Presumed Bartonella-Associated Spondylodiscitis in a 3-Year-Old Child: A Case Report and Review of the Literature
Abstract
1. Introduction
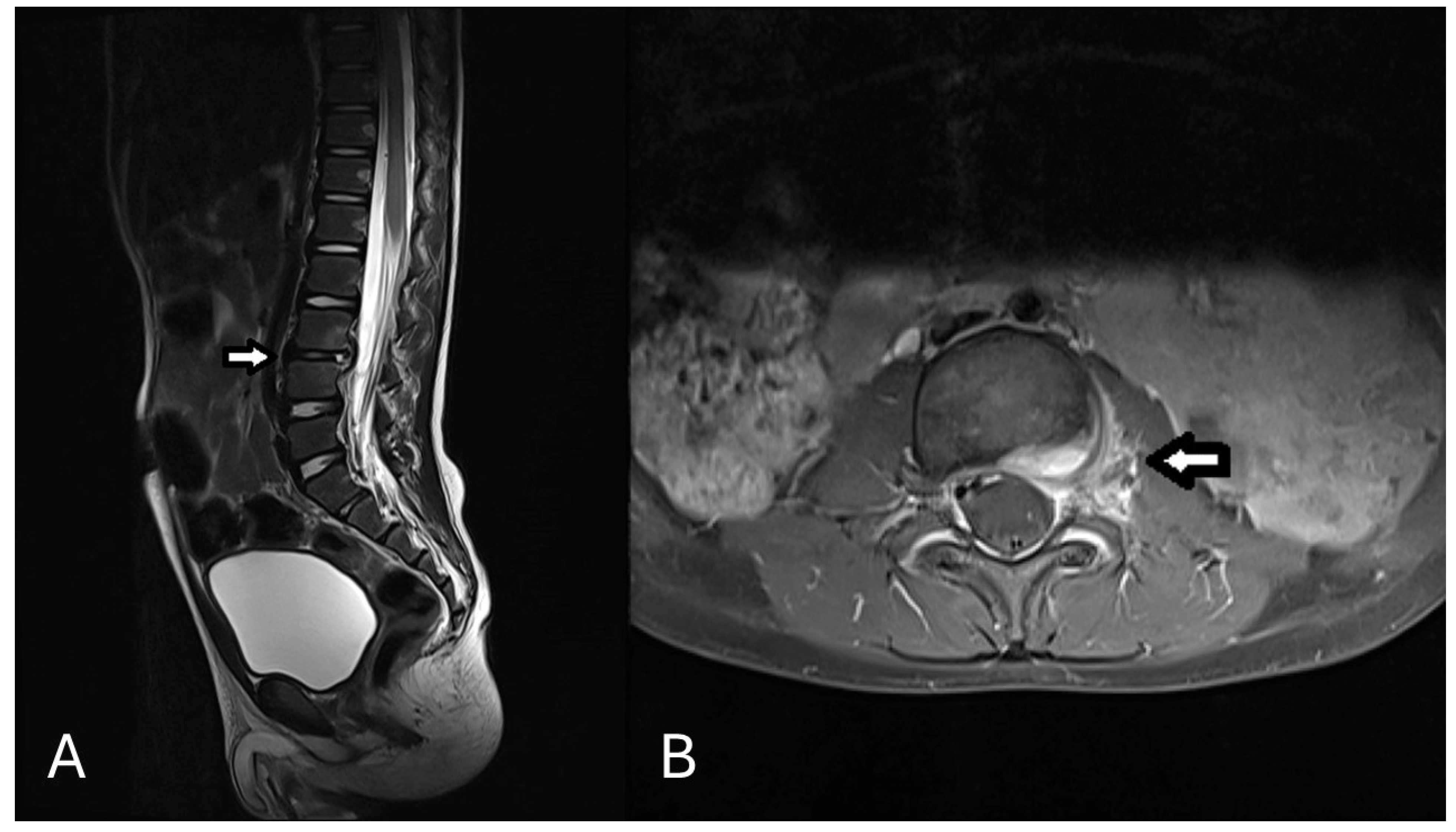
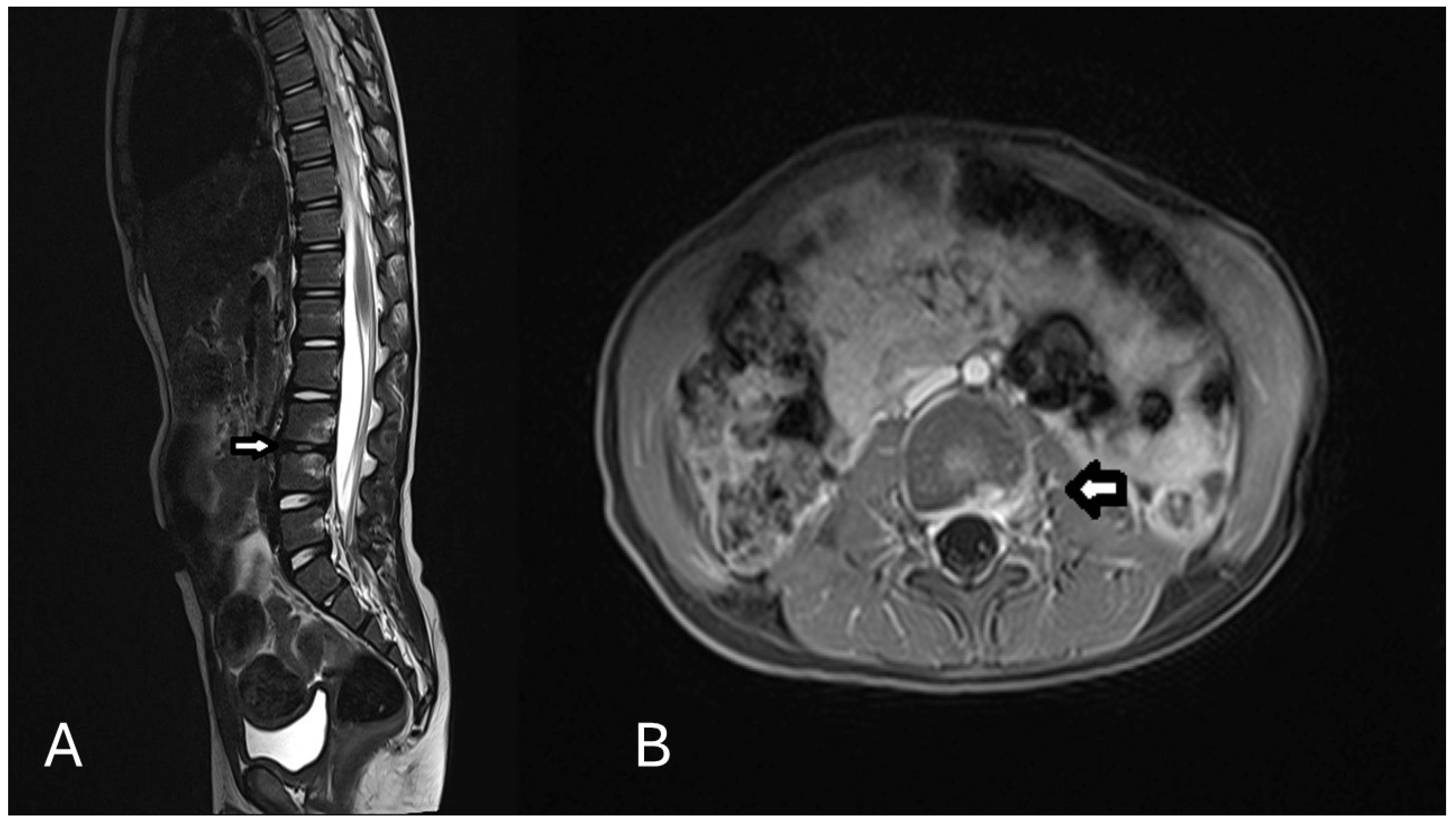
2. Case Presentation
3. Literature Review
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavalieri, S.; Pessina, B.; Indolfi, G.; Galli, L.; Trapani, S. Spondylodiscitis in Pediatric Age: A Retrospective Cohort Study. Pediatr. Infect. Dis. J. 2022, 41, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Infectious Discitis and Spondylodiscitis in Children. Int. J. Mol. Sci. 2016, 17, 539. [Google Scholar] [CrossRef] [PubMed]
- Grammatico, L.; Baron, S.; Rusch, E.; Lepage, B.; Surer, N.; Desenclos, J.C.; Besnier, J.M. Epidemiology of vertebral osteomyelitis (VO) in France: Analysis of hospital-discharge data 2002–2003. Epidemiol. Infect. 2008, 136, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65 (Suppl. S3), iii11–iii24. [Google Scholar] [CrossRef]
- Jacomo, V.; Kelly, P.J.; Raoult, D. Natural History of Bartonella Infections (an Exception to Koch’s Postulate). Clin. Diagn. Lab. Immunol. 2002, 9, 8–18. [Google Scholar] [CrossRef]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef]
- Carithers, H.A. Cat-scratch disease. An overview based on a study of 1200 patients. Am. J. Dis. Child. 1985, 139, 1124–1133. [Google Scholar] [CrossRef]
- Margileth, A.M. Cat scratch disease. Adv. Pediatr. Infect. Dis. 1993, 8, 1–21. [Google Scholar]
- Hajjaji, N.; Hocqueloux, L.; Kerdraon, R.; Bret, L. Bone infection in cat-scratch disease: A review of the literature. J. Infect. 2007, 54, 417–421. [Google Scholar] [CrossRef]
- Vermeulen, M.J.; Rutten, G.J.; Verhagen, I.; Peeters, M.F.; van Dijken, P.J. Transient paresis associated with cat-scratch disease: Case report and literature review of vertebral osteomyelitis caused by Bartonella henselae. Pediatr. Infect. Dis. J. 2006, 25, 1177–1181. [Google Scholar] [CrossRef]
- Topçu, B.; Gönüllü, H.U.; Yeşilbaş, O.; Suma, P.P.; Soysal, A. Multifocal Osteomyelitis in an Adolescent Patient with Cat Scratch Disease. Case Rep. Infect. Dis. 2024, 2024, 9562634. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Beltrami, G.; Di Maurizio, M.; Indolfi, G.; Trapani, S. Bartonella henselae vertebral osteomyelitis in a pediatric patient: A case report. Clin. Case Rep. 2022, 10, e6117. [Google Scholar] [CrossRef] [PubMed]
