Caffeine and Clinical Outcomes in Premature Neonates
Abstract
1. Introduction
2. Caffeine for Treating Apnea of Prematurity
3. Pharmacology of Caffeine in Premature Neonates
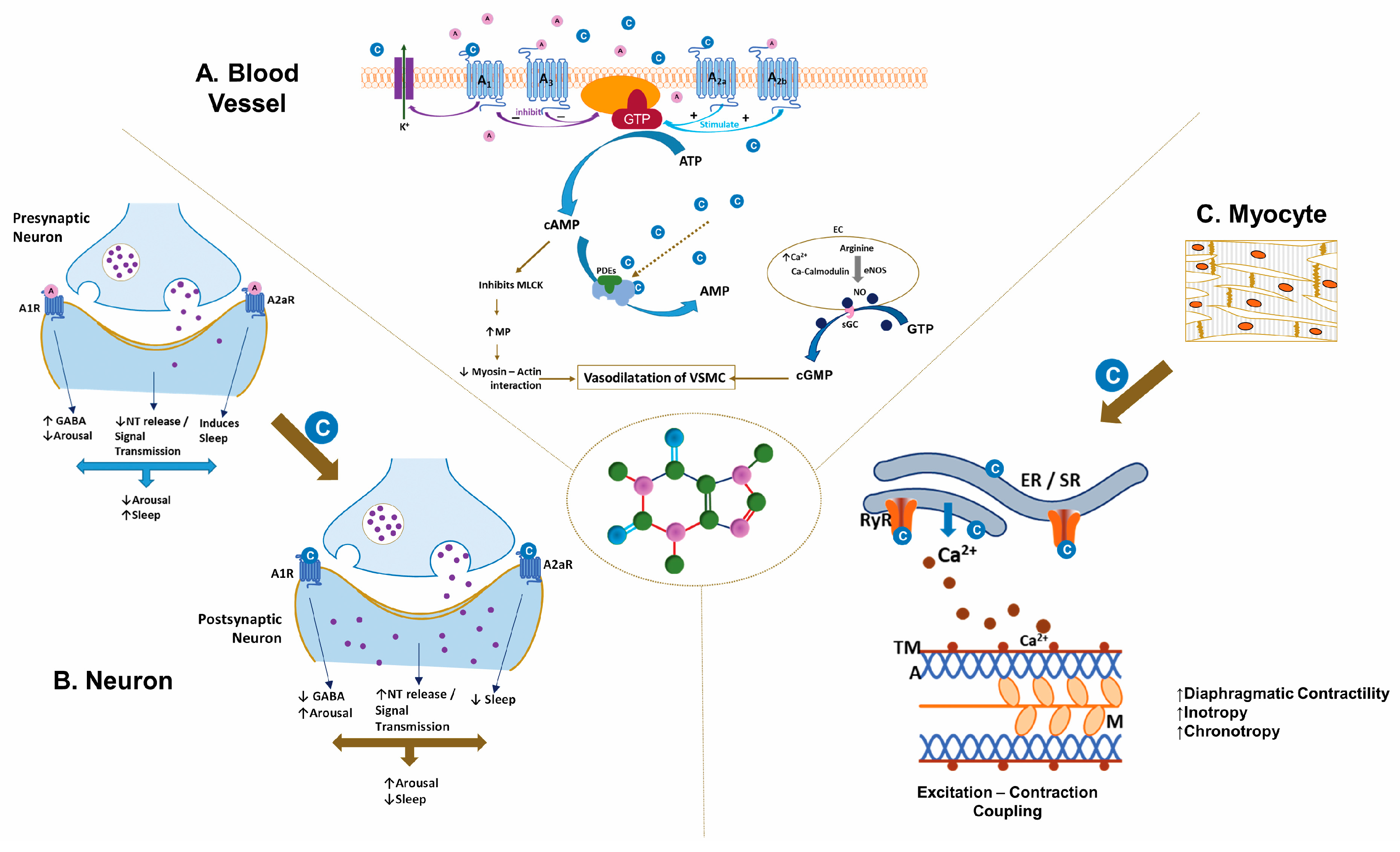
4. Caffeine’s Mechanism of Action
5. Caffeine, Bronchopulmonary Dysplasia, and Lung Protection
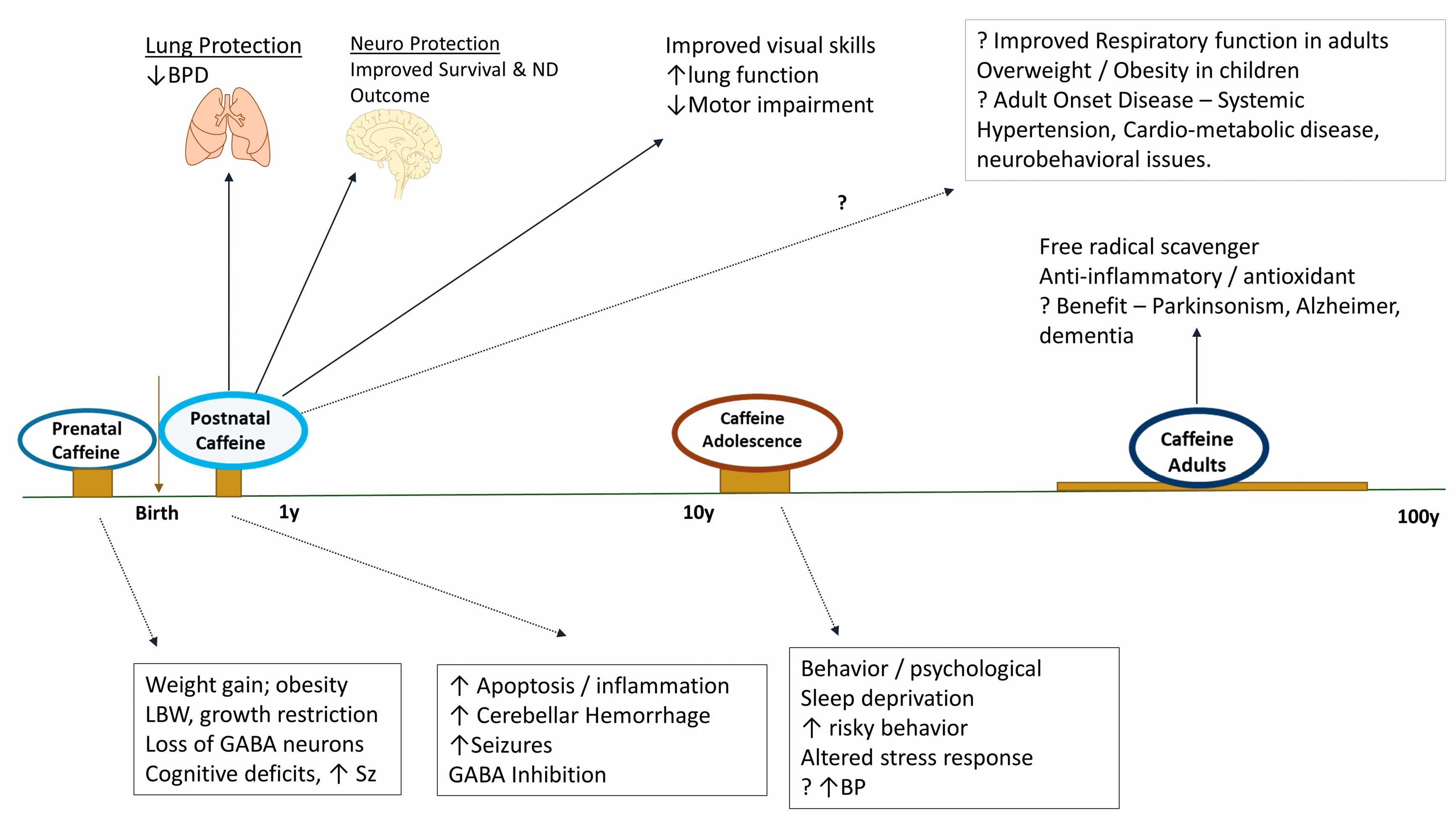
6. Caffeine and Neuroprotection
7. The Long-Term Effects of Neonatal Caffeine Exposure
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Temple, J.L. Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci. Biobehav. Rev. 2009, 33, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Branum, A.M.; Rossen, L.M.; Schoendorf, K.C. Trends in caffeine intake among U.S. children and adolescents. Pediatrics 2014, 133, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Warzak, W.J.; Evans, S.; Floress, M.T.; Gross, A.C.; Stoolman, S. Caffeine Consumption in Young Children. J. Pediatr. 2011, 158, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Burnham, L.; Matlak, S.; Makrigiorgos, G.; Braun, N.; Knapp, B.P.; Merewood, A. Breastfeeding and coffee consumption in children younger than 2 years in Boston, Massachusetts, USA. J. Hum. Lact. 2015, 31, 267–272. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- A Grandner, M.; Knutson, K.L.; Troxel, W.; Hale, L.; Jean-Louis, G.; E Miller, K. Implications of sleep and energy drink use for health disparities. Nutr. Rev. 2014, 72, 14–22. [Google Scholar] [CrossRef]
- Richards, G.; Smith, A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef]
- Mathew, O.P. Apnea of prematurity: pathogenesis and management strategies. J. Perinatol. 2011, 31, 302–310. [Google Scholar] [CrossRef]
- Richmond, G.H. Action of Caffeine and Aminophylline as Respiratory Stimulants in Man. J. Appl. Physiol. 1949, 2, 16–23. [Google Scholar] [CrossRef]
- Aranda, J.V.; Gorman, W.; Bergsteinsson, H.; Gunn, T. Efficacy of caffeine in treatment of apnea in the low-birth-weight infant. J. Pediatr. 1977, 90, 467–472. [Google Scholar] [CrossRef]
- Aranda, J.V.; Sitar, D.S.; Parsons, W.D.; Loughnan, P.M.; Neims, A.H. Pharmacokinetic Aspects of Theophylline in Premature Newborns. New Engl. J. Med. 1976, 295, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Mitenko, P.A.; Ogilvie, R.I. Rational Intravenous Doses of Theophylline. New Engl. J. Med. 1973, 289, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Bairam, A.; Boutroy, M.-J.; Badonnel, Y.; Vert, P. Theophylline versus caffeine: Comparative effects in treatment of idiopathic apnea in the preterm infant. J. Pediatr. 1987, 110, 636–639. [Google Scholar] [CrossRef]
- Brouard, C.; Moriette, G.; Murat, I.; Flouvat, B.; Pajot, N.; Walti, H.; De Gamarra, E.; Relier, J.-P. Comparative Efficacy of Theophylline and Caffeine in the Treatment of Idiopathic Apnea in Premature Infants. Arch. Pediatr. Adolesc. Med. 1985, 139, 698. [Google Scholar] [CrossRef] [PubMed]
- Erenberg, A.; Leff, R.D.; Haack, D.G.; Mosdell, K.W.; Hicks, G.M.; Wynne, B.A. Caffeine Citrate Study Group Caffeine Citrate for the Treatment of Apnea of Prematurity: A Double-Blind, Placebo-Controlled Study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 644–652. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; De Paoli, A.G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, CD000140. [Google Scholar] [CrossRef]
- Steer, P.A.; Henderson-Smart, D.J. Caffeine versus theophylline for apnea in preterm infants. Cochrane Database Syst. Rev. 2010, CD000273. [Google Scholar]
- Bhatt-Mehta, V.; Schumacher, R.E. Treatment of apnea of prematurity. Paediatr. Drugs 2003, 5, 195–210. [Google Scholar] [CrossRef]
