Lifestyle and SSRI Interventions in Pediatric Cyclic Vomiting Syndrome: Rethinking First-Line Management
Abstract
1. Introduction
- Unhealthy dietary habits, including
- ○
- Frequent consumption of junk food (defined as more than twice per week),
- ○
- Skipping meals,
- ○
- Meals inconsistent with a healthy Mediterranean-style diet,
- ○
- Inadequate hydration
- Poor sleep hygiene, defined as failure to achieve a minimum of 8 h of regular nighttime sleep
- Excessive screen time, defined as more than 2 h per day outside of school hours
- Presence of anxiety symptoms, as reported by the patient, family, or school staff
2. Materials and Methods
- -
- Laboratory tests: Kidney function tests, liver transaminases, C-reactive protein, erythrocyte sedimentation rate, serum amyloid A, fibrinogen, celiac screening (tissue transglutaminase and/or endomysial antibodies), food allergy tests (when clinically indicated), urinary organic acid analysis, and metabolic screening with tandem mass spectrometry.
- -
- Radiological tests: Abdominal ultrasonography and abdominal X-ray performed both during and between attacks.
- -
- Upper gastrointestinal endoscopy: Performed with biopsies, which revealed no abnormalities.
2.1. Treatment Protocol
- Acute Attack Management [6]:
- Lifestyle Modifications:
- ○
- Maintaining regular meal patterns with three main meals consistent with a healthy Mediterranean-style diet, supplemented by 1–2 fruit-based snacks per day,
- ○
- Ensuring adequate hydration appropriate for age and body weight,
- ○
- Achieving at least 8 h of nighttime sleep in a dark, quiet environment (sleep hygiene),
- ○
- Limiting screen time to fewer than 2 h per day (outside of school hours),
- ○
- Adhering to psychiatric treatment and follow-up recommendations.
- Prophylaxis with Cyproheptadine [6]:
- Treatment Based on Psychiatric Indication [8]:
- ○
- Sertraline, initiated at 12.5–25 mg/day in the first week and increased to 50 mg/day thereafter,
- ○
- Fluoxetine, initiated at 5–10 mg/day, titrated up to a maximum of 20 mg/day.
2.2. 12-Week Follow-Up and Lifestyle Adherence Assessment
- No skipped meals; three regular and nutritious main meals consistent with the Mediterranean diet, plus 1–2 fruit-based snacks daily,
- Adequate hydration tailored to the child’s age and weight,
- At least 8 h of nighttime sleep in a dark environment (good sleep hygiene),
- Screen time limited to ≤2 h per day (outside school hours),
- Full compliance with psychiatric department recommendations for therapy or follow-up.
2.3. Definition of Treatment Outcomes
- Complete cessation of vomiting attacks,
- No attacks requiring hospitalization,
- No school or work absenteeism due to attacks.
- Attacks requiring hospitalization,
- No reduction or an increase in attack frequency or intensity,
- Attacks resulting in school absenteeism for the child or work absenteeism for the caregiver,
- Any single attack lasting longer than 24 h.
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAM | Acute Attack Management |
| BMI | Body mass index |
| CVS | Cyclic vomiting syndrome |
| EEG | Electroencephalogram |
