Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond
Abstract
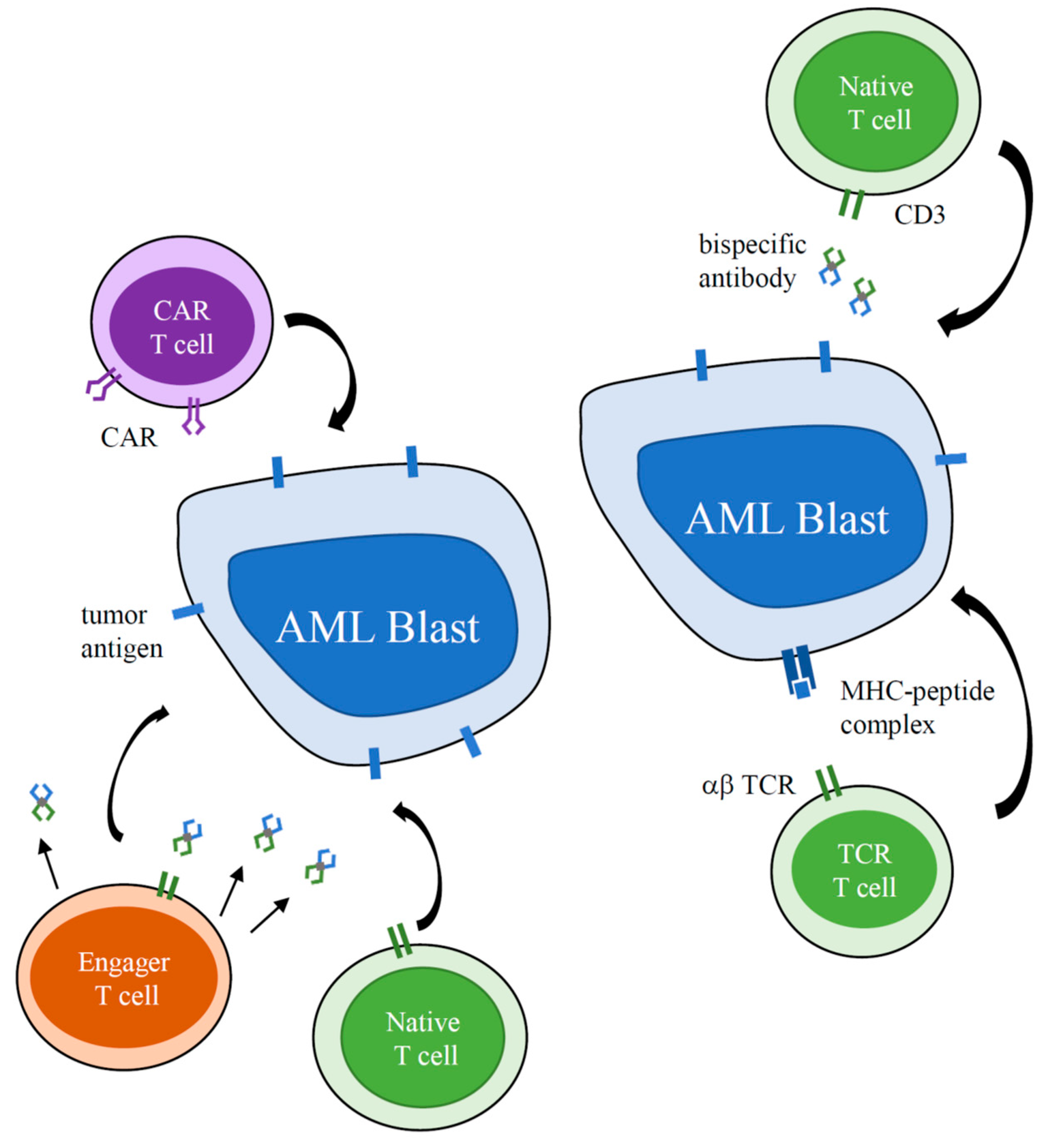
:1. Introduction
2. Identifying Target Antigens
2.1. Antigens with Overlapping Expression in AML Blasts, Leukemic Stem Cells and Normal Hematopoietic Progenitor Cells
2.2. Antigens Primarily Overlapping with Mature Hematopoietic Cells
2.3. Antigens Present on Multiple Tumor Types
2.4. Epitope-Specific Antigens
2.5. Antigens Present in Distinct AML Subsets
2.6. Intracellular Antigens
3. Bispecific Antibody Clinical Development
4. CAR T Cell Clinical Development
5. Additional T Cell-Based Immunotherapy Strategies
5.1. Bispecific T-Cell Engager Secreting Cells
5.2. T Cell Receptor Engineered T Cells
5.3. Tumor-Associated Antigen Specific T Cells
6. Challenges of Bispecific Antibody and CAR T-Cell Therapies
6.1. Toxicity
6.2. Immune Escape
6.3. AML Microenvironment
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zwaan, C.M.; Kolb, E.A.; Reinhardt, D.; Abrahamsson, J.; Adachi, S.; Aplenc, R.; De Bont, E.S.; De Moerloose, B.; Dworzak, M.; Gibson, B.E.; et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J. Clin. Oncol. 2015, 33, 2949–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jen, E.Y.; Xu, Q.; Schetter, A.; Przepiorka, D.; Shen, Y.L.; Roscoe, D.; Sridhara, R.; Deisseroth, A.; Philip, R.; Farrell, A.T.; et al. FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin. Cancer Res. 2019, 25, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, M.C.; Lu, X.; Huang, Y.; Lin, X.; Mahmood, I.; Przepiorka, D.; Gavin, D.; Lee, S.; Liu, K.; George, B.; et al. FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin. Cancer Res. 2019, 25, 1142–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchkouj, N.; Kasamon, Y.L.; de Claro, R.A.; George, B.; Lin, X.; Lee, S.; Blumenthal, G.M.; Bryan, W.; McKee, A.E.; Pazdur, R. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-cell Lymphoma. Clin. Cancer Res. 2019, 25, 1702–1708. [Google Scholar] [CrossRef] [Green Version]
- Mahalleh, M.; Shabani, M.; Rayzan, E.; Rezaei, N. Reinforcing the primary immunotherapy modulators against acute leukemia; monoclonal antibodies in AML. Immunotherapy 2019. [Google Scholar] [CrossRef]
- Davis, K.L.; Agarwal, A.M.; Verma, A.R. Checkpoint inhibition in pediatric hematologic malignancies. Pediatr. Hematol. Oncol. 2017, 34, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Hansrivijit, P.; Gale, R.P.; Barrett, J.; Ciurea, S.O. Cellular therapy for acute myeloid Leukemia—Current status and future prospects. Blood Rev. 2019, 37, 100578. [Google Scholar] [CrossRef]
- Liu, Y.; Bewersdorf, J.P.; Stahl, M.; Zeidan, A.M. Immunotherapy in acute myeloid leukemia and myelodysplastic syndromes: The dawn of a new era? Blood Rev. 2019, 34, 67–83. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Velasquez, M.P.; Gottschalk, S. Advances in immunotherapy for pediatric acute myeloid leukemia. Expert Opin. Biol. Ther. 2018, 18, 51–63. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Riviere, I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perna, F.; Berman, S.; Soni, R.; Mansilla-Soto, J.; Eyguem, J.; Hamieh, M.; Hendrickson, R.; Brennan, C.; Sadelain, M. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell 2017, 32, 506–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riviere, I.; Sadelain, M. Chimeric antigen receptors: A cell and gene therapy perspective. Mol. Ther. 2017, 25, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Salter, A.I.; Pont, M.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood 2018, 131, 2621–2629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehninger, A.; Kramer, M.; Rollig, C.; Thiede, C.; Bornhauser, M.; von Bonin, M.; Wermke, M.; Feldmann, A.; Bachmann, M.; Ehninger, G.; et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014, 4, e218. [Google Scholar] [CrossRef] [Green Version]
- Jordan, C.T.; Upchurch, D.; Szilvassy, S.J.; Guzman, M.L.; Howard, D.S.; Pettigrew, A.L.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.A.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, N.; Caux, C.; Kitamura, T.; Watanabe, Y.; Arai, K.; Banchereau, J.; Miyajima, A. Expression and factor-dependent modulation of the interleukin-3 receptor subunits on human hematopoietic cells. Blood 1993, 82, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Testa, U.; Fossati, C.; Samoggia, P.; Masciulli, R.; Mariani, G.; Hassan, H.J.; Sposi, N.M.; Guerriero, R.; Rosato, V.; Gabbianelli, M.; et al. Expression of growth factor receptors in unilineage differentiation culture of purified hematopoietic progenitors. Blood 1996, 88, 3391–3406. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, S.; Zhao, L.; Zhang, B.; Chen, H. IFN-γ andTNF-α aggravate endothelial damage caused by CD123-targeted CAR T cell. Onco Targets Ther. 2019, 12, 4907–4925. [Google Scholar] [CrossRef] [Green Version]