- Rhein, L.M.; Dobson, N.R.; Darnall, R.A.; Corwin, M.J.; Heeren, T.C.; Poets, C.F.; McEntire, B.L.; Hunt, C.E. Caffeine Pilot Study Group. Effects of caffeine on intermittent hypoxia in infants born prematurely: A randomized clinical trial. JAMA Pediatr. 2014, 168, 250–257. [Google Scholar] [CrossRef]
- Poets, C.F.; Roberts, R.S.; Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Bader, D.; Bairam, A.; Moddemann, D.; Peliowski, A.; Rabi, Y.; et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA 2015, 314, 595. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Anderson, P.J.; Doyle, L.W.; Dewey, D.; Grunau, R.E.; Asztalos, E.V.; Davis, P.G.; Tin, W.; Moddemann, D.; Solimano, A.; et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012, 307, 275–282. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Anderson, P.J.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Doyle, L.W.; Grunau, R.E.; et al. Academic Performance, Motor Function, and Behavior 11 Years After Neonatal Caffeine Citrate Therapy for Apnea of Prematurity: An 11-Year Follow-up of the CAP Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Murner-Lavanchy, I.M.; Doyle, L.W.; Schmidt, B.; Roberts, R.S.; Asztalos, E.V.; Costantini, L.; Davis, P.G.; Dewey, D.; D’Ilario, J.; Grunau, R.E.; et al. Neurobehavioral Outcomes 11 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. Pediatrics 2018, 141, e20174047. [Google Scholar] [CrossRef]
- Marcus, C.L.; Meltzer, L.J.; Roberts, R.S.; Traylor, J.; Dix, J.; D’Ilario, J.; Asztalos, E.; Opie, G.; Doyle, L.W.; Biggs, S.N.; et al. Long-term effects of caffeine therapy for apnea of prematurity on sleep at school age. Am. J. Respir. Crit. Care Med. 2014, 190, 791–799. [Google Scholar] [CrossRef]
- Ranganathan, S.; Doyle, L.W.; Cheong, J.L.Y. Neonatal Caffeine Treatment and Respiratory Function at 11 Years in Children under 1,251 g at Birth. Am. J. Respir. Crit. Care Med. 2017, 196, 1318–1324. [Google Scholar]
- Dobson, N.R.; Hunt, C.E. Caffeine: an evidence-based success story in VLBW pharmacotherapy. Pediatr. Res. 2018, 84, 333–340. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Abbracchio, M.P.; Burnstock, G.; Daly, J.W.; Harden, T.K.; Jacobson, K.A.; Leff, P.; Williams, M.; Harden, K.T. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994, 46, 143–156. [Google Scholar]
- Blanchard, J.; Sawers, S.J.A. The absolute bioavailability of caffeine in man. Eur. J. Clin. Pharmacol. 1983, 24, 93–98. [Google Scholar] [CrossRef]
- Natarajan, G.; Botica, M.-L.; Thomas, R.; Aranda, J.V. Therapeutic Drug Monitoring for Caffeine in Preterm Neonates: An Unnecessary Exercise? Pediatrics 2007, 119, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, M.J. The pharmacology of caffeine. Prog. Drug Res. 1987, 31, 273–313. [Google Scholar] [PubMed]
- Lee, T.C.; Charles, B.; Steer, P.; Flenady, V.; Shearman, A. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity*. Clin. Pharmacol. Ther. 1997, 61, 628–640. [Google Scholar] [CrossRef]
- Sachse, K.T.; Jackson, E.K.; Wisniewski, S.R.; Gillespie, D.G.; Puccio, A.M.; Clark, R.S.; Dixon, C.E.; Kochanek, P.M. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J. Cereb. Blood Flow Metab. 2008, 28, 395–401. [Google Scholar] [CrossRef]
- Kimmel, C.A.; White, C.G.; Grafton, T.F.; Young, J.F.; Nelson, C.J. Blood Flow Changes and Conceptal Development in Pregnant Rats in Response to Caffeine. Toxicol. Sci. 1984, 4, 240–247. [Google Scholar] [CrossRef]
- Gilbert, S.G.; Stavric, B.; Klassen, R.D.; Rice, D.C. The Fate of Chronically Consumed Caffeine in the Monkey (Macaca fascicularis). Toxicol. Sci. 1985, 5, 578–587. [Google Scholar] [CrossRef]
