Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
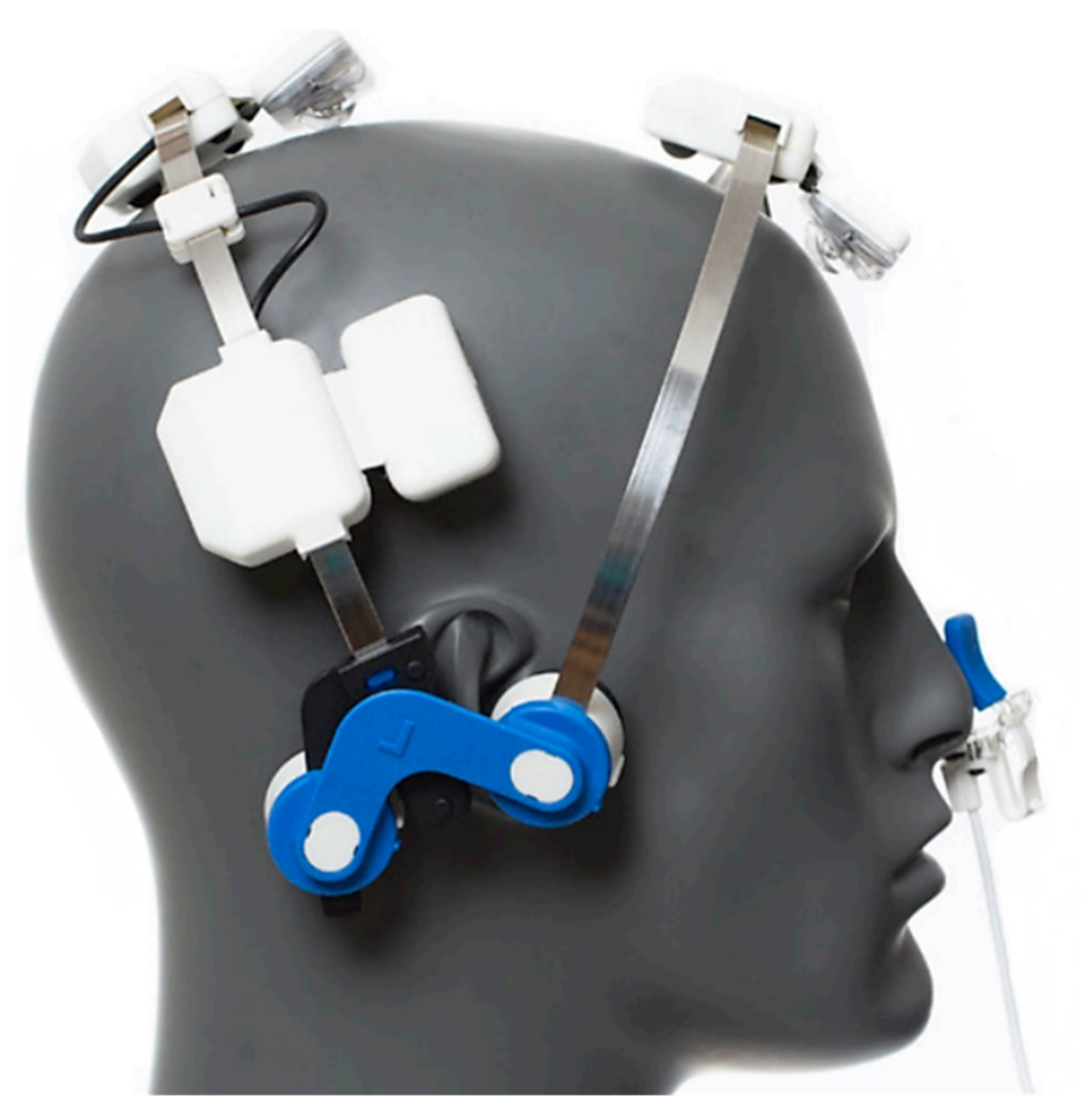
2.2. Stimulation
2.3. Procedure of Administration
2.4. Baseline and Follow-Up Assessments and Outcome Measures
2.5. Statistical Analysis
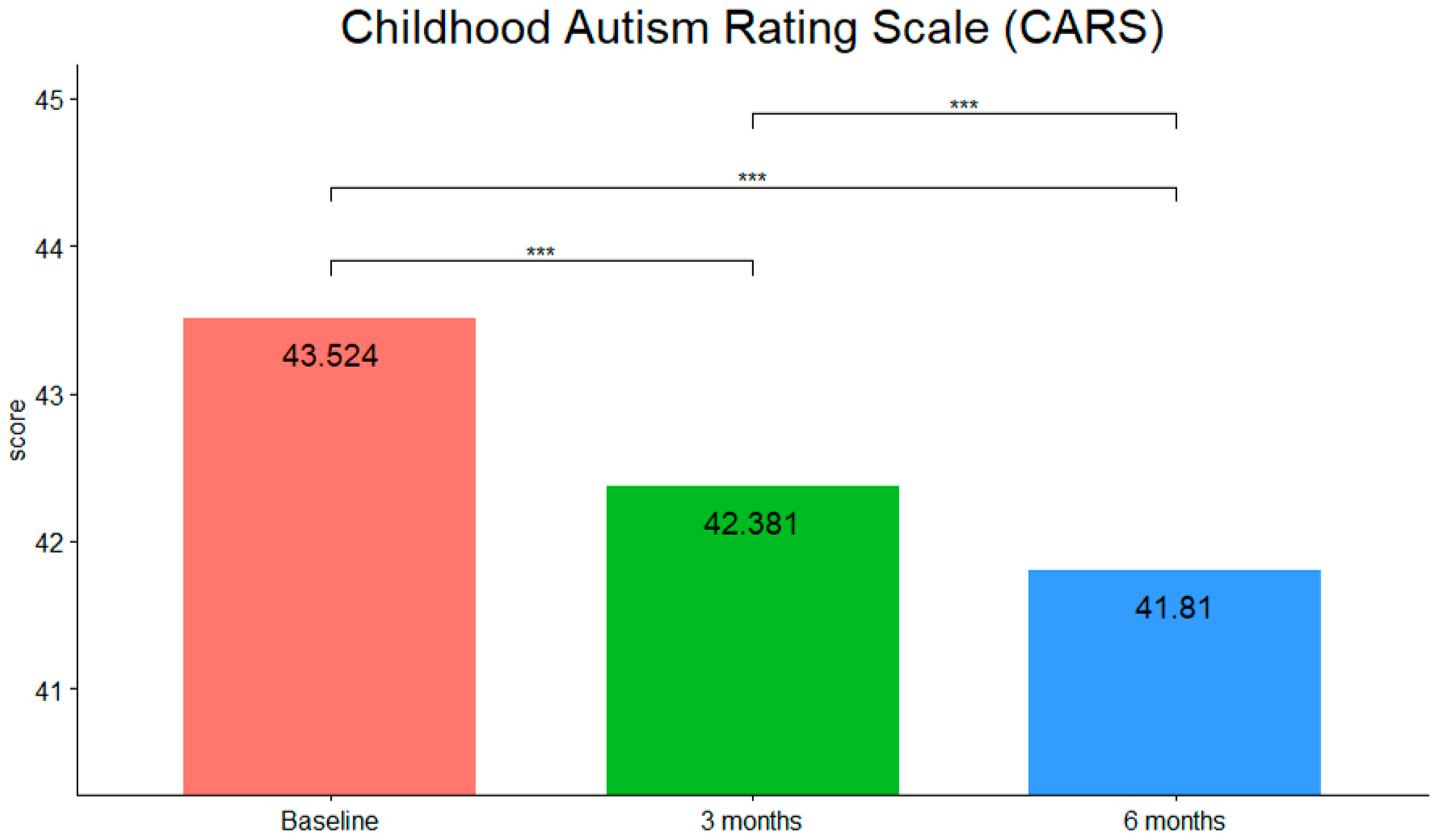
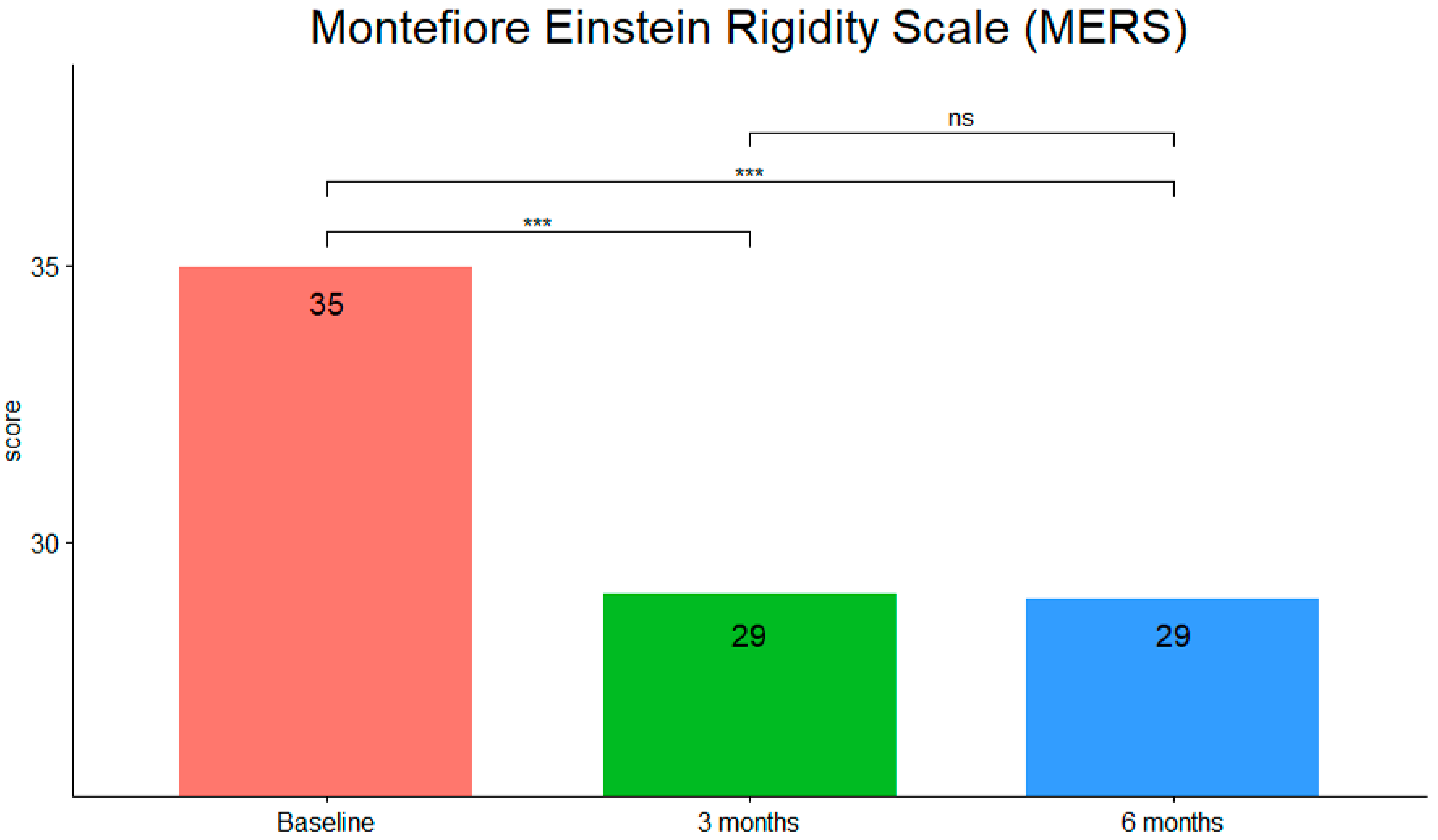
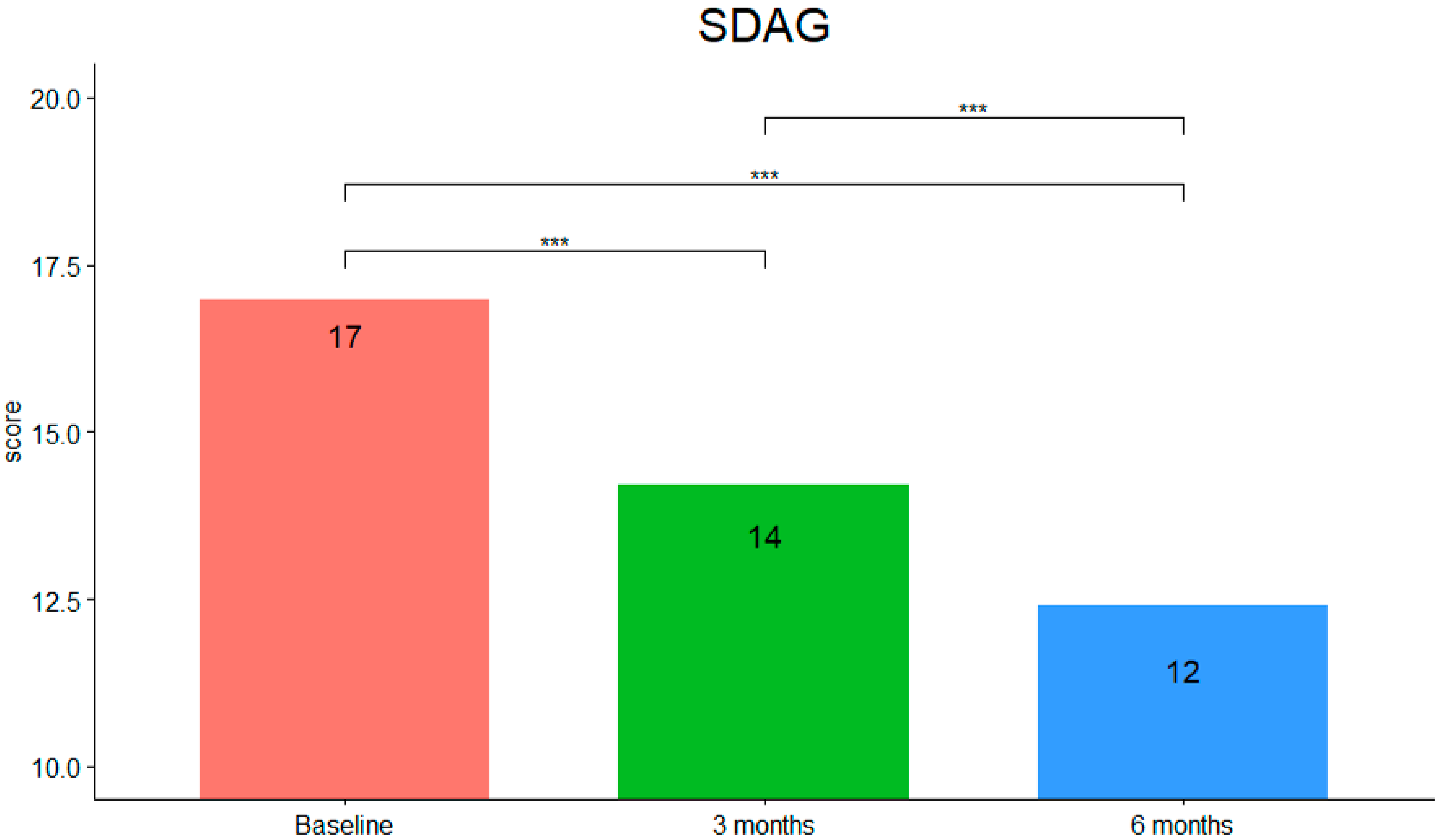
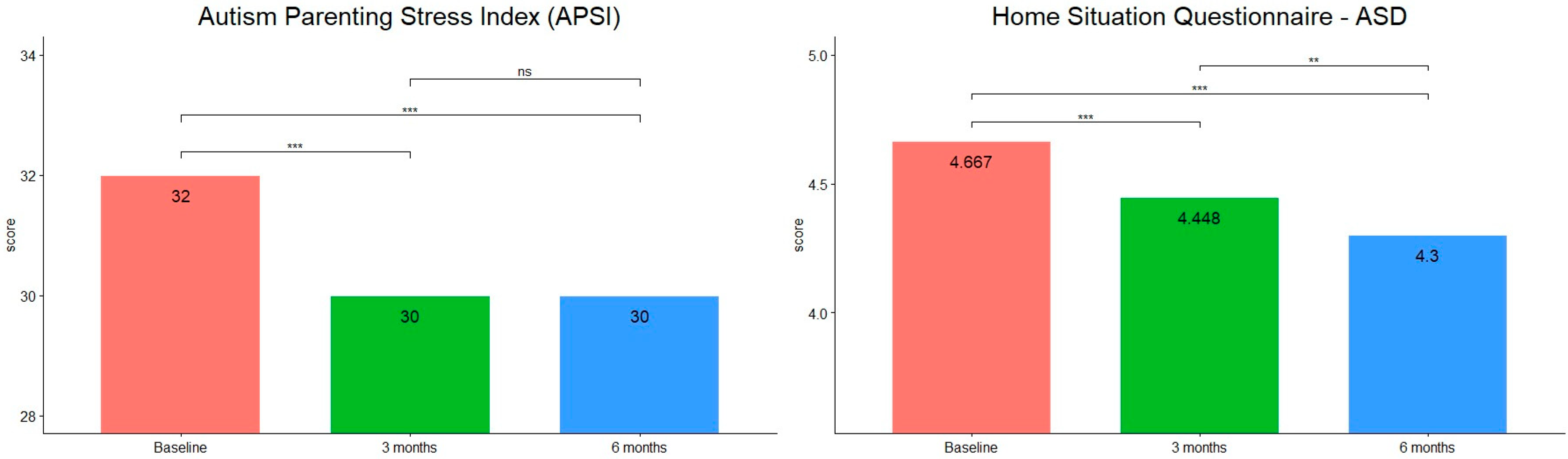
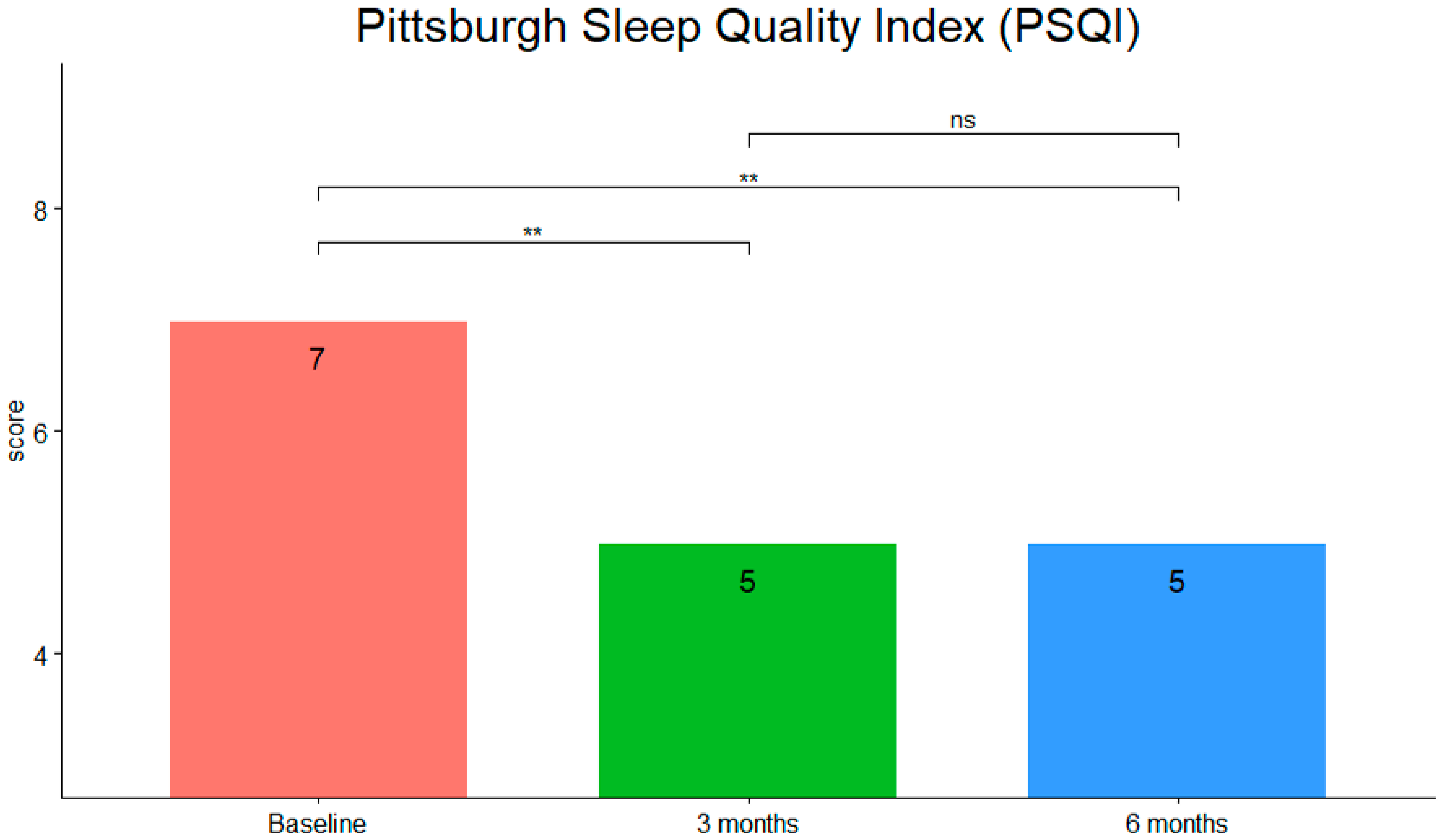
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Hill, A.P.; Zuckerman, K.E.; Hagen, A.D.; Kriz, D.J.; Duvall, S.W.; van Santen, J.; Nigg, J.; Fair, D.; Fombonne, E. Aggressive Behavior Problems in Children with Autism Spectrum Disorders: Prevalence and Correlates in a Large Clinical Sample. Res. Autism Spectr. Disord. 2014, 8, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soke, G.N.; Rosenberg, S.A.; Hamman, R.F.; Fingerlin, T.; Robinson, C.; Carpenter, L.; Giarelli, E.; Lee, L.C.; Wiggins, L.D.; Durkin, M.S.; et al. Brief Report: Prevalence of Self-injurious Behaviors among Children with Autism Spectrum Disorder-A Population-Based Study. J. Autism Dev. Disord. 2016, 46, 3607–3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Reynolds, C.F.; Riemann, D. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Gargaro, B.A.; Rinehart, N.J.; Bradshaw, J.L.; Tonge, B.J.; Sheppard, D.M. Autism and ADHD: How far have we come in the comorbidity debate? Neurosci. Biobehav. Rev. 2011, 35, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, E.A.; DeMayo, M.M.; Guastella, A.J. Executive Function in Autism Spectrum Disorder: History, Theoretical Models, Empirical Findings, and Potential as an Endophenotype. Front. Psychiatry 2010, 10, 753. [Google Scholar] [CrossRef] [Green Version]
- Barttfeld, P.; Wicker, B.; Cukier, S.; Navarta, S.; Lew, S.; Sigman, M. A big-world network in ASD: Dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 2011, 49, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikumar, A.; Evans, D.W.; Dougherty, C.C.; Carpenter, K.; Michael, A.M. A Review of the Default Mode Network in Autism Spectrum Disorders and Attention Deficit Hyperactivity Disorder. Brain Connect. 2021, 11, 253–263. [Google Scholar] [CrossRef]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Chahin, S.S.; Apple, R.W.; Kuo, K.H.; Dickson, C.A. Autism spectrum disorder: Psychological and functional assessment, and behavioral treatment approaches. Transl. Pediatr. 2020, 9 (Suppl. S1), S66–S75. [Google Scholar] [CrossRef]
- Eissa, N.; Al-Houqani, M.; Sadeq, A.; Ojha, S.K.; Sasse, A.; Sadek, B. Current Enlightenment About Etiology and Pharmacological Treatment of Autism Spectrum Disorder. Front. Neurosci. 2018, 12, 304. [Google Scholar] [CrossRef] [Green Version]
