Unique Bone Marrow Findings of FDG-PET/CT in Acute Leukemia in Children: Comparison to Inflammatory Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. FDG PET/CT Scanning
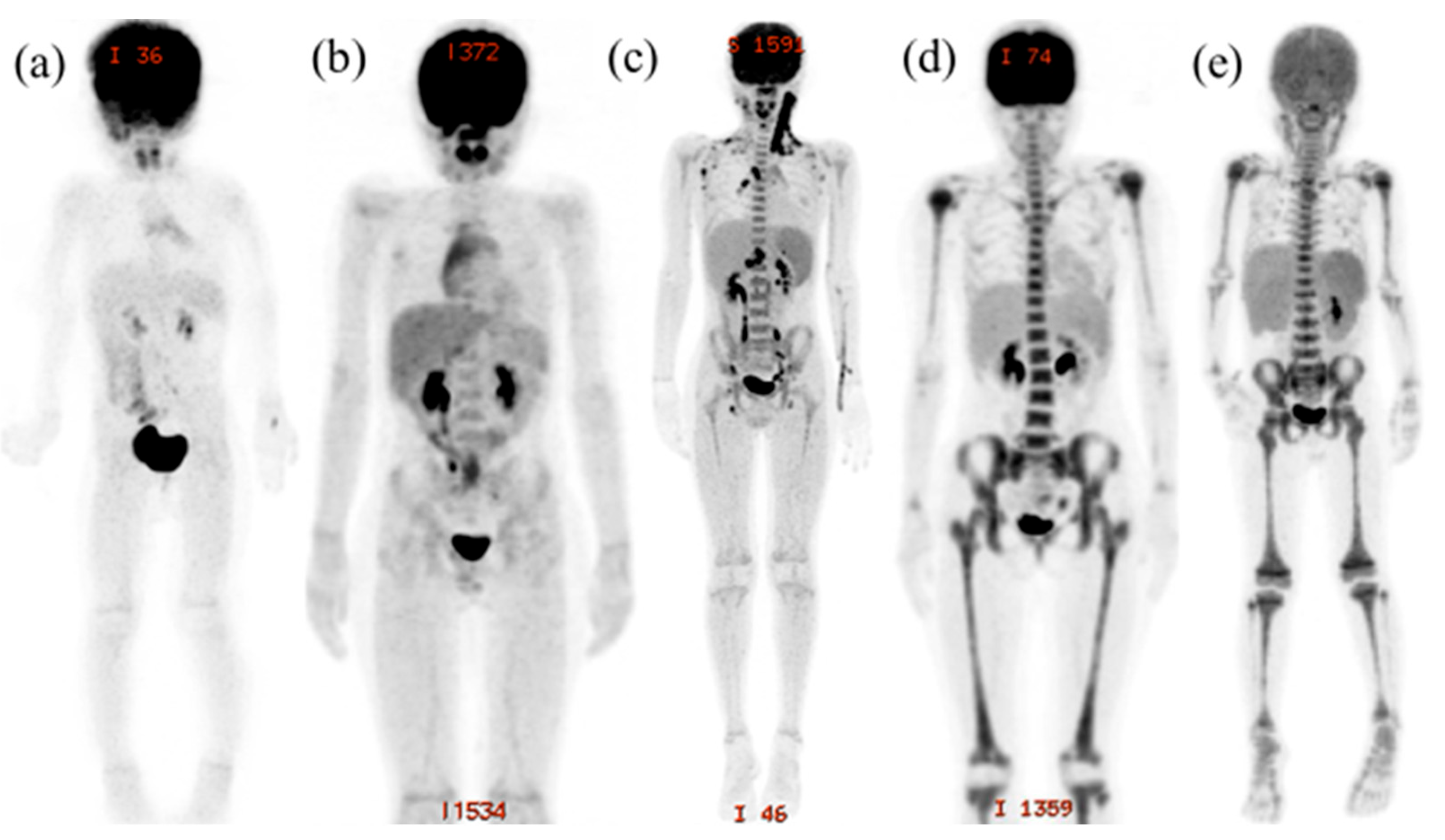
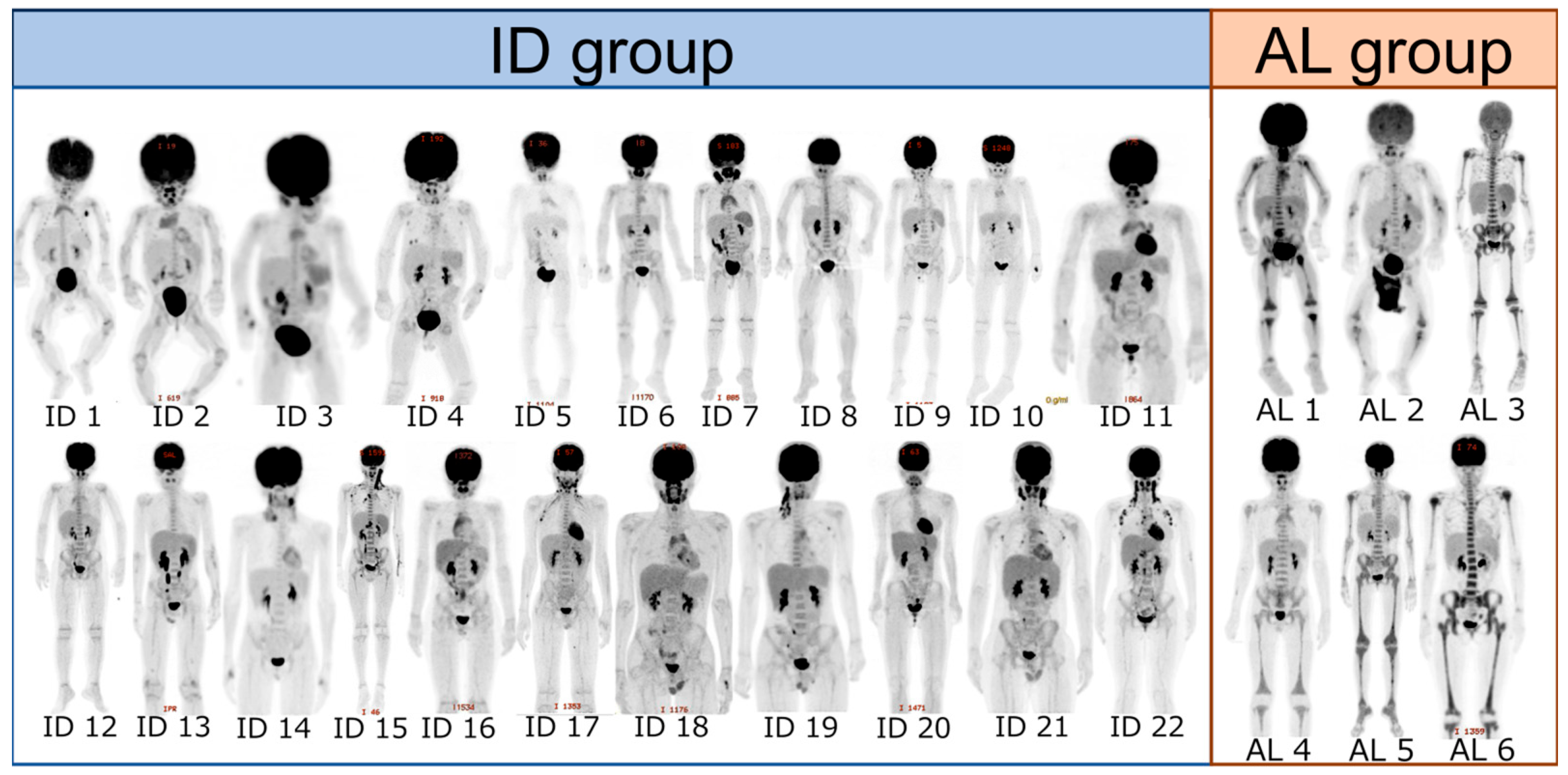
2.3. Evaluation of FDG-PET/CT Imaging
2.4. Statistics
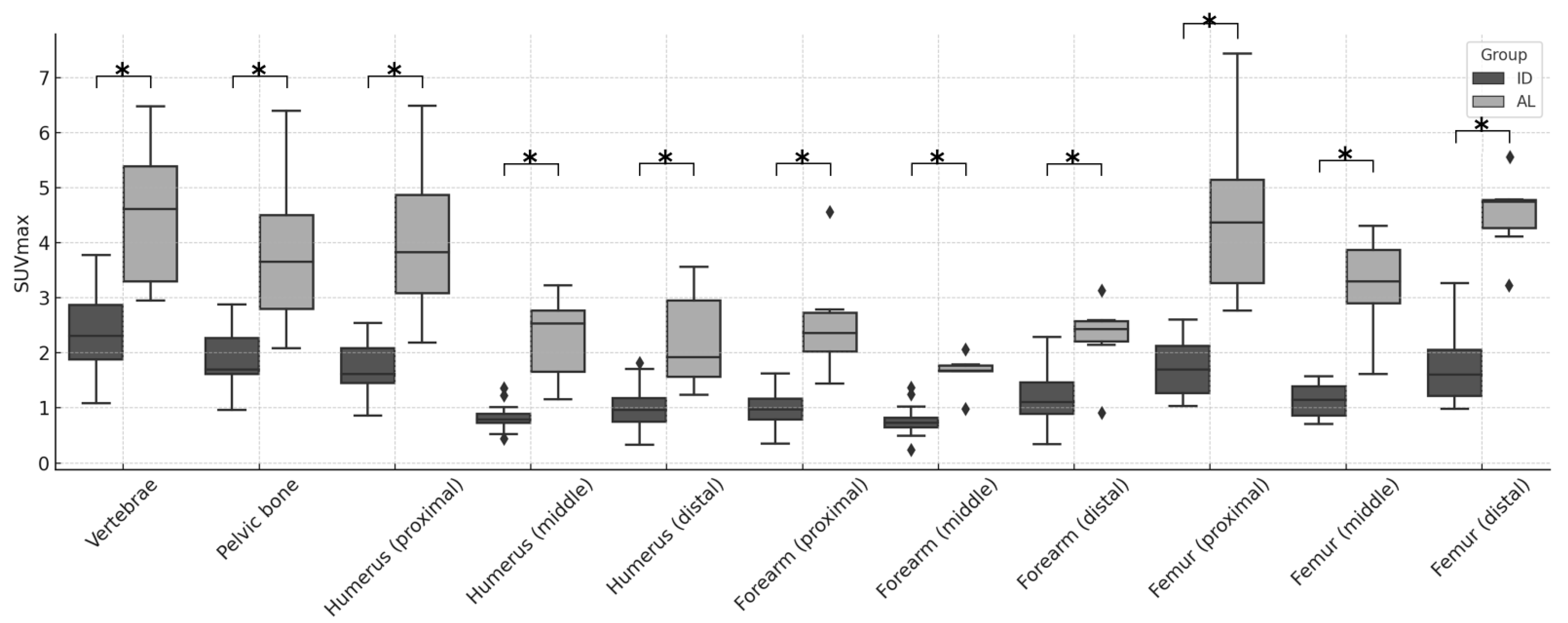
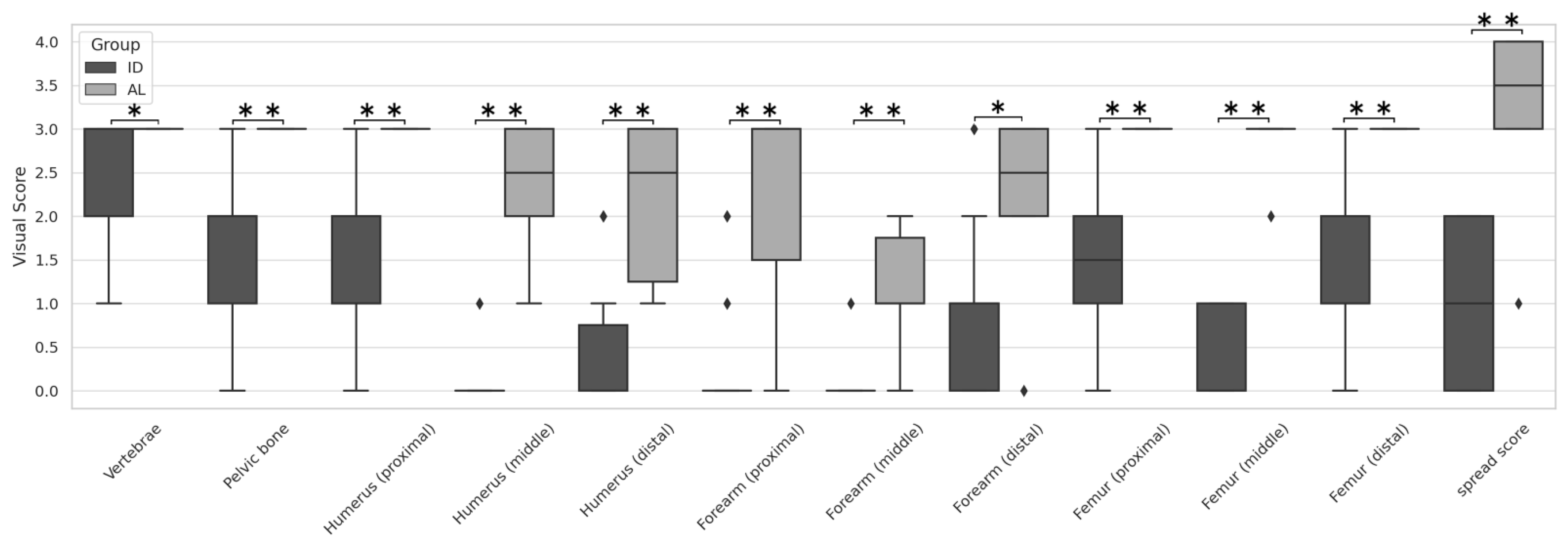
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDG-PET/CT | Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography |
| FUO | fever of unknown origin |
| AL | acute leukemia |
| ID | inflammatory disease |
| SUV max | maximum standardized uptake value |
| VS | visual score |
| SS | spread score |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| NCGM | National Center for Global Health and Medicine |
| MIP | maximum intensity projection |
| Hb | Hemoglobin |
| Plt | Platelet |
| WBC | White blood cell |
| CRP | C-reactive protein |
| sIL-2R | soluble interleukin-2 receptor |
| ESR | erythrocyte sedimentation rate |
References
- Ward, E.; DeSantis, C.; Robbins, A.; Kohler, B.; Jemal, A. Childhood and adolescent cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 83–103. [Google Scholar] [CrossRef]