- Thorn, C.F.; Aklillu, E.; McDonagh, E.M.; Klein, T.E.; Altman, R.B. PharmGKB summary: Caffeine pathway. Pharmacogenet. Genomics 2012, 22, 389–395. [Google Scholar] [CrossRef]
- Al-Alaiyan, S.; al-Rawithi, S.; Raines, D.; Yusuf, A.; Legayada, E.; Shoukri, M.M.; el-Yazigi, A. Caffeine metabolism in premature infants. J. Clin. Pharmacol. 2001, 41, 620–627. [Google Scholar] [CrossRef]
- Aranda, J.V.; Collinge, J.M.; Zinman, R.; Watters, G. Maturation of caffeine elimination in infancy. Arch. Dis. Child. 1979, 54, 946–949. [Google Scholar] [CrossRef]
- Le Guennec, J.C.; Billon, B.; Pare, C. Maturational changes of caffeine concentrations and disposition in infancy during maintenance therapy for apnea of prematurity: influence of gestational age, hepatic disease, and breast-feeding. Pediatrics 1985, 76, 834–840. [Google Scholar]
- Begas, E.; Kouvaras, E.; Tsakalof, A.; Papakosta, S.; Asprodini, E.K. In vivo evaluation of CYP1A2, CYP2A6, NAT-2 and xanthine oxidase activities in a Greek population sample by the RP-HPLC monitoring of caffeine metabolic ratios. Biomed. Chromatogr. 2007, 21, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar]
- Rivkees, S.A. The ontogeny of cardiac and neural A1 adenosine receptor expression in rats. Dev. Brain Res. 1995, 89, 202–213. [Google Scholar] [CrossRef]
- Janusz, C.A.; Berman, R.F. The adenosine binding enhancer, PD 81,723, inhibits epileptiform bursting in the hippocampal brain slice. Brain Res. 1993, 619, 131–136. [Google Scholar] [CrossRef]
- A Cunha, R.; Milusheva, E.; Vizi, E.S.; A Ribeiro, J.; Sebastião, A.M. Excitatory and inhibitory effects of A1 and A2A adenosine receptor activation on the electrically evoked [3H]acetylcholine release from different areas of the rat hippocampus. J. Neurochem. 1994, 63, 207–214. [Google Scholar] [CrossRef]
- Wojcik, W.J.; Neff, N.H. Differential location of adenosine A1 and A2 receptors in striatum. Neurosci. Lett. 1983, 41, 55–60. [Google Scholar] [CrossRef]
- Matos, M.; Augusto, E.; Schwarzschild, M.A.; Cunha, R.A.; Agostinho, P.; Dos Santos-Rodrigues, A.; Chen, J.-F.; Dos Santos-Rodrigues, A.; Chen, J. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 2012, 60, 702–716. [Google Scholar] [CrossRef]
- Owolabi, J.; Olatunji, S.; Olanrewaju, A. Caffeine and Cannabis Effects on Vital Neurotransmitters and Enzymes in the Brain Tissue of Juvenile Experimental Rats. Ann. Neurosci. 2017, 24, 65–73. [Google Scholar] [CrossRef]
- Persad, L.A.B. Energy Drinks and the Neurophysiological Impact of Caffeine. Front. Mol. Neurosci. 2011, 5. [Google Scholar] [CrossRef]
- McHill, A.W.; Smith, B.J.; Wright, K.P. Effects of Caffeine on Skin and Core Temperatures, Alertness, and Recovery Sleep During Circadian Misalignment. J. Boil. Rhythm. 2014, 29, 131–143. [Google Scholar] [CrossRef]
- McLellan, T.M.; Bell, D.G.; Kamimori, G.H. Caffeine improves physical performance during 24 h of active wakefulness. Aviat. Space, Environ. Med. 2004, 75, 666–672. [Google Scholar]
- Fredholm, B.B.; Yang, J.; Wang, Y. Low, but not high, dose caffeine is a readily available probe for adenosine actions. Mol. Asp. Med. 2017, 55, 20–25. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.E.; Zurzola, F.J. Rapid Quantitative Liquid Chromatographic Determination of Caffeine Levels in Plasma after Oral Dosing. J. Pharm. Sci. 1984, 73, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.; Lindsey, T. Fatal caffeine overdose: Two case reports. Forensic Sci. Int. 2005, 153, 67–69. [Google Scholar] [CrossRef]