| MELAS | Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes |
| NSAID | Non-steroidal anti-inflammatory drug |
| Rome IV | Rome IV Diagnostic Criteria for Functional Gastrointestinal Disorders |
| SDS | Standard deviation score |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
References
- Karrento, K.; Wu, M.; Rodriguez, D.; Coyne, K.S.; Tahir, M.J.; Richmond, C.A.; Chen, Y.J.; Williams, J.; Venkatesan, T. Understanding the Adult and Adolescent Patient Experience with Cyclic Vomiting Syndrome: A Concept Elicitation Study. BMC Gastroenterol. 2025, 25, 85. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.S.; Di Lorenzo, C.; Saps, M.; Shulman, R.J.; Staiano, A.; van Tilburg, M. Functional Disorders: Children and Adolescents. Gastroenterology 2016, 150, 1456–1468.e2. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Liu, S.; Tao, F. Current Trends in Pediatric Migraine: Clinical Insights and Therapeutic Strategies. Brain Sci. 2025, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, V.; Bozza, F.; Beatrice, A.; Cameli, N.; Cinnante, E.M.C.; Lentini, G.; Faedda, N.; Natalucci, G.; Guidetti, V. Non-Pharmacological Treatments in Paediatric Migraine. J. Clin. Med. 2024, 13, 1278. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.A.; Douglas, I.; VanTieghem, M.; Tottenham, N.; Callaghan, B. Using Gastrointestinal Distress Reports to Predict Youth Anxiety Risk: Implications for Mental Health Literacy and Community Care. Dev. Psychobiol. 2021, 63, e22126. [Google Scholar] [CrossRef] [PubMed]
- Karrento, K.; Rosen, J.M.; Tarbell, S.E.; Issenman, R.M.; Gelfand, A.A.; Gamboa, H.; Parikh, S.; Adams, K.; Wiercioch, W.; Li, B.U.K. North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition 2025 Guidelines for Management of Cyclic Vomiting Syndrome in Children. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 1028–1061. [Google Scholar] [CrossRef] [PubMed]
- Li, B.U.K. Managing Cyclic Vomiting Syndrome in Children: Beyond the Guidelines. Eur. J. Pediatr. 2018, 177, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.S.; Dobson, E.T.; Strawn, J.R. Pharmacologic Treatment of Pediatric Anxiety Disorders. Curr. Treat. Options Psychiatry 2016, 3, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Hasler, W.L.; Li, B.U.K.; Levinthal, D.J.; Venkatesan, T. Cyclic Vomiting Syndrome: Future Clinical and Research Priorities For: Special Supplement/Proceedings of 3rd International Symposium. Neurogastroenterol. Motil. 2025, 37, e14825. [Google Scholar] [CrossRef] [PubMed]
- Gosalvez-Tejada, A.; Li, B.U.K.; Simpson, P.; Zhang, L.; Kovacic, K. Natural History of Pediatric Cyclic Vomiting Syndrome: Progression to Dysautonomia. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Princic, N.; Winer, I.; Richmond, C.; Williams, J.; Thavamani, A.; Levinthal, D.J.; Venkatesan, T. Epidemiology, Comorbidities, and Treatment of Cyclic Vomiting Syndrome in the United States. Am. J. Gastroenterol. 2024, 119, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castillo, R.A.; Frazier, R.; Venkatesan, T.; Remes-Troche, J.M. Cyclic Vomiting Syndrome: From Pathophysiology to Treatment. Rev. Gastroenterol. Mex. 2024, 89, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Raucci, U.; Borrelli, O.; Di Nardo, G.; Tambucci, R.; Pavone, P.; Salvatore, S.; Baldassarre, M.E.; Cordelli, D.M.; Falsaperla, R.; Felici, E.; et al. Cyclic Vomiting Syndrome in Children. Front. Neurol. 2020, 11, 583425. [Google Scholar] [CrossRef] [PubMed]