- Mathkour, M.; Chu, J.; Scullen, T.; Ibrahim, N.; Werner, C.; Carr, C.J.; Huang, B.; Abou-Al-Shaar, H.; Dallapiazza, R.F.; Maulucci, C.M.; et al. Atlantoaxial instability secondary to Bartonella henselae osteomyelitis managed surgically by atlantoaxial instrumentation: A case report and systematic review. J. Craniovertebral Junction Spine 2022, 13, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Erdem, G.; Watson, J.R.; Hunt, W.G.; Young, C.; Souverbielle, C.T.; Honegger, J.R.; Cassady, K.A.; Ilgenfritz, M.; Napolitano, S.; Koranyi, K. Clinical and Radiologic Manifestations of Bone Infection in Children with Cat Scratch Disease. J. Pediatr. 2018, 201, 274–280.e12. [Google Scholar] [CrossRef]
- Akbari, S.H.A.; Averill, C.E.; Roland, J.L.; Orscheln, R.; Strahle, J. Bartonella henselae infection presenting as cervical spine osteomyelitis: Case report. J. Neurosurg. Pediatr. 2018, 22, 189–194. [Google Scholar] [CrossRef]
- Kopsidas, I.; Margariti, R.; Gavra, M.; Syngelou, A.; Zambakidis, C.; Tsolia, M.; Nikolaidis, N.; Spyridis, N. An 11-Year-Old Male With Vertebral Osteomyelitis and a Paraspinal Abscess. Pediatr. Infect. Dis. J. 2018, 37, e341–e343. [Google Scholar] [CrossRef]
- Rafferty, J.R.; Janopaul-Naylor, E.; Riese, J. Torticollis and Fever in a Young Boy: A Unique Presentation of Cat-Scratch Disease with Vertebral Osteomyelitis and Epidural Phlegmon. Pediatr. Emerg. Care 2017, 33, e164–e166. [Google Scholar] [CrossRef]
- Dornbos, D.; Morin, J.; Watson, J.R.; Pindrik, J. Thoracic osteomyelitis and epidural abscess formation due to cat scratch disease: Case report. J. Neurosurg. Pediatr. 2016, 18, 713–716. [Google Scholar] [CrossRef]
- Zepeda, T.J.; Morales, S.J.; Letelier, A.H.; Delpiano, M.L. Osteomielitis vertebral por Bartonella henselae: A propósito de un caso. Rev. Chil. Pediatr. 2016, 87, 53–58. [Google Scholar] [CrossRef]
- Al-Rahawan, M.M.; Gray, B.M.; Mitchell, C.S.; Smith, S.D. Thoracic vertebral osteomyelitis with paraspinous mass and intraspinal extension: An atypical presentation of cat-scratch disease. Pediatr. Radiol. 2012, 42, 116–119. [Google Scholar] [CrossRef]
- Tasher, D.; Armarnik, E.; Mizrahi, A.; Liat, B.S.; Constantini, S.; Grisaru-Soen, G. Cat scratch disease with cervical vertebral osteomyelitis and spinal epidural abscess. Pediatr. Infect. Dis. J. 2009, 28, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rathore, M.H. Cat Scratch Disease with Epidural Extension while on Antimicrobial Treatment. Pediatr. Neurosurg. 2007, 43, 164–166. [Google Scholar] [CrossRef] [PubMed]
