Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review
Abstract
Simple Summary
Abstract
1. Introduction
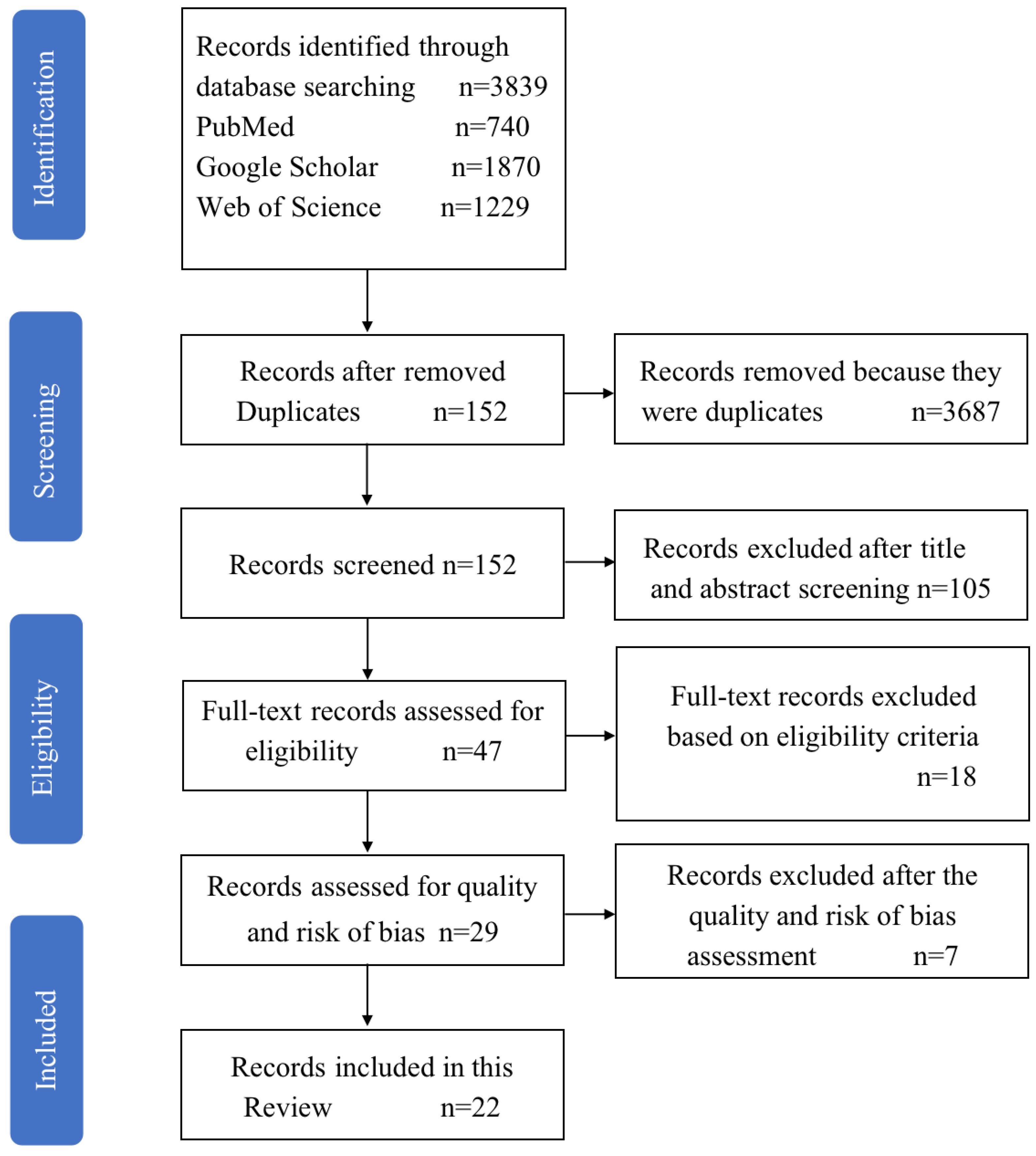
2. Method
2.1. Information Sources
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Risk of Bias in Individual Studies
3. Results
4. Discussion
4.1. Spatio-Temporal Characteristics of Typically Developing Toddler Gait
4.1.1. Temporal Parameters of the Toddler Gait
4.1.2. Spatial Parameters of the Toddler Gait
4.2. Kinematic Characteristics of Typically Developing Toddler Gait
4.3. Kinetic Characteristics of Typically Developing Toddler Gait
4.3.1. Ground Reaction Force Related Parameters of the Toddler Gait
4.3.2. Plantar Pressures of the Toddler Gait
5. Limitations and Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whittle, M.W. Normal Gait. In Gait Analysis; Elsevier: Amsterdam, The Netherlands, 1991; pp. 48–90. [Google Scholar]
- Wells, J.P.; Hyler-Both, D.L.; Danley, T.D.; Wallace, G.H. Biomechanics of growth and development in the healthy human infant: A pilot study. J. Am. Osteopath. Assoc. 2002, 102, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C. Order of appearance of the ossification centers in the foot during the period of intrauterine life in human material. Investig. Clin. 1997, 38, 127–138. [Google Scholar]
- Gould, N.; Moreland, M.; Alvarez, R.; Trevino, S.; Fenwick, J. Development of the child’s arch. Foot Ankle 1989, 9, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Islam, G.M.R. Association of socioeconomic status with childhood anemia among infant, toddler, and preschool children in bangladesh. Value Health Reg. Issues 2020, 21, 141–148. [Google Scholar] [CrossRef]
- Onis, M. WHO Motor Development Study: Windows of achievement for six gross motor development milestones. Acta Paediatr. 2007, 95, 86–95. [Google Scholar] [CrossRef]
- Adolph, K.E.; Avolio, A.M. Walking infants adapt locomotion to changing body dimensions. J. Exp. Psychol. Hum. Percept. Perform. 2000, 26, 1148–1166. [Google Scholar] [CrossRef] [PubMed]
- Zeininger, A.; Schmitt, D.; Jensen, J.L.; Shapiro, L.J. Ontogenetic changes in foot strike pattern and calcaneal loading during walking in young children. Gait Posture 2018, 59, 18–22. [Google Scholar] [CrossRef]
- Holt, K.G.; Saltzman, E.; Ho, C.-L.; Kubo, M.; Ulrich, B.D. Discovery of the pendulum and spring dynamics in the early stages of walking. J. Mot. Behav. 2006, 38, 206–218. [Google Scholar] [CrossRef]
- Adolph, K.E.; Vereijken, B.; Shrout, P.E. What changes in infant walking and why. Child Dev. 2003, 74, 475–497. [Google Scholar] [CrossRef] [PubMed]
- Thelen, E.; Ulrich, B.D.; Wolff, P.H. Hidden skills: A dynamic systems analysis of treadmill stepping during the first year. Monogr. Soc. Res. Child Dev. 1991, 56, 1–98. [Google Scholar] [CrossRef]
- Sutherland, D. The development of mature gait. Gait Posture 1997, 6, 163–170. [Google Scholar] [CrossRef]
- Sutherland, D.; Olsen, R.; Biden, E.; Wyatt, M. The Development of Mature Walking; Mac Keith Press: London, UK, 2011; ISBN 521412218. [Google Scholar]
- Ledebt, A.; Bril, B. Acquisition of upper body stability during walking in toddlers. Dev. Psychobiol. 2000, 36, 311–324. [Google Scholar] [CrossRef]
- Kyeong, D.; Whitney, L.; Laura, G.C.; Karen, G. The cost of simplifying complex developmental phenomena: A new perspective on learning to walk. Dev. Sci. 2017, 21, e12615. [Google Scholar] [CrossRef]
