Moving Away from One-Size-Fits-All: Assessing the Use of Pharmacogenetic-Guided Medication Therapy in Pediatric Patients with Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Setting
2.4. Recruitment
2.5. Data Collection
2.6. PGx Testing Methods
PGx Report
2.7. Measures
2.7.1. Demographics
2.7.2. Medication Use History Questionnaire
2.7.3. PGx Testing Implementation/Acceptability Questionnaire—Prescriber
2.7.4. PGx Testing Follow-Up Questionnaire—Patient and Caregiver
2.7.5. PGx Testing Overall Feedback Questionnaire—Prescriber
2.8. Analytical and Statistical Approach
3. Results
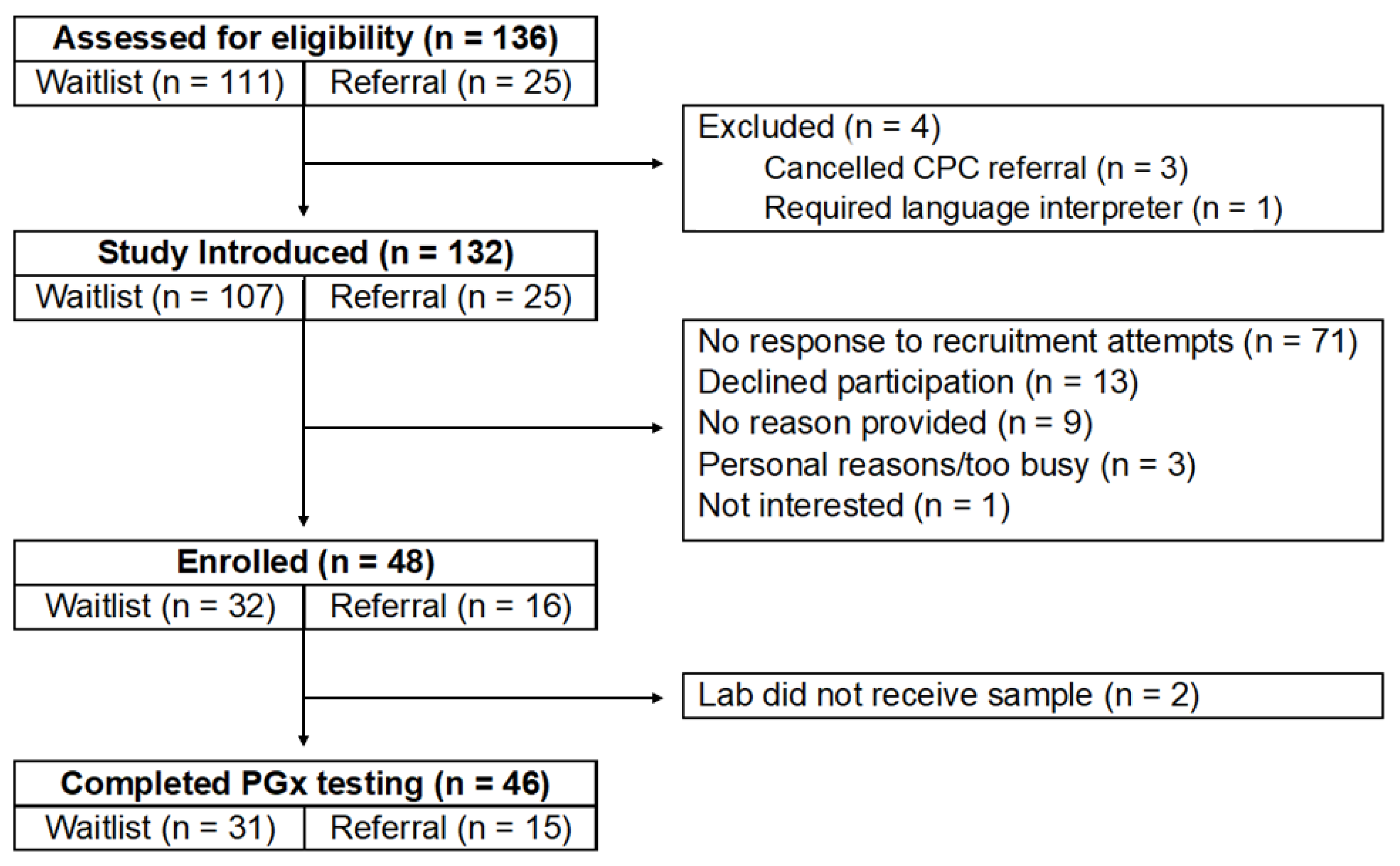
3.1. Recruitment and Retention
3.2. Participant Characteristics
3.3. Medication Usage
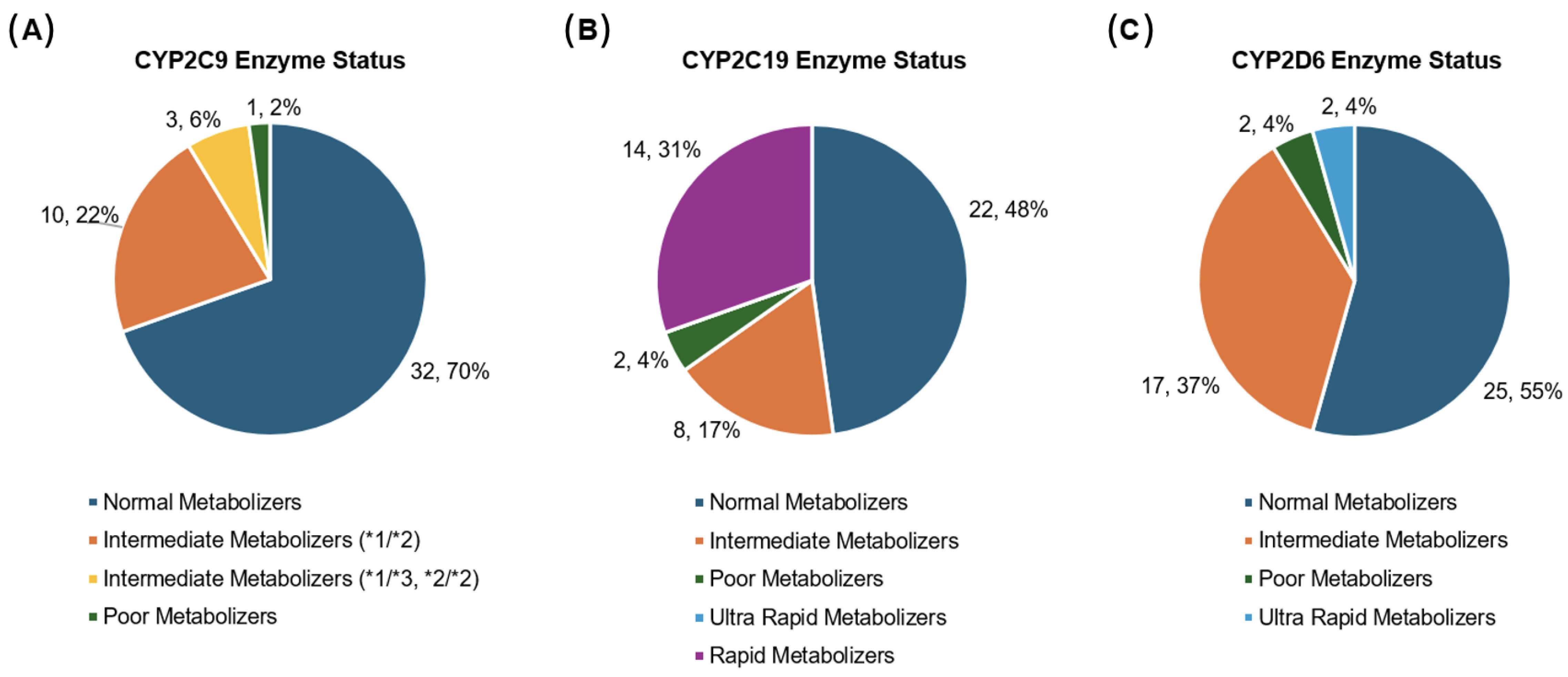
3.4. Drug-Metabolizing Enzyme Status
3.5. Primary Objective
Rate of Deviance from Standard Dosing Regimens Based on PGx Testing Results
3.6. Secondary Objective
3.6.1. Feasibility of PGx Testing
3.6.2. Acceptability of PGx Testing
3.6.3. Implementation of PGx Testing
3.7. Exploratory Objective
Predicting Therapeutic Response Based on PGx Results
4. Discussion
4.1. Clinical Considerations
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGx | Pharmacogenetics |
| ADRs | Adverse Drug Reactions |
| HCPs | Health Care Providers |
| CPC | Chronic Pain Clinic |
| EMR | Electronic Medical Record |
| POC | Point of Care |
Appendix A
|
|
|
|
Appendix B
| Please indicate how the following statements apply to you: |
Completely Agree/Strongly Agree/Agree/Somewhat Agree/Somewhat Disagree/Disagree/Strongly Disagree/Completely Disagree
|
|
|
|
Appendix C
| Chronic Pain Sample |
|
|
|
Appendix D
|
|
|
|
|
Appendix E
| Please indicate how the following statements apply to you: |
Completely Agree Strongly Agree/Agree/Somewhat Agree/Somewhat Disagree/Disagree/Strongly Disagree/Completely Disagree
|
Appendix F
| Gene | Predicted * Allele | Functional Status |
|---|---|---|
| CYP2C9 | * 1 | Normal Function |
| * 2 | Decreased Function | |
| * 3 | No Function | |
| * 4 | Decreased Function | |
| * 5 | Decreased Function | |
| * 6 | No Function | |
| * 8 | Decreased Function | |
| * 11 | Decreased Function | |
| * 12 | Decreased Function | |
| * 13 | No Function | |
| * 15 | No Function | |
| * 25 | No Function | |
| * 27 | Uncertain Function | |
| CYP2C19 | * 1 | Normal Function |
| * 2 | No Function | |
| * 3 | No Function | |
| * 4A | No Function | |
| * 4B | No Function | |
| * 5 | No Function | |
| * 6 | No Function | |
| * 7 | No Function | |
| * 8 | No Function | |
| * 17 | Increased Function | |
| CYP2D6 ** | * 1 | Normal |
| * 2 | Normal Function | |
| * 3 | No Function | |
| * 4 | No Function | |