- Pallanti, S.; Bencini, L.; Cantisani, A.; Hollander, E. Psychotropic treatment of autism. In Autism Spectrum Disorders; Karger Publishers: London, UK, 2015; Volume 180, pp. 151–165. [Google Scholar] [CrossRef]
- DeVane, C.L.; Charles, J.M.; Abramson, R.K.; Williams, J.E.; Carpenter, L.A.; Raven, S.; Gwynette, F.; Stuck, C.A.; Geesey, M.E.; Bradley, C.; et al. Pharmacotherapy of autism spectrum disorder: Results from the randomized BAART clinical trial. Pharmacotherapy 2019, 39, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finisguerra, A.; Borgatti, R.; Urgesi, C. Non-invasive Brain Stimulation for the Rehabilitation of Children and Adolescents with Neurodevelopmental Disorders: A Systematic Review. Front. Psychol. 2019, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enticott, P.G.; Pallanti, S.; Hollander, E. Transcranial magnetic resonance and noninvasive brain stimulation. In Autism Spectrum Disorders; Hollander, E., Hagerman, R.J., Fein, D., Eds.; American Psychiatric Association Publishing: Washington DC, USA, 2018. [Google Scholar]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanis, J.; Henderson, L.A. How and why does photobiomodulation change brain activity? Neural Regen. Res. 2020, 15, 2243–2244. [Google Scholar] [CrossRef]
- Mochizuki-Oda, N.; Kataoka, Y.; Cui, Y.; Yamada, H.; Heya, M.; Awazu, K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002, 323, 207–210. [Google Scholar] [CrossRef]