- Horibe, K.; Saito, A.; Takimoto, T.; Tsuchida, M.; Manabe, A.; Shima, M.; Ohara, A.; Mizutani, S. Incidence and survival rates of hematological malignancies in Japanese children and adolescents (2006–2010): Based on registry data from the Japanese Society of Pediatric Hematology. Int. J. Hematol. 2013, 98, 74–88. [Google Scholar] [CrossRef]
- Matsui, M.; Yamanaka, J.; Shichino, H.; Sato, N.; Kubota, K.; Matsushita, T. FDG-PET/CT for detection of Extramedullary Disease in 2 Pediatric Patients With AML. J. Pediatr. Hematol. Oncol. 2016, 38, 398–401. [Google Scholar] [CrossRef]
- Su, K.; Nakamoto, Y.; Nakatani, K.; Kurihara, K.; Hayakawa, N.; Togashi, K. Diffuse homogeneous bone marrow uptake of FDG in patients with acute lymphoblastic leukemia. Clin. Nucl. Med. 2013, 38, e33–e34. [Google Scholar] [CrossRef]
- Arimoto, M.; Nakamoto, Y.; Nakatani, K.; Ishimori, T.; Yamashita, K.; Takaori-Kondo, A.; Togashi, K. Increased bone marrow uptake of 18F-FDG in leukemia patients: Preliminary findings. Springerplus 2015, 4, 521. [Google Scholar] [CrossRef]
- Kouijzer, I.J.E.; Mulders-Manders, C.M.; Bleeker-Rovers CPOyen, W.J.G. Fever of Unknown Origin: The Value of FDG-PET/CT. Semin. Nucl. Med. 2018, 48, 100–107. [Google Scholar] [CrossRef]