| * 5 | No Function | |
| * 6 | No Function | |
| * 7 | No Function | |
| * 8 | No Function | |
| * 9 | Decreased Function | |
| * 10 | Decreased Function | |
| * 11 | No Function | |
| * 12 | No Function | |
| * 14A | Decreased Function | |
| * 14B | Decreased Function | |
| * 15 | No Function | |
| * 17 | Decreased Function | |
| * 18 | No Function | |
| * 19 | No Function | |
| * 20 | No Function | |
| * 29 | Decreased Function | |
| * 41 | Decreased Function | |
| * 69 | No Function |
References
- Huguet, A.; Miró, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 2000, 9, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Perquin, C.W.; Hazebroek-Kampschreur, A.; Hunfeld, J.; Bohnen, A.M.; van Suijlekom-Smit, L.; Passchier, J.; van der Wouden, J.C. Pain in children and adolescents: A common experience. Pain 2000, 87, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Lau, E.; Palozzi, L.; Campbell, F. Pain management in children: Part 1—Pain assessment tools and a brief review of nonpharmacological and pharmacological treatment options. Can. Pharm. J. 2012, 145, 222–225. [Google Scholar] [CrossRef]
- Taylor, E.M.; Boyer, K.; Campbell, F.A. Pain in hospitalized children: A prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Res. Manag. 2008, 13, 25–32. [Google Scholar] [CrossRef]
- Bateman, S.; Caes, L.; Eccleston, C.; Noel, M.; Jordan, A. Co-occurring chronic pain and primary psychological disorders in adolescents: A scoping review. Paediatr. Neonatal Pain 2023, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Baweja, R.; Arshad, S.H.; Samsel, C.; Friedberg, R.D. Chronic pain and its impact on child mental health: Management challenges and clinical guidance for child and adolescent psychiatrists. J. Am. Acad. Child Adolesc. Psychiatry 2022, 61, 1411–1414. [Google Scholar] [CrossRef]
- DiGiusto, M.; Bhalla, T.; Martin, D.; Foerschler, D.; Jones, M.J.; Tobias, J.D. Patient-controlled analgesia in the pediatric population: Morphine versus hydromorphone. J. Pain Res. 2014, 7, 471–475. [Google Scholar]
- O’Donnell, F.T.; Rosen, K.R. Pediatric pain management: A review. Mo. Med. 2014, 111, 231–237. [Google Scholar]
- Thiesen, S.; Conroy, E.J.; Bellis, J.R.; Bracken, L.E.; Mannix, H.L.; Bird, K.A.; Duncan, J.C.; Cresswell, L.; Kirkham, J.J.; Peak, M.; et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children—A prospective observational cohort study of 6601 admissions. BMC Med. 2013, 11, 237. [Google Scholar] [CrossRef]
- Titchen, T.; Cranswick, N.; Beggs, S. Adverse drug reactions to nonsteroidal anti-inflammatory drugs, COX-2 inhibitors and paracetamol in a paediatric hospital. Br. J. Clin. Pharmacol. 2005, 59, 718–723. [Google Scholar] [CrossRef]