- Lamble, A.; Brodersen, L.; Alonzo, T.A.; Wang, J.; Gerbing, R.; Pardo, L.; Sung, L.; Tasian, S.; Cooper, T.; Kolb, E.; et al. Correlation of CD123 expression lebel with disease characteristics and outcomes in pediatric acute myeloid leukemia: A report from the children’s oncology group. Blood 2019, 134, 459. [Google Scholar] [CrossRef]
- Rosnet, O.; Buhring, H.J.; Marchetto, S.; Rappold, I.; Lavagna, C.; Sainty, D.; Arnoulet, C.; Chabannon, C.; Kanz, L.; Hannum, C.; et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia 1996, 10, 238–248. [Google Scholar] [PubMed]
- Kuchenbauer, F.; Kern, W.; Schoch, C.; Kohlmann, A.; Hiddemann, W.; Haferlach, T.; Schnittger, S. Detailed analysis of FLT3 expression levels in acute myeloid leukemia. Haematologica 2005, 90, 1617–1625. [Google Scholar] [PubMed]
- Kikushige, Y.; Yoshimoto, G.; Miyamoto, T.; Iino, T.; Mori, Y.; Iwasaki, H.; Niiro, H.; Takenaka, K.; Nagafuji, K.; Harada, M.; et al. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J. Immunol. 2008, 180, 7358–7367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenderian, S.; Ruella, M.; Shestova, O.; Klichinsky, M.; Aikawa, V.; Morrissette, J.; Scholler, J.; Song, D.; Porter, D.; Carroll, M.; et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 2015, 29, 1637–1647. [Google Scholar] [CrossRef]
- Jitschin, R.; Saul, D.; Braun, M.; Tohumeken, S.; Volkl, S.; Kischel, R.; Lutteropp, M.; Dos Santos, C.; Mackensen, A.; Mougiakakos, D. CD33/CD3-bispecific T-cell engaging (BiTE®) antibody construct targets monocytic AML myeloid-derived suppressor cells. J. Immunother. Cancer 2018, 6, 116. [Google Scholar] [CrossRef]
- Godwin, C.D.; McDonald, G.B.; Walter, R.B. Sinusoidal obstruction syndrome following CD33-targeted therapy in acute myeloid leukemia. Blood 2017, 129, 2330–2332. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, S.; Xiao, W.; Li, W.; Wang, L.; Yang, S.; Wang, W.; Xu, L.; Liao, S.; Liu, W.; et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Riether, C.; Schurch, C.M.; Buhrer, E.D.; Hinterbrandner, M.; Huguenin, A.L.; Hoepner, S.; Zlobec, I.; Pabst, T.; Radpour, R.; Ochsenbein, A.F. CD70/CD27 signaling promotes blast stemness and is a viable therapeutic target in acute myeloid leukemia. J. Exp. Med. 2017, 214, 359–380. [Google Scholar] [CrossRef]
- Leick, M.; Scarfo, I.; Choi, B.; Larson, R.; Bouffard, A.; Castano, A.; Cabral, M.; Schmidts, A.; Frigault, M.; Maus, M. Use of CD70 targeted chimeric antigen receptor T cells for the treatment of acute myeloid leukemia. Blood 2019, 134, 4443. [Google Scholar] [CrossRef]
- Sauer, T.; Parikh, K.; Rooney, C.; Omer, B.; Gottschalk, S.; Sharma, S. CD70-specific CAR T cells have potent activity against acute myeloid leukemia (AML) without HSC toxicity. Blood 2019, 134, 1932. [Google Scholar] [CrossRef]
- Shen, J.; Putt, K.S.; Visscher, D.W.; Murphy, L.; Cohen, C.; Singhal, S.; Sandusky, G.; Feng, Y.; Dimitrov, D.S.; Low, P.S. Assessment of folate receptor-beta expression in human neoplastic tissues. Oncotarget 2015, 6, 14700–14709. [Google Scholar] [CrossRef] [Green Version]
- Lynn, R.C.; Feng, Y.; Schutsky, K.; Poussin, M.; Kalota, A.; Dimitrov, D.S.; Powell, D.J., Jr. High-affinity FRbeta-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia 2016, 30, 1355–1364. [Google Scholar] [CrossRef]
- Lynn, R.C.; Poussin, M.; Kalota, A.; Feng, Y.; Low, P.S.; Dimitrov, D.S.; Powell, D.J., Jr. Targeting of folate receptor beta on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood 2015, 125, 3466–3476. [Google Scholar] [CrossRef]
- Lu, Y.J.; Chu, H.; Wheeler, L.W.; Nelson, M.; Westrick, E.; Matthaei, J.F.; Cardle, I.I.; Johnson, A.; Gustafson, J.; Parker, N.; et al. Preclinical evaluation of bispecific adaptor molecule controlled folate receptor CAR-T cell therapy with special focus on pediatric malignancies. Front. Oncol. 2019, 9, 151. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I trial of autologous CAR T cells targeting NKG2D ligands in patients with AML/MDS and multiple myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef]
