Pediatric-Onset Multiple Sclerosis and Primary Headache: Is There a Link?
Abstract
1. Introduction
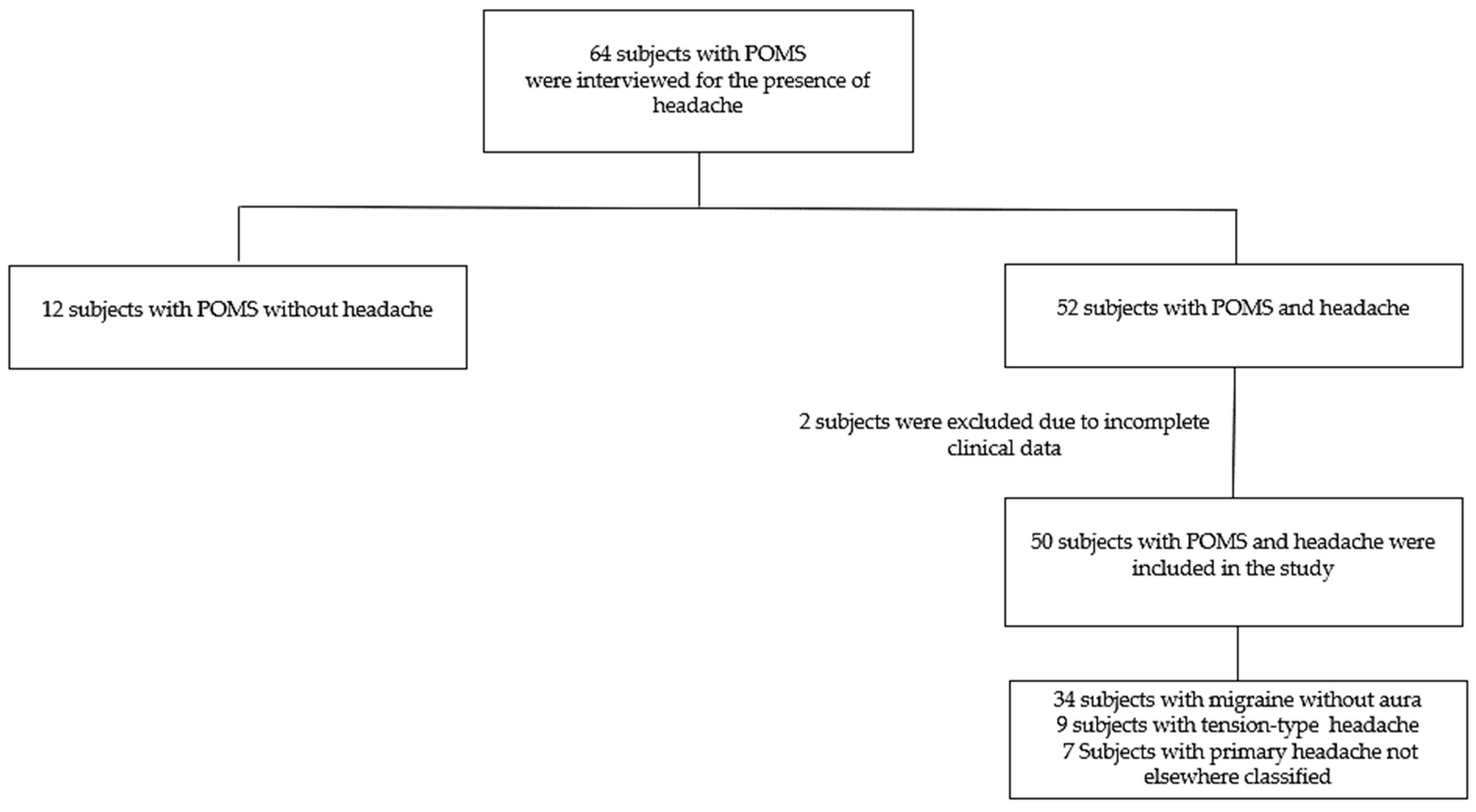
2. Materials and Methods
3. Results
3.1. All POMS Patients
3.2. POMS Patients with Headaches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLD | Cladribine |
| CNS | Central nervous system |
| DMF | Dimethyl fumarate |
| DMT | Disease-modifying therapy |
| FNG | Fingolimod |
| GLM | Generalized linear model |
| ICHD-3 | The International Classification of Headache Disorders, 3rd edition |
| IQR | Interquartile range |
| INFb-im | Intramuscular interferon beta |
| INFb-sc | Subcutaneous interferon beta |
| HFE | High-frequency episodic |
| LFE | Low-frequency episodic |
| MHDs | Monthly headache days |
| MRI | Magnetic Resonance Imaging |
| MS | Multiple sclerosis |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| NTZ | Natalizumab |
| OCZ | Ocrelizumab |
| POMS | Pediatric-onset MS |
| RTX | Rituximab |
| SDS | Standard deviation score |
| TACs | Trigeminal autonomic cephalalgias |
References
- Moisset, X.; Ouchchane, L.; Guy, N.; Bayle, D.J.; Dallel, R.; Clavelou, P. Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain 2013, 154, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Fisk, J.D.; Fitzgerald, K.; Kowalec, K.; Maxwell, C.; Rotstein, D.; Salter, A.; Tremlett, H. Etiology, effects and management of comorbidities in multiple sclerosis: Recent advances. Front. Immunol. 2023, 14, 1197195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Deng, Z.R.; Zu, M.D.; Wang, Y. The epidemiology of primary headaches in patients with multiple sclerosis. Brain Behav. 2021, 11, e01830. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, F.L.A.; Ng, B.C.; Wijnands, J.M.A.; Kingwell, E.; Marrie, R.A.; Tremlett, H. A systematic review of morbidities suggestive of the multiple sclerosis prodrome. Expert Rev. Neurother. 2020, 20, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Kister, I.; Munger, K.L.; Herbert, J.; Ascherio, A. Increased risk of multiple sclerosis among women with migraine in the Nurses’ Health Study II. Mult. Scler. 2012, 18, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Schoeps, V.A.; Smith, J.B.; Langer-Gould, A. Migraines, the multiple sclerosis prodrome, and multiple sclerosis susceptibility. Mult. Scler. 