- de Kort, J.G.; Robben, S.G.; Schrander, J.J.; van Rhijn, L.W. Multifocal osteomyelitis in a child: A rare manifestation of cat scratch disease: A case report and systematic review of the literature. J. Pediatr. Orthop. B 2006, 15, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, N.; Abuhammour, W.; Al-Tatari, H.; Asmar, B. Disseminated Cat Scratch Disease with Vertebral Osteomyelitis and Epidural Abscess. South. Med. J. 2005, 98, 1142–1145. [Google Scholar] [CrossRef]
- Del Santo, M.; Malorgiol, C.; Not, T.; Maranzana, G.; Cerasoli, G.; Facchini, S.; Zennaro, F.; Ventura, A. Vertebral Osteomyelitis in 2 Children. Clin. Pediatr. 2002, 41, 711–713. [Google Scholar] [CrossRef]
- Pocheville, I.; Morteruel, E.; Álvarez, J.; Pérez-Irezabal, J. Osteomielitis vertebral asociada a enfermedad por arañazo de gato. Enfermedades Infecc. Microbiol. Clin. 2002, 20, 538–539. [Google Scholar] [CrossRef]
- Robson, J.M.B.; Harte, G.J.; Osborne, D.R.S.; McCormack, J.G. Cat-Scratch Disease with Paravertebral Mass and Osteomyelitis. Clin. Infect. Dis. 1999, 28, 274–278. [Google Scholar] [CrossRef][Green Version]
- Herren, C.; Jung, N.; Pishnamaz, M.; Breuninger, M.; Siewe, J.; Sobottke, R. Spondylodiscitis: Diagnosis and Treatment Options. Dtsch. Arztebl. Int. 2017, 114, 875–882. [Google Scholar] [CrossRef]
- Gök, Ş.E.; Kaptanoĝlu, E.; Çelikbaş, A.; Ergönül, Ö.; Baykam, N.; Eroĝlu, M.; Dokuzoĝuz, B. Vertebral osteomyelitis: Clinical features and diagnosis. Clin. Microbiol. Infect. 2014, 20, 1055–1060. [Google Scholar] [CrossRef]
- Bergmans, A.M.C.; Groothedde, J.-W.; Schellekens, J.F.P.; van Embden, J.D.A.; Ossewaarde, J.M.; Schouls, A.L.M. Etiology of Cat Scratch Disease: Comparison of Polymerase Chain Reaction Detection of Bartonella (Formerly Rochalimaea) and Afipia felis DNA with Serology and Skin Tests. J. Infect. Dis. 1995, 171, 916–923. [Google Scholar] [CrossRef]