- Zhang, T.; Barber, A.; Sentman, C.L. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006, 66, 5927–5933. [Google Scholar] [CrossRef] [Green Version]
- Marklin, M.; Hagelstein, I.; Koerner, S.P.; Rothfelder, K.; Pfluegler, M.S.; Schumacher, A.; Grosse-Hovest, L.; Jung, G.; Salih, H.R. Bispecific NKG2D-CD3 and NKG2D-CD16 fusion proteins for induction of NK and T cell reactivity against acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Peinert, S.; Prince, H.M.; Guru, P.M.; Kershaw, M.H.; Smyth, M.J.; Trapani, J.A.; Gambell, P.; Harrison, S.; Scott, A.M.; Smyth, F.E.; et al. Gene-modified T cells as immunotherapy for multiple myeloma and acute myeloid leukemia expressing the lewis Y antigen. Gene Ther. 2010, 17, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Westwood, J.A.; Smyth, M.J.; Teng, M.W.; Moeller, M.; Trapani, J.A.; Scott, A.M.; Smyth, F.E.; Cartwright, G.A.; Power, B.E.; Honemann, D.; et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 19051–19056. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, D.S.; Neeson, P.J.; Khot, A.; Peinert, S.; Tai, T.; Tainton, K.; Chen, K.; Shin, M.; Wall, D.M.; Honemann, D.; et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. 2013, 21, 2122–2129. [Google Scholar] [CrossRef] [Green Version]
- Casucci, M.; Nicolis di Robilant, B.; Falcone, L.; Camisa, B.; Norelli, M.; Genovese, P.; Gentner, B.; Gullotta, F.; Ponzoni, M.; Bernardi, M.; et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood 2013, 122, 3461–3472. [Google Scholar] [CrossRef] [PubMed]
- Gillissen, M.A.; de Jong, G.; Kedde, M.; Yasuda, E.; Levie, S.E.; Moiset, G.; Hensbergen, P.J.; Bakker, A.Q.; Wagner, K.; Villaudy, J.; et al. Patient-derived antibody recognizes a unique CD43 epitope expressed on all AML and has antileukemia activity in mice. Blood Adv. 2017, 1, 1551–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, L.; de Jong, G.; Gillissen, M.A.; Yasuda, E.; Kattler, V.; Bru, C.; Fatmawati, C.; van Hal-van Veen, S.E.; Cercel, M.G.; Moiset, G.; et al. A chemo-enzymatically linked bispecific antibody retargets T cells to a sialylated epitope on CD43 in acute myeloid leukemia. Cancer Res. 2019, 79, 3372–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, B.F.; Eisenbarth, G.S.; Fauci, A.S. Human lymphocyte antigens: Production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc. Natl. Acad. Sci. USA 1979, 76, 5829–5833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalidi, H.S.; Chang, K.L.; Medeiros, L.J.; Brynes, R.K.; Slovak, M.L.; Murata-Collins, J.L.; Arber, D.A. Acute lymphoblastic leukemia. Survey of immunophenotype, French-American-British classification, frequency of myeloid antigen expression, and karyotypic abnormalities in 210 pediatric and adult cases. Am. J. Clin. Pathol. 1999, 111, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.L.; Smith, L.M.; Anderson, J.; Abromowitch, M.; Campana, D.; Jacobsen, J.; Lones, M.A.; Gross, T.G.; Cairo, M.S.; Perkins, S.L. The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: A Children’s Oncology Group report. Br. J. Haematol. 2012, 159, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kita, K.; Miwa, H.; Nakase, K.; Kawakami, K.; Kobayashi, T.; Shirakawa, S.; Tanaka, I.; Ohta, C.; Tsutani, H.; Oguma, S.; et al. Clinical importance of CD7 expression in acute myelocytic leukemia. The Japan Cooperative Group of Leukemia/Lymphoma. Blood 1993, 81, 2399–2405. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Yeung, J.; Brandwein, J.; Yi, Q.L. CD7 expression predicts poor disease free survival and post-remission survival in patients with acute myeloid leukemia and normal karyotype. Leuk. Res. 2007, 31, 157–162. [Google Scholar] [CrossRef]
- Del Poeta, G.; Stasi, R.; Venditti, A.; Cox, C.; Aronica, G.; Masi, M.; Bruno, A.; Simone, M.D.; Buccisano, F.; Papa, G. CD7 expression in acute myeloid leukemia. Leuk. Lymphoma 1995, 17, 111–119. [Google Scholar] [CrossRef]
- Saito, T.; Usui, N.; Dobashi, N.; Maki, N.; Asai, O.; Yano, S.; Kato, A.; Watanabe, H.; Katori, M.; Nagamine, M.; et al. Prognostic significance of CD7 expression in adult acute myeloid leukemia. Rinsho Ketsueki 1998, 39, 481–486. [Google Scholar]
- Saxena, A.; Sheridan, D.P.; Card, R.T.; McPeek, A.M.; Mewdell, C.C.; Skinnider, L.F. Biologic and clinical significance of CD7 expression in acute myeloid leukemia. Am. J. Hematol. 1998, 58, 278–284. [Google Scholar] [CrossRef]