- Murata, Y.; Kubota, K.; Yukihiro, M.; Ito, M.; Watanabe, H.; Shibuya, H. Correlation between 18F-FDG uptake by bone marrow and hematological parameters; measurements by PET/CT. Nucl. Med. Biol. 2006, 33, 999–1004. [Google Scholar] [CrossRef]
- Cremers, J.P.; Van Kroonenburgh, M.J.; Mostard, R.L.; Vöö, S.A.; Wijnen, P.A.; Koek, G.H.; Drent, M. Extent of disease activity assessed by 18FFDG PET/CT in a Dutch sarcoidosis population. Sarcoidosis Vasc. Diffus. Lung Dis. 2014, 31, 37–45. [Google Scholar]
- Zhang, J.; Dong, M.J.; Liu, K.F.; Xu, L.M.; Zhao, K.; Yang, J.; Weng, W.W.; Qiu, H.; Lin, L.L.; Zhu, Y.J. (18)F-Fluorodeoxyglucose positron emission tomography/computed tomography in patients with Kikuchi-Fujimoto disease: A nine-case series in China. Int. J. Clin. Exp. Med. 2015, 8, 21034–21043. [Google Scholar] [PubMed]
- Koizumi, K.; Masaki, H.; Matsuda, H.; Uchiyama, M.; Okuno, M.; Oguma, E.; Onuma, H.; Kanegawa, K.; Kanaya, S.; Kamiyama, H.; et al. Japanese consensus guidelines for pediatric nuclear medicine. Part 1: Pediatric radiopharmaceutical administered doses (JSNM pediatric dosage card). Part 2: Technical considerations for pediatric nuclear medicine imaging procedures. Ann. Nucl. Med. 2014, 28, 498–503. [Google Scholar] [PubMed]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hyun, S.H.; Lee, K.S.; Kim, B.T.; Kim, J.; Shim, Y.M.; Ahn, M.J.; Kim, T.S.; Yi, C.A.; Chung, M.J. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: Prediction of therapeutic response and prognostic implications. Ann. Surg. Oncol. 2010, 17, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Muto, G.; Yamashita, H.; Takahashi, Y.; Miyata, Y.; Morooka, M.; Minamimoto, R.; Kubota, K.; Kaneko, H.; Kano, T.; Mimori, A. Large vessel vasculitis in elderly patients: Early diagnosis and steroid-response evaluation with FDG-PET/CT and contrast-enhanced CT. Rheumatol. Int. 2014, 34, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Fu, L.; Ren, Y.Y.; Wu, H.B.; Wang, Q.S.; Han, Y.J.; Zhou, W.L.; Li, H.S.; Wang, Z. 18F-FDG super bone marrow uptake: A highly potent indicator for the malignant infiltration. Medicine 2016, 95, e5579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Yang, F.; Wang, H.; Li, P. Assessment of diffuse bone marrow involvement on 18F-fluoro-D-glucose PET/computed tomography. Clin. Transl. Imaging 2024, 12, 423–429. [Google Scholar] [CrossRef]
- Subhas, N.; Patel, P.V.; Pannu, H.K.; Jacene, H.A.; Fishman, E.K.; Wahl, R.L. Imaging of Pelvic Malignancies with In-Line FDG PET-CT: Case Examples and Common Pitfalls of FDG PET. RadioGraphics 2005, 25, 1031–1043. [Google Scholar] [CrossRef]