2025, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Belbasis, L.; Bellou, V.; Evangelou, E.; Tzoulaki, I. Environmental factors and risk of multiple sclerosis: Findings from meta-analyses and Mendelian randomization studies. Mult. Scler. 2020, 26, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Daghlas, I.; Larsson, S.C. Alcohol, coffee consumption, and smoking in relation to migraine: A bidirectional Mendelian randomization study. Pain 2022, 163, e342–e348. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, F.; Nash, M.C.; Bakour, C.; Kip, K. Adverse Childhood Experiences (ACEs) and Headaches Among Children: A Cross-Sectional Analysis. Headache 2020, 60, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Eid, K.; Torkildsen, Ø.; Aarseth, J.; Aalstad, M.; Bhan, A.; Celius, E.G.; Cortese, M.; Daltveit, A.K.; Holmøy, T.; Myhr, K.M.; et al. Association of adverse childhood experiences with the development of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.K.; Robinson, S.C.; Shao, X.; Quach, H.; Quach, D.; Choudhary, V.; Bellesis, K.H.; Dorin, P.; Mei, J.; Chinn, T.; et al. Cross-Trait Mendelian Randomization Study to Investigate Whether Migraine Is a Risk Factor for Multiple Sclerosis. Neurology 2023, 100, e1353–e1362. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.R.; Chang, J.; Dublin, A.B.; Vijayan, N. The association of brainstem lesions with migraine-like headache: An imaging study of multiple sclerosis. Headache 2005, 45, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, M.; Kropp, P.; Hoffmann, F.; Zettl, U.K. Headache in the course of multiple sclerosis: A prospective study. J. Neural Transm. 2019, 126, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.; Pardo, G.; Rabadi, M. Headache and Its Management in Patients with Multiple Sclerosis. Curr. Treat. Options Neurol. 2018, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- International Headache Society. The International Classification of Headache Disorders, 3rd edition (ICHD-3). Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Banwell, B.L.; Barkhof, F.; Ciccarelli, O.; De Stefano, N.; Geurts, J.J.G.; Paul, F.; Reich, D.S.; Toosy, A.T.; et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: Practical guidelines. Brain A J. Neurol. 