- Venditti, A.; Del Poeta, G.; Buccisano, F.; Tamburini, A.; Cox-Froncillo, M.C.; Aronica, G.; Bruno, A.; Del Moro, B.; Epiceno, A.M.; Battaglia, A.; et al. Prognostic relevance of the expression of Tdt and CD7 in 335 cases of acute myeloid leukemia. Leukemia 1998, 12, 1056–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrs, S.; Scherr, M.; Romani, J.; Zaborski, M.; Drexler, H.G.; Quentmeier, H. CD7 in acute myeloid leukemia: Correlation with loss of wild-type CEBPA, consequence of epigenetic regulation. J. Hematol. Oncol. 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Silva, D.; Atilla, E.; Atilla, P.A.; Mo, F.; Tashiro, H.; Srinivasan, M.; Lulla, P.; Rouce, R.H.; Cabral, J.M.S.; Ramos, C.A.; et al. CD7 Car T cells for the therapy of acute myeloid leukemia. Mol. Ther. 2019, 27, 272–280. [Google Scholar] [CrossRef]
- Dobrowolska, H.; Gill, K.Z.; Serban, G.; Ivan, E.; Li, Q.; Qiao, P.; Suciu-Foca, N.; Savage, D.; Alobeid, B.; Bhagat, G.; et al. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytom. B Clin. Cytom. 2013, 84, 21–29. [Google Scholar] [CrossRef]
- John, S.; Chen, H.; Deng, M.; Gui, X.; Wu, G.; Chen, W.; Li, Z.; Zhang, N.; An, Z.; Zhang, C.C. A novel anti-lilrb4 CAR-T cell for the treatment of monocytic AML. Mol. Ther. 2018, 26, 2487–2495. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Wang, Y.; Ahmed, T.; Zaslav, A.L.; Hogan, L.; Avila, C.; Wada, M.; Salman, H. Anti-CD19 chimeric antigen receptor targeting of CD19+acute myeloid leukemia. Leuk. Res. Rep. 2018, 9, 42–44. [Google Scholar] [CrossRef]
- Molldrem, J.J.; Lee, P.P.; Wang, C.; Felio, K.; Kantarjian, H.M.; Champlin, R.E.; Davis, M.M. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat. Med. 2000, 6, 1018–1023. [Google Scholar] [CrossRef]
- Herrmann, A.C.; Im, J.S.; Pareek, S.; Ruiz-Vasquez, W.; Lu, S.; Sergeeva, A.; Mehrens, J.; He, H.; Alatrash, G.; Sukhumalchandra, P.; et al. A Novel T-Cell Engaging Bi-specific Antibody Targeting the Leukemia Antigen PR1/HLA-A2. Front. Immunol. 2018, 9, 3153. [Google Scholar] [CrossRef]
- Rafiq, S.; Purdon, T.J.; Daniyan, A.F.; Koneru, M.; Dao, T.; Liu, C.; Scheinberg, D.A.; Brentjens, R.J. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms tumor 1 antigen. Leukemia 2017, 31, 1788–1797. [Google Scholar] [CrossRef]
- Chapuis, A.G.; Egan, D.N.; Bar, M.; Schmitt, T.M.; McAfee, M.S.; Paulson, K.G.; Voillet, V.; Gottardo, R.; Ragnarsson, G.B.; Bleakley, M.; et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 2019, 25, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.R.; Sukumaran, S.; Hristopoulos, M.; Totpal, K.; Stainton, S.; Lu, E.; Wong, A.; Tam, L.; Newman, R.; Vuillemenot, B.R.; et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood 2017, 129, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, M.; Henn, A.; Raum, T.; Bajtus, M.; Matthes, K.; Hendrich, L.; Wahl, J.; Hoffmann, P.; Kischel, R.; Kvesic, M.; et al. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol. Cancer Ther. 2014, 13, 1549–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chichili, G.R.; Huang, L.; Li, H.; Burke, S.; He, L.; Tang, Q.; Jin, L.; Gorlatov, S.; Ciccarone, V.; Chen, F.; et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: Preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 2015, 7, 289ra282. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.S.; Guo, H.; Wu, Z.; Hatano, M.N.; Cheung, N.V. A potent tetravalent T-cell-engaging bispecific antibody against CD33 in acute myeloid leukemia. Blood Adv. 2018, 2, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Reusch, U.; Harrington, K.H.; Gudgeon, C.J.; Fucek, I.; Ellwanger, K.; Weichel, M.; Knackmuss, S.H.; Zhukovsky, E.A.; Fox, J.A.; Kunkel, L.A.; et al. Characterization of CD33/CD3 tetravalent bispecific tandem diabodies (tandabs) for the treatment of acute myeloid leukemia. Clin. Cancer Res. 2016, 22, 5829–5838. [Google Scholar] [CrossRef] [Green Version]
- Harrington, K.H.; Gudgeon, C.J.; Laszlo, G.S.; Newhall, K.J.; Sinclair, A.M.; Frankel, S.R.; Kischel, R.; Chen, G.; Walter, R.B. The broad anti-aml activity of the CD33/CD3 bite antibody construct, AMG 330, is impacted by disease stage and risk. PLoS ONE 2015, 10, e0135945. [Google Scholar] [CrossRef]