- Falsaperla, R.; Scalia, B.; Collotta, A.D.; Giacchi, V.; Cimino, C.; Ruggieri, M. Treatment Options for Cyclic Vomiting Syndrome: A Real-World, Single-Center Experience with Systematic Literature Review and Meta-Analysis. J. Clin. Pharmacol. 2024, 64, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, A.; Velayuthan, S.; Patel, D.; Al-Hammadi, N.; Sferra, T.J.; Sankararaman, S. Association of Anxiety and Gastrointestinal Comorbidities in Repeat Hospital Admissions in Pediatric Cyclic Vomiting Syndrome. Am. J. Gastroenterol. 2023, 118, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Zar-Kessler, C.A.M.; Belkind-Gerson, J.; Bender, S.; Kuo, B.M. Treatment of Functional Abdominal Pain with Antidepressants: Benefits, Adverse Effects, and the Gastroenterologist’s Role. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Baumel, W.T.; Mills, J.A.; Schroeder, H.K.; Neptune, Z.; Levine, A.; Strawn, J.R. Gastrointestinal Symptoms in Pediatric Patients with Anxiety Disorders and Their Relationship to Selective Serotonin Reuptake Inhibitor Treatment or Placebo. Child Psychiatry Hum. Dev. 2025, 56, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Mills, J.A.; Poweleit, E.A.; Ramsey, L.B.; Croarkin, P.E. Adverse Effects of Antidepressant Medications and Their Management in Children and Adolescents. Pharmacotherapy 2023, 43, 675–690. [Google Scholar] [CrossRef] [PubMed]

| 1- Two or more periods of intense, unremitting nausea and paroxysmal vomiting lasting for hours to days within a 6-month period. |
| 2- Stereotypical pattern of episodes in each patient. |
| 3- Episodes separated by weeks to months, with a return to baseline health between attacks. |
| 4- Symptoms that cannot be attributed to another medical condition following appropriate evaluation. |
| n = 119 | |
|---|---|
| Age (years) * | 8.7 ± 3.9 |
| Range of age (years) | 1.2–17.5 |
| Gender | |
| Female | 61 (51.3%) |
| Male | 58 (48.7%) |
| Number of attacks per 6 months ** | 5 (2–15) |
| Duration of attacks (days) ** | 1 (1–8) |
| Hospitalization | 62 (52.1%) |
| Potential triggers | |
| Defined anxiety | 93 (78.2%) |
| Screen time > 2 h | 89 (74.8%) |
| Junk food consumption | 58 (48.7%) |
| Irregular sleep | 50 (42.0%) |
| Family history of migraine | 26 (21.8%) |
| Treatment | |
| Cyproheptadine | 66 (55.5%) |
| Attack | 19 (16.0%) |
| SSRI | 34 (28.6%) |
| Lifestyle adherence | 88 (73.9%) |
| Improvement at the 3rd month | 107 (89.9%) |
| Treatment Failure (n = 12) | Treatment Success (n = 107) | p-Value | |
|---|---|---|---|
| Age (years) * | 8.1 ± 3.7 | 8.8 ± 4.0 | 0.542 A |
| Gender | >0.999 B | ||
| Female | 6 (50.0%) | 55 (51.4%) | |
| Male | 6 (50.0%) | 52 (48.6%) | |
| Number of attacks per 6 months ** | 6 (3–10) | 5 (2–15) | 0.379 C |