- Kong, H.; Jones, P.P.; Koop, A.; Zhang, L.; Duff, H.J.; Chen, S.R. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem. J. 2008, 414, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Porta, M.; Zima, A.V.; Nani, A.; Diaz-Sylvester, P.L.; Copello, J.A.; Ramos-Franco, J.; Blatter, L.A.; Fill, M. Single ryanodine receptor channel basis of caffeine’s action on Ca2+ sparks. Biophys. J. 2011, 100, 931–938. [Google Scholar] [CrossRef]
- Michel, P.P.; Alvarez-Fischer, D.; Guerreiro, S.; Hild, A.; Hartmann, A.; Hirsch, E.C. Role of activity-dependent mechanisms in the control of dopaminergic neuron survival. J. Neurochem. 2007, 101, 289–297. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galan, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int. J. Vasc. Med. 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Hashiguchi, W.; Nagatomo, I.; Akasaki, Y.; Uchida, M.; Tominaga, M.; Takigawa, M. Influences of caffeine to nitric oxide production and zonisamide concentration in the brain of seizure-susceptible EL mice. Psychiatry Clin. Neurosci. 2001, 55, 319–324. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Effect of caffeine on the neuromuscular system — potential as an ergogenic aid. Appl. Physiol. Nutr. Metab. 2008, 33, 1284–1289. [Google Scholar] [CrossRef]
- Umemura, T.; Ueda, K.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Jitsuiki, D.; Soga, J.; Goto, C.; Chayama, K.; et al. Effects of Acute Administration of Caffeine on Vascular Function. Am. J. Cardiol. 2006, 98, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- A Beavo, J.; Rogers, N.L.; Crofford, O.B.; Hardman, J.G.; Sutherland, E.W.; Newman, E.V. Effects of xanthine derivatives on lipolysis and on adenosine 3’,5’-monophosphate phosphodiesterase activity. Mol. Pharmacol. 1970, 6, 597–603. [Google Scholar] [PubMed]
- Sattin, A. Increase in the Content of Adenosine 3’5’-Monophosphate in Mouse Forebrain During Seizures and Prevention of the Increase by Methylxanthines. J. Neurochem. 1971, 18, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Dalal, N.; Jain, A. Antioxidant behaviour of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem. Toxicol. 1991, 29, 1–6. [Google Scholar] [CrossRef]
- Devasagayam, T.; Kamat, J.; Mohan, H.; Kesavan, P. Caffeine as an antioxidant: inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. et Biophys. Acta (BBA) - Biomembr. 1996, 1282, 63–70. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauß, E.; Schlör, A.; Sifringer, M.; Scheuer, T.; Bührer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef]
- Chavez-Valdez, R.; Wills-Karp, M.; Ahlawat, R.; Cristofalo, E.A.; Nathan, A.; Gauda, E.B. Caffeine modulates TNF-alpha production by cord blood monocytes: The role of adenosine receptors. Pediatr. Res. 2009, 65, 203–208. [Google Scholar] [CrossRef]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 Adenosine Receptor Upregulation and Activation Attenuates Neuroinflammation and Demyelination in a Model of Multiple Sclerosis. J. Neurosci. 2004, 24, 1521–1529. [Google Scholar] [CrossRef]
- Henderson-Smart, D.J.; Davis, P.G.; Henderson-Smart, D.J. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Steer, P.; Flenady, V.; Shearman, A.; Charles, B.; Gray, P.H.; Henderson-Smart, D.; Bury, G.; Fraser, S.; Hegarty, J.; Rogers, Y.; et al. High dose caffeine citrate for extubation of preterm infants: A randomised controlled trial. Arch Dis. Child Fetal Neonatal Ed. 2004, 89, F499–F503. [Google Scholar] [CrossRef]
- Mohammed, S.; Nour, I.; Shabaan, A.E.; Shouman, B.; Abdel-Hady, H.; Nasef, N. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur. J. Nucl. Med. Mol. Imaging 2015, 174, 949–956. [Google Scholar] [CrossRef] [PubMed]