2019, 142, 1858–1875. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, B.; Cordano, C.; Asteggiano, C.; Ruprecht, K.; Otto, C.; Rutatangwa, A.; Lui, A.; Hart, J.; Flanagan, E.P.; James, J.A.; et al. Multiple Sclerosis Is Rare in Epstein-Barr Virus-Seronegative Children with Central Nervous System Inflammatory Demyelination. Ann. Neurol. 2021, 89, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Barzegar, M.; Nehzat, N.; Shaygannejad, V.; Sahraian, M.A.; Ghajarzadeh, M. The prevalence of migraine in multiple sclerosis (MS): A systematic review and meta-analysis. J. Clin. Neurosci. 2020, 79, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Munger, K.L.; Makhani, N. The Multiple Sclerosis Prodrome: Evidence to Action. Front. Neurol. 2022, 12, 761408. [Google Scholar] [CrossRef] [PubMed]
- Gklinos, P.; Mitsikostas, D.D. Headache disorders in multiple sclerosis: Is there an association? A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2024, 85, 105536. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, P.; Nociti, V.; Cianfoni, A.; Stefanini, C.; De Rose, P.; Martinelli, D.; Dittoni, S.; Vollono, C.; Batocchi, A.P.; Della Marca, G. Migraine-like headache and status migrainosus as attacks of multiple sclerosis in a child. Pediatrics 2010, 126, e459–e464. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, P.; Rocca, M.A.; Colombo, B.; Annovazzi, P.; Comi, G.; Filippi, M. Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. J. Neurol. Sci. 2006, 244, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Makhani, N.; Lebrun, C.; Siva, A.; Brassat, D.; Carra Dallière, C.; de Seze, J.; Du, W.; Durand Dubief, F.; Kantarci, O.; Langille, M.; et al. Radiologically isolated syndrome in children: Clinical and radilogic outcomes. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e395. [Google Scholar] [CrossRef] [PubMed]