- Vermeulen, M.J.; Herremans, M.; Verbakel, H.; Bergmans, A.M.C.; Roord, J.J.; Van Dijken, P.J.; Peeters, M.F. Serological testing for Bartonella henselae infections in The Netherlands: Clinical evaluation of immunofluorescence assay and ELISA. Clin. Microbiol. Infect. 2007, 13, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, A.M.; Peeters, M.F.; Schellekens, J.F.; Vos, M.C.; Sabbe, L.J.; Ossewaarde, J.M.; Verbakel, H.; Hooft, H.J.; Schouls, L.M. Pitfalls and fallacies of cat scratch disease serology: Evaluation of Bartonella henselae-based indirect fluorescence assay and enzyme-linked immunoassay. J. Clin. Microbiol. 1997, 35, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Metzkor-Cotter, E.; Kletter, Y.; Avidor, B.; Varon, M.; Golan, Y.; Ephros, M.; Giladi, M. Long-term serological analysis and clinical follow-up of patients with cat scratch disease. Clin. Infect. Dis. 2003, 37, 1149–1154. [Google Scholar] [CrossRef]
- Bayart, J.-L.; Gusbin, C.; Lardinois, B.; Scohy, A.; Kabamba-Mukadi, B. Analytical and clinical evaluation of new automated chemiluminescent immunoassays for the detection of IgG and IgM anti-Bartonella henselae antibodies. Diagn. Microbiol. Infect. Dis. 2020, 98, 115203. [Google Scholar] [CrossRef]
- Kusaba, N.; Yoshida, H.; Sumino, M.; Sata, M. Longitudinal Study of Serological Response to Bartonella henselae by Indirect Fluorescence Assay in Cat Scratch Disease. J. Jpn. Assoc. Infect. Dis. 2001, 75, 557–561. [Google Scholar] [CrossRef][Green Version]
- Carithers, H.A. Cat-scratch disease associated with an osteolytic lesion. Am. J. Dis. Child. 1983, 137, 968–970. [Google Scholar] [CrossRef]
- Fretzayas, A.; Tapratzi, P.; Kavazarakis, E.; Sinaniotis, C. Multiorgan involvement in systemic cat-scratch disease. Scand. J. Infect. Dis. 1993, 25, 145–148. [Google Scholar] [CrossRef]
- Bass, J.W.; Freitas, B.C.; Freitas, A.D.; Sisler, C.L.; Chan, D.S.; Vincent, J.M.; Person, D.A.; Claybaugh, J.R.; Wittler, R.R.; Weisse, M.E.; et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr. Infect. Dis. J. 1998, 17, 447–452. [Google Scholar] [CrossRef]
- Margileth, A.M. Antibiotic therapy for cat-scratch disease: Clinical study of therapeutic outcome in 268 patients and a review of the literature. Pediatr. Infect. Dis. J. 1992, 11, 474–478. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Mader, J.T.; Calhoun, J. Oral rifampin plus azithromycin or clarithromycin to treat osteomyelitis in rabbits. Clin. Orthop. Relat. Res. 1999, 359, 229–236. [Google Scholar] [CrossRef]