- Bisi, M.C.; Stagni, R. Evaluation of toddler different strategies during the first six-months of independent walking: A longitudinal study. Gait Posture 2015, 41, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; De Clercq, D.; Aerts, P. Changes in 3D joint dynamics during the first 5 months after the onset of independent walking: A longitudinal follow-up study. Gait Posture 2006, 24, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; Verbecque, E.; Dumas, R.; Cheze, L.; Van Hamme, A.; Robert, T. Developmental changes in spatial margin of stability in typically developing children relate to the mechanics of gait. Gait Posture 2018, 63, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, M.; Hallemans, A.; Truijen, S.; Aerts, P. A cross-sectional study about the relationship between morphology and step-time parameters in children between 15 and 36 months. Gait Posture 2010, 32, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Guffey, K.; Regier, M.; Mancinelli, C.; Pergami, P. Gait parameters associated with balance in healthy 2- to 4-year-old children. Gait Posture 2016, 43, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Looper, J.; Chandler, L.S. How do toddlers increase their gait velocity? Gait Posture 2013, 37, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Marencakova, J.; Price, C.; Maly, T.; Zahalka, F.; Nester, C. How do novice and improver walkers move in their home environments? An open-sourced infant’s gait video analysis. PLoS ONE 2019, 14, e0218665. [Google Scholar] [CrossRef]
- Adolph, K.E.; Cole, W.G.; Komati, M.; Garciaguirre, J.S.; Badaly, D.; Lingeman, J.M.; Chan, G.L.Y.; Sotsky, R.B. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol. Sci. 2012, 23, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Van Hamme, A.; El Habachi, A.; Samson, W.; Dumas, R.; Chèze, L.; Dohin, B. Gait parameters database for young children: The influences of age and walking speed. Clin. Biomech. 2015, 30, 572–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hallemans, A.; De Clercq, D.; Otten, B.; Aerts, P. 3D joint dynamics of walking in toddlers. Gait Posture 2005, 22, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.; Van Hamme, A.; Desroches, G.; Dohin, B.; Dumas, R.; Chèze, L. Biomechanical maturation of joint dynamics during early childhood: Updated conclusions. J. Biomech. 2013, 46, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhou, N.; Xu, B.; Chen, W.; Wu, J.; Zhou, J. The mechanism of force transference in feet of children ages two to six. Gait Posture 2017, 54, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Dulai, S.; Ramadi, A.; Lewicke, J.; Watkins, B.; Prowse, M.; Vette, A.H. Functional characterization of plantar pressure patterns in gait of typically developing children using dynamic pedobarography. Gait Posture 2021, 84, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bosch, K.; Rosenbaum, D. Gait symmetry improves in childhood-A 4-year follow-up of foot loading data. Gait Posture 2010, 32, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Cowgill, L.W.; Warrener, A.; Pontzer, H.; Ocobock, C. Waddling and toddling: The biomechanical effects of an immature gait. Am. J. Phys. Anthropol. 2010, 143, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.; Dohin, B.; Desroches, G.; Chaverot, J.L.; Dumas, R.; Cheze, L. Foot mechanics during the first six years of independent walking. J. Biomech. 2011, 44, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; Aerts, P.; Otten, B.; De Deyn, P.P.; De Clercq, D. Mechanical energy in toddler gait A trade-off between economy and stability? J. Exp. Biol. 2004, 207, 2417–2431. [Google Scholar] [CrossRef][Green Version]