- Laszlo, G.S.; Gudgeon, C.J.; Harrington, K.H.; Dell’Aringa, J.; Newhall, K.J.; Means, G.D.; Sinclair, A.M.; Kischel, R.; Frankel, S.R.; Walter, R.B. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 2014, 123, 554–561. [Google Scholar] [CrossRef] [Green Version]
- Arndt, C.; von Bonin, M.; Cartellieri, M.; Feldmann, A.; Koristka, S.; Michalk, I.; Stamova, S.; Bornhauser, M.; Schmitz, M.; Ehninger, G.; et al. Redirection of T cells with a first fully humanized bispecific CD33-CD3 antibody efficiently eliminates AML blasts without harming hematopoietic stem cells. Leukemia 2013, 27, 964–967. [Google Scholar] [CrossRef]
- Westervelt, P.; Roboz, G.; Cortes, J.; Altman, J.; Oehler, V.; Long, M.; Kantarjian, H.; Lee, S.; Han, T.; Guenot, J.; et al. Safety and clinical activity of AMV564, a CD33/CD3 T-cell engager, in patients with relapsed/refractory acute myeloid leukemia (AML): Updated results from the phase I first-in-human trial. HemaSphere 2019, 3, 393–394. [Google Scholar] [CrossRef]
- Al-Hussaini, M.; Rettig, M.P.; Ritchey, J.K.; Karpova, D.; Uy, G.L.; Eissenberg, L.G.; Gao, F.; Eades, W.C.; Bonvini, E.; Chichili, G.R.; et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood 2016, 127, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Zhou, Q.; Deshmukh, V.; Phull, H.; Ma, J.; Tardif, V.; Naik, R.R.; Bouvard, C.; Zhang, Y.; Choi, S.; et al. Targeting human C-type lectin-like molecule-1 (CLL1) with a bispecific antibody for immunotherapy of acute myeloid leukemia. Angew Chem. Int. Ed. Engl. 2014, 53, 9841–9845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loo, P.F.; Hangalapura, B.N.; Thordardottir, S.; Gibbins, J.D.; Veninga, H.; Hendriks, L.J.A.; Kramer, A.; Roovers, R.C.; Leenders, M.; de Kruif, J.; et al. MCLA-117, a CLEC12AxCD3 bispecific antibody targeting a leukaemic stem cell antigen, induces T cell-mediated AML blast lysis. Expert Opin. Biol. Ther. 2019, 19, 721–733. [Google Scholar] [CrossRef]
- Durben, M.; Schmiedel, D.; Hofmann, M.; Vogt, F.; Nubling, T.; Pyz, E.; Buhring, H.J.; Rammensee, H.G.; Salih, H.R.; Grosse-Hovest, L.; et al. Characterization of a bispecific FLT3 X CD3 antibody in an improved, recombinant format for the treatment of leukemia. Mol. Ther. 2015, 23, 648–655. [Google Scholar] [CrossRef] [Green Version]
- DeRenzo, C.; Gottschalk, S. Genetic modification strategies to enhance CAR T cell persistence for patients with solid tumors. Front. Immunol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Arcangeli, S.; Rotiroti, M.C.; Bardelli, M.; Simonelli, L.; Magnani, C.F.; Biondi, A.; Biagi, E.; Tettamanti, S.; Varani, L. Balance of anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol. Ther. 2017, 25, 1933–1945. [Google Scholar] [CrossRef]
- Tasian, S.K.; Kenderian, S.S.; Shen, F.; Ruella, M.; Shestova, O.; Kozlowski, M.; Li, Y.; Schrank-Hacker, A.; Morrissette, J.J.D.; Carroll, M.; et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood 2017, 129, 2395–2407. [Google Scholar] [CrossRef] [Green Version]
- Thokala, R.; Olivares, S.; Mi, T.; Maiti, S.; Deniger, D.; Huls, H.; Torikai, H.; Singh, H.; Champlin, R.E.; Laskowski, T.; et al. Redirecting specificity of T cells using the sleeping beauty system to express chimeric antigen receptors by mix-and-matching of VL and VH domains targeting CD123+ tumors. PLoS ONE 2016, 11, e0159477. [Google Scholar] [CrossRef]
- Cartellieri, M.; Feldmann, A.; Koristka, S.; Arndt, C.; Loff, S.; Ehninger, A.; von Bonin, M.; Bejestani, E.P.; Ehninger, G.; Bachmann, M.P. Switching CAR T cells on and off: A novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016, 6, e458. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Liu, X.; Wang, X.; Sun, Z.; Song, X.T. CD123 redirected multiple virus-specific T cells for acute myeloid leukemia. Leuk. Res. 2016, 41, 76–84. [Google Scholar] [CrossRef]
- Gill, S.; Tasian, S.K.; Ruella, M.; Shestova, O.; Li, Y.; Porter, D.L.; Carroll, M.; Danet-Desnoyers, G.; Scholler, J.; Grupp, S.A.; et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014, 123, 2343–2354. [Google Scholar] [CrossRef] [Green Version]
- Pizzitola, I.; Anjos-Afonso, F.; Rouault-Pierre, K.; Lassailly, F.; Tettamanti, S.; Spinelli, O.; Biondi, A.; Biagi, E.; Bonnet, D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target pirmary acute myeloid leukemia cells in vivo. Leukemia 2014, 28, 1596–1605. [Google Scholar] [CrossRef]