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M., 3rd; Petermann, G.W.; et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.R.; Bradley, J.S.; Chatterjee, A.; Copley, L.A.; Robinson, J.; Kronman, M.P.; Arrieta, A.; Fowler, S.L.; Harrison, C.; Carrillo-Marquez, M.A.; et al. Clinical Practice Guideline by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: 2021 Guideline on Diagnosis and Management of Acute Hematogenous Osteomyelitis in Pediatrics. J. Pediatr. Infect. Dis. Soc. 2021, 10, 801–844. [Google Scholar] [CrossRef]
- Lang, S.; Walter, N.; Neumann, C.; Bärtl, S.; Simon, M.; Ehrenschwender, M.; Hitzenbichler, F.; Alt, V.; Rupp, M. Aktuelle Praxis der empirischen Antibiotikatherapie bei Spondylodiszitis [Current practice of empiric antibiotic treatment for spondylodiscitis]. Orthopadie 2022, 51, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Landes, M.; Maor, Y.; Mercer, D.; Habot-Wilner, Z.; Bilavsky, E.; Chazan, B.; Cohen, R.; Glikman, D.; Strahilevitz, J.; Katzir, M.; et al. Cat Scratch Disease Presenting as Fever of Unknown Origin Is a Unique Clinical Syndrome. Clin. Infect. Dis. 2020, 71, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A. Ocular complications of cat scratch disease. Br. J. Ophthalmol. 2020, 104, 1640–1646. [Google Scholar] [CrossRef]
- Cucchi, D.G.; Govers, A.; Janse, F.H.; van Dalen, B.M. Acute perimyocarditis associated with Bartonella henselae infection. BMJ Case Rep. 2023, 16, e255928. [Google Scholar] [CrossRef]
- Lewis, D.W.; Tucker, S.H. Central nervous system involvement in cat scratch disease. Pediatrics 1986, 77, 714–721. [Google Scholar] [CrossRef]
- Ramírez, D.R.; del Castillo, L.O.; Guardado, M.G.; Quintero, M.Q.; García, A.M.; Pérez, C.M. [Cat scratch disease with hepatosplenic involvement]. An. Pediatr. 2004, 61, 450–451. [Google Scholar] [CrossRef]
- Verma, S.K.; Martin, A.; Montero, J.A. Atypical Cat Scratch Disease With Hepatosplenic Involvement. Clin. Gastroenterol. Hepatol. 2017, 15, e5–e6. [Google Scholar] [CrossRef][Green Version]
| Year | Age (Years) | Location of Osteomyelitis | CSD Diagnosis | Management Plan | |
|---|---|---|---|---|---|
| Topçu et al. [11] | 2024 | 15 | Lumbar vertebral |
|
|
| Abu-Rumeileh et al. [12] | 2022 | 10 | Lumbar vertebral |
|
|
| Mathkour et al. [13] | 2022 | 2 | Cervical vertebral |
|
|
| Erdem et al. [14] | 2018 * | 5 | Cervical, thoracic and sacral vertebral + femur + tibia |
|
|
| 12 | Thoracic vertebral + sacrum |
|
| ||
| 7 | Lumbar vertebral (L2) |
|
| ||
| 5 | Thoracic vertebral |
|
| ||
| 7 | Thoracic and sacral vertebral |
|
| ||