- Ivanenko, Y.P.; Dominici, N.; Cappellini, G.; Dan, B.; Cheron, G.; Lacquaniti, F. Development of pendulum mechanism and kinematic coordination from the first unsupported steps in toddlers. J. Exp. Biol. 2004, 207, 3797–3810. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Dominici, N.; Cappellini, G.; Lacquaniti, F. Kinematics in newly walking toddlers does not depend upon postural stability. J. Neurophysiol. 2005, 94, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Morrison, S.C.; Hashmi, F.; Phethean, J.; Nester, C. Biomechanics of the infant foot during the transition to independent walking: A narrative review. Gait Posture 2018, 59, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, E.V.; Patrick, S.K.; Yang, J.F. Gait Transitions in Human Infants: Coping with Extremes of Treadmill Speed. PLoS ONE 2016, 11, e0148124. [Google Scholar] [CrossRef] [PubMed]
- Dusing, S.C.; Thorpe, D.E. A normative sample of temporal and spatial gait parameters in children using the GAITRite® electronic walkway. Gait Posture 2007, 25, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Rose-Jacobs, R. Development of gait at slow, free, and fast speeds in 3- and 5-year-old children. Phys. Ther. 1983, 63, 1251–1259. [Google Scholar] [CrossRef][Green Version]
- Sala, D.A.; Cohen, E. Gait component changes observed during independent ambulation in young children. Indian J. Pediatr. 2013, 80, 397–403. [Google Scholar] [CrossRef]
- Badaly, D.; Adolph, K.E. Beyond the average: Walking infants take steps longer than their leg length. Infant Behav. Dev. 2008, 31, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Assaiante, C.; Mallau, S.; Viel, S.; Jover, M.; Schmitz, C. Development of postural control in healthy children: A functional approach. Neural Plast. 2005, 12, 109–118. [Google Scholar] [CrossRef]
- Thelen, E.; Ridley-Johnson, R.; Fisher, D.M. Shifting patterns of bilateral coordination and lateral dominance in the leg movements of young infants. Dev. Psychobiol. 1983, 16, 29–46. [Google Scholar] [CrossRef]
- Thelen, E.; Ulrich, B.D.; Niles, D. Bilateral coordination in human infants: Stepping on a split-belt treadmill. J. Exp. Psychol. Hum. Percept. Perform. 1987, 13, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F. Split-Belt treadmill stepping in infants suggests autonomous pattern generators for the left and right leg in humans. J. Neurosci. 2005, 25, 6869–6876. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.E.; Yang, J.F. Loading the limb during rhythmic leg movements lengthens the duration of both flexion and extension in human infants. J. Neurophysiol. 2007, 97, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.E.; Yang, J.F. Interlimb coordination in rhythmic leg movements: Spontaneous and training-induced manifestations in human infants. J. Neurophysiol. 2008, 100, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Phillips, S.J. A longitudinal study of intralimb coordination in the first year of independent walking: A dynamical systems analysis. Child Dev. 1993, 64, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Bril, B.; Brenière, Y. Postural requirements and progression velocity in young walkers. J. Mot. Behav. 1992, 24, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.P.; Dominici, N.; Lacquaniti, F. Development of independent walking in toddlers. Exerc. Sport Sci. Rev. 2007, 35, 67–73. [Google Scholar] [CrossRef]