- Ferland, C.E.; Vega, E.; Ingelmo, P.M. Acute pain management in children: Challenges and recent improvements. Curr. Opin. Anaesthesiol. 2018, 31, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Světlík, S.; Hronová, K.; Bakhouche, H.; Matoušková, O.; Slanař, O. Pharmacogenetics of chronic pain and its treatment. Mediat. Inflamm. 2013, 2013, 864319. [Google Scholar] [CrossRef] [PubMed]
- Patrinos, G.P.; Shuldiner, A.R. Pharmacogenomics: The low-hanging fruit in the personalized medicine tree. Hum. Genet. 2022, 141, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Hicks, J.K.; Pui, C.H.; Relling, M.V.; Evans, W.E. Pharmacogenomics and individualized medicine: Translating science into practice. Clin. Pharmacol. Ther. 2012, 92, 467–475. [Google Scholar] [CrossRef]
- Relling, M.V.; Klein, T.E. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin. Pharmacol. Ther. 2011, 89, 464–467. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics knowledge for personalized medicine. Clin. Pharm. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef]
- Magarbeh, L.; Gorbovskaya, I.; Le Foll, B.; Jhirad, R.; Müller, D.J. Reviewing pharmacogenetics to advance precision medicine for opioids. Biomed. Pharmacother. 2021, 142, 112060. [Google Scholar] [CrossRef]
- Rodriguez Cairoli, F.; Appiani, F.; Sambade, J.M.; Comandé, D.; Camacho Arteaga, L.; Ciapponi, A. Efficacy and safety of opioid therapy guided by pharmacogenetics: A systematic review. Pharmacogenomics 2021, 22, 573–586. [Google Scholar] [CrossRef]
- Zobdeh, F.; Eremenko, I.I.; Akan, M.A.; Tarasov, V.V.; Chubarev, V.N.; Schiöth, H.B.; Mwinyi, J. Pharmacogenetics and pain treatment with a focus on non-steroidal anti-inflammatory drugs (NSAIDS) and antidepressants: A systematic review. Pharmaceutics 2022, 14, 1190. [Google Scholar] [CrossRef]
- Agulló, L.; Aguado, I.; Muriel, J.; Margarit, C.; Gómez, A.; Escorial, M.; Sánchez, A.; Fernández, A.; Peiró, A.M. Pharmacogenetic guided opioid therapy improves chronic pain outcomes and comorbid mental health: A randomized, double-blind, controlled study. Int. J. Mol. Sci. 2023, 24, 10754. [Google Scholar] [CrossRef]
- Hamilton, W.G.; Gargiulo, J.M.; Reynolds, T.R.; Parks, N.L. Prospective randomized study using pharmacogenetics to customize postoperative pain medication following hip and knee arthroplasty. J. Arthroplast. 2022, 37, S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Loh, F.H.; Azzi, B.; Weingarten, A.; Loewy, Z.G. Pharmacogenomic testing and patient perception inform pain pharmacotherapy. J. Pers. Med. 2021, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Kunselman, A.R. A brief overview of pilot studies and their sample size justification. Fertil. Steril. 2024, 121, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Day, S.J. Internal pilot studies for estimating sample size. Stat. Med. 1994, 13, 2455–2463. [Google Scholar] [CrossRef]