- Mardiros, A.; Dos Santos, C.; McDonald, T.; Brown, C.E.; Wang, X.; Budde, L.E.; Hoffman, L.; Aguilar, B.; Chang, W.C.; Bretzlaff, W.; et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 2013, 122, 3138–3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tettamanti, S.; Marin, V.; Pizzitola, I.; Magnani, C.F.; Giordano Attianese, G.M.; Cribioli, E.; Maltese, F.; Galimberti, S.; Lopez, A.F.; Biondi, A.; et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br. J. Haematol. 2013, 161, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.; Song, J.; Kim, Y.; Blanchard, S.; Wagner, J.; Stein, A.; AWeng, L.; Del Real, M.; Hernandez, R.; Marucci, E.; et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: A first-in-human clinical trial. Blood 2017, 130, 811. [Google Scholar]
- Wang, Q.; Wang, Y.; Lv, H.; Han, Q.; Fan, H.; Guo, B.; Wang, L.; Han, W. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tao, Z.; Xu, Y.; Liu, J.; An, N.; Wang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. CD33-specific chimeric antigen receptor T cells with different co-stimulators showed potent anti-leukemia efficacy and different phenotype. Hum. Gene Ther. 2018, 29, 626–639. [Google Scholar] [CrossRef]
- Minagawa, K.; Jamil, M.O.; Al-Obaidi, M.; Pereboeva, L.; Salzman, D.; Erba, H.P.; Lamb, L.S.; Bhatia, R.; Mineishi, S.; Di Stasi, A. In vitro pre-clinical validation of suicide gene modified anti-CD33 redirected chimeric antigen receptor T-cells for acute myeloid leukemia. PLoS ONE 2016, 11, e0166891. [Google Scholar] [CrossRef] [Green Version]
- O’Hear, C.; Heiber, J.; Schubert, I.; Fey, G.; Geiger, T. Anti-CD33 chimeric antigen receptor targeting of acute myeloid leukemia. Haematologica 2015, 100, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Dutour, A.; Marin, V.; Pizzitola, I.; Valsesia-Wittmann, S.; Lee, D.; Yvon, E.; Finney, H.; Lawson, A.; Brenner, M.; Biondi, A.; et al. In Vitro and In Vivo Antitumor Effect of Anti-CD33 Chimeric Receptor-Expressing EBV-CTL against CD33 Acute Myeloid Leukemia. Adv. Hematol. 2012, 2012, 683065. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Yu, K.R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell 2018, 173, 1439–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murad, J.M.; Baumeister, S.H.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Reder, J.; Sentman, C.L.; Gilham, D.; Lehmann, F.; Snykers, S.; et al. Manufacturing development and clinical production of NKG2D chimeric antigen receptor-expressing T cells for autologous adoptive cell therapy. Cytotherapy 2018, 20, 952–963. [Google Scholar] [CrossRef]
- Sallman, D.A.; Brayer, J.; Sagatys, E.M.; Lonez, C.; Breman, E.; Agaugue, S.; Verma, B.; Gilham, D.E.; Lehmann, F.F.; Davila, M.L. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica 2018, 103, e424–e426. [Google Scholar] [CrossRef] [Green Version]
- Laborda, E.; Mazagova, M.; Shao, S.; Wang, X.; Quirino, H.; Woods, A.K.; Hampton, E.N.; Rodgers, D.T.; Kim, C.H.; Schultz, P.G.; et al. Development of A chimeric antigen receptor targeting C-type lectin-like molecule-1 for human acute myeloid leukemia. Int. J. Mol. Sci. 2017, 18, 2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Cao, Y.; Pinz, K.; Ma, Y.; Wada, M.; Chen, K. First-in-human CLL1-CD33 compound CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia: Update on phase 1 clinical trial. Blood 2018, 132, 901. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Li, S.; Liu, J.; Xing, Y.; Xing, H.; Tian, Z.; Tang, K.; Rao, Q.; Wang, M.; et al. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J. Hematol. Oncol. 2018, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Jetani, H.; Garcia-Cadenas, I.; Nerreter, T.; Thomas, S.; Rydzek, J.; Meijide, J.B.; Bonig, H.; Herr, W.; Sierra, J.; Einsele, H.; et al. CAR T-cells targeting FLT3 have potent activity against FLT3(-)ITD(+) AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia 2018, 32, 1168–1179. [Google Scholar] [CrossRef]
- Reiter, K.; Polzer, H.; Krupka, C.; Maiser, A.; Vick, B.; Rothenberg-Thurley, M.; Metzeler, K.H.; Dorfel, D.; Salih, H.R.; Jung, G.; et al. Tyrosine kinase inhibition increases the cell surface localization of FLT3-ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia 2018, 32, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, E.; Zolov, S.N.; Huang, K.; Bonifant, C.L. T-cell Activity against AML Improved by Dual-Targeted T Cells Stimulated through T-cell and IL7 Receptors. Cancer Immunol. Res. 2019, 7, 683–692. [Google Scholar] [CrossRef]
