A Case of Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma Successfully Treated with Immunosuppressive Therapy
Abstract
Highlights
- A 14-year-old female with SPTCL (without HLH) was successfully treated using immunosuppressive therapy alone (methylprednisolone and cyclosporine A), leading to rapid symptom resolution, the normalization of laboratory markers (e.g., ferritin, CRP, ESR), and sustained remission without relapse for over 1 year after tapering and discontinuation.
- A heterozygous C9 gene mutation (c.346C>T, p.Arg116Ter) was identified via next-generation sequencing in a pediatric SPTCL patient, suggesting a potential link between partial complement system dysfunction (impaired MAC formation) and immune dysregulation contributing to SPTCL pathogenesis.
- This case supports considering immunosuppressive therapy as an initial treatment option for pediatric SPTCL without HLH, highlighting its efficacy in achieving long-term remission and adding to the evidence that genetic factors like C9 mutations may play a role in disease presentation resembling autoimmune conditions.
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, Y.; Gao, H.; Zhou, C.; Jin, L.; Yang, J.; Huang, S.; Zhang, M.; Zhang, Y.; Wang, T. A retrospective study of 18 children with subcutaneous panniculitis-like T-cell lymphoma: Multidrug combination chemotherapy or immunomodulatory therapy? Orphanet J. Rare Dis. 2022, 17, 432. [Google Scholar] [CrossRef]
- Bradford, P.T.; Devesa, S.S.; Anderson, W.F.; Toro, J.R. Cutaneous lymphoma incidence patterns in the United States: A population-based study of 3884 cases. Blood 2009, 113, 5064–5073. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.L.; Medeiros, L.J.; Braziel, R.M.; Jaffe, E.S. T-cell lymphoma involving subcutaneous tissue. A clinicopathologic entity commonly associated with hemophagocytic syndrome. Am. J. Surg. Pathol. 1991, 15, 17–27. [Google Scholar] [CrossRef]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef]
- Ou, W.; Zhao, Y.; Wei, A.; Ma, H.; Zhang, L.; Lian, H.; Zhang, Q.; Wang, D.; Li, Z.; Wang, T.; et al. Subcutaneous panniculitis-like T-cell lymphoma associated with hemophagocytic lymphohistiocytosis: A systematic review of 63 patients reported in the literature. Clin. Exp. Med. 2023, 23, 4575–4583. [Google Scholar] [CrossRef]