- Tanaka, O.; Kurasawa, M.; Sugiura, M.; Aihara, T. MR Imaging of Bone Marrow Disorders in Children. J. Jpn. Soc. Pediatr. Radiol. 2000, 16, 112–121. [Google Scholar]
- Vogler, J.B.; Murphy, W.A. Bone marrow imaging. Radiology 1988, 168, 670–693. [Google Scholar] [CrossRef]
- Moore, S.G.; Dawson, K.L. Red and yellow marrow in femur; age-related changes in appearance at MR imaging. Radiology 1990, 175, 219–223. [Google Scholar] [CrossRef]
- Laor, T.; Jaramillo, D. MR imaging insights into skeletal maturation: What is normal? Radiology 2009, 250, 28–38. [Google Scholar] [CrossRef]
- Michigami, T. Regulatory mechanisms for the development of growth plate cartilage. Cell Mol. Life Sci. 2013, 70, 4213–4221. [Google Scholar] [CrossRef] [PubMed]


| ID (Range) | AL (Range) | p-Value | |
|---|---|---|---|
| Median age (years) | 6.5 (0.6–16) | 7 (2–17) | 0.74 |
| Sex (boys; girls) | 13; 8 | 4; 2 | 0.83 |
| WBC (/μL) | 6135 (1910–30,670) | 9870 (2550–19,000) | 0.11 |
| Hb (g/dL) | 12.8 (8.9–15.8) | 8.8 (3.5–13.1) | <0.01 |
| Plt (×104/μL) | 24.1 (2.8–62.2) | 10.4 (1.6–34.0) | 0.03 |
| CRP (mg/dL) * | 0.28 (0.03–29.3) | 1.15 (0.01–3.52) | 0.89 |
| Ferritin (ng/mL) | 208 (20.5–653) | 270 (111–565) | 0.64 |
| sIL-2R (U/mL) | 863 (707–1011) | 1855 (488–22,869) | 0.825 |