- McPherson, C.; Neil, J.J.; Tjoeng, T.H.; Pineda, R.; Inder, T.E. A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr. Res. 2015, 78, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jin, L.; Chen, X. Efficacy and Safety of Different Maintenance Doses of Caffeine Citrate for Treatment of Apnea in Premature Infants: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9061234. [Google Scholar] [CrossRef] [PubMed]
- A Steer, P.; Flenady, V.J.; Shearman, A.; Lee, T.C.; I Tudehope, D.; Charles, B.G. Periextubation caffeine in preterm neonates: a randomized dose response trial. J. Paediatr. Child Heal. 2003, 39, 511–515. [Google Scholar] [CrossRef]
- Brattstrom, P.; Russo, C.; Ley, D.; Bruschettini, M. High-versus low-dose caffeine in preterm infants: A systematic review and meta-analysis. Acta Paediatr. 2019, 108, 401–410. [Google Scholar] [CrossRef]
- Patel, R.M.; Leong, T.; Carlton, D.P.; Vyas-Read, S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J. Perinatol. 2013, 33, 134–140. [Google Scholar] [CrossRef]
- Taha, D.; Kirkby, S.; Nawab, U.; Dysart, K.C.; Genen, L.; Greenspan, J.S.; Aghai, Z.H. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J. Matern. Neonatal Med. 2014, 27, 1698–1702. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, Y.S.; Shin, J.H.; Choi, B.M. Hemodynamic Effects on Systemic Blood Flow and Ductal Shunting Flow after Loading Dose of Intravenous Caffeine in Preterm Infants according to the Patency of Ductus Arteriosus. J. Korean Med. Sci. 2018, 33, e25. [Google Scholar] [CrossRef]
- Nagatomo, T.; Jimenez, J.; Richter, J.; De Baere, S.; Vanoirbeek, J.; Naulaers, G.; Allegaert, K.; Croubels, S.; Deprest, J.A.; Toelen, J. Caffeine Prevents Hyperoxia-Induced Functional and Structural Lung Damage in Preterm Rabbits. Neonatology 2016, 109, 274–281. [Google Scholar] [CrossRef]
- Weichelt, U.; Cay, R.; Schmitz, T.; Strauss, E.; Sifringer, M.; Buhrer, C.; Endesfelder, S. Prevention of hyperoxia-mediated pulmonary inflammation in neonatal rats by caffeine. Eur. Respir. J. 2013, 41, 966–973. [Google Scholar] [CrossRef]
- Teng, R.-J.; Jing, X.; Michalkiewicz, T.; Afolayan, A.J.; Wu, T.-J.; Konduri, G.G. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L586–L598. [Google Scholar] [CrossRef] [PubMed]
- Dayanim, S.; Lopez, B.; Maisonet, T.M.; Grewal, S.; Londhe, V.A. Caffeine induces alveolar apoptosis in the hyperoxia-exposed developing mouse lung. Pediatr. Res. 2014, 75, 395–402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rath, P.; Nardiello, C.; Surate Solaligue, D.E.; Agius, R.; Mizikova, I.; Huhn, S.; Mayer, K.; Vadasz, I.; Herold, S.; Runkel, F.; et al. Caffeine administration modulates TGF-beta signaling but does not attenuate blunted alveolarization in a hyperoxia-based mouse model of bronchopulmonary dysplasia. Pediatr. Res. 2017, 81, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, V.; Nielsen, L.; Wang, H.; Kumar, V.H.S. Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury. BMC Pulm. Med. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Hu, J.-L.; Fu, X.-H.; Zeng, Y.-J.; Zhou, Y.-G.; Xiong, G.; Yang, N.; Dai, S.-S.; He, F.-T. Chronic or high dose acute caffeine treatment protects mice against oleic acid-induced acute lung injury via an adenosine A2A receptor-independent mechanism. Eur. J. Pharmacol. 2011, 654, 295–303. [Google Scholar] [CrossRef]
- Walsh, M.C.; Morris, B.H.; Wrage, L.A.; Vohr, B.R.; Poole, W.K.; Tyson, J.E.; Wright, L.L.; Ehrenkranz, R.A.; Stoll, B.J.; Fanaroff, A.A. Extremely Low Birthweight Neonates with Protracted Ventilation: Mortality and 18-Month Neurodevelopmental Outcomes. J. Pediatr. 2005, 146, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lodha, A.; Entz, R.; Synnes, A.; Creighton, D.; Yusuf, K.; Lapointe, A.; Yang, J.; Shah, P.S. investigators of the Canadian Neonatal Network (CNN) and the Canadian Neonatal Follow-up Network (CNFUN). Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics 2019, 143, e20181348. [Google Scholar] [CrossRef]