- Konstantinovic, L.M.; Jelic, M.B.; Jeremic, A.; Stevanovic, V.B.; Milanovic, S.D.; Filipovic, S.R. Transcranial application of near-infrared low-level laser can modulate cortical excitability. Lasers Surg. Med. 2013, 45, 648–653. [Google Scholar] [CrossRef]
- Maiello, M.; Losiewicz, O.M.; Bui, E.; Spera, V.; Hamblin, M.R.; Marques, L.; Cassano, P. Transcranial Photobiomodulation with Near-Infrared Light for Generalized Anxiety Disorder: A Pilot Study. Photobiomodulation Photomed. Laser Surg. 2019, 37, 644–650. [Google Scholar] [CrossRef]
- Yang, L.; Tucker, D.; Dong, Y.; Wu, C.; Lu, Y.; Li, Y.; Zhang, J.; Liu, T.C.; Zhang, Q. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp. Neurol. 2018, 299 Pt A, 86–96. [Google Scholar] [CrossRef]
- Figueiro Longo, M.G.; Tan, C.O.; Chan, S.T.; Welt, J.; Avesta, A.; Ratai, E.; Mercaldo, N.D.; Yendiki, A.; Namati, J.; Chico-Calero, I.; et al. Effect of Transcranial Low-Level Light Therapy vs Sham Therapy among Patients with Moderate Traumatic Brain Injury: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2017337. [Google Scholar] [CrossRef]
- Askalsky, P.; Iosifescu, D.V. Transcranial Photobiomodulation for The Management of Depression: Current Perspectives. Neuropsychiatr. Dis. Treat. 2019, 15, 3255–3272. [Google Scholar] [CrossRef] [Green Version]