- Singh, S.; Philip, C.C.; John, M.J. Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma with Hemophagocytosis Showing Complete Resolution with the BFM90 Protocol: Case Report and Review of Literature. J. Pediatr. Hematol. Oncol. 2019, 41, 478–481. [Google Scholar] [CrossRef]
- Merritt, B.Y.; Curry, J.L.; Duvic, M.; Vega, F.; Sheehan, A.M.; Curry, C.V. Pediatric subcutaneous panniculitis-like T-cell lymphoma with features of hemophagocytic syndrome. Pediatr. Blood Cancer 2013, 60, 1916–1917. [Google Scholar] [CrossRef]
- Tirachotikul, T.; Rattanathammethee, T.; Makruasi, N.; Chintabanyat, A.; Julamanee, J.; Khuhapinant, A.; Chuncharunee, S.; Kanitsap, N.; Wongkhantee, S.; Wong, P.; et al. Treatment outcomes between cyclosporin and chemotherapy in adult subcutaneous panniculitis-like T-cell lymphoma: A report from nation-wide Thai lymphoma study group registry. Ann. Hematol. 2024, 103, 5741–5748. [Google Scholar] [CrossRef]
- Huppmann, A.R.; Xi, L.; Raffeld, M.; Pittaluga, S.; Jaffe, E.S. Subcutaneous panniculitis-like T-cell lymphoma in the pediatric age group: A lymphoma of low malignant potential. Pediatr. Blood Cancer 2013, 60, 1165–1170. [Google Scholar] [CrossRef]
- Johnston, E.E.; LeBlanc, R.E.; Kim, J.; Chung, J.; Balagtas, J.; Kim, Y.H.; Link, M.P. Subcutaneous panniculitis-like T-cell lymphoma: Pediatric case series demonstrating heterogeneous presentation and option for watchful waiting. Pediatr. Blood Cancer 2015, 62, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.L.; Sang, H.; Deng, L.; Li, Z. Subcutaneous Panniculitis-Like T-Cell Lymphoma in Children: A Review of the Literature. Pediatr. Dermatol. 2015, 32, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Nakano, N.; Iwai, T.; Iwai, A.; Tauchi, H.; Ohshima, K.; Ishii, E. Pediatric subcutaneous panniculitis-like T-cell lymphoma with favorable result by immunosuppressive therapy: A report of two cases. Pediatr. Hematol. Oncol. 2014, 31, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Frederiks, A.J.; Spagnolo, D.V.; Ramachandran, S.; Brand, R. Subcutaneous panniculitis-like T-cell lymphoma in a 14-year-old female homozygous for HAVCR2 mutation. Australas. J. Dermatol. 2021, 62, e576–e579. [Google Scholar] [CrossRef]