- Silva, C.G.; Métin, C.; Fazeli, W.; Machado, N.J.; Darmopil, S.; Launay, P.-S.; Ghestem, A.; Nesa, M.-P.; Bassot, E.; Szabó, E.; et al. Adenosine Receptor Antagonists Including Caffeine Alter Fetal Brain Development in Mice. Sci. Transl. Med. 2013, 5, 197ra104. [Google Scholar] [CrossRef]
- Kabir, Z.D.; McCarthy, D.M.; Bhide, P.G.; Kosofsky, B.E. Cup of Joe: A brain development “no”? Sci. Transl. Med. 2013, 5, 197fs130. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Zuchora, M.; Sawicka, K.M.; Kozińska, J.; Czuczwar, S.J. Acute exposure to caffeine decreases the anticonvulsant action of ethosuximide, but not that of clonazepam, phenobarbital and valproate against pentetrazole-induced seizures in mice. Pharmacol. Rep. 2006, 58, 652–659. [Google Scholar]
- Van Koert, R.R.; Bauer, P.R.; Schuitema, I.; Sander, J.W.; Visser, G.H. Caffeine and seizures: A systematic review and quantitative analysis. Epilepsy Behav. 2018, 80, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Banner, W.; Czajka, P.A. Acute Caffeine Overdose in the Neonate. Arch. Pediatr. Adolesc. Med. 1980, 134, 495. [Google Scholar] [CrossRef] [PubMed]
- Vesoulis, Z.A.; McPherson, C.; Neil, J.J.; Mathur, A.M.; Inder, T.E. Early High-Dose Caffeine Increases Seizure Burden in Extremely Preterm Neonates: A Preliminary Study. J. Caffeine Res. 2016, 6, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Atik, A.; Cheong, J.; Harding, R.; Rees, S.; De Matteo, R.; Tolcos, M. Impact of daily high-dose caffeine exposure on developing white matter of the immature ovine brain. Pediatr. Res. 2014, 76, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.; Divakaran, P.; Wiggins, R. The effect of postnatal caffeine administration on brain myelination. Brain Res. 1982, 249, 189–191. [Google Scholar] [CrossRef]
- Black, A.M.; Pandya, S.; Clark, D.; Armstrong, E.A.; Yager, J.Y. Effect of caffeine and morphine on the developing pre-mature brain. Brain Res. 2008, 1219, 136–142. [Google Scholar] [CrossRef]
- Wentz, C.T.; Magavi, S.S. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology 2009, 56, 994–1000. [Google Scholar] [CrossRef]
- Desfrere, L.; Olivier, P.; Schwendimann, L.; Verney, C.; Gressens, P. Transient Inhibition of Astrocytogenesis in Developing Mouse Brain Following Postnatal Caffeine Exposure. Pediatr. Res. 2007, 62, 604–609. [Google Scholar] [CrossRef]
- Singh, A.P.; Xu, Y.; Wang, H.; Kumar, V.H.S. The Beneficial Effects of Postnatal Caffeine on Spatial Learning in Adult Mice. J. Caffeine Adenosine Res. 2019, 9. [Google Scholar] [CrossRef]
- Geiger, J.; Labella, F.; Nagy, J.I. Ontogenesis of adenosine receptors in the central nervous system of the rat. Dev. Brain Res. 1984, 13, 97–104. [Google Scholar] [CrossRef]
- Johansson, B.; Georgiev, V.; Fredholm, B. Distribution and postnatal ontogeny of adenosine A2A receptors in rat brain: comparison with dopamine receptors. Neuroscience 1997, 80, 1187–1207. [Google Scholar] [CrossRef]
- Descombes, S.; Avoli, M.; Psarropoulou, C. A comparison of the adenosine-mediated synaptic inhibition in the CA3 area of immature and adult rat hippocampus. Dev. Brain Res. 1998, 110, 51–59. [Google Scholar] [CrossRef]