- Hacke, W.; Schellinger, P.D.; Albers, G.W.; Bornstein, N.M.; Dahlof, B.L.; Fulton, R.; Kasner, S.E.; Shuaib, A.; Richieri, S.P.; Dilly, S.G.; et al. Transcranial laser therapy in acute stroke treatment: Results of neurothera effectiveness and safety trial 3, a phase III clinical end point device trial. Stroke 2014, 45, 3187–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, P.; Petrie, S.R.; Mischoulon, D.; Cusin, C.; Katnani, H.; Yeung, A.; De Taboada, L.; Archibald, A.; Bui, E.; Baer, L.; et al. Transcranial Photobiomodulation for the Treatment of Major Depressive Disorder. The ELATED-2 Pilot Trial. Photomed. Laser Surg. 2018, 36, 634–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassano, P.; Caldieraro, M.A.; Norton, R.; Mischoulon, D.; Trinh, N.H.; Nyer, M.; Dording, C.; Hamblin, M.R.; Campbell, B.; Iosifescu, D.V. Reported Side Effects, Weight and Blood Pressure, after Repeated Sessions of Transcranial Photobiomodulation. Photobiomodulation Photomed. Laser Surg. 2019, 37, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Caldieraro, M.A.; Cassano, P. Transcranial and systemic photobiomodulation for major depressive disorder: A systematic review of efficacy, tolerability and biological mechanisms. J. Affect. Disord. 2019, 243, 262–273. [Google Scholar] [CrossRef]
- Ceranoglu, T.A.; Cassano, P.; Hoskova, B.; Green, A.; Dallenbach, N.; DiSalvo, M.; Biederman, J.; Joshi, G. Transcranial Photobiomodulation in Adults with High-Functioning Autism Spectrum Disorder: Positive Findings from a Proof-of-Concept Study. Photobiomodul Photomed. Laser Surg. 2022, 40, 4–12. [Google Scholar] [CrossRef]