- Whitall, J.; Getchell, N. From walking to running: Applying a dynamical systems approach to the development of locomotor skills. Child Dev. 1995, 66, 1541–1553. [Google Scholar] [CrossRef]
- Bril, B.; Dupuy, L.; Dietrich, G.; Corbetta, D. Learning to tune the antero-posterior propulsive forces during walking: A necessary skill for mastering upright locomotion in toddlers. Exp. Brain Res. 2015, 233, 2903–2912. [Google Scholar] [CrossRef]
- Yaguramaki, N.; Kimura, T. Acquirement of stability and mobility in infant gait. Gait Posture 2002, 16, 69–77. [Google Scholar] [CrossRef]
- Burnett, C.N.; Johnson, E.W. Development of gait in childhood: Part II. Dev. Med. Child Neurol. 2008, 13, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Okamoto, K.; Andrew, P.D. Electromyographic developmental changes in one individual from newborn stepping to mature walking. Gait Posture 2003, 17, 18–27. [Google Scholar] [CrossRef]
- Moe-Nilssen, R.; Helbostad, J.L. Estimation of gait cycle characteristics by trunk accelerometry. J. Biomech. 2004, 37, 121–126. [Google Scholar] [CrossRef]
- Pang, M.Y.C.; Yang, J.F. Interlimb co-ordination in human infant stepping. J. Physiol. 2001, 533, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.P.; Carman, R. Developmental profiles of spontaneous movements in infants. Early Hum. Dev. 1994, 39, 109–126. [Google Scholar] [CrossRef]
- Cheron, G.; Bengoetxea, A.; Bouillot, E.; Lacquaniti, F.; Dan, B. Early emergence of temporal co-ordination of lower limb segments elevation angles in human locomotion. Neurosci. Lett. 2001, 308, 123–127. [Google Scholar] [CrossRef]
- Cheron, G.; Bouillot, E.; Dan, B.; Bengoetxea, A.; Draye, J.-P.; Lacquaniti, F. Development of a kinematic coordination pattern in toddler locomotion: Planar covariation. Exp. Brain Res. 2001, 137, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Bisi, M.; Riva, F.; Stagni, R. Measures of gait stability: Performance on adults and toddlers at the beginning of independent walking. J. Neuroeng. Rehabil. 2014, 11, 131. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Phillips, S.J. The step cycle organization of infant walkers. J. Mot. Behav. 1987, 19, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Czerniecki, J.M. Foot and ankle biomechanics in walking and running. A review. Am. J. Phys. Med. Rehabil. 1988, 67, 246–252. [Google Scholar] [PubMed]
- Elftman, H. A cinematic study of the distribution of pressure in the human foot. Anat. Rec. 1934, 59, 481–491. [Google Scholar] [CrossRef]
- Elftman, H.; Manter, J. Chimpanzee and human feet in bipedal walking. Am. J. Phys. Anthropol. 1935, 20, 69–79. [Google Scholar] [CrossRef]
- Grundy, M.; Tosh, P.A.; McLeish, R.D.; Smidt, L. An investigation of the centres of pressure under the foot while walking. J. Bone Jt. Surg. Br. 1975, 57, 98–103. [Google Scholar] [CrossRef]
- Bertsch, C.; Unger, H.; Winkelmann, W.; Rosenbaum, D. Evaluation of early walking patterns from plantar pressure distribution measurements. First year results of 42 children. Gait Posture 2004, 19, 235–242. [Google Scholar] [CrossRef]