- Sonigo, G.; Battistella, M.; Beylot-Barry, M.; Ingen-Housz-Oro, S.; Franck, N.; Barete, S.; Boulinguez, S.; Dereure, O.; Bonnet, N.; Socié, G.; et al. HAVCR2 mutations are associated with severe hemophagocytic syndrome in subcutaneous panniculitis-like T-cell lymphoma. Blood 2020, 135, 1058–1061. [Google Scholar] [CrossRef]
- Willemze, R.; Jansen, P.M.; Cerroni, L.; Berti, E.; Santucci, M.; Assaf, C.; Canninga-van Dijk, M.R.; Carlotti, A.; Geerts, M.L.; Hahtola, S.; et al. Subcutaneous panniculitis-like T-cell lymphoma: Definition, classification, and prognostic factors: An EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood 2008, 111, 838–845. [Google Scholar] [CrossRef]
- Miranda, R.N.; Amador, C.; Chan, J.K.C.; Guitart, J.; Rech, K.L.; Medeiros, L.J.; Naresh, K.N.; WHO Fifth Edition Classification Project. Fifth Edition of the World Health Organization Classification of Tumors of the Hematopoietic and Lymphoid Tissues: Mature T-Cell, NK-Cell, and Stroma-Derived Neoplasms of Lymphoid Tissues. Mod. Pathol. 2024, 37, 100512. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, F.; Zhou, L.; Liu, L. Pediatric subcutaneous panniculitis-like T-cell lymphoma: A case report and review of the literature. Pediatr. Radiol. 2025. advance online publication. [Google Scholar] [CrossRef]
- Lee, W.S.; Hwang, J.H.; Kim, M.J.; Go, S.I.; Lee, A.; Song, H.N.; Lee, M.J.; Kang, M.H.; Kim, H.G.; Lee, J.H. Cyclosporine A as a Primary Treatment for Panniculitis-like T Cell Lymphoma: A Case with a Long-Term Remission. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2014, 46, 312–316. [Google Scholar] [CrossRef][Green Version]
- Michonneau, D.; Petrella, T.; Ortonne, N.; Ingen-Housz-Oro, S.; Franck, N.; Barete, S.; Battistella, M.; Beylot-Barry, M.; Vergier, B.; Maynadié, M.; et al. Subcutaneous Panniculitis-like T-cell Lymphoma: Immunosuppressive Drugs Induce Better Response than Polychemotherapy. Acta Derm. Venereol. 2017, 97, 358–364. [Google Scholar] [CrossRef]
- Lin, E.C.; Liao, J.B.; Fang, Y.H.; Hong, C.H. The pathophysiology and current treatments for the subcutaneous panniculitis-like T cell lymphoma: An updated review. Asia Pac. J. Clin. Oncol. 2023, 19, 27–34. [Google Scholar] [CrossRef]
- Khajoee, V.; Ihara, K.; Kira, R.; Takemoto, M.; Torisu, H.; Sakai, Y.; Guanjun, J.; Hee, P.M.; Tokunaga, K.; Hara, T. Founder effect of the C9 R95X mutation in Orientals. Hum. Genet. 2003, 112, 244–248. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, H.S.; Lee, Y.K.; Cho, H.C. High Incidence of Complement C9 Deficiency in Koreans. Ann. Clin. Lab. Sci. 2005, 35, 144–148. [Google Scholar]