- Leisman, G.; Machado, C.; Machado, Y.; Chinchilla-Acosta, M. Effects of Low-Level Laser Therapy in Autism Spectrum Disorder. Adv. Exp. Med. Biol. 2018, 1116, 111–130. [Google Scholar] [CrossRef]
- Mannu, P.; Maiello, M.; Spera, V.; Cassano, P. Transcranial Photobiomodulation for Down Syndrome. Photobiomodulation Photomed. Laser Surg. 2019, 37, 579–580. [Google Scholar] [CrossRef]
- Salgueiro, M.D.C.C.; Kobayashi, F.Y.; Motta, L.J.; Gonçalves, M.L.L.; Horliana, A.C.R.T.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Gomes, A.O.; Junior, A.B.; Bussadori, S.K. Effect of Photobiomodulation on Salivary Cortisol, Masticatory Muscle Strength, and Clinical Signs in Children with Sleep Bruxism: A Randomized Controlled Trial. Photobiomodul Photomed. Laser Surg. 2021, 39, 23–29. [Google Scholar] [CrossRef]
- Noirrit-Esclassan, E.; Valera, M.C.; Vignes, E.; Munzer, C.; Bonal, S.; Daries, M.; Vaysse, F.; Puiseux, C.; Castex, M.P.; Boulanger, C.; et al. Photobiomodulation with a combination of two wavelengths in the treatment of oral mucositis in children: The PEDIALASE feasibility study. Arch. Pédiatrie 2019, 26, 268–274. [Google Scholar] [CrossRef]
- Santos, M.; Nascimento, K.S.; Carazzato, S.; Barros, A.O.; Mendes, F.M.; Diniz, M.B. Efficacy of photobiomodulation therapy on masseter thickness and oral health-related quality of life in children with spastic cerebral palsy. Lasers Med. Sci. 2017, 32, 1279–1288. [Google Scholar] [CrossRef]
- Faulhaber, F.; Procianoy, R.S.; Silveira, R.C. Side Effects of Phototherapy on Neonates. Am. J. Perinatol. 2019, 36, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodulation Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gavish, L.; Houreld, N.N. Therapeutic Efficacy of Home-Use Photobiomodulation Devices: A Systematic Literature Review. Photobiomodulation Photomed. Laser Surg. 2019, 37, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Vielight. Available online: https://www.vielight.com/wp-content/uploads/2020/06/Neuros-AG-info-sheet-2020-A-v3.pdf (accessed on 3 March 2022).
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef]
- Jadgeo, J.R.; Adams, L.E.; Brody, N.I.; Siegel, D.M. Transcranial red and near-infrared light trans-mission in a cadaveric model. PLoS ONE 2012, 7, e47460. [Google Scholar]
- Tedford, C.E.; DeLapp, S.; Jacques, S.; Anders, J. Quantitative analysis of transcranial and intra-parenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015, 47, 312–322. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health. General Wellness: Policy for Low Risk Devices Guidance for Industry and Food and Drug Administration Staff. Document issued on 27 September 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/general-wellness-policy-low-risk-devices (accessed on 8 April 2022).