- Ruskin, D.; Borsatto, J.; Szczech, K.; Tremblay, M.; D’Alessandro, L.N.; Mesaroli, G.; Sun, N.; Munns, C.; Stinson, J. “Working together”: Perspectives of healthcare professionals in providing virtual care to youth with chronic pain during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2023, 20, 4757. [Google Scholar] [CrossRef]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Whirl-Carrillo, M.; Huddart, R.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Whaley, R.; Klein, T.E. An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2021, 110, 563–572. [Google Scholar] [CrossRef]
- Kim, J.A.; Ceccarelli, R.; Lu, C.Y. Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020). J. Pers. Med. 2021, 11, 179. [Google Scholar] [CrossRef]
- Hurst, H.A.; Bolton, J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J. Manip. Physiol. Ther. 2004, 27, 26–35. [Google Scholar] [CrossRef]
- Theken, K.N.; Lee, C.R.; Gong, L.; Caudle, K.E.; Formea, C.M.; Gaedigk, A.; Klein, T.E.; Agúndez, J.A.G.; Grosser, T. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clin. Pharmacol. Ther. 2020, 108, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.J.; Thomas, C.D.; Barbarino, J.; Desta, Z.; Van Driest, S.L.; El Rouby, N.; Johnson, J.A.; Cavallari, L.H.; Shakhnovich, V.; Thacker, D.L.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin. Pharmacol. Ther. 2021, 109, 1417–1423. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Yukselogl, A.; Ross, C.J.; Rosychuk, R.J.; Drendel, A.L.; Manaloor, R.; Johnson, D.W.; Le May, S.; Carleton, B. Effects of pharmacogenetic profiles on pediatric pain relief and adverse events with ibuprofen and oxycodone. Pain Rep. 2023, 8, e1113. [Google Scholar] [CrossRef]
- Manworren, R.C.; Stinson, J. Pediatric pain measurement, assessment, and evaluation. Semin. Pediatr. Neurol. 2016, 23, 189–200. [Google Scholar] [CrossRef]
- Jin, J. Risks of codeine and tramadol in children. JAMA 2017, 318, 1514. [Google Scholar] [CrossRef]
- Dean, L.; Kane, M. Codeine Therapy and CYP2D6 Genotype. In Medical Genetics Summaries [Internet], 3rd ed.; Pratt, V.M., Scott, S.A., Pirmohamed, M., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2021. [Google Scholar]
- Kane, M. CYP2D6 Overview: Allele and Phenotype Frequencies. In Medical Genetics Summaries [Internet], 2nd ed.; Pratt, V.M., Scott, S.A., Pirmohamed, M., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2021. [Google Scholar]