- Ursitti, F.; Valeriani, M. Migraine in childhood: Gender differences. Eur. J. Paediatr. Neurol. 2023, 42, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, S.L.; Ludwick, A.M.; Lebel, A.; Borsook, D. Age- and sex-related differences in the presentation of paediatric migraine: A retrospective cohort study. Cephalalgia Int. J. Headache 2018, 38, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. Hormonal influences in migraine—Interactions of oestrogen, oxytocin and CGRP. Nat. Rev. Neurol. 2021, 17, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kopruszinski, C.M.; Watanabe, M.; Dodick, D.W.; Navratilova, E.; Porreca, F. Female-selective mechanisms promoting migraine. J. Headache Pain 2024, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.M.; Rutatangwa, A.; Francisco, C. Pediatric Multiple Sclerosis: A Review. Adv. Pediatr. 2019, 66, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Giagnoni, F.; Lorefice, L.; Caria, P.; Dettori, T.; D’Alterio, M.N.; Angioni, S.; Hendren, A.J.; Caboni, P.; Pibiri, M.; et al. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines 2022, 10, 3107. [Google Scholar] [CrossRef] [PubMed]
- Graziano, E.; Hagemeier, J.; Weinstock-Guttman, B.; Ramasamy, D.P.; Zivadinov, R. Increased contrast enhancing lesion activity in relapsing-remitting multiple sclerosis migraine patients. Neuroimage Clin. 2015, 9, 110–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappa, R.; Theroux, L.; Brenton, J.N. Pediatric Multiple Sclerosis: Genes, Environment, and a Comprehensive Therapeutic Approach. Pediatr. Neurol. 2017, 75, 17–28. [Google Scholar] [CrossRef] [PubMed]
| Variable | POMS with Headache | POMS Without Headache | Sig. |
|---|---|---|---|
| Sex (female, male %) | 74%, 26% | 42.9%, 26% | * |
| Age at onset of POMS (mean ± SDS) | 13.7 ± 2.6 | 13.4 ± 2.6 | – |
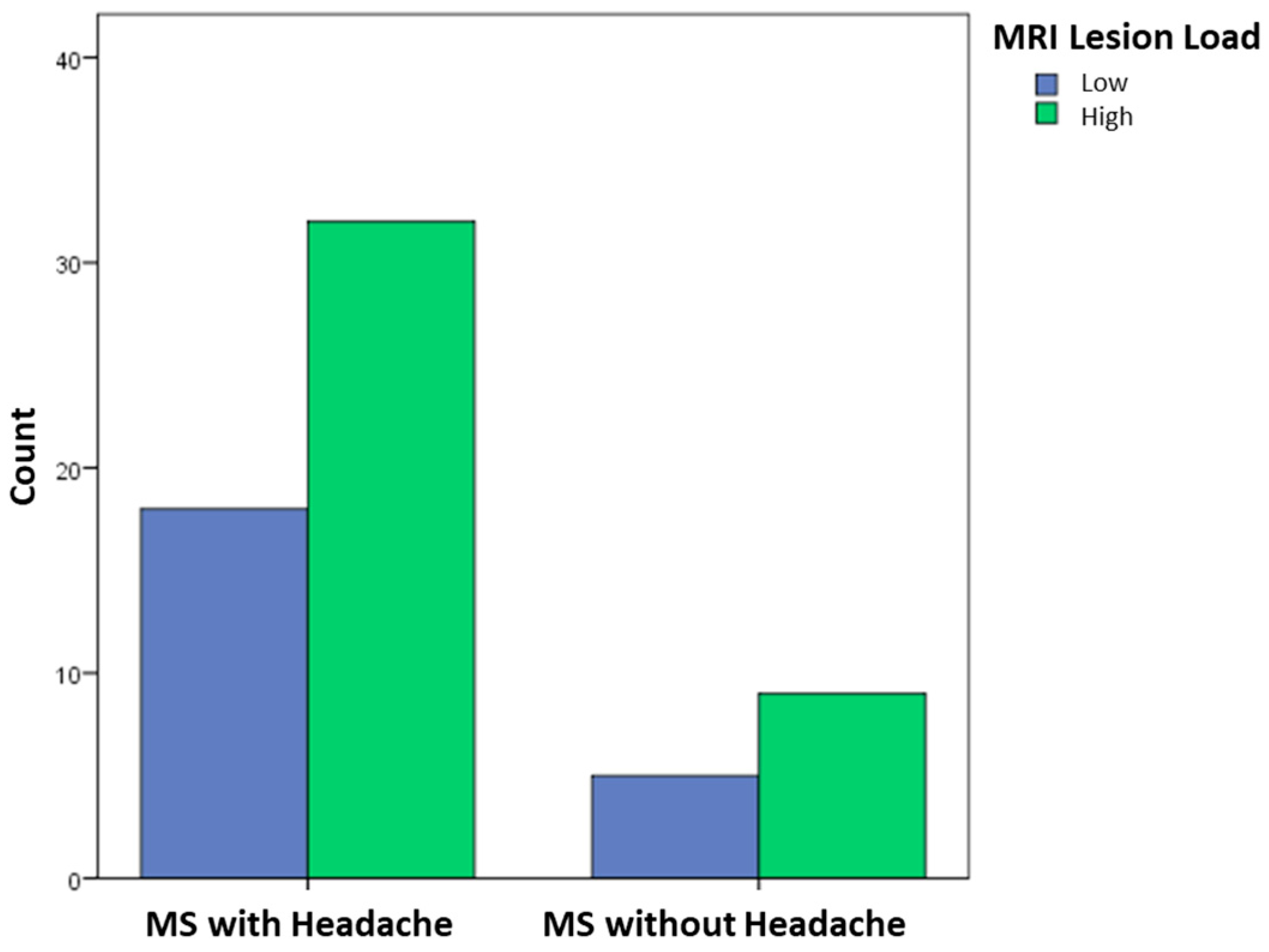
| Lesion count on MRI (low, high) | 36%, 64% | 35.7%, 64.3% | – |
| Gadolinium-enhancing lesions (mean ± SDS) | 0.9 ± 0.6 | 0.7 ± 0.3 | – |
| Familiarity with headaches | 72% | 42.9% | * |
| DMT (%) | – | ||
| INFb sc | 0% | 7.1% | |
| INFb im | 2% | 7.1% | |
| DMF | 4% | 7.1% | |
| NTZ | 24% | 42.9% | |
| RTX | 16% | 14.3% | |
| FNG | 28% | 7.1% | |
| OCZ | 24% | 14.3% | |
| CLD | 2% | 1.6% |
| Feature of Headache | Frequency and Percentage (Total 50 Subjects) |
|---|---|
| Female/Male | 37, 74%: 13, 26% |
| Mean age at onset of headache | 14.6 years, SD ± 2.3 |
| Diagnosis | Migraine without aura: 34, 68% Tension-type headache: 9, 18% Primary headache not elsewhere classified: 7, 14% |