- Fabera, P.; Parizkova, M.; Uttl, L.; Vondrakova, K.; Kubova, H.; Tsenov, G.; Mares, P. Adenosine A1 Receptor Agonist 2-chloro-N6-cyclopentyladenosine and Hippocampal Excitability During Brain Development in Rats. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- A Cunha, R.; Constantino, M.C.; Sebastião, A.M.; A Ribeiro, J. Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. NeuroReport 1995, 6, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Constantino, M.D.; Johansson, B.; Fredholm, B.B. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1996, 353, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106, 1–16. [Google Scholar] [CrossRef]
- Babikian, T.; Prins, M.L.; Cai, Y.; Barkhoudarian, G.; Hartonian, I.; Hovda, D.A.; Giza, C.C. Molecular and physiological responses to juvenile traumatic brain injury: focus on growth and metabolism. Dev. Neurosci. 2010, 32, 431–441. [Google Scholar] [CrossRef]
- Chu, Y.F.; Chang, W.H.; Black, R.M.; Liu, J.R.; Sompol, P.; Chen, Y.; Wei, H.; Zhao, Q.; Cheng, I.H. Crude caffeine reduces memory impairment and amyloid beta(1–42) levels in an Alzheimer’s mouse model. Food Chem. 2012, 135, 2095–2102. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Wong, L.Y.; Harris, S.L.; Richardson, J.R.; Khobahy, I.; Li, W.; Gadad, B.S.; German, D.C. Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson’s disease. Exp. Neurol. 2012, 234, 482–487. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and caffeine consumption and depression: A meta-analysis of observational studies. Aust. N Z J Psychiatry 2016, 50, 228–242. [Google Scholar] [CrossRef]
- Li, D.K.; Ferber, J.R.; Odouli, R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: A prospective cohort study. Int. J. Obes. 2015, 39, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Botton, J.; Brantsæter, A.-L.; Haugen, M.; Alexander, J.; Meltzer, H.M.; Bacelis, J.; Elfvin, A.; Jacobsson, B.; Sengpiel, V. Maternal caffeine intake during pregnancy and childhood growth and overweight: Results from a large Norwegian prospective observational cohort study. BMJ Open 2018, 8, e018895. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Odouli, R.; Li, D. Maternal Caffeine Consumption During Pregnancy and the Risk of Miscarriage: A Prospective Cohort Study. Obstet. Anesthesia Dig. 2008, 28, 229–230. [Google Scholar] [CrossRef]
- Bakker, R.; Steegers, E.A.; Obradov, A.; Raat, H.; Hofman, A.; Jaddoe, V.W. Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the Generation R Study. Am. J. Clin. Nutr. 2010, 91, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- CARE Study Group. Maternal caffeine intake during pregnancy and risk of fetal growth restriction: A large prospective observational study. BMJ 2008, 337, a2332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, H.; Wu, Y.; He, Z.; Zhang, L.; Guo, Y.; Ma, L.; Magdalou, J.; Chen, L.; Wang, H. Gender-specific increase in susceptibility to metabolic syndrome of offspring rats after prenatal caffeine exposure with post-weaning high-fat diet. Toxicol. Appl. Pharmacol. 2015, 284, 345–353. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, B.; Liang, G.; Ping, J.; Kou, H.; Li, X.; Xiong, J.; Hu, D.; Chen, L.; Magdalou, J.; et al. Caffeine-Induced Activated Glucocorticoid Metabolism in the Hippocampus Causes Hypothalamic-Pituitary-Adrenal Axis Inhibition in Fetal Rats. PLOS ONE 2012, 7, e44497. [Google Scholar] [CrossRef]
- Buscariollo, D.L.; Fang, X.; Greenwood, V.; Xue, H.; Rivkees, S.A.; Wendler, C.C. Embryonic Caffeine Exposure Acts via A1 Adenosine Receptors to Alter Adult Cardiac Function and DNA Methylation in Mice. PLOS ONE 2014, 9, e87547. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Wendler, C.C. Adverse and protective influences of adenosine on the newborn and embryo: implications for preterm white matter injury and embryo protection. Pediatr. Res. 2011, 69, 271–278. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Luo, H.-W.; Kou, H.; Wen, Y.-X.; Shen, L.; Pei, L.-G.; Zhou, J.; Zhang, Y.-Z.; Wang, H. Prenatal caffeine exposure induced a lower level of fetal blood leptin mainly via placental mechanism. Toxicol. Appl. Pharmacol. 2015, 289, 109–116. [Google Scholar] [CrossRef]