- Hallemans, A.; De Clercq, D.; Van Dongen, S.; Aerts, P. Changes in foot-function parameters during the first 5 months after the onset of independent walking: A longitudinal follow-up study. Gait Posture 2006, 23, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hallemans, A.; D’Août, K.; De Clercq, D.; Aerts, P. Pressure distribution patterns under the feet of new walkers: The first two months of independent walking. Foot Ankle Int. 2003, 24, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Diop, M.; Rahmani, A.; Belli, A.; Gautheron, V.; Geyssant, A.; Cottalorda, J. Influence of speed variation and age on ground reaction forces and stride parameters of children’s normal gait. Int. J. Sports Med. 2005, 26, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Takegami, Y. Wave pattern of ground reaction force of growing children. J. Pediatr. Orthop. 1992, 12, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Samson, W.; Desroches, G.; Cheze, L.; Dumas, R. 3D joint dynamics analysis of healthy children’s gait. J. Biomech. 2009, 42, 2447–2453. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, N. Evaluation of the medial longitudinal arch: A comparison between the dynamic plantar pressure measurement system and radiographic analysis. Acta Orthop. Traumatol. Turc. 2010, 44, 241–245. [Google Scholar] [CrossRef]
- Bosch, K.; Gerss, J.; Rosenbaum, D. Preliminary normative values for foot loading parameters of the developing child. Gait Posture 2007, 26, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Hennig, E.M.; Staats, A.; Rosenbaum, D. Plantar Ppressure distribution patterns of young school children in comparison to adults. Foot Ankle Int. 1994, 15, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Chen, W.; Xu, B. Investigation of children’s foot arch based on the variation between static and dynamic footprint. Leather Footwear J. 2014, 14, 205–216. [Google Scholar] [CrossRef]
- Unger, H.; Rosenbaum, P.D.D.D. Gender-specific differences of the foot during the first year of walking. Foot Ankle Int. 2004, 25, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Steele, J.R.; Munro, B.J. The feet of overweight and obese young children: Are they flat or fat? Obesity 2006, 14, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, Y.; Xu, B.; Chen, W. Features of plantar pressure distribution of chinese children aged between two and eleven. Leather Footwear J. 2014, 14, 135–146. [Google Scholar] [CrossRef]
- Alvarez, C.; De Vera, M.; Chhina, H.; Black, A. Normative data for the dynamic pedobarographic profiles of children. Gait Posture 2008, 28, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wheelwright, E.F.; Minns, R.A.; Law, H.T.; Elton, R.A. Temporal and spatial parameters of gait in children: Normal control data. Dev. Med. Child Neurol. 2008, 35, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Brenière, Y.; Bril, B. Development of postural control of gravity forces in children during the first 5 years of walking. Exp. Brain Res. 1998, 121, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Okamoto, K. Electromyographic characteristics at the onset of independent walking in infancy. Electromyogr. Clin. Neurophysiol. 2001, 41, 33–41. [Google Scholar]
- Mei, Q.; Gu, Y.; Sun, D.; Li, J.; Justin, F. Progress on biomechanical research of image-based subject-specific OpenSim lower extremity musculoskeletal model. J. Med. Biomech. 2020, 35, 259–264. [Google Scholar] [CrossRef]
- Voskuil, V.R.; Stroup, S.; Leyden, M. Acceptability and usability of a wearable activity tracker and application among inactive adolescent girls. Phys. Act. Health 2020, 4, 52–61. [Google Scholar] [CrossRef]
- Mei, Q.; Xiang, L.; Li, J.; Fernande, J.; Gu, Y. Analysis of ground reaction forces during running based on one-dimensional statistical parametric mapping. J. Med. Biomech. 2021, 36, 684–691. [Google Scholar] [CrossRef]