- Bonifant, C.L.; Szoor, A.; Torres, D.; Joseph, N.; Velasquez, M.P.; Iwahori, K.; Gaikwad, A.; Nguyen, P.; Arber, C.; Song, X.T.; et al. CD123-Engager T Cells as a Novel Immunotherapeutic for Acute Myeloid Leukemia. Mol. Ther. 2016, 24, 1615–1626. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, M.P.; Szoor, A.; Vaidya, A.; Thakkar, A.; Nguyen, P.; Wu, M.F.; Liu, H.; Gottschalk, S. CD28 and 41BB costimulation enhances the effector function of CD19-specific engager T cells. Cancer Immunol. Res. 2017, 5, 860–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliperta, R.; Cartellieri, M.; Feldmann, A.; Arndt, C.; Koristka, S.; Michalk, I.; von Bonin, M.; Ehninger, A.; Bachmann, J.; Ehninger, G.; et al. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015, 5, e348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliperta, R.; Welzel, P.B.; Bergmann, R.; Freudenberg, U.; Berndt, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Stanzione, M.; Cartellieri, M.; et al. Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy. Sci. Rep. 2017, 7, 42855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Hu, X.; Wang, J.; Sahu, A.D.; Cohen, D.; Song, L.; Ouyang, Z.; Fan, J.; Wang, B.; Fu, J.; et al. Immune receptor repertoires in pediatric and adult acute myeloid leukemia. Genome Med. 2019, 11, 73. [Google Scholar] [CrossRef]
- Xue, L.; Hu, Y.; Wang, J.; Liu, X.; Wang, X. T cells targeting multiple tumor-associated antigens as a postremission treatment to prevent or delay relapse in acute myeloid leukemia. Cancer Manag. Res. 2019, 11, 6467–6476. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro-Vornhagen, A.; Godel, P.; Subklewe, M.; Stemmler, H.J.; Schlosser, H.A.; Schlaak, M.; Kochanek, M.; Boll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Orlando, E.J.; Han, X.; Tribouley, C.; Wood, P.A.; Leary, R.J.; Riester, M.; Levine, J.E.; Qayed, M.; Grupp, S.A.; Boyer, M.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef]
- Gardner, R.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef]
- Klco, J.M.; Spencer, D.H.; Miller, C.A.; Griffith, M.; Lamprecht, T.L.; O’Laughlin, M.; Fronick, C.; Magrini, V.; Demeter, R.T.; Fulton, R.S.; et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell 2014, 25, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Petrov, J.C.; Wada, M.; Pinz, K.G.; Yan, L.E.; Chen, K.H.; Shuai, X.; Liu, H.; Chen, X.; Leung, L.H.; Salman, H.; et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia 2018, 32, 1317–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamble, A.J.; Lind, E.F. Targeting the Immune Microenvironment in Acute Myeloid Leukemia: A Focus on T Cell Immunity. Front. Oncol. 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, M.; Szajnik, M.; Czystowska, M.; Mandapathil, M.; Strauss, L.; Welsh, A.; Foon, K.; Whiteside, T.; Boyiadzis, M. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin. Cancer Rev. 2009, 15, 3325–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Bucher, C.; Munger, M.; Highfill, S.; Tolar, J.; Munn, D.; Levine, B.; Riddle, M.; June, C.; Vallera, D.; et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood 2009, 114, 3793–3802. [Google Scholar] [CrossRef]
- Pyzer, A.; Stroopinsky, D.; Rajabi, H.; Washington, A.; Tagde, A.; Coll, M.; Fung, J.; Bryant, M.; Cole, L.; Palmer, K.; et al. MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood 2017, 129, 1791–1801. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Li, Y.; Zhang, Z.; Ju, Y.; Li, L.; Zhang, B.; Liu, B. Increase in myeloid-derived suppressor cells (MDSCs) associated with minimal residual disease (MRD) detection in adult acute myeloid leukemia. Int. J. Hematol. 2015, 102, 579–586. [Google Scholar] [CrossRef]
- Rickmann, M.; Macke, L.; Sundarasetty, B.; Stamer, K.; Figueiredo, C.; Blasczyk, R.; Heuser, M.; Krauter, J.; Ganser, A.; Stripecke, R. Monitoring dendritic cell and cytokine biomarkersduring remission prior to relapse in patients with FLT3-ITD acute myeloid leukemia. Ann. Hematol. 2013, 92, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Dong, Y.; Yang, Q.; Xu, W.; Jiang, S.; Yu, Z.; Yu, K.; Zhang, S. Acute myeloid leukemia cells express ICOS ligand to promote the expansion of regulatory T cells. Front. Immunol. 2018, 9, 2227. [Google Scholar] [CrossRef] [Green Version]