| Duration (days) ** | 1.5 (1–7) | 1 (1–8) | 0.182 C |
| Hospitalization | 8 (66.7%) | 54 (50.5%) | 0.447 B |
| Potential triggers | |||
| Defined anxiety | 9 (75.0%) | 84 (78.5%) | 0.723 D |
| Screen time > 2 h | 6 (50.0%) | 83 (77.6%) | 0.072 D |
| Junk food consumption | 4 (33.3%) | 54 (50.5%) | 0.411 B |
| Irregular sleep | 7 (58.3%) | 43 (40.2%) | 0.369 B |
| Family history of migraine | 3 (25.0%) | 23 (21.5%) | 0.723 D |
| Treatment | 0.024 E | ||
| Cyproheptadine | 9 (75.0%) | 57 (53.3%) | |
| Attack | 3 (25.0%) | 16 (15.0%) | |
| SSRI | 0 (0.0%) a | 34 (31.8%) a | |
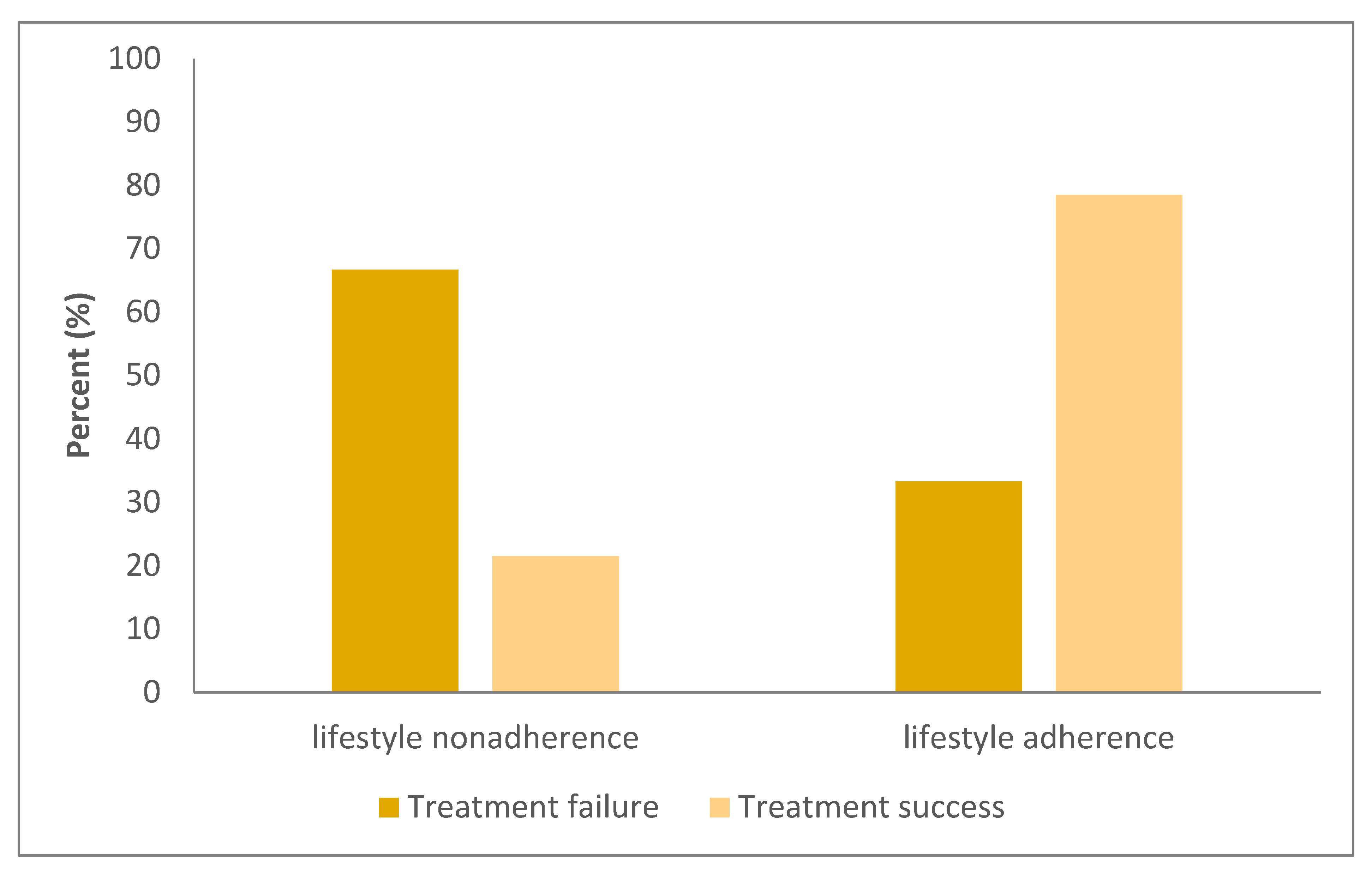
| Lifestyle adherence | 4 (33.3%) | 84 (78.5%) | 0.002 D |
| Cyproheptadine (n = 66) | Attack (n = 19) | SSRI (n = 34) | p-Value | |
|---|---|---|---|---|
| Age (years) * | 6.7 ± 3.0 a | 8.5 ± 3.1 b | 12.8 ± 2.5 a,b | <0.001 A |
| Gender | 0.226 B | |||
| Female | 33 (50.0%) | 13 (68.4%) | 15 (44.1%) | |
| Male | 33 (50.0%) | 6 (31.6%) | 19 (55.9%) | |
| Number of attacks per 6 months ** | 6 (2–15) c | 3 (2–8) b,c | 8 (2–12) b | 0.007 C |
| Duration of attacks (days) ** | 1 (1–7) a | 1 (1–3) b | 2 (1–8) a,b | 0.002 C |
| Hospitalization | 29 (43.9%) a | 7 (36.8%) b | 26 (76.5%) a,b | 0.003 B |
| Potential triggers | ||||
| Defined anxiety | 50 (75.8%) a | 10 (52.6%) b | 34 (100%) a,b | <0.001 B |
| Screen time > 2 h | 42 (63.6%) a | 14 (73.7%) b | 33 (97.1%) a,b | <0.001 B |
| Junk food consumption | 28 (42.4%) c | 15 (78.9%) b,c | 15 (44.1%) b | 0.016 B |
| Irregular sleep | 39 (59.1%) a | 8 (42.1%) b | 3 (8.8%) a,b | <0.001 B |
| Lifestyle adherence | 53 (80.3%) a | 16 (84.2%) | 19 (55.9%) a | 0.017 B |
| Treatment success | 57 (86.4%) a | 16 (84.2%) b | 34 (100.0%) a,b | 0.024 D |
| Cyproheptadine | Attack | SSRI | p-Value A | |
|---|---|---|---|---|
| Lifestyle nonadherence group | <0.001 | |||
| Treatment failure | 5 (38.5%) a | 3 (100.0%) b | 0 (0.0%) a,b | |
| Treatment success | 8 (61.5%) a | 0 (0.0%) b | 15 (100.0%) a,b | |
| Lifestyle adherence group | 0.469 | |||
| Treatment failure | 4 (7.5%) | 0 (0.0%) | 0 (0.0%) | |
| Treatment success | 49 (92.5%) | 16 (100.0%) | 19 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuntaş, C.; Sevinçok, D.; Hilal Dolu, M.; Gültekin, E. Lifestyle and SSRI Interventions in Pediatric Cyclic Vomiting Syndrome: Rethinking First-Line Management. Children 2025, 12, 964. https://doi.org/10.3390/children12080964
Altuntaş C, Sevinçok D, Hilal Dolu M, Gültekin E. Lifestyle and SSRI Interventions in Pediatric Cyclic Vomiting Syndrome: Rethinking First-Line Management. Children. 2025; 12(8):964. https://doi.org/10.3390/children12080964
Chicago/Turabian StyleAltuntaş, Cansu, Doğa Sevinçok, Merve Hilal Dolu, and Ece Gültekin. 2025. "Lifestyle and SSRI Interventions in Pediatric Cyclic Vomiting Syndrome: Rethinking First-Line Management" Children 12, no. 8: 964. https://doi.org/10.3390/children12080964
APA StyleAltuntaş, C., Sevinçok, D., Hilal Dolu, M., & Gültekin, E. (2025). Lifestyle and SSRI Interventions in Pediatric Cyclic Vomiting Syndrome: Rethinking First-Line Management. Children, 12(8), 964. https://doi.org/10.3390/children12080964