- Logan, D.E.; Claar, R.L.; Scharff, L. Social desirability response bias and self-report of psychological distress in pediatric chronic pain patients. Pain 2008, 136, 366–372. [Google Scholar] [CrossRef]
- Lenko, R.; Voepel-Lewis, T. To relieve pain or avoid opioid-related risk? A comparison of parents’ analgesic trade-off preferences and decision-making in 2019 versus 2013 in a single U.S. pediatric hospital. Paediatr. Anaesth. 2021, 31, 878–884. [Google Scholar] [CrossRef]
- McGrath, P.J.; Finley, G.A. Attitudes and beliefs about medication and pain management in children. J. Palliat. Care 1996, 12, 46–50. [Google Scholar] [CrossRef]
- Rony, R.Y.; Fortier, M.A.; Chorney, J.M.; Perret, D.; Kain, Z.N. Parental postoperative pain management: Attitudes, assessment, and management. Pediatrics 2010, 125, e1372–e1378. [Google Scholar] [CrossRef] [PubMed]
- Ferreira do Couto, M.L.; Fonseca, S.; Pozza, D.H. Pharmacogenetic approaches in personalized medicine for postoperative pain management. Biomedicines 2024, 12, 729. [Google Scholar] [CrossRef] [PubMed]
- Rassekh, S.R.; Rieder, M.; ‘t Jong, G. Gene-based drug therapy in children. Paediatr. Child Health 2023, 28, 205–251. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, R.H.J.; Cohn, I.; Feldman, M.E.; Gorman, D.; Ito, S. Gene-based drug therapy for children and youth treated with psychoactive medications. Paediatr. Child Health 2024, 29, 311–323. [Google Scholar] [CrossRef]
- Kaye, A.D.; Garcia, A.J.; Hall, O.M.; Jeha, G.M.; Cramer, K.D.; Granier, A.L.; Kallurkar, A.; Cornett, E.M.; Urman, R.D. Update on the pharmacogenomics of pain management. Pharmgenomics Pers. Med. 2019, 12, 125–143. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef]
| Demographics | N = 46 |
|---|---|
| Age (years) | |
| Mean (SD) | 14.5 (2.28) |
| [Range] | [8, 17] |
| Sex | n (%) |
| Female | 35 (76.1) |
| Male | 11 (23.9) |
| Pain Duration (years) | |
| Mean (SD) | 3.58 (3.24) |
| [Range] | [0.5, 13] |
| Type of Chronic Pain * | n (%) |
| Musculoskeletal | 33 (71.7) |
| Headache | 9 (19.6) |
| Abdominal | 8 (17.4) |
| Neuropathic | 4 (8.7) |
| Pelvic | 2 (4.3) |
| Psychological Diagnoses * | n (%) |
| Anxiety disorder | 28 (60.9) |
| ADHD | 11 (23.9) |
| Intellectual/learning disability | 10 (21.7) |
| Depression | 7 (15.2) |
| Autism spectrum disorder | 5 (10.9) |
| Tourette syndrome | 2 (4.3) |
| Somatoform disorder | 2 (4.3) |
| Other (PTSD, psychotic disorder) | 2 (4.3) |
| Other Concurrent Diagnoses * | |
| Orthopedic | 8 (17.4) |
| Cardiac | 6 (13.0) |
| Neurological | 6 (13.0) |
| Gastrointestinal | 6 (13.0) |
| Musculoskeletal/Connective tissue | 5 (10.9) |
| Other | 8 (17.3) |
| Baseline Scores at CPC Intake † | Mean (SD) |