- Nabe, S.; Yamada, T.; Suzuki, J.; Toriyama, K.; Yasuoka, T.; Kuwahara, M.; Shiraishi, A.; Takenaka, K.; Yasukawa, M. Reinforce the antitumor activity of CD8+ T cells via glutamine restriction. Cancer Sci. 2018, 12, 3737–3750. [Google Scholar] [CrossRef] [Green Version]
- Mussai, F.; De Santo, C.; Abu-Dayyeh, I.; Booth, S.; Quek, L.; McEwen-Smith, R.; Qureshi, A.; Dazzi, F.; Vyas, P.; Cerundolo, V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood 2013, 122, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Krupka, C.; Kufer, P.; Kischel, R.; Zugmaier, G.; Lichtenegger, F.S.; Kohnke, T.; Vick, B.; Jeremias, I.; Metzeler, K.H.; Altmann, T.; et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: Reversing a T-cell-induced immune escape mechanism. Leukemia 2016, 30, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Gokbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Faul, C.; Diedrich, H.; Topp, M.S.; Bruggemann, M.; Horst, H.A.; et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018, 131, 1522–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Moreau, A.; Melchiorri, D.; Camarero, J.; Josephson, F.; Olimpier, O.; Bergh, J.; Karres, D.; Tzogani, K.; Gisselbrecht, C.; et al. Blinatumomab for acute lymphoblastic leukemia: The first bispecific T-cell engager antibody to be approved by the EMA for minimal residual disease. Oncologist 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Target | NCT | Institution/Sponsor | Product | Ages |
|---|---|---|---|---|
| CD123 | NCT04158739 | Children’s Oncology Group | flotetuzumab (MGD006) | <21 |
| NCT02715011 | Janssen Research & Development, LLC | JNJ-63709178 | 18+ | |
| NCT02152956 | MacroGenics | flotetuzumab (MGD006) | 18+ | |
| CD33 | NCT02520427 | Amgen | AMG330 | 18+ |
| NCT03144245 | Amphivena | AMV564 | 18+ | |
| NCT03915379 | Janssen Research & Development, LLC | JNJ-67571244 | 18+ | |
| NCT03516760 | GEMoaB Monoclonals GmbH | GEM333 | 18+ | |
| CLEC12A (CLL1) | NCT03038230 | Merus N. V. | MCLA-117 | 18+ |
| Target | NCT | Institution/Sponsor | Ages |
|---|---|---|---|
| United States | |||
| CD123 | NCT02159495 | City of Hope Medical Center | 12+ |
| NCT03766126 | University of Pennsylvania | 18+ | |
| NCT04109482 | Mustang Bio | 18+ | |
| NCT03190278 | Cellectis S. A. | 18–64 | |
| pending | St. Jude Children’s Research Hospital | <21 | |
| CD33 | NCT03971799 | Center for International Blood and Marrow Transplant Research (National Cancer Institute, Children’s Hospital of Philadelphia) | 1–30 |
| NKG2D | NCT04167696 NCT03018405 NCT02203825 | Celyad | 18+ |
| FLT3 | NCT03904069 | Amgen | 12+ |
| International | |||
| CD123 | NCT03556982 | Affiliated Hospital of the Chinese Academy of Military Medical Sciences, China | 14–75 |
| NCT03796390 | Hebei Senlang Biotechnology, China | 2–65 | |
| NCT04014881 | Wuhan Union Hospital, China | 18–70 | |
| NCT03114670 | Affiliated Hospital to Academy of Military Medical Sciences, China | 18+ | |
| NCT04106076 | Cellectis S. A., United Kingdom | ||
| CD7 | NCT04033302 | Shenzhen Geno-Immune Medical Institute, China | 6 mos-75 |
| CD44v6 | NCT04097301 | MolMed, Horizon 2020, Italy | I: 18–75 II: 1–75 |
| Lewis Y | NCT01716364 | Peter MacCallum Cancer Center, Australia | 18+ |
| CD19 | NCT03896854 | Shanghai Unicar-Therapy Bio-medicine Technology Co, Ltd., China | |
| CD123/CLL1 | NCT03631576 | Fujian Medical University, China | <70 |
| CD123/CD33 | NCT04156256 | iCell Gene Therapeutics, China | child, adult |
| CCL1/CD33/CD123 | NCT04010877 | Shenzhen Geno-Immune Medical Institute, China | 2–75 |
| Muc1/CLL1/CD33/ CD38/CD56/CD123 | NCT03222674 | Shenzhen Geno-Immune Medical Institute, China | 2–75 |
| CD33/CD28/CD56/ CD123/CD117/CD133/CD34/MucI | NCT03473457 | Zhujiang Hospital, China | 6 mos+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epperly, R.; Gottschalk, S.; Velasquez, M.P. Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond. Children 2020, 7, 14. https://doi.org/10.3390/children7020014
Epperly R, Gottschalk S, Velasquez MP. Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond. Children. 2020; 7(2):14. https://doi.org/10.3390/children7020014
Chicago/Turabian StyleEpperly, Rebecca, Stephen Gottschalk, and Mireya Paulina Velasquez. 2020. "Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond" Children 7, no. 2: 14. https://doi.org/10.3390/children7020014
APA StyleEpperly, R., Gottschalk, S., & Velasquez, M. P. (2020). Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond. Children, 7(2), 14. https://doi.org/10.3390/children7020014