- Zoppi, M.; Weiss, M.; Nydegger, U.E.; Hess, T.; Späth, P.J. Recurrent meningitis in a patient with congenital deficiency of the C9 component of complement. First case of C9 deficiency in Europe. Arch. Intern. Med. 1990, 150, 2395–2399. [Google Scholar] [CrossRef]
- Horiuchi, T.; Tsukamoto, H.; Sawabe, T.; Harashima, S.; Morita, C.; Kashiwagi, Y.; Himeji, D.; Masumoto, K.; Otsuka, T.; Kusaba, T.; et al. Behçet’s disease associated with complement component 9 (C9) deficiency. Mod. Rheumatol. 2000, 10, 276–278. [Google Scholar] [CrossRef]
- Sugimoto, M.; Nishikai, M.; Sato, A.; Suzuki, Y.; Nihei, M.; Uchida, J.; Mimura, N. SLE-like and sicca symptoms in late component (C9) complement deficiency. Ann. Rheum. Dis. 1987, 46, 153–155. [Google Scholar] [CrossRef]
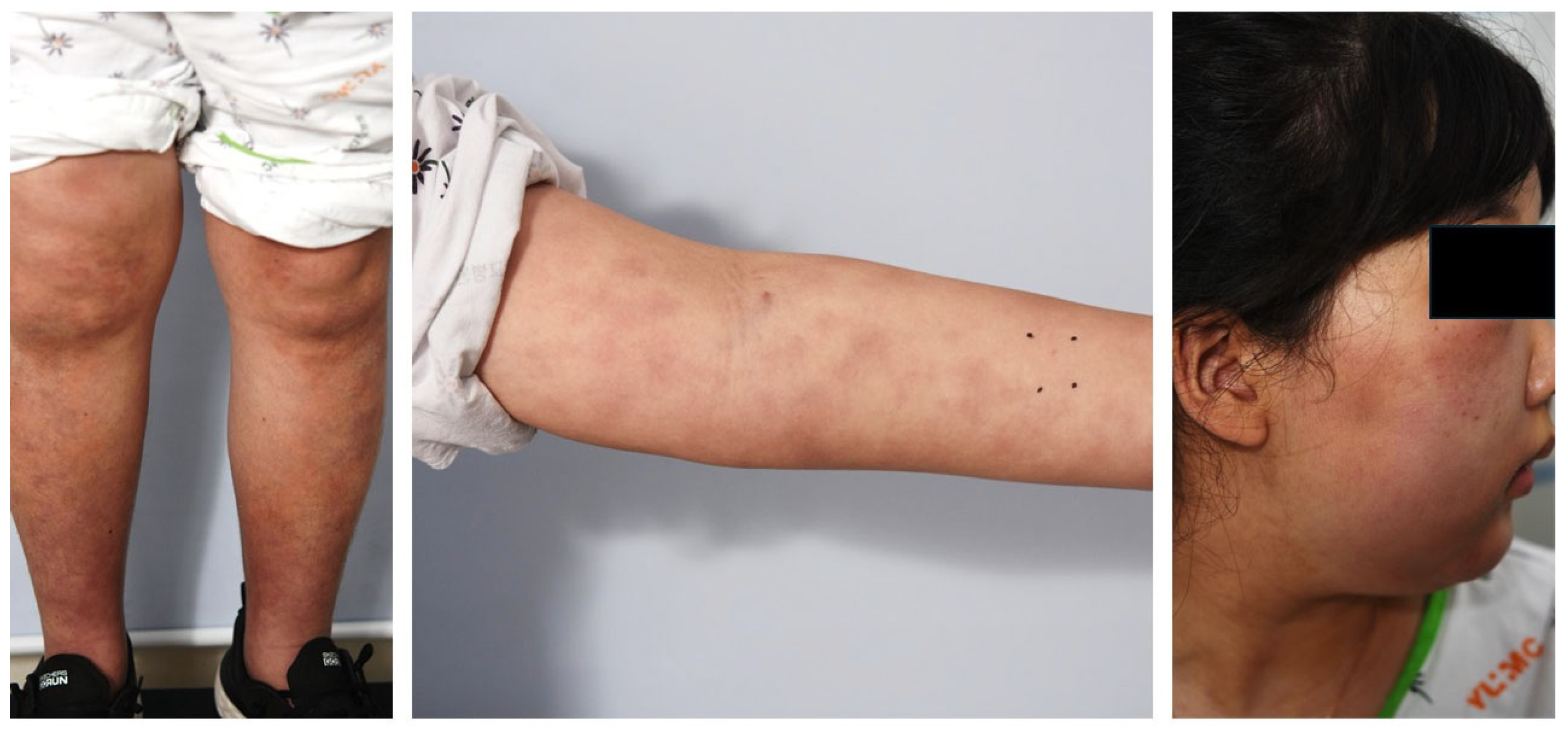

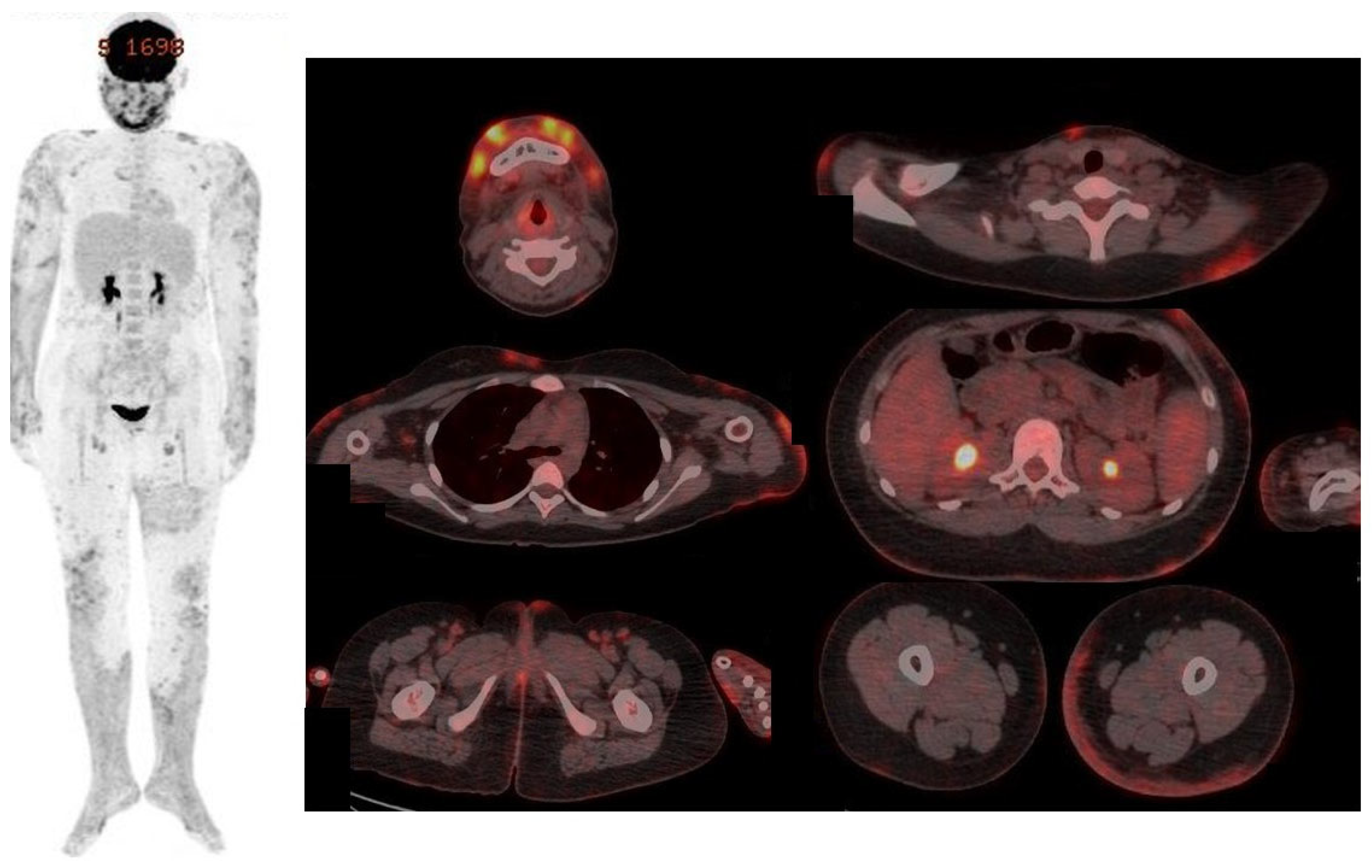
| Days After Admission | 1 | 7 | 13 | 22 | 36 | 50 |
|---|---|---|---|---|---|---|
| ESR (mm/H) | 27 | 8 | 4 | 2 | 2 | 4 |
| Ferritin (ng/mL) | 3612.31 | 2249.2 | 856.95 | 307.2 | 101.38 | 45.09 |
| WBC (K/μL) | 4.58 | 2.68 | 10.95 | 16.1 | 12.06 | 10.41 |
| LDH (IU/L) | 636 | 687 | 349 | 245 | 208 | 204 |
| CRP (mg/dL) | 2.69 | 6.2 | 0.12 | 1.06 | 0.03 | 0.04 |
| D-dimer (μg/mL FEU) | 4.16 | 1.74 | 1.25 | 1.06 | 0.36 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.C.; Shin, D.H.; Lee, J.M. A Case of Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma Successfully Treated with Immunosuppressive Therapy. Children 2025, 12, 1029. https://doi.org/10.3390/children12081029
Kim MC, Shin DH, Lee JM. A Case of Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma Successfully Treated with Immunosuppressive Therapy. Children. 2025; 12(8):1029. https://doi.org/10.3390/children12081029
Chicago/Turabian StyleKim, Min Chong, Dong Hoon Shin, and Jae Min Lee. 2025. "A Case of Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma Successfully Treated with Immunosuppressive Therapy" Children 12, no. 8: 1029. https://doi.org/10.3390/children12081029
APA StyleKim, M. C., Shin, D. H., & Lee, J. M. (2025). A Case of Pediatric Subcutaneous Panniculitis-like T-Cell Lymphoma Successfully Treated with Immunosuppressive Therapy. Children, 12(8), 1029. https://doi.org/10.3390/children12081029