| PROMIS Pain Interference | 65.87 (7.56) |
| PROMIS Anxiety | 60.46 (8.77) |
| PROMIS Depressive Symptoms | 58.16 (10.78) |
| PROMIS Mobility | 36.09 (7.25) |
| Pain Catastrophizing | |
| Patient | 64.28 (12.06) |
| Caregiver | 61.18 (12.34) |
| Medication Use at Study Enrollment * | N (%) | PGx-Listed Medication Use at Time of PGx Report † | N (%) |
|---|---|---|---|
Taking medication(s) as part of treatment
| 29/46 (63.0) 15/29 (51.7) 14/29 (48.2) | Taking PGx medication(s) as part of treatment
| 24/46 (52.2) 18/24 (75.0) 6/24 (25.0) |
Taking analgesic
| 25/29 (86.2) 15/25 (60.0) 5/15 (33.3) 2/15 (13.3) 5/15 (33.3) 3/15 (20.0) 2/25 (8.0) 1/2 (50.0) 1/2 (50.0) 7/25 (28.0) 6/7 (85.7) 1/7 (14.3) 6/25 (24.0) 1/25 (4.0) | Taking PGx analgesic
| 13/24 (54.2) 12/13 (92.3) 9/13 (69.2) 3/13 (23.1) 1/13 (7.7) |
Taking psychotropic
| 13/29 (44.8) 11/13 (84.6) 4/11 (36.4) 4/11 (36.4) 2/11 (18.2) 1/11 (9.1) 2/13 (14.4) 1/2 (50.0) 1/2 (50.0) 1/13 (7.7) | Taking psychotropic
| 12/24 (50.0) 9/24 (37.5) 4/9 (44.4) 4/9 (44.4) 1/9 (11.1) 1/24 (4.2) 1/24 (4.2) 1/24 (4.2) 4/24 (16.7) 2/24 (8.3) 1/2 (50.0) 1/2 (50.0) 2/24 (8.3) 1/24 (4.2) |
| Study Group | PGx Report Uploaded to Medical Chart Prior to POC Visit | Had PGx Consultation Prior to POC Visit |
|---|---|---|
Cohort
| 38/46 (82.6%) | 33/46 (71.7%) |
| 23/31 (74.2%) | 18/31 (58.1%) | |
| 15/15 (100%) | 15/15 (100%) |
| Immediately After PGx Testing Consultation: | Agreed n (%) | |
|---|---|---|
| Patients (N = 38) * | Caregivers (N = 40) * | |
| I felt safe and supported throughout the PGx testing process. | 38 (100) | 40 (100) |
| The results from my/my child’s test were explained to me in an understandable way. | 36 (94.7) | 39 (97.5) |
| Being part of the PGx study helped me understand how I/my child would respond to the medications listed. | 35 (92.1) | 39 (97.5) |
| Being part of the PGx study reassured me that my/my child’s healthcare team is trying to pick the correct medication therapy for me. | 36 (94.7) | 38 (95) |
| PGx testing helped me understand myself/my child better. | 35 (92.1) | 38 (95) |
| PGx testing was fun for me to learn about. | 36 (94.7) | 40 (100) |
| PGx testing will help guide my/my child’s treatment decisions. | 35 (92.1) | 40 (100) |
| PGx testing helps my/my child’s physician make better decisions. | 35 (92.1) | 40 (100) |
| PGx testing is worth the waiting time. | 36 (94.7) | 39 (97.5) |
| PGx testing was not an invasion of my/my child’s privacy. | 38 (100) | 39 (97.5) |
| PGx testing reduced uncertainty about my/my child’s response to medications. | 34 (89.5) | 34 (85) |
| PGx testing increased my confidence in the effect of the medication for pain control. | 31 (81.6) | 38 (95) |