- Singh, A.P.; Lakshminrusimha, S.; Nielsen, L.; Wang, H.; Gugino, S.; Kumar, V.H.S. Effects of neonatal caffeine on blood pressure, vessel reactivity & systemic stress in adult mice. In Proceedings of the Pediatric Academic Societies Meeting, San Francisco, CA, USA, 8 May 2017. [Google Scholar]
- O’Neill, C.E.; Newsom, R.J.; Stafford, J.; Scott, T.; Archuleta, S.; Levis, S.C.; Spencer, R.L.; Campeau, S.; Bachtell, R.K. Adolescent caffeine consumption increases adulthood anxiety-related behavior and modifies neuroendocrine signaling. Psychoneuroendocrinology 2016, 67, 40–50. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.C.; McCoy, T.P.; Rhodes, S.D.; Wagoner, A.; Wolfson, M. Caffeinated Cocktails: Energy Drink Consumption, High-risk Drinking, and Alcohol-related Consequences among College Students. Acad. Emerg. Med. 2008, 15, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ardais, A.; Borges, M.; Rocha, A.; Sallaberry, C.; Cunha, R.; Porciúncula, L.; Cunha, R. Caffeine triggers behavioral and neurochemical alterations in adolescent rats. Neuroscience 2014, 270, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Calamaro, C.J.; A Mason, T.B.; Ratcliffe, S.J. Adolescents living the 24/7 lifestyle: effects of caffeine and technology on sleep duration and daytime functioning. Pediatrics 2009, 123. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Guyton, K.Z.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; et al. Carcinogenicity of drinking coffee, mate, and very hot beverages. Lancet Oncol. 2016, 17, 877–878. [Google Scholar] [CrossRef]
- Ugai, T.; Matsuo, K.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Goto, A.; Inoue, M.; Kanda, Y.; Tsugane, S.; et al. Coffee and green tea consumption and subsequent risk of acute myeloid leukemia and myelodysplastic syndromes in Japan. Int. J. Cancer 2018, 142, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Kueser, T.J.; Walker, M.W.; Southgate, W.M.; Huckaby, J.L.; Roy, B.J.; Kinsella, J.P.; Pérez, J.A.; Keszler, M. Low-Dose Nitric Oxide Therapy for Persistent Pulmonary Hypertension of the Newborn. New Engl. J. Med. 2000, 342, 469–474. [Google Scholar] [CrossRef]
- Roberts, J.D., Jr.; Fineman, J.R.; Morin, F.C., III; Shaul, P.W.; Rimar, S.; Schreiber, M.D.; Polin, R.A.; Zwass, M.S.; Zayek, M.M.; Gross, I.; et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N. Engl. J. Med. 1997, 336, 605–610. [Google Scholar] [CrossRef]
- Soll, R.F.; Blanco, F.; Soll, R. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst. Rev. 2001, CD000144. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.H.S.; Lipshultz, S.E. Caffeine and Clinical Outcomes in Premature Neonates. Children 2019, 6, 118. https://doi.org/10.3390/children6110118
Kumar VHS, Lipshultz SE. Caffeine and Clinical Outcomes in Premature Neonates. Children. 2019; 6(11):118. https://doi.org/10.3390/children6110118
Chicago/Turabian StyleKumar, Vasantha H.S., and Steven E. Lipshultz. 2019. "Caffeine and Clinical Outcomes in Premature Neonates" Children 6, no. 11: 118. https://doi.org/10.3390/children6110118
APA StyleKumar, V. H. S., & Lipshultz, S. E. (2019). Caffeine and Clinical Outcomes in Premature Neonates. Children, 6(11), 118. https://doi.org/10.3390/children6110118