- Schopler, E.; Reichler, R.; Rochen Renner, B. The Childhood Autism Rating Scale; Western Psychological Services: Los Angeles, CA, USA, 1988. [Google Scholar]
- Chowdhury, M.; Aman, M.G.; Lecavalier, L.; Smith, T.; Johnson, C.; Swiezy, N.; McCracken, J.T.; King, B.; McDougle, C.J.; Bearss, K.; et al. Factor structure and psychometric properties of the revised Home Situations Questionnaire for autism spectrum disorder: The Home Situations Questionnaire-Autism Spectrum Disorder. Autism 2015, 54, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.; Schalock, M. Autism Parenting Stress Index: Initial psychometric evidence. J. Autism Dev. Disord. 2012, 42, 566–574. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef] [Green Version]
- Brochetti, R.A.; Leal, M.P.; Rodrigues, R.; da Palma, R.K.; de Oliveira, L.; Horliana, A.; Damazo, A.S.; de Oliveira, A.; Paula Vieira, R.; Lino-Dos-Santos-Franco, A. Photobiomodulation therapy improves both I nflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med. Sci. 2017, 32, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Jahan, A.; Nazari, M.A.; Mahmoudi, J.; Salehpour, F.; Salimi, M.M. Transcranial near-infrared photobiomodulation could modulate brain electrophysiological features and attentional performance in healthy young adults. Lasers Med. Sci. 2019, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Barstein, J.; Ethridge, L.E.; Mosconi, M.W.; Takarae, Y.; Sweeney, J.A. Resting state EEG abnormalities in autism spectrum disorders. J. Neurodev. Disord. 2013, 5, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Dmochowski, J.P.; Zeng, L.; Kallioniemi, E.; Husain, M.; Gonzalez-Lima, F.; Liu, H. Transcranial photobiomodulation with 1064-nm laser modulates brain electroencephalogram rhythms. Neurophotonics 2019, 6, 025013. [Google Scholar] [CrossRef]
- Zomorrodi, R.; Loheswaran, G.; Pushparaj, A.; Lim, L. Pulsed Near Infrared Transcranial and Intranasal Photobiomodulation Significantly Modulates Neural Oscillations: A pilot exploratory study. Sci. Rep. 2019, 9, 6309. [Google Scholar] [CrossRef] [Green Version]
- Brody, H. Meaning and an Overview of the Placebo Effect. Perspect. Biol. Med. 2018, 61, 353–360. [Google Scholar] [CrossRef]
- Kjær, S.W.; Rice, A.; Wartolowska, K.; Vase, L. Neuromodulation: More than a placebo effect? Pain 2020, 161, 491–495. [Google Scholar] [CrossRef]
| PATIENT | AGE | GENDER | COMORBIDITIES | MEDICATION | OTHER TREATMENTS |
|---|---|---|---|---|---|
| 1 | 9 | M | ADHD | Omega-3; Melatonin; Probiotics; Phosphatidylserine | CBT; Speech therapy |
| 2 | 5 | M | ADHD; SAD | Melatonin; Probiotics; Phosphatidylserine | CBT; Parent Training |
| 3 | 7 | F | ODD | Probiotics | CBT; Speech Therapy |
| 4 | 6 | F | SAD | Omega-3 | CBT |
| 5 | 12 | M | ADHD | Omega-3; Probiotics; Phosphatidylserine | CBT; Speech Therapy |
| 6 | 7 | F | ADHD | Melatonin; Phosphatidylserine | CBT; Speech Therapy |
| 7 | 15 | M | Omega-3; Melatonin; Probiotics | CBT; Speech Therapy | |
| 8 | 14 | M | SAD | Melatonin | CBT; Speech Therapy |
| 9 | 7 | M | ODD | CBT; Speech Therapy; Parent Training | |
| 10 | 7 | M | ODD; SAD | Omega-3; Melatonin; Probiotics | |
| 11 | 8 | M | Melatonin | ||
| 12 | 5 | F | SAD | CBT; Speech Therapy | |
| 13 | 8 | F | ODD | Omega-3 | CBT |
| 14 | 8 | M | ADHD | Phosphatidylserine | CBT; Speech Therapy |
| 15 | 10 | M | ADHD | Probiotics; Phosphatidylserine | |
| 16 | 11 | F | ADHD; SAD | Omega-3; Phosphatidylserine | CBT; Speech Therapy |
| 17 | 7 | F | ADHD | Omega-3; Melatonin; Probiotics; Phosphatidylserine | CBT; Speech Therapy |
| 18 | 7 | M | Omega-3; Melatonin | CBT; Speech Therapy; Parent Training | |
| 19 | 14 | F | ADHD; SAD | Omega-3; Probiotics; Phosphatidylserine | CBT; Speech Therapy |
| 20 | 14 | M | CBT; Speech Therapy | ||
| 21 | 10 | M | ADHD | Melatonin; Phosphatidylserine | CBT; Speech Therapy |
| Device Parameter | LED | ||
|---|---|---|---|
| Posterior Transcranial LEDs | Anterior Transcranial LED | Intranasal LED | |
| Power output | 100 mW | 75 mW | 25 mW |
| Power density | 100 mW/cm2 | 75 mW/cm2 | 25 mW/cm2 |
| Energy delivered | 60 J | 45 J | 15 J |
| Energy density per LED | 60 J/cm2 | 45 J/cm2 | 15 J/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallanti, S.; Di Ponzio, M.; Grassi, E.; Vannini, G.; Cauli, G. Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study. Children 2022, 9, 755. https://doi.org/10.3390/children9050755
Pallanti S, Di Ponzio M, Grassi E, Vannini G, Cauli G. Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study. Children. 2022; 9(5):755. https://doi.org/10.3390/children9050755
Chicago/Turabian StylePallanti, Stefano, Michele Di Ponzio, Eleonora Grassi, Gloria Vannini, and Gilla Cauli. 2022. "Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study" Children 9, no. 5: 755. https://doi.org/10.3390/children9050755
APA StylePallanti, S., Di Ponzio, M., Grassi, E., Vannini, G., & Cauli, G. (2022). Transcranial Photobiomodulation for the Treatment of Children with Autism Spectrum Disorder (ASD): A Retrospective Study. Children, 9(5), 755. https://doi.org/10.3390/children9050755