| ESR (mm/hr) | 31 (6–111) | 140 | 0.147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suenaga, Y.; Kubota, K.; Matsui, M.; Makimoto, A.; Yamanaka, J.; Mochizuki, S.; Hotta, M.; Chikanishi, M.M.; Shichino, H. Unique Bone Marrow Findings of FDG-PET/CT in Acute Leukemia in Children: Comparison to Inflammatory Diseases. Children 2025, 12, 1218. https://doi.org/10.3390/children12091218
Suenaga Y, Kubota K, Matsui M, Makimoto A, Yamanaka J, Mochizuki S, Hotta M, Chikanishi MM, Shichino H. Unique Bone Marrow Findings of FDG-PET/CT in Acute Leukemia in Children: Comparison to Inflammatory Diseases. Children. 2025; 12(9):1218. https://doi.org/10.3390/children12091218
Chicago/Turabian StyleSuenaga, Yuta, Kazuo Kubota, Motohiro Matsui, Atsushi Makimoto, Junko Yamanaka, Shinji Mochizuki, Masatoshi Hotta, Miyako Morooka Chikanishi, and Hiroyuki Shichino. 2025. "Unique Bone Marrow Findings of FDG-PET/CT in Acute Leukemia in Children: Comparison to Inflammatory Diseases" Children 12, no. 9: 1218. https://doi.org/10.3390/children12091218
APA StyleSuenaga, Y., Kubota, K., Matsui, M., Makimoto, A., Yamanaka, J., Mochizuki, S., Hotta, M., Chikanishi, M. M., & Shichino, H. (2025). Unique Bone Marrow Findings of FDG-PET/CT in Acute Leukemia in Children: Comparison to Inflammatory Diseases. Children, 12(9), 1218. https://doi.org/10.3390/children12091218