| 3 | Lumbar vertebral |
|
| ||
| 5 | Thoracic vertebral (T3) |
|
| ||
| 5 | Lumbar vertebral (L2) |
|
| ||
| 10 | Entire spine |
|
| ||
| 10 | Sacral vertebral (S3-4) + femur |
|
| ||
| Akbari et al. [15] | 2018 | 7 | Cervical vertebral (C3) paravertebral abscess (C2-4) |
|
|
| Kopsidas et al. [16] | 11 | Thoracic vertebral + paraspinal abscess |
|
| |
| Rafferty et al. [17] | 2017 | 5 | Vertebral (C7-T2) |
|
|
| Dornbos et al. [18] | 2016 | 5 | Thoracic vertebral (T8) |
|
|
| Zepeda et al. [19] | 2016 | 8 | Thoracolumbar Vertebral |
|
|
| Al-Rahawan [20] | 2012 | 7 | Thoracic vertebral (T5 -T9) |
|
|
| Tasher et al. [21] | 2009 | 5 | Cervical vertebral |
|
|
| Hussain et al. [22] | 2007 | 3 | Thoracolumbar vertebral |
|
|
| Vermeulen et al. [10] | 2006 | 9 | Cervical vertebral |
|
|
| De Kort et al. [23] | 2006 | 9 | Lumbosacral vertebral, multifocal osteomyelitis |
|
|
| Abdel-Haq et al. [24] | 2005 | 5 | Thoracic vertebral (T4-7) |
|
|
| Santo et al. [25] | 2002 | 2 | Lumbar vertebral (L4-5) |
|
|
| 2 | Lumbar vertebral (L2-3) |
|
| ||
| Pocheville et al. [26] | 2002 | 12 | Thoracic vertebral |
|
|
| Robson et al. [27] | 1999 | 9 | Thoracic vertebral (T9) + paravertebral mass |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Assaad, H.; Schumann, E.; Klemann, C.; Dietze-Jergus, N.; Heyde, C.-E.; Pieroh, P. Presumed Bartonella-Associated Spondylodiscitis in a 3-Year-Old Child: A Case Report and Review of the Literature. Children 2025, 12, 649. https://doi.org/10.3390/children12050649
El Assaad H, Schumann E, Klemann C, Dietze-Jergus N, Heyde C-E, Pieroh P. Presumed Bartonella-Associated Spondylodiscitis in a 3-Year-Old Child: A Case Report and Review of the Literature. Children. 2025; 12(5):649. https://doi.org/10.3390/children12050649
Chicago/Turabian StyleEl Assaad, Hadi, Eckehard Schumann, Christian Klemann, Nadine Dietze-Jergus, Christoph-Eckhard Heyde, and Philipp Pieroh. 2025. "Presumed Bartonella-Associated Spondylodiscitis in a 3-Year-Old Child: A Case Report and Review of the Literature" Children 12, no. 5: 649. https://doi.org/10.3390/children12050649
APA StyleEl Assaad, H., Schumann, E., Klemann, C., Dietze-Jergus, N., Heyde, C.-E., & Pieroh, P. (2025). Presumed Bartonella-Associated Spondylodiscitis in a 3-Year-Old Child: A Case Report and Review of the Literature. Children, 12(5), 649. https://doi.org/10.3390/children12050649





