440779 | 0.01701494 | 2.99693118 | 0.03003288 | 0.02521299 |
| 17 | 13.0555521 | 0.01702826 | 2.90428816 | 0.03000283 | 0.02534075 |
| 18 | 13.6925743 | 0.01700749 | 2.97734302 | 0.03003171 | 0.0252365 |
| 19 | 13.70686 | 0.0170153 | 2.99584673 | 0.0300342 | 0.02521118 |
| 20 | 15.5920049 | 0.01707421 | 2.97423509 | 0.03002545 | 0.0252366 |
| 21 | 16.5123639 | 0.01700549 | 2.99958726 | 0.03006071 | 0.02519454 |
| 22 | 16.6602285 | 0.01708459 | 2.99414794 | 0.03002029 | 0.02520413 |
| 23 | 17.0696906 | 0.01700989 | 2.98707433 | 0.03003351 | 0.02520774 |
| 24 | 18.7182166 | 0.01700966 | 2.98791986 | 0.03000627 | 0.02519679 |
| 25 | 18.8514308 | 0.01704943 | 2.9998606 | 0.03006069 | 0.02518672 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Argaez, M.A.; Abreú-López, D.; Gracia-Fadrique, J.; Dutta, A. Numerical Study of Electrostatic Desalting: A Detailed Parametric Study. Processes 2022, 10, 2118. https://doi.org/10.3390/pr10102118
Ramirez-Argaez MA, Abreú-López D, Gracia-Fadrique J, Dutta A. Numerical Study of Electrostatic Desalting: A Detailed Parametric Study. Processes. 2022; 10(10):2118. https://doi.org/10.3390/pr10102118
Chicago/Turabian StyleRamirez-Argaez, Marco A., Diego Abreú-López, Jesús Gracia-Fadrique, and Abhishek Dutta. 2022. "Numerical Study of Electrostatic Desalting: A Detailed Parametric Study" Processes 10, no. 10: 2118. https://doi.org/10.3390/pr10102118
APA StyleRamirez-Argaez, M. A., Abreú-López, D., Gracia-Fadrique, J., & Dutta, A. (2022). Numerical Study of Electrostatic Desalting: A Detailed Parametric Study. Processes, 10(10), 2118. https://doi.org/10.3390/pr10102118