| Lee et al. [15] | Bisi et al. [16] | Hallemans et al. [17] | Hallemans et al. [18] | Van Dam et al. [19] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study site | America | Italy | Belgium | Belgium | Belgium | ||||||
| Participants | 97 | 20 | 10 | 84 | 100 | ||||||
| Age | 10.75 to 19.99 months | 10 to 16 months | 12.6 ± 1.64 to 17.2 ± 1.64 months | 1–10 years (10–36 months included) | 15–36 months | ||||||
| Design | Cross-sectional | Longitudinal | Longitudinal | Cross-sectional | Cross-sectional | ||||||
| Measurement frequency and timing | Once: 67 infants. 2 or 3 times: 30 infants. | 5 times: 0, 1, 2, 3, 6 months after walking onset. | Every 6 months for 9 measurements scheduled. | Once: tested age ranges from 1 to 10 years. | Once: tested age ranges from 15 to 36 months. | ||||||
| Data Collected | 1 walking trial per participant with free play task, 6 consecutive steps per participant with straight-path task. | 1 walking trial per participant along the corridor spontaneously. | 336 trials of all individuals. | Clear foot strikes on the force plates and full marker visibility for at least 2 consecutive strides. | 2 walking trials per participant. | ||||||
| Anatomic sites | Foot. | Trunk and leg. | Full body (focusing on the lower extremities). | Ankle and foot. | Foot. | ||||||
| Walking speed and Style | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | ||||||
| Footwear/Attire | Not reported | Not reported | Not reported | Bare foot | Soft leather shoes | ||||||
| Instruments | Pressure-sensitive mat (4 sensors/in2). Three video cameras. | Tri-axial wireless inertial sensors. Video camera. | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | Optometric movement registration system. Force plate. | Strip of paper of 5 m long and 0.84 m wide. Leather shoes with stamps. Ink. | ||||||
| Variables | Speed, step length, and step width. | Stride time, swing time, stance time, cadence, acceleration, regularity. | Vertical ground reaction forces, cadence, walking speed, double support time, stride length and step width, joint kinematics and kinetics. | Spatial margin of stability, walking speed, stride length, step width, swing. | Step-time parameters: step length, step width, cadence and walking speed. | ||||||
| Guffey et al. [20] | Looper et al. [21] | Marencakova et al. [22] | Adolph et al. [23] | Hamme et al. [24] | Hallemans et al. [25] | ||||||
| Study site | America | America | Czech | America | France | Belgium | |||||
| Participants | 84 | 8 | 20 | 151 | 106 | 10 | |||||
| Age | 2–4.9 years | 6–11 months | 7–13 months | 11.8–19.3 months | 1 to 7 years (10–36 months included) | 13.5 to 18.5 months | |||||
| Design | Cross-sectional | Longitudinal | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | |||||
| Measurement frequency and timing | Once: 6 age groups split every 6 months. | 5 times: 1, 2, 3, 4, 5 months after walking onset. | Once: analyzed age ranges from 7 to 13 months. | Once: tested age ranges from 11.8 to 19.3 months. | several times | Once: tested age ranges from 13.5 to 18.5 months. | |||||
| Data Collected | 3 walking trials per participant. | 4 trials of each individual. | 1 walking bout consisting of a minimum of 5 complete gait cycles. | 1 trials of each individual with free play task and straight-path task. | 1 to 6 gait trials per gait analysis. | 3–5 trials of each individual. | |||||
| Anatomic sites | Foot. | Foot. | Leg. | Foot. | Full body (focusing on the lower extremities). | Full body (focusing on the lower extremities). | |||||