| At 3-month follow-up | Patients (N = 33) * | Caregivers (N = 33) * |
| Did having PGx testing increase your confidence in taking medication? Median, IQR † | 7, 3 | 8, 5 |
| Did having PGx testing improve your willingness to take medication more regularly (i.e., at the dosing schedule prescribed by your/your child’s physician)? Median, IQR † | 5, 6 | 7, 6 |
| Participant | Medication | Side Effects | Symptom Change * | PGx Metabolizer Status | PGx Response/ADR Prediction |
|---|---|---|---|---|---|
| A | Sertraline | No | Better | CYP2C19 NM | Normal metabolism |
| Ondansetron | No | Moderately better | CYP2D6 NM | Normal metabolism | |
| B | Codeine | No | No change | CYP2D6 PM | Reduced conversion to morphine leading to reduced clinical effect (analgesia) |
| C | Pantoprazole | No | No change | CYP2C19 RM | Increased metabolism (decreased drug plasma levels) leading to reduced drug response and/or therapeutic failure |
| D | Lansoprazole | No | Little better | CYP2C19 IM | Decreased metabolism (increased drug plasma levels) leading to increased chance of efficacy |
| E | Codeine | Yes—drowsy | Moderately better | CYP2D6 UM | Increased metabolism to morphine leading to higher risk of side effects |
| F | Lansoprazole | No | Moderately better | CYP2C19 IM | Decreased metabolism (increased drug plasma levels) leading to increased chance of efficacy |
| G | Citalopram | No | Almost the same | CYP2C19 RM | Increased metabolism leading to decreased clinical benefit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruskin, D.; Szczech, K.; Scodellaro, S.; Sun, N.; Cohn, I. Moving Away from One-Size-Fits-All: Assessing the Use of Pharmacogenetic-Guided Medication Therapy in Pediatric Patients with Chronic Pain. Children 2025, 12, 721. https://doi.org/10.3390/children12060721
Ruskin D, Szczech K, Scodellaro S, Sun N, Cohn I. Moving Away from One-Size-Fits-All: Assessing the Use of Pharmacogenetic-Guided Medication Therapy in Pediatric Patients with Chronic Pain. Children. 2025; 12(6):721. https://doi.org/10.3390/children12060721
Chicago/Turabian StyleRuskin, Danielle, Klaudia Szczech, Sierra Scodellaro, Naiyi Sun, and Iris Cohn. 2025. "Moving Away from One-Size-Fits-All: Assessing the Use of Pharmacogenetic-Guided Medication Therapy in Pediatric Patients with Chronic Pain" Children 12, no. 6: 721. https://doi.org/10.3390/children12060721
APA StyleRuskin, D., Szczech, K., Scodellaro, S., Sun, N., & Cohn, I. (2025). Moving Away from One-Size-Fits-All: Assessing the Use of Pharmacogenetic-Guided Medication Therapy in Pediatric Patients with Chronic Pain. Children, 12(6), 721. https://doi.org/10.3390/children12060721