| Frequency of MHDs | LFE: 27, 54% HFE: 16, 32% Chronic: 7, 14% |
| Quality of pain | Throbbing: 31, 62% Constrictive: 17, 34% Stabbing: 2, 4% |
| Pain side | Unilateral with side preference: 5, 10% Unilateral with no side preference: 11, 22% Bilateral: 34, 68% |
| Location of pain | Frontal: 9, 18% Temporal: 37, 74% Widespread: 4, 8% |
| Associated symptoms | Photophobia: 28, 56% Phonophobia: 22, 44% Nausea or vomiting: 12, 32% |
| Drugs for treating the attacks | Acetaminophen: 14, 28% Ibuprofen: 14, 38% Ketoprofen: 9, 18% Nimesulide: 1, 2% Ketoralac: 1, 2% |
| Prophylactic therapy for chronic subjects | Amitriptyline: 3, 100% |
| Variable | Beta Coefficient | Standard Deviation | T | Sig. |
|---|---|---|---|---|
| Sex | 1.3 | 0.6 | 3.9 | 0.04 |
| Type of headache (migraine vs. TTH) | −0.217 | 0.14 | −1.4 | 0.14 |
| Frequency of headache attacks per month (high, low) | 0.14 | 0.1 | 0.21 | 1.36 |
| Photophobia | −0.72 | 0.17 | −0.4 | 0.68 |
| Phonophobia | 0.37 | 0.18 | 1.9 | 0.06 |
| Nausea or vomiting | 0.16 | 0.16 | 0.96 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiralongo, G.; Monte, G.; Ferilli, M.A.N.; Ursitti, F.; Sforza, G.; Ruscitto, C.; Mazzeo, G.; Borrelli, A.; Valeriani, M.; Papetti, L. Pediatric-Onset Multiple Sclerosis and Primary Headache: Is There a Link? Children 2025, 12, 963. https://doi.org/10.3390/children12080963
Tiralongo G, Monte G, Ferilli MAN, Ursitti F, Sforza G, Ruscitto C, Mazzeo G, Borrelli A, Valeriani M, Papetti L. Pediatric-Onset Multiple Sclerosis and Primary Headache: Is There a Link? Children. 2025; 12(8):963. https://doi.org/10.3390/children12080963
Chicago/Turabian StyleTiralongo, Giuseppe, Gabriele Monte, Michela A. N. Ferilli, Fabiana Ursitti, Giorgia Sforza, Claudia Ruscitto, Giuseppe Mazzeo, Alessandro Borrelli, Massimiliano Valeriani, and Laura Papetti. 2025. "Pediatric-Onset Multiple Sclerosis and Primary Headache: Is There a Link?" Children 12, no. 8: 963. https://doi.org/10.3390/children12080963
APA StyleTiralongo, G., Monte, G., Ferilli, M. A. N., Ursitti, F., Sforza, G., Ruscitto, C., Mazzeo, G., Borrelli, A., Valeriani, M., & Papetti, L. (2025). Pediatric-Onset Multiple Sclerosis and Primary Headache: Is There a Link? Children, 12(8), 963. https://doi.org/10.3390/children12080963