| Walking speed and Style | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | |||||
| Footwear/Attire | Bare foot | Not reported | Not reported | Not reported | Not reported | Not reported | |||||
| Instruments | GAITRite system. | GAITRite system. | Free accessible tool Tracker. | Video camera. GAITRite system. | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | |||||
| Variables | Step and stride length, velocity, cadence, step time, cycle time, stance time, swing time, single support time and double support time. | Velocity, cadence, step length, step length and cadence normalized by leg length and single support. | Falls frequency, stops frequency, cadence, time of stance phase, swing phase and double support phase. | Step length, step width, time walking, steps/hour, distance/hour. | Vertical ground reaction forces, angle, moment, power. | Vertical ground reaction forces, cadence, stride time, single support time, double support time, stride length and step width. | |||||
| Zeininger et al. [8] | Hallemans et al. [17] | Hallemans et al. [25] | Samson et al. [26] | Hu et al. [27] | Dulai et al. [28] | |
|---|---|---|---|---|---|---|
| Study site | America | Belgium | Belgium | France | China | Canada |
| Participants | 18 | 10 | 10 | 75 | 319 | 102 |
| Age | 11.5 to 43.1 months | 12.6 ± 1.64 to 17.2 ± 1.64 months | 13.5 to 18.5 months | 1 to 6 years (10–36 months included) | 2–6 years (10–36 months included) | 2–17 years (10–36 months included) |
| Design | Cross-sectional | Longitudinal | Cross-sectional | Longitudinal | Cross-sectional | Cross-sectional |
| Measurement frequency and timing | Once: tested age ranges from 11.5 to 43.1 months. | Every 6 months for 9 measurements scheduled. | Once: tested age ranges from 13.5 to 18.5 months. | 4 times, 2 times and 1 time per year after 1, 2 and more than 3 of independent walking. | Once: tested age ranges from 2 to 6 years. | Once: 5 age groups (2–3, 4–6, 7–10, 11–14, 15–17 years) |
| Data Collected | 4 trials of each individual. | 336 trials of all individuals. | 3–5 trials of each individual. | 6 trials of each foot on force plate. | 3 walks for each foot. | 3 trials of each individual. |
| Anatomic sites | Total foot. | Full body (focusing on the lower extremities). | Full body (focusing on the lower extremities). | Ankle, knee and hip joints | Hallux, toes, metatarsal heads, mid-foot, medial and lateral heel. | Hallux, heel, lesser toes, medial and lateral forefoot, midfoot. |
| Walking speed and Style | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Not reported. |
| Footwear/Attire | Bare feet | Not reported | Not reported | Not reported | Not reported | Bare feet |
| Instruments | Vicon MX motion analysis system synchronized with Bertec force plates. | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | Motion Analysiss system with Eagles cameras, force platform, Footscans plantar pressure platform. | RSscan (4 sensors cm2). | emed-x platform (4 sensors cm2). |
| Dynamic variables | Location of the center of pressure relative to the calcaneus, the orientation and magnitude of ground reaction forces during foot contact. | Vertical ground reaction forces, cadence, walking speed, double support time, stride length and step width, joint kinematics and kinetics. | Vertical ground reaction forces, cadence, stride time, single support time, double support time, stride length and step width. | Moment, speed. | Relative force-time integral (FTIrel) (%). | Foot impulse, regional percent impulses, impulse ratios. |
| Bosch et al. [29] | Cowgill et al. [30] | Samson et al. [31] | Hallemans et al. [32] | Ivanenko et al. [33] | Ivanenko et al. [34] | |
| Study site | Germany | America | France | Belgium | Saint Lucia | Saint Lucia |
| Participants | 62 | 10 | 42 | 9 | 26 | 7 |
| Age | 15.1 ± 2.4 to 63.2 ± 2.4 months | 1 to 3.9 years | 1 to 6 years (10–36 months included) | 12 to 18 months | 11 to 153 months (10–36 months included) | 12 to 15 months |
| Design | Longitudinal | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional |
| Measurement frequency and timing | Every 6 months for 9 measurements scheduled. | Once: tested age ranges from 1 to 3.9 years. | Once: tested age ranges from 1 to 6 years. | Once: tested age ranges from 12 to 18 months. | Once: tested age ranges from 13.5 to 18.5 months. | Once: tested age ranges from 12 to 15 months. |
| Data Collected | 5 trials of each individual. | 1 trail of 1 foot on force plate and speed changed less than 20%. | 1 trials of each foot on force plate. | 5 trials of each individual. | 10 trials of each individual. | 10 trials of each individual. |
| Anatomic sites | Total foot. | Total foot, greater trochanter, lateral femoral condyle and lateral malleolus. | Total foot, metatarsophalangeal and ankle joint. | Full body. | Trunk, pelvis, thigh, shank, and foot. | Trunk, pelvis, thigh, shank, and foot. |
| Walking speed and Style | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. | Self-selected speed. |
| Footwear/Attire | Bare feet | Bare feet | Not reported | Not reported | Not reported | Not reported |
| Instruments | capacitive pressure distribution platform (4 sensors cm2). | infrared cameras (Vicon MX4) with skin marker attachment and embedded force plate (AMTI). | Motion Analysiss system with Eagles cameras, force platform, Footscans plantar pressure platform. | Instrumented walkway surrounded, infrared cameras (Vicon Motion Systems), force platforms (AMTI). | ELITE or VICON motion analysis systems, force platform. | VICON motion analysis systems, force platform. |
| Dynamic variables | Contact area, peak pressure, force-time integral, relative maximum force, contact time and the force-time integral. | Peak ground reaction forces and ground reaction forces impulse. | Vertical ground reaction forces, moment. | Vertical ground reaction forces, speed, moment. | Vertical ground reaction forces, speed, moment. | Vertical ground reaction forces, speed, step length and width. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Mei, Q.; Yu, P.; Gao, Z.; Hu, Q.; Fekete, G.; István, B.; Gu, Y. Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review. Children 2022, 9, 406. https://doi.org/10.3390/children9030406
Liu W, Mei Q, Yu P, Gao Z, Hu Q, Fekete G, István B, Gu Y. Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review. Children. 2022; 9(3):406. https://doi.org/10.3390/children9030406
Chicago/Turabian StyleLiu, Wei, Qichang Mei, Peimin Yu, Zixiang Gao, Qiuli Hu, Gustav Fekete, Bíró István, and Yaodong Gu. 2022. "Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review" Children 9, no. 3: 406. https://doi.org/10.3390/children9030406
APA StyleLiu, W., Mei, Q., Yu, P., Gao, Z., Hu, Q., Fekete, G., István, B., & Gu, Y. (2022). Biomechanical Characteristics of the Typically Developing Toddler Gait: A Narrative Review. Children, 9(3), 406. https://doi.org/10.3390/children9030